Multimodal Imaging of the Corneal Endothelial Transition Zone Reveals Progenitor Cell Population
Highlights
- Central endothelial cell (EC) density decreases with donor age and storage time, but the transition zone (TZ) remains structurally and quantitatively stable.
- The TZ shows distinct morphology and expression of progenitor markers, indicating stem cell–like regenerative potential.
- TZ may play a role in corneal endothelial regeneration and could therefore be considered in further donor cornea assessments.
- Non-invasive imaging (HRTII/RCM) could enable better evaluation of TZ health and may improve donor cornea selection in the future.
Abstract
1. Introduction
2. Materials and Methods
2.1. Ex Vivo Human Donor Corneas
2.2. Phase Contrast Microscopy
2.3. Reflectance Confocal Microscopy with Intrinsic Contrast Using Heidelberg Retina Tomograph Equipped with the Rostock Cornea Module (HRTII/RCM)
2.4. Scanning Electron Microscopy
2.5. Whole Mount Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Human Donor Characteristics and Corneal Endothelial Cell Density Distribution
3.2. Imaging Modified Reflectance Microscopy
3.3. Influence of Storage Time on Corneal Endothelial Cell Density, Transition Zone Width, and Cell Count
3.4. Influence of Age on EC Density, TZ Width, and Cell Count
3.5. Ultrastructural Examination of the Endothelial Transition Zone
3.6. Expression Analysis of Endothelial Transition Zone
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TZ | Transition Zone |
| iTZ | Inner Transition Zone |
| oTZ | Outer Transition Zone |
| EC | Endothelial Cell |
| PE | Peripheral Endothelium |
| TM | Trabecular Meshwork |
| HRTII/RCM | Heidelberg Retina Tomograph equipped with Rostock Cornea Module |
| SEM | Scanning Electron Microscopy |
| TGF-β | Transforming Growth Factor-Beta |
| FECD | Fuchs’endothelial corneal dystrophy |
| DM | Descemet’s membrane |
| SL | Schwalbe’s line |
