Targeting Astrocytic Connexin 43 Mitigates Glutamate-Driven Motor Neuron Stress in Late-Onset Spinal Muscular Atrophy
Highlights
- Astrocytic Cx43 is markedly upregulated in late-onset SMA, in both mouse spinal cord tissue and SMN-deficient murine and human astrocytes.
- Cx43 overactivation drives excessive glutamate release and aberrant Ca2+ responses in motor neurons, thereby promoting excitotoxic stress.
- Inhibition of Cx43 hemichannels (Gap27) normalizes glutamate release from astrocytes and restores motor neuron Ca2+ homeostasis, demonstrating a direct functional contribution of Cx43 to SMA-related neurotoxicity.
- Targeting astrocytic dysfunction, specifically Cx43 hemichannels, represents a promising SMN-independent therapeutic strategy for late-onset SMA, complementing current SMN-restoring treatments.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation of the Spinal Cord
2.3. Spinal Cord Slice Preparation
2.4. Culture of Spinal Astrocytes from WT Mice
2.5. Human Tissue Samples
2.6. Generation of hiAstrocytes from Skin Fibroblasts
2.7. Induction of SMN Deficiency in Cultured Spinal Astrocytes
2.8. Immunostaining
2.9. Western Blots
2.10. Real-Time Quantitative Polymerase Chain Reactions
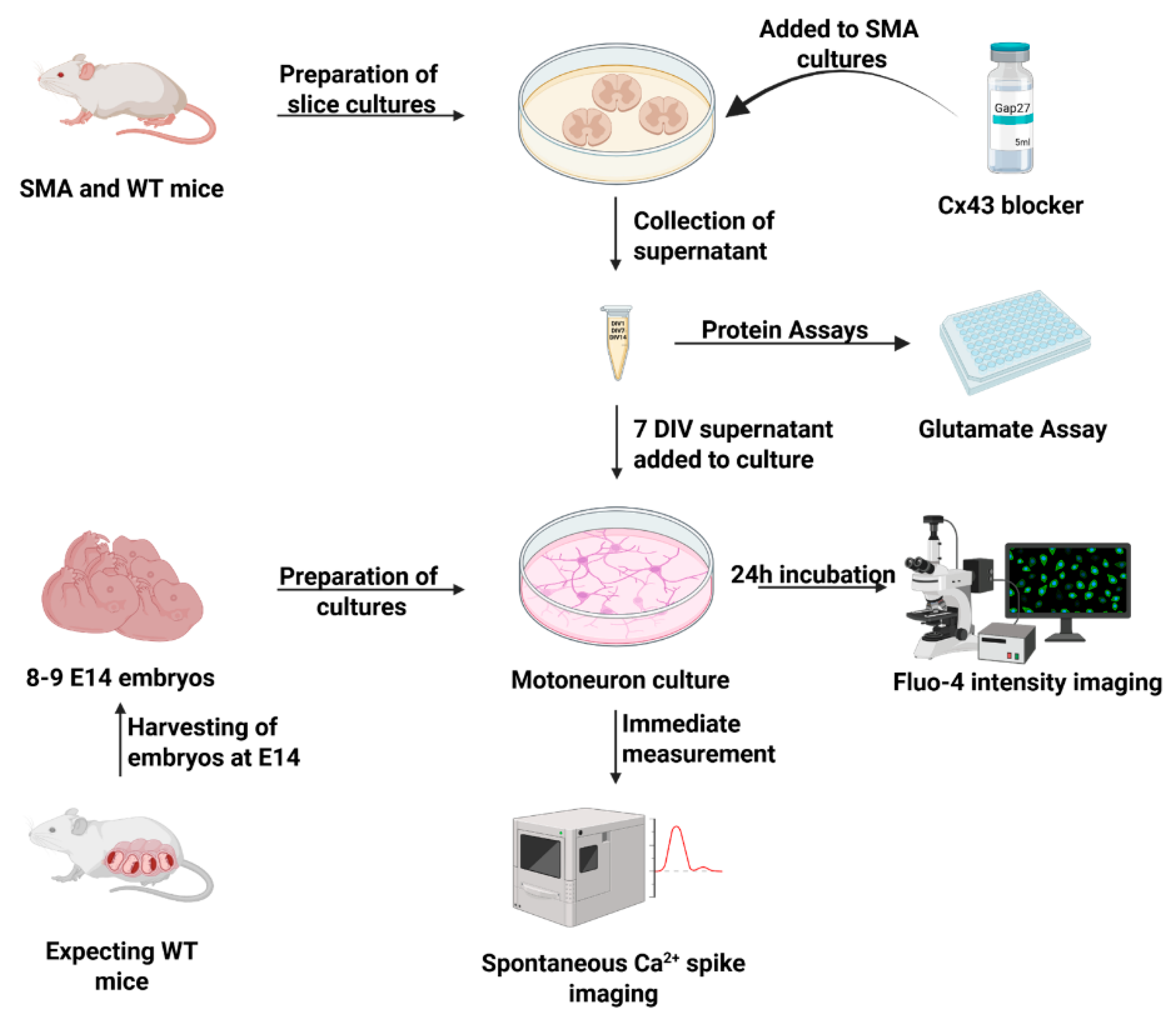
2.11. Isolation and Culture of Organotypic Spinal Cord Slice Cultures from WT and Late-Onset SMA-Mice
2.12. Isolation and Culture of Spinal Motor Neurons from Embryonic WT Mice
2.13. Calcium Imaging
2.14. Glutamate Assay
2.15. Statistical Analysis
3. Results
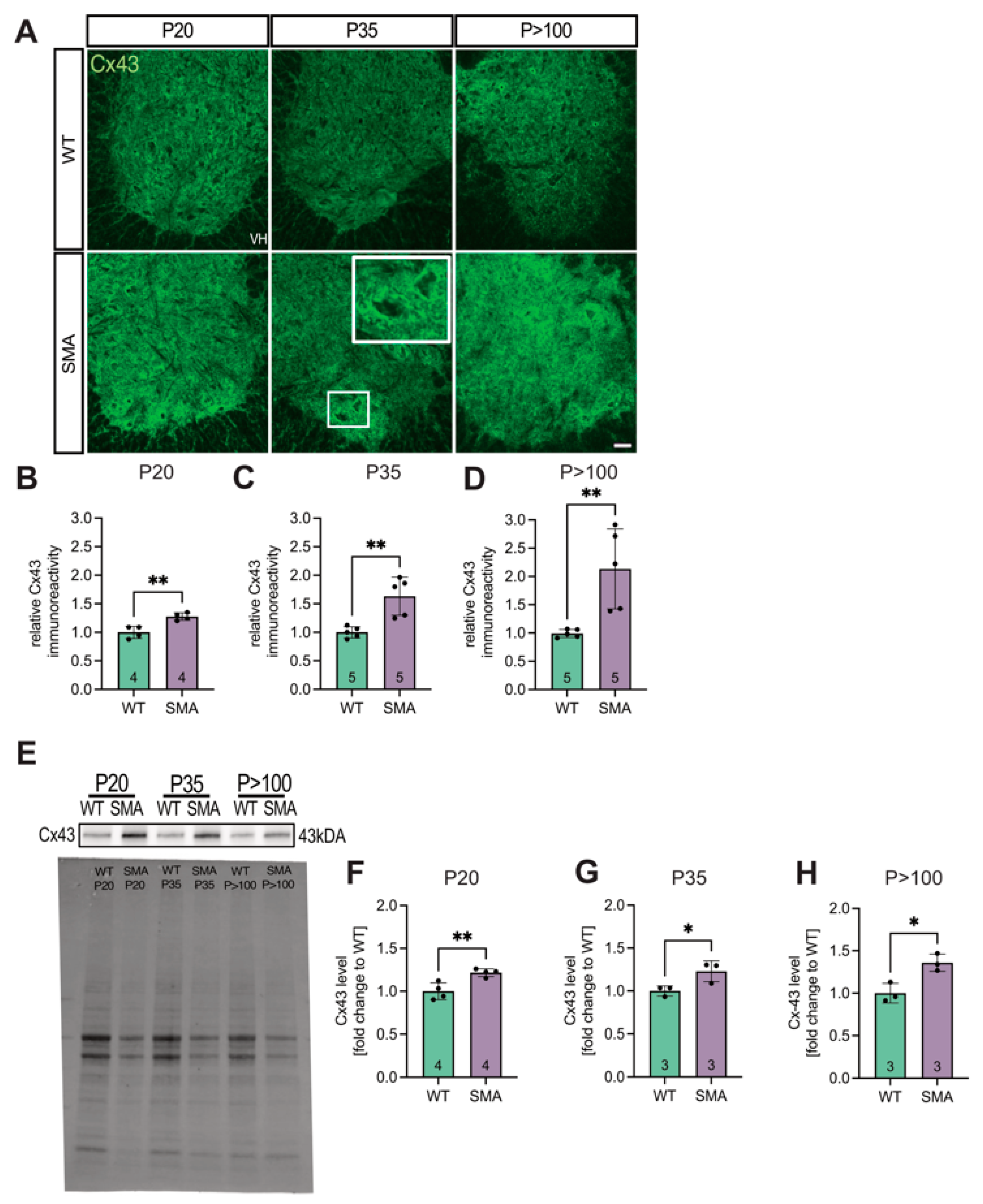
3.1. Spinal Cord Tissue Exhibited Increased Expression of Cx43 in Late-Onset SMA Mice
3.2. Cx43 mRNA Expression Is Post-Transcriptionally Modulated
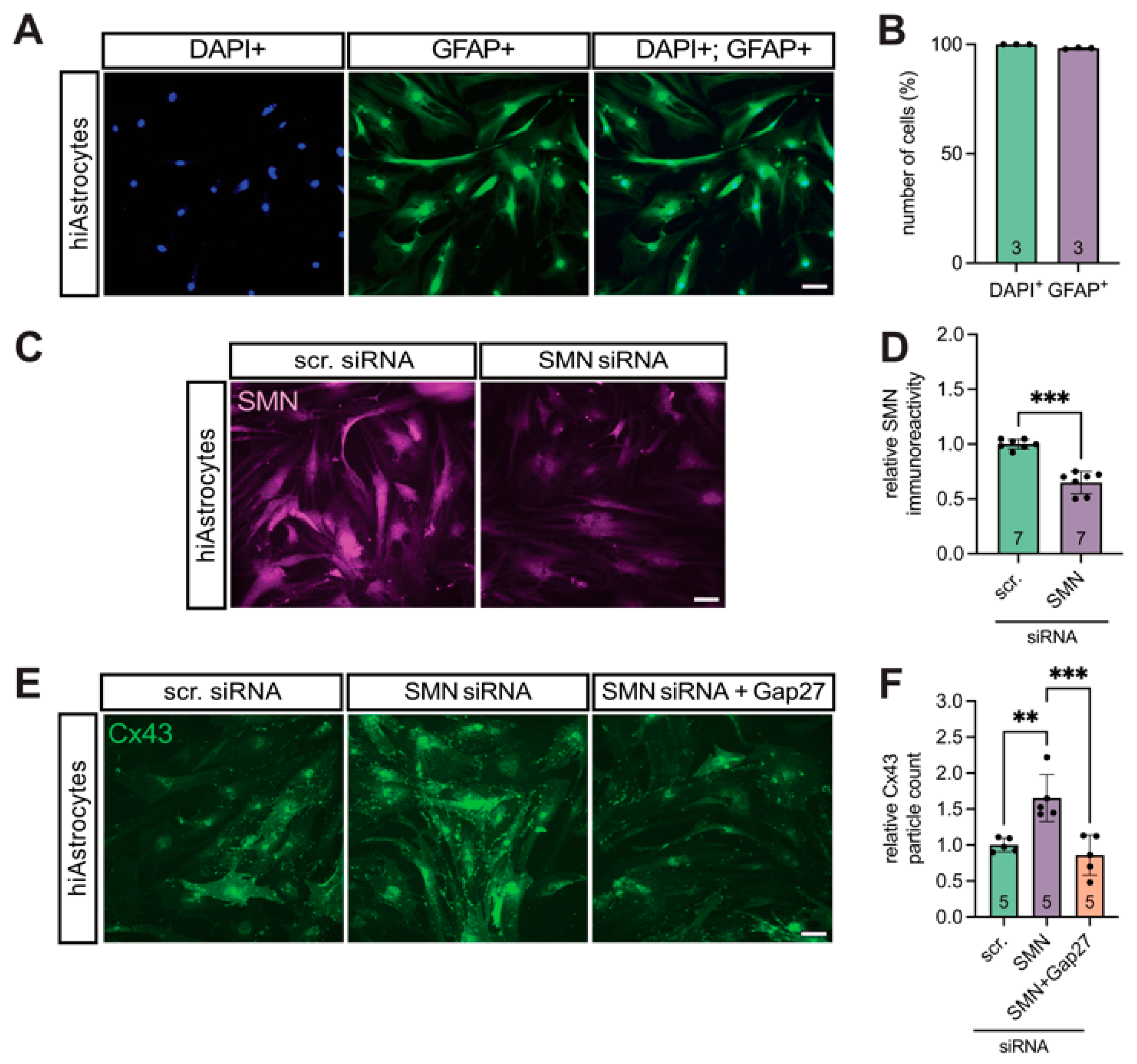