| RT | Room temperature |
| CTCF | Corrected total cell fluorescence |
| ECM | Extracellular matrix |
| SD | Standard deviation |
| DGFG | German Society for Tissue Transplantation |
References
- Vaiciuliene, R.; Rylskyte, N.; Baguzyte, G.; Jasinskas, V. Risk factors for fluctuations in corneal endothelial cell density (review). Exp. Ther. Med. 2022, 23, 129. [Google Scholar] [CrossRef]
- Schroeter, J.; Rieck, P. Endothelial evaluation in the cornea bank. Dev. Ophthalmol. 2009, 43, 47–62. [Google Scholar] [CrossRef]
- Bu, J.B.; Grabitz, S.D.; Schon, F.; Apel, M.; Pusch, T.; Gericke, A.; Poplawski, A.; Pfeiffer, N.; Wasielica-Poslednik, J. Utility of a Model to Predict Endothelial Cell Density of Donor Corneas to Determine Suitability for Transplantation. Transl. Vis. Sci. Technol. 2024, 13, 21. [Google Scholar] [CrossRef]
- Whikehart, D.R.; Parikh, C.H.; Vaughn, A.V.; Mishler, K.; Edelhauser, H.F. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol. Vis. 2005, 11, 816–824. [Google Scholar]
- Lee, J.S.; Lee, S.Y.; Chin, H.S.; Kim, N.R.; Jung, J.W. Microstructure of the corneal endothelial transition zone in different laboratory animals. Mol. Vis. 2024, 30, 107–113. [Google Scholar]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Joyce, N.C.; Harris, D.L.; Mello, D.M. Mechanisms of mitotic inhibition in corneal endothelium: Contact inhibition and TGF-β2. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2152–2159. [Google Scholar]
- Paull, A.C.; Whikehart, D.R. Expression of the p53 family of proteins in central and peripheral human corneal endothelial cells. Mol. Vis. 2005, 11, 328–334. [Google Scholar] [PubMed]
- Xiao, Y.; McGhee, C.N.J.; Zhang, J. Adult stem cells in the eye: Identification, characterisation, and therapeutic application in ocular regeneration—A review. Clin. Exp. Ophthalmol. 2024, 52, 148–166. [Google Scholar] [CrossRef]
- Breazzano, M.P.; Fikhman, M.; Abraham, J.L.; Barker-Griffith, A.E. Analysis of Schwalbe’s Line (Limbal Smooth Zone) by Scanning Electron Microscopy and Optical Coherence Tomography in Human Eye Bank Eyes. J. Ophthalmic Vis. Res. 2013, 8, 9–16. [Google Scholar]
- Katikireddy, K.R.; Schmedt, T.; Price, M.O.; Price, F.W.; Jurkunas, U.V. Existence of Neural Crest-Derived Progenitor Cells in Normal and Fuchs Endothelial Dystrophy Corneal Endothelium. Am. J. Pathol. 2016, 186, 2736–2750. [Google Scholar] [CrossRef]
- Tripathi, B.J.; Tripathi, R.C. Neural crest origin of human trabecular meshwork and its implications for the pathogenesis of glaucoma. Am. J. Ophthalmol. 1989, 107, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Ham, L.; Verschoor, C.A.; Ong, T.S.; van der Wees, J.; Melles, G.R. Spontaneous corneal clearance despite graft detachment in descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2009, 148, 227–234.e1. [Google Scholar] [CrossRef]
- Arbelaez, J.G.; Price, M.O.; Price, F.W., Jr. Long-term follow-up and complications of stripping descemet membrane without placement of graft in eyes with Fuchs endothelial dystrophy. Cornea 2014, 33, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.A.; Grierson, I. Morphological effects of argon laser trabeculoplasty upon the glaucomatous human meshwork. Eye 1989, 3 Pt 6, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Seah, X.; Yusoff, N.; Setiawan, M.; Wahlig, S.; Htoon, H.M.; Peh, G.S.L.; Kocaba, V.; Mehta, J.S. Characterization of Human Transition Zone Reveals a Putative Progenitor-Enriched Niche of Corneal Endothelium. Cells 2019, 8, 1244. [Google Scholar] [CrossRef]
- Braunger, B.M.; Ademoglu, B.; Koschade, S.E.; Fuchshofer, R.; Gabelt, B.T.; Kiland, J.A.; Hennes-Beann, E.A.; Brunner, K.G.; Kaufman, P.L.; Tamm, E.R. Identification of adult stem cells in Schwalbe’s line region of the primate eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7499–7507. [Google Scholar] [CrossRef]