3.3. SMN Deficiency Increased Astrocytic Cx43 Expression
3.4. Translational Validation of Cx43 Dysregulation Using a Human iPSC-Based Model
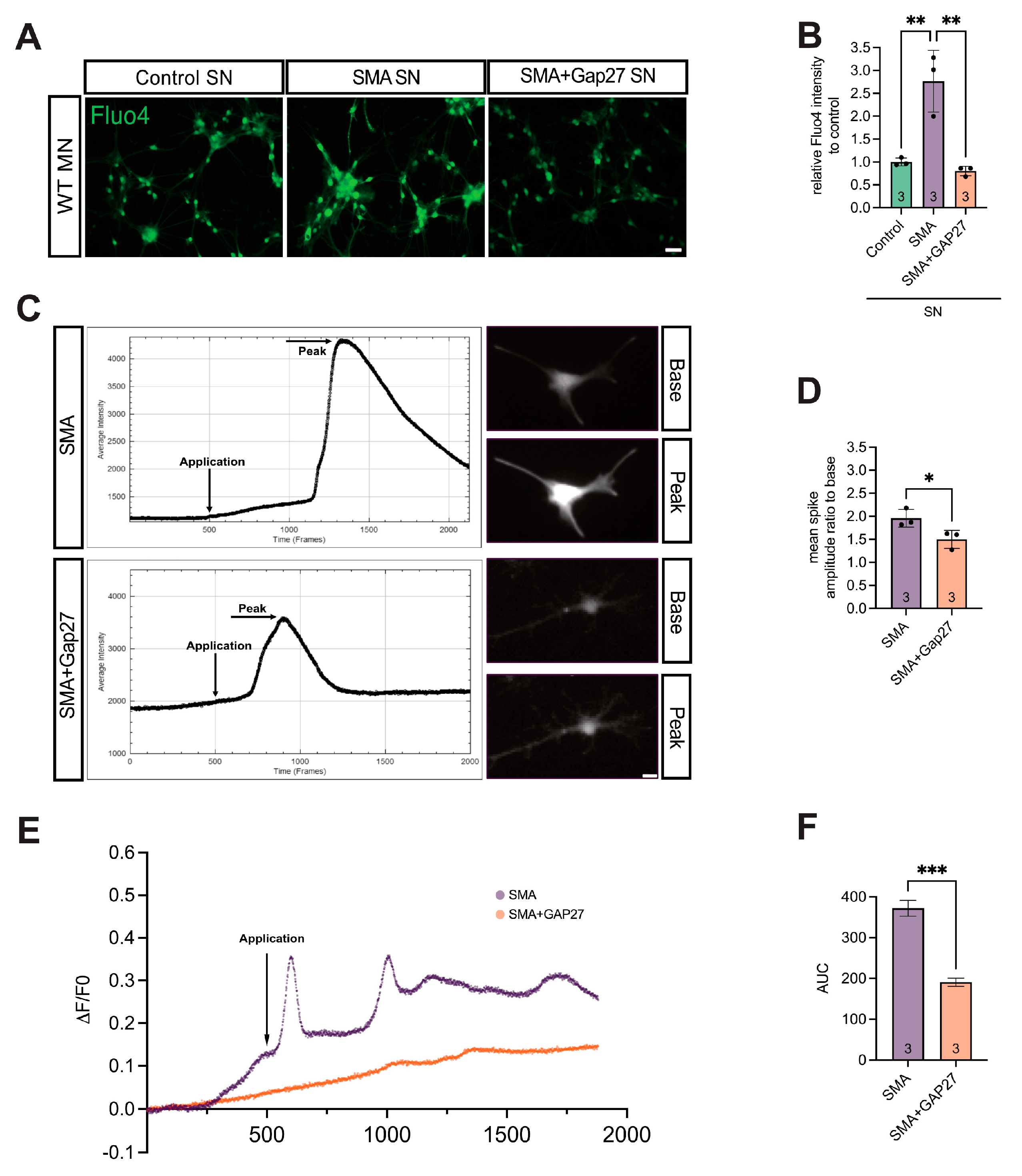
3.5. Cx43 Inhibition Reduces SMA-Associated Calcium Levels in MNs
3.6. SMA Cultures Showed Elevated Glutamate Levels, Reversed by Inhibiting Cx43
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| ATP | adenosine triphosphate |
| BCA | bicinchoninic acid protein assay |
| Ca2+ | calcium ion |
| Cx43 | connexin 43 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DIV | days in vitro |
| DMD | Duchenne muscular dystrophy |
| E | embryonal day |
| EGF | epidermal growth factor |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| FGF | fibroblast growth factor |
| iPSC | induced pluripotent stem cells |
| GFAP | glial fibrillary acidic protein |
| GJA1 | gap junction alpha-1 |
| hiAstrocytes | human induced astrocytes |
| late-onset SMA | late-onset spinal muscular atrophy |
| P | postnatal day |
| PBS | phosphate-buffered saline |
| PDL | poly-d-lysine |
| qPCR | quantitative polymerase chain reactions |
| RT | room temperature |
| scr | scrambled |
| SMA | spinal muscular atrophy |
| SMI-32 | non-phosphorylated neurofilament H |
| SMN1 | survival of motor neuron-1 |
| SMN2 | survival of motor neuron-2 |
| SMN | survival of motor neuron |
| TUJ1 | βIII-tubulin |
| WB | Western blot |
| WT | wild-type |
References
- Crawford, T.O.; Pardo, C.A. The Neurobiology of Childhood Spinal Muscular Atrophy. Neurobiol. Dis. 1996, 3, 97–110. [Google Scholar] [CrossRef]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Monani, U.R. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn-/- mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000, 9, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative Analyses of SMN1 and SMN2 Based on Real-Time LightCycler PCR: Fast and Highly Reliable Carrier Testing and Prediction of Severity of Spinal Muscular Atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, P.E.; Parsons, D.W.; Simard, L.R.; Rochette, C.; Ray, P.N.; Mendell, J.R.; Prior, T.W.; Burghes, A.H.M. Identification of Proximal Spinal Muscular Atrophy Carriers and Patients by Analysis of SMNT and SMNC Gene Copy Number. Am. J. Hum. Genet. 1997, 60, 1411–1422. [Google Scholar] [CrossRef]
- Chaytow, H.; Faller, K.M.E.; Huang, Y.-T.; Gillingwater, T.H. Spinal muscular atrophy: From approved therapies to future therapeutic targets for personalized medicine. Cell Rep. Med. 2021, 2, 100346. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Hagenacker, T.; Wurster, C.D.; Günther, R.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Weiler, M.; Ziegler, A.; Kuttler, J.; Koch, J.C.; et al. Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020, 19, 317–325. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Swoboda, K.J.; Darras, B.T.; Iannaccone, S.T.; Montes, J.; De Vivo, D.C.; Norris, D.A.; Bennett, C.F.; Bishop, K.M. Results from a phase 1 study of nusinersen (ISIS-SMN Rx) in children with spinal muscular atrophy. Neurology 2016, 86, 890–897. [Google Scholar] [CrossRef]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Mercuri, E.; Muntoni, F.; Baranello, G.; Masson, R.; Boespflug-Tanguy, O.; Bruno, C.; Corti, S.; Daron, A.; Deconinck, N.; Servais, L.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 832–841. [Google Scholar] [CrossRef]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022, 21, 42–52. [Google Scholar] [CrossRef]