- Du, Y.; Roh, D.S.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L.; Schuman, J.S. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1566–1575. [Google Scholar] [CrossRef]
- Stachs, O.; Guthoff, R.F.; Aumann, S. In Vivo Confocal Scanning Laser Microscopy. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 263–284. [Google Scholar]
- Eckard, A.; Stave, J.; Guthoff, R.F. In vivo investigations of the corneal epithelium with the confocal Rostock Laser Scanning Microscope (RLSM). Cornea 2006, 25, 127–131. [Google Scholar] [CrossRef]
- Stachs, O.; Zhivov, A.; Kraak, R.; Stave, J.; Guthoff, R. In vivo three-dimensional confocal laser scanning microscopy of the epithelial nerve structure in the human cornea. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 569–575. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Islam, Q.U.; Saeed, M.K.; Mehboob, M.A. Age related changes in corneal morphological characteristics of healthy Pakistani eyes. Saudi J. Ophthalmol. 2017, 31, 86–90. [Google Scholar] [CrossRef]
- Wahlig, S.; Yam, G.H.; Chong, W.; Seah, X.Y.; Kocaba, V.; Ang, M.; Htoon, H.M.; Tun, T.A.; Ong, H.S.; Mehta, J.S. Quantification of the Posterior Cornea Using Swept Source Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Goh, Y.W.; Patel, D.V.; Riley, A.F.; McGhee, C.N. Corneal microstructural changes in nerve fiber, endothelial and epithelial density after cataract surgery in patients with diabetes mellitus. Cornea 2015, 34, 177–181. [Google Scholar] [CrossRef]
- Kohler, B.; Allgeier, S.; Bartschat, A.; Guthoff, R.F.; Bohn, S.; Reichert, K.M.; Stachs, O.; Winter, K.; Mikut, R. In vivo imaging of the corneal nerve plexus: From single image to large scale map. Ophthalmologe 2017, 114, 601–607. [Google Scholar] [CrossRef]
- McCarey, B.E.; Edelhauser, H.F.; Lynn, M.J. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Panda-Jonas, S.; Mehta, J.S.; Jonas, R.A. Anatomic Relationship Among Descemet’s Membrane, Trabecular Meshwork, Scleral Spur, and Ciliary Muscle. Investig. Ophthalmol. Vis. Sci. 2025, 66, 8. [Google Scholar] [CrossRef] [PubMed]
- Hirata-Tominaga, K.; Nakamura, T.; Okumura, N.; Kawasaki, S.; Kay, E.P.; Barrandon, Y.; Koizumi, N.; Kinoshita, S. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells 2013, 31, 1396–1407. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J. Stem Cells 2014, 6, 305–311. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, J.; Bostrom, K.I. SOX Transcription Factors in Endothelial Differentiation and Endothelial-Mesenchymal Transitions. Front. Cardiovasc. Med. 2019, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Li, F.; Han, B.; Tighe, S.; Zhang, S.; Chen, S.Y.; Liu, X.; Tseng, S.C. Activation of RhoA-ROCK-BMP signaling reprograms adult human corneal endothelial cells. J. Cell Biol. 2014, 206, 799–811. [Google Scholar] [CrossRef]
- McGowan, S.L.; Edelhauser, H.F.; Pfister, R.R.; Whikehart, D.R. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 2007, 13, 1984–2000. [Google Scholar]
- Yun, H.; Zhou, Y.; Wills, A.; Du, Y. Stem Cells in the Trabecular Meshwork for Regulating Intraocular Pressure. J. Ocul. Pharmacol. Ther. 2016, 32, 253–260. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ross, D.D.; Nakanishi, T.; Bailey-Dell, K.; Zhou, S.; Mercer, K.E.; Sarkadi, B.; Sorrentino, B.P.; Schuetz, J.D. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004, 279, 24218–24225. [Google Scholar] [CrossRef]
- Zhou, S.; Morris, J.J.; Barnes, Y.; Lan, L.; Schuetz, J.D.; Sorrentino, B.P. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 12339–12344. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Shimmura, S.; Miyashita, H.; Kawashima, M.; Kawakita, T.; Tsubota, K. The anti-oxidative role of ABCG2 in corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5617–5622. [Google Scholar] [CrossRef]