- Ponath, G.; Ramanan, S.; Mubarak, M.; Housley, W.; Lee, S.; Sahinkaya, F.R.; Vortmeyer, A.; Raine, C.S.; Pitt, D. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain 2017, 140, 399–413. [Google Scholar] [CrossRef]
- Yamanaka, K.; Chun, S.J.; Boillee, S.; Fujimori-Tonou, N.; Yamashita, H.; Gutmann, D.H.; Takahashi, R.; Misawa, H.; Cleveland, D.W. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008, 11, 251–253. [Google Scholar] [CrossRef]
- McGivern, J.V.; Patitucci, T.N.; Nord, J.A.; Barabas, M.A.; Stucky, C.L.; Ebert, A.D. Spinal muscular atrophy astrocytes exhibit abnormal calcium regulation and reduced growth factor production. Glia 2013, 61, 1418–1428. [Google Scholar] [CrossRef]
- Schmitt, L.I.; David, C.; Steffen, R.; Hezel, S.; Roos, A.; Schara-Schmidt, U.; Kleinschnitz, C.; Leo, M.; Hagenacker, T. Spinal astrocyte dysfunction drives motor neuron loss in late-onset spinal muscular atrophy. Acta Neuropathol. 2023, 145, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Schmitt, L.I.; Fleischer, M.; Steffen, R.; Osswald, C.; Kleinschnitz, C.; Hagenacker, T. Induction of Survival of Motor Neuron (SMN) Protein Deficiency in Spinal Astrocytes by Small Interfering RNA as an In Vitro Model of Spinal Muscular Atrophy. Cells 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Konietzko, U.; Müller, C.M. Astrocytic dye coupling in rat hippocampus: Topography, developmental onset, and modulation by protein kinase C. Hippocampus 2004, 4, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Musil, L.S.; Goodenough, D.A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 1991, 115, 1357–1374. [Google Scholar] [CrossRef]
- Soares, A.R.; Martins-Marques, T.; Ribeiro-Rodrigues, T.; Ferreira, J.V.; Catarino, S.; Pinho, M.J.; Zuzarte, M.; Isabel Anjo, S.; Manadas, B.; Sluijter, J.P.G.; et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 2015, 5, 13243. [Google Scholar] [CrossRef]
- Wang, X.; Veruki, M.L.; Bukoreshtliev, N.V.; Hartveit, E.; Gerdes, H.-H. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. USA 2010, 107, 17194–17199. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Liu, X. From a glial syncytium to a more restricted and specific glial networking. J. Physiol.-Paris 2012, 106, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.; Chever, O.; Rollenhagen, A.; Quenech’du, N.; Ezan, P.; Lübke, J.H.R.; Rouach, N. Astroglial Connexin 43 Regulates Synaptic Vesicle Release at Hippocampal Synapses. Cells 2023, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yuan, H.; Duan, L.; Cao, R.; Gao, B.; Xiong, Y.-F.; Rao, Z.-R. Glutamate release through connexin 43 by cultured astrocytes in a stimulated hypertonicity model. Brain Res. 2011, 1392, 8–15. [Google Scholar] [CrossRef]
- Almad, A.A.; Doreswamy, A.; Gross, S.K.; Richard, J.P.; Huo, Y.; Haughey, N.; Maragakis, N.J. Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis. Glia 2016, 64, 1154–1169. [Google Scholar] [CrossRef]
- Decrock, E.; De Vuyst, E.; Vinken, M.; Van Moorhem, M.; Vranckx, K.; Wang, N.; Van Laeken, L.; De Bock, M.; D’Herde, K.; Lai, C.P.; et al. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ. 2009, 16, 151–163. [Google Scholar] [CrossRef]
- Balint, V.; Peric, M.; Dacic, S.; Stanisavljevic Ninkovic, D.; Marjanovic, J.; Popovic, J.; Stevanovic, M.; Lazic, A. The Role of SOX2 and SOX9 Transcription Factors in the Reactivation-Related Functional Properties of NT2/D1-Derived Astrocytes. Biomedicines 2024, 12, 796. [Google Scholar] [CrossRef]