- Tong, Z.; Yin, Z. Distribution, contribution and regulation of nestin+ cells. J. Adv. Res. 2024, 61, 47–63. [Google Scholar] [CrossRef]
- Sivagurunathan, S.; Vahabikashi, A.; Yang, H.; Zhang, J.; Vazquez, K.; Rajasundaram, D.; Politanska, Y.; Abdala-Valencia, H.; Notbohm, J.; Guo, M.; et al. Expression of vimentin alters cell mechanics, cell-cell adhesion, and gene expression profiles suggesting the induction of a hybrid EMT in human mammary epithelial cells. Front. Cell Dev. Biol. 2022, 10, 929495. [Google Scholar] [CrossRef]
- Park, D.; Xiang, A.P.; Mao, F.F.; Zhang, L.; Di, C.G.; Liu, X.M.; Shao, Y.; Ma, B.F.; Lee, J.H.; Ha, K.S.; et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells 2010, 28, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, C.W.; Harkey, M.A.; Torok-Storb, B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 2002, 99, 507–512. [Google Scholar] [CrossRef]
- Eckes, B.; Colucci-Guyon, E.; Smola, H.; Nodder, S.; Babinet, C.; Krieg, T.; Martin, P. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 2000, 113 Pt 13, 2455–2462. [Google Scholar] [CrossRef]
- Zhang, J.; Ahmad, A.M.; Ng, H.; Shi, J.; McGhee, C.N.J.; Patel, D.V. Successful culture of human transition zone cells. Clin. Exp. Ophthalmol. 2020, 48, 689–700. [Google Scholar] [CrossRef]
- Yam, G.H.; Pi, S.; Du, Y.; Mehta, J.S. Posterior corneoscleral limbus: Architecture, stem cells, and clinical implications. Prog. Retin. Eye Res. 2023, 96, 101192. [Google Scholar] [CrossRef]
- Roy, O.; Leclerc, V.B.; Bourget, J.M.; Theriault, M.; Proulx, S. Understanding the process of corneal endothelial morphological change in vitro. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Schimmelpfennig, B.H. Direct and indirect determination of nonuniform cell density distribution in human corneal endothelium. Investig. Ophthalmol. Vis. Sci. 1984, 25, 223–229. [Google Scholar]