- Reyes-Ortiz, A.M.; Abud, E.M.; Burns, M.S.; Wu, J.; Hernandez, S.J.; McClure, N.; Wang, K.Q.; Schulz, C.J.; Miramontes, R.; Lau, A.; et al. Single-nuclei transcriptome analysis of Huntington disease iPSC and mouse astrocytes implicates maturation and functional deficits. iScience 2023, 26, 105732. [Google Scholar] [CrossRef]
- Orellana, J.A.; Hernández, D.E.; Ezan, P.; Velarde, V.; Bennett, M.V.L.; Giaume, C.; Sáez, J.C. Hypoxia in high glucose followed by reoxygenation in normal glucose reduces the viability of cortical astrocytes through increased permeability of connexin 43 hemichannels. Glia 2009, 58, 329–343. [Google Scholar] [CrossRef]
- Wang, N.; De Bock, M.; Antoons, G.; Gadicherla, A.K.; Bol, M.; Decrock, E.; Evans, W.H.; Sipido, K.R.; Bukauskas, F.F.; Leybaert, L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res. Cardiol. 2012, 107, 304. [Google Scholar] [CrossRef]
- Laird, D.W.; Puranam, K.L.; Revel, J.P. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem. J. 1991, 273 Pt 1, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kjenseth, A.; Fykerud, T.; Rivedal, E.; Leithe, E. Regulation of gap junction intercellular communication by the ubiquitin system. Cell. Signal. 2010, 22, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O. Posttranscriptional regulation of connexin-43 expression. Arch Biochem. Biophys. 2012, 524, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.E.; Coller, J. The many routes to regulating mRNA translation. Genome Biol. 2006, 7, 332. [Google Scholar] [CrossRef]
- Pellizzoni, L.; Kataoka, N.; Charroux, B.; Dreyfuss, G. A Novel Function for SMN, the Spinal Muscular Atrophy Disease Gene Product, in Pre-mRNA Splicing. Cell 1998, 95, 615–624. [Google Scholar] [CrossRef]
- Markoullis, K.; Sargiannidou, I.; Schiza, N.; Hadjisavvas, A.; Roncaroli, F.; Reynolds, R.; Kleopa, K.A. Gap junction pathology in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol. 2012, 123, 873–886. [Google Scholar] [CrossRef]
- Mercuri, E.; Pera, M.C.; Scoto, M.; Finkel, R.; Muntoni, F. Spinal muscular atrophy—Insights and challenges in the treatment era. Nat. Rev. Neurol. 2020, 16, 706–715. [Google Scholar] [CrossRef]
- Welby, E.; Ebert, A.D. Diminished motor neuron activity driven by abnormal astrocytic EAAT1 glutamate transporter activity in spinal muscular atrophy is not fully restored after lentiviral SMN delivery. Glia 2023, 71, 1311–1332. [Google Scholar] [CrossRef]
- Abudara, V.n.; Bechberger, J.; Freitas-Andrade, M.; De Bock, M.; Wang, N.; Bultynck, G.; Naus, C.C.; Leybaert, L.; Giaume, C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front. Cell. Neurosci. 2014, 8, 306. [Google Scholar] [CrossRef]
- Grek, C.L.; Prasad, G.M.; Viswanathan, V.; Armstrong, D.G.; Gourdie, R.G.; Ghatnekar, G.S. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair Regen. 2015, 23, 203–212. [Google Scholar] [CrossRef]
- Ghatnekar, G.S.; Grek, C.L.; Armstrong, D.G.; Desai, S.C.; Gourdie, R.G. The Effect of a Connexin43-Based Peptide on the Healing of Chronic Venous Leg Ulcers: A Multicenter, Randomized Trial. J. Investig. Dermatol. 2015, 135, 289–298. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Chawdhary, B.; de Souza, A.; Meyer, E.; Kaye, A.H.; Green, C.R.; Stylli, S.S.; Danesh-Meyer, H. Tonabersat Significantly Reduces Disease Progression in an Experimental Mouse Model of Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 17454. [Google Scholar] [CrossRef]