- Daus, W.; Volcker, H.E.; Meysen, H.; Bundschuh, W. Vital staining of the corneal endothelium--increased possibilities of diagnosis. Fortschr. Ophthalmol. 1989, 86, 259–264. [Google Scholar] [PubMed]
- Trivedi, R.H.; Nutaitis, M.; Vroman, D.; Crosson, C.E. Influence of race and age on aqueous humor levels of transforming growth factor-beta 2 in glaucomatous and nonglaucomatous eyes. J. Ocul. Pharmacol. Ther. 2011, 27, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Acott, T.S.; Samples, J.R.; Bradley, J.M.; Bacon, D.R.; Bylsma, S.S.; Van Buskirk, E.M. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am. J. Ophthalmol. 1989, 107, 1–6. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Kay, E.P.; Ueno, M.; Sakamoto, Y.; Nakamura, S.; Hamuro, J.; Kinoshita, S. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2493–2502. [Google Scholar] [CrossRef]
- Okumura, N.; Kay, E.P.; Nakahara, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Inhibition of TGF-β signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS ONE 2013, 8, e58000. [Google Scholar] [CrossRef] [PubMed]
- Eveleth, D.; Pizzuto, S.; Weant, J.; Jenkins-Eveleth, J.; Bradshaw, R.A. Proliferation of Human Corneal Endothelia in Organ Culture Stimulated by Wounding and the Engineered Human Fibroblast Growth Factor 1 Derivative TTHX1114. J. Ocul. Pharmacol. Ther. 2020, 36, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Curtis, E.; Ramarapu, R.; Marshall, M.; Yoo, H.; Park, S.; Leonard, B.; Thomasy, S.M. Unlocking the therapeutic potential of the corneal endothelium: Intravital imaging reveals endogenous regenerative capabilities. Investig. Ophthalmol. Vis. Sci. 2025, 66, 2947. [Google Scholar]

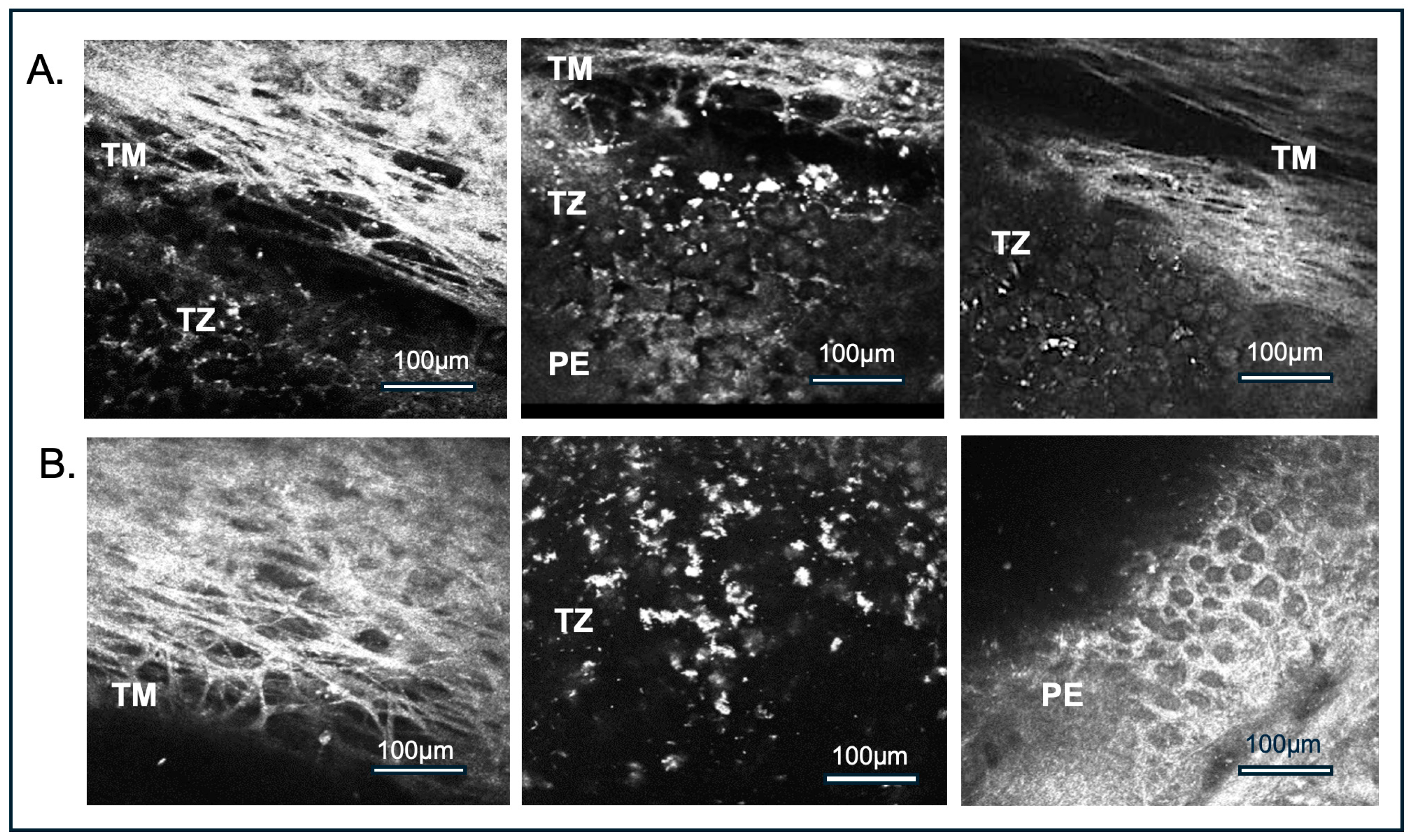
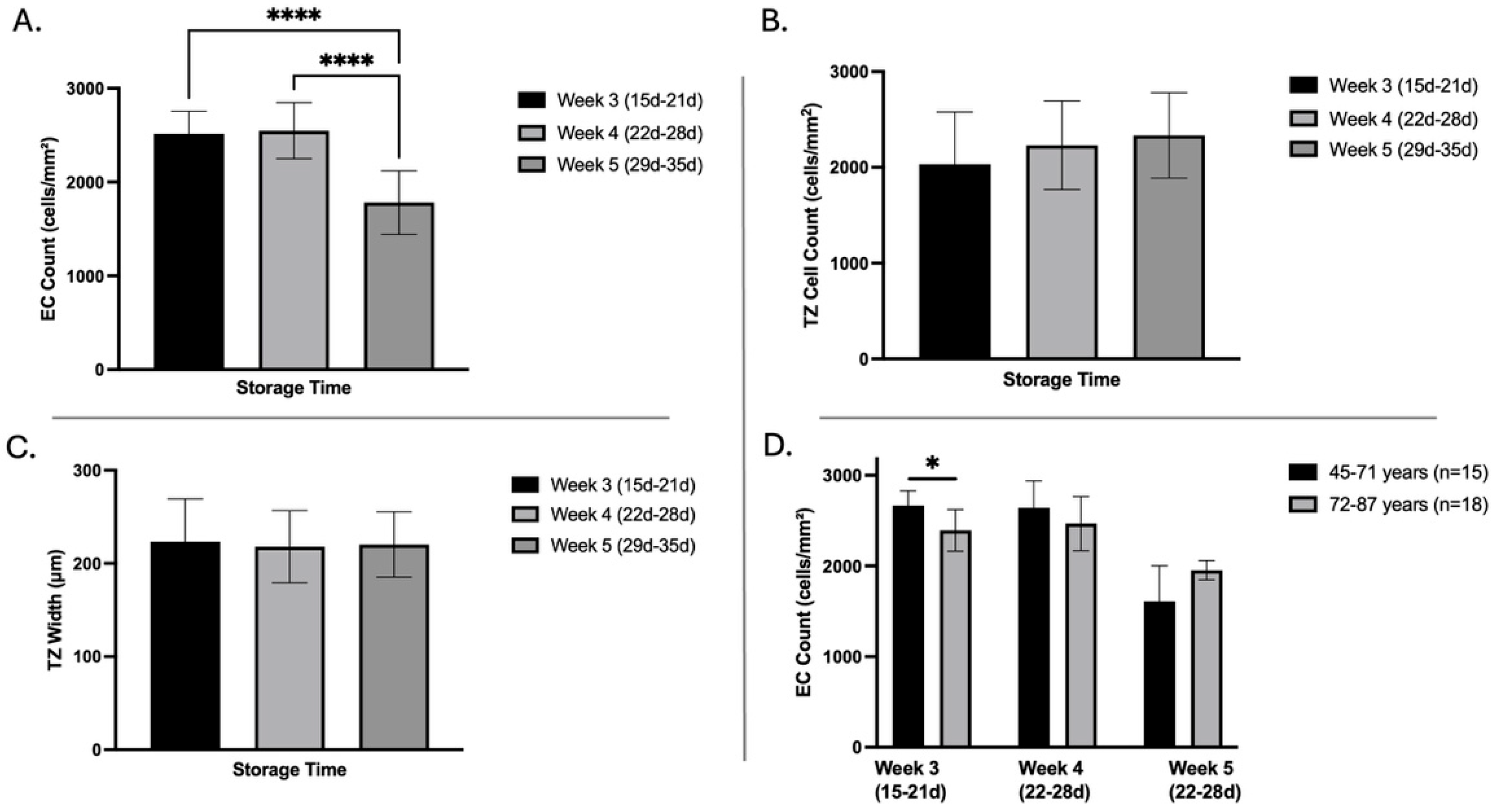

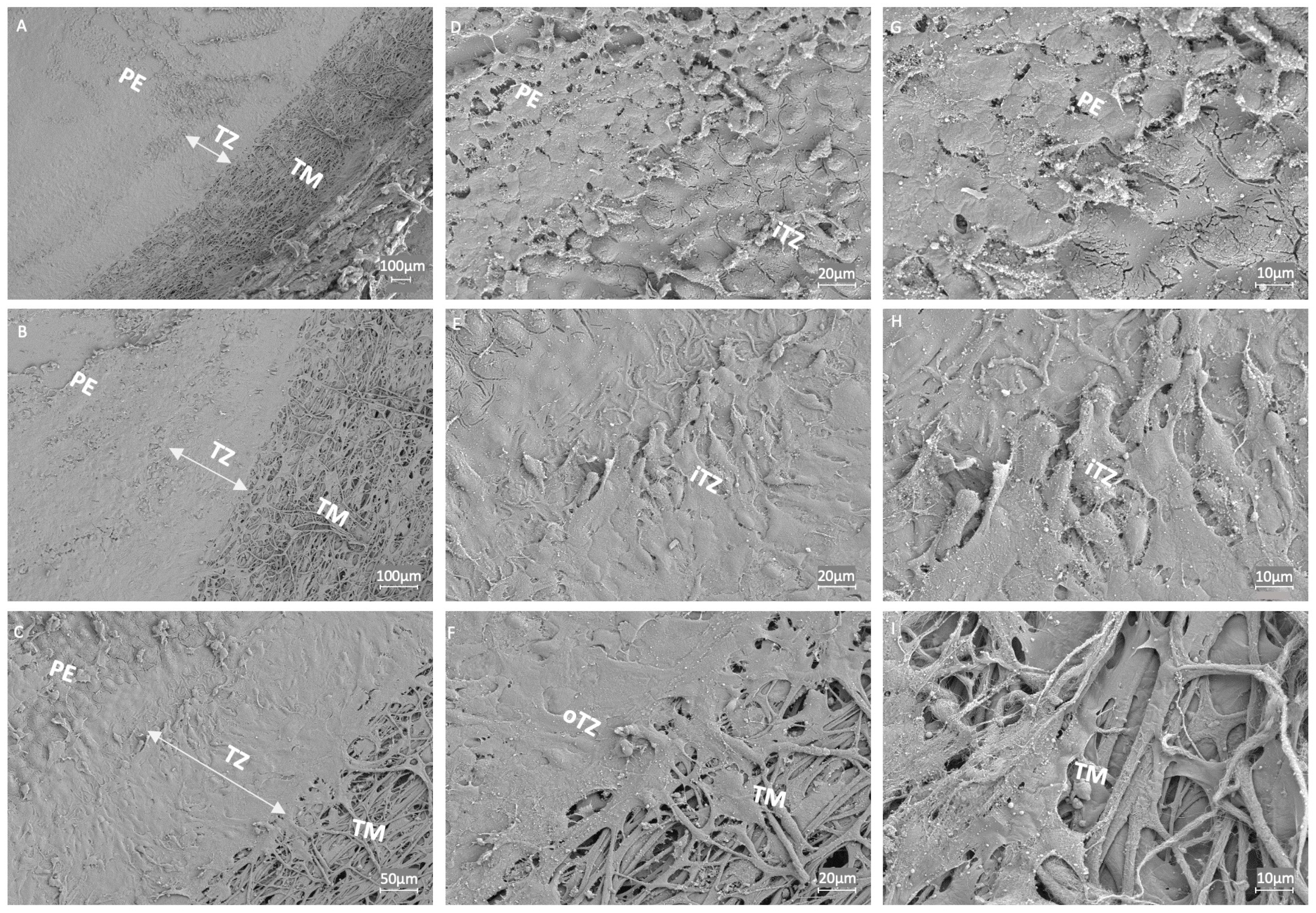
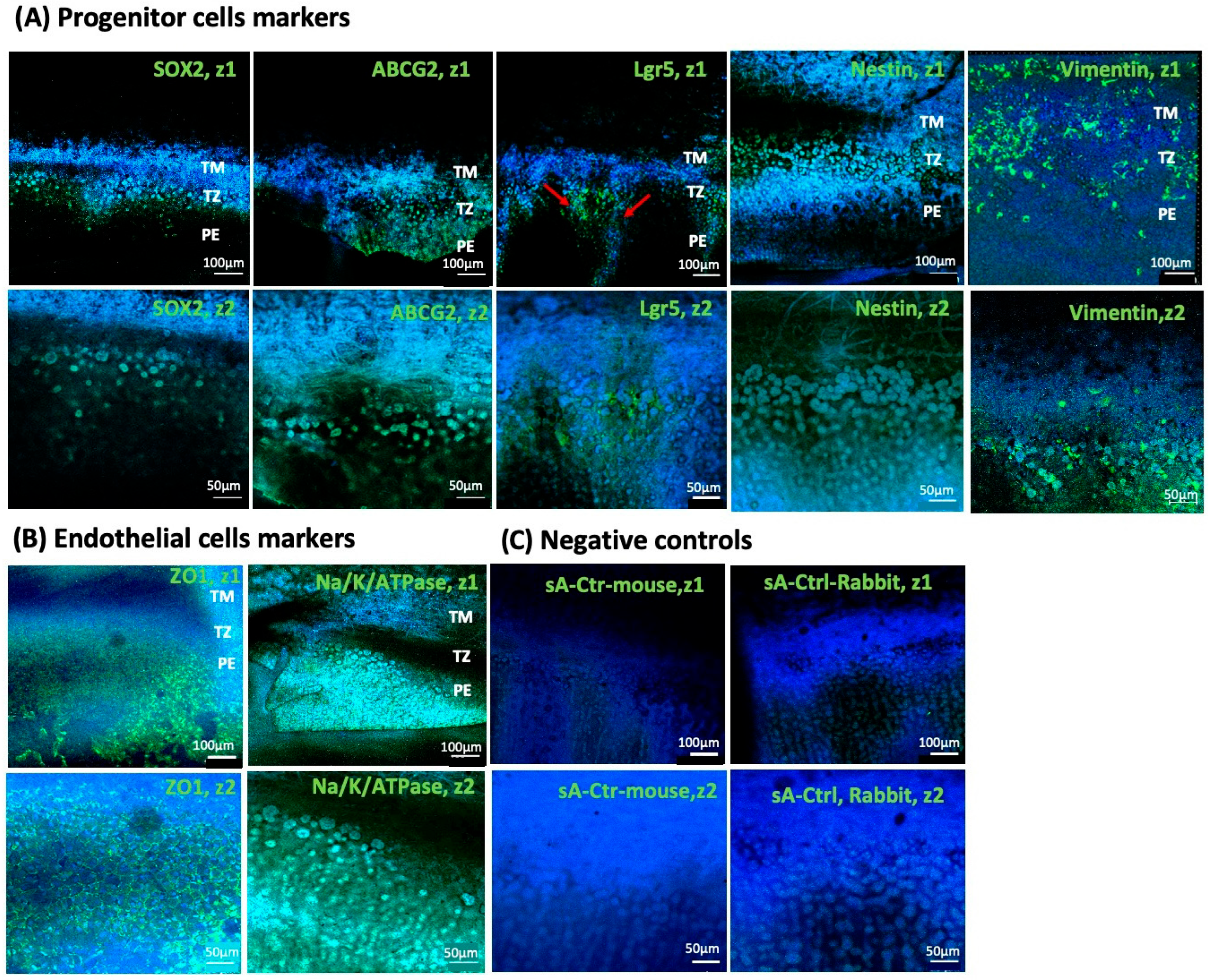