- Wei, H.; Deng, F.; Chen, Y.; Qin, Y.; Hao, Y.; Guo, X. Ultrafine carbon black induces glutamate and ATP release by activating connexin and pannexin hemichannels in cultured astrocytes. Toxicology 2014, 323, 32–41. [Google Scholar] [CrossRef]
- Mugisho, O.O.; Green, C.R.; Kho, D.T.; Zhang, J.; Graham, E.S.; Acosta, M.L.; Rupenthal, I.D. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2018, 1862, 385–393. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmanian, S.; Schmitt, L.-I.; Liebig, K.C.; Hezel, S.; Roos, A.; Schara-Schmidt, U.; Kleinschnitz, C.; Leo, M.; Hagenacker, T. Targeting Astrocytic Connexin 43 Mitigates Glutamate-Driven Motor Neuron Stress in Late-Onset Spinal Muscular Atrophy. Cells 2025, 14, 1852. https://doi.org/10.3390/cells14231852
Salmanian S, Schmitt L-I, Liebig KC, Hezel S, Roos A, Schara-Schmidt U, Kleinschnitz C, Leo M, Hagenacker T. Targeting Astrocytic Connexin 43 Mitigates Glutamate-Driven Motor Neuron Stress in Late-Onset Spinal Muscular Atrophy. Cells. 2025; 14(23):1852. https://doi.org/10.3390/cells14231852
Chicago/Turabian StyleSalmanian, Schahin, Linda-Isabell Schmitt, Kai Christine Liebig, Stefanie Hezel, Andreas Roos, Ulrike Schara-Schmidt, Christoph Kleinschnitz, Markus Leo, and Tim Hagenacker. 2025. "Targeting Astrocytic Connexin 43 Mitigates Glutamate-Driven Motor Neuron Stress in Late-Onset Spinal Muscular Atrophy" Cells 14, no. 23: 1852. https://doi.org/10.3390/cells14231852
APA StyleSalmanian, S., Schmitt, L.-I., Liebig, K. C., Hezel, S., Roos, A., Schara-Schmidt, U., Kleinschnitz, C., Leo, M., & Hagenacker, T. (2025). Targeting Astrocytic Connexin 43 Mitigates Glutamate-Driven Motor Neuron Stress in Late-Onset Spinal Muscular Atrophy. Cells, 14(23), 1852. https://doi.org/10.3390/cells14231852