| Donor Corneas (n = 41) | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Age (years) | 45 | 87 | 71.56 ± 10.90 |
| Storage time (days) | 15 | 35 | 23.39 ± 5.72 |
| EC count (per mm2) | 1110 | 2980 | 2382.80 ± 405.64 |
| TZ cell count (per mm2) | 925 | 2975 | 2107.81 ± 505.03 |
| TZ width (µm) | 142.25 | 299.00 | 221.11 ± 40.93 |
| Markers | No. of Sample | Expression | ||
|---|---|---|---|---|
| TM | TZ | PE | ||
| ZO1 | 4 | − | − | +++ |
| Na/K-ATPase | 4 | + | − | +++ |
| Lgr5 | 4 | − | ++ | ++ |
| ABCG2 | 3 | + | +++ | ++ |
| SOX2 | 3 | − | +++ | − |
| Nestin | 4 | ++ | +++ | + |
| Vimentin | 4 | ++ | +++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathi, S.; Hülse, P.; Staehlke, S.; Walckling, M.; Anwar, M.; Trosan, P.; Bohn, S.; Stachs, O.; Peh, G.S.L.; Yam, G.H.-F.; et al. Multimodal Imaging of the Corneal Endothelial Transition Zone Reveals Progenitor Cell Population. Cells 2025, 14, 1851. https://doi.org/10.3390/cells14231851
Rathi S, Hülse P, Staehlke S, Walckling M, Anwar M, Trosan P, Bohn S, Stachs O, Peh GSL, Yam GH-F, et al. Multimodal Imaging of the Corneal Endothelial Transition Zone Reveals Progenitor Cell Population. Cells. 2025; 14(23):1851. https://doi.org/10.3390/cells14231851
Chicago/Turabian StyleRathi, Sonika, Patricia Hülse, Susanne Staehlke, Marcus Walckling, Mahmoud Anwar, Peter Trosan, Sebastian Bohn, Oliver Stachs, Gary S. L. Peh, Gary Hin-Fai Yam, and et al. 2025. "Multimodal Imaging of the Corneal Endothelial Transition Zone Reveals Progenitor Cell Population" Cells 14, no. 23: 1851. https://doi.org/10.3390/cells14231851
APA StyleRathi, S., Hülse, P., Staehlke, S., Walckling, M., Anwar, M., Trosan, P., Bohn, S., Stachs, O., Peh, G. S. L., Yam, G. H.-F., Mehta, J. S., Hofmann, N., Börgel, M., & Fuchsluger, T. A. (2025). Multimodal Imaging of the Corneal Endothelial Transition Zone Reveals Progenitor Cell Population. Cells, 14(23), 1851. https://doi.org/10.3390/cells14231851











