Advancements in Cellular Therapeutics in Corneal Diseases
Abstract
1. Introduction
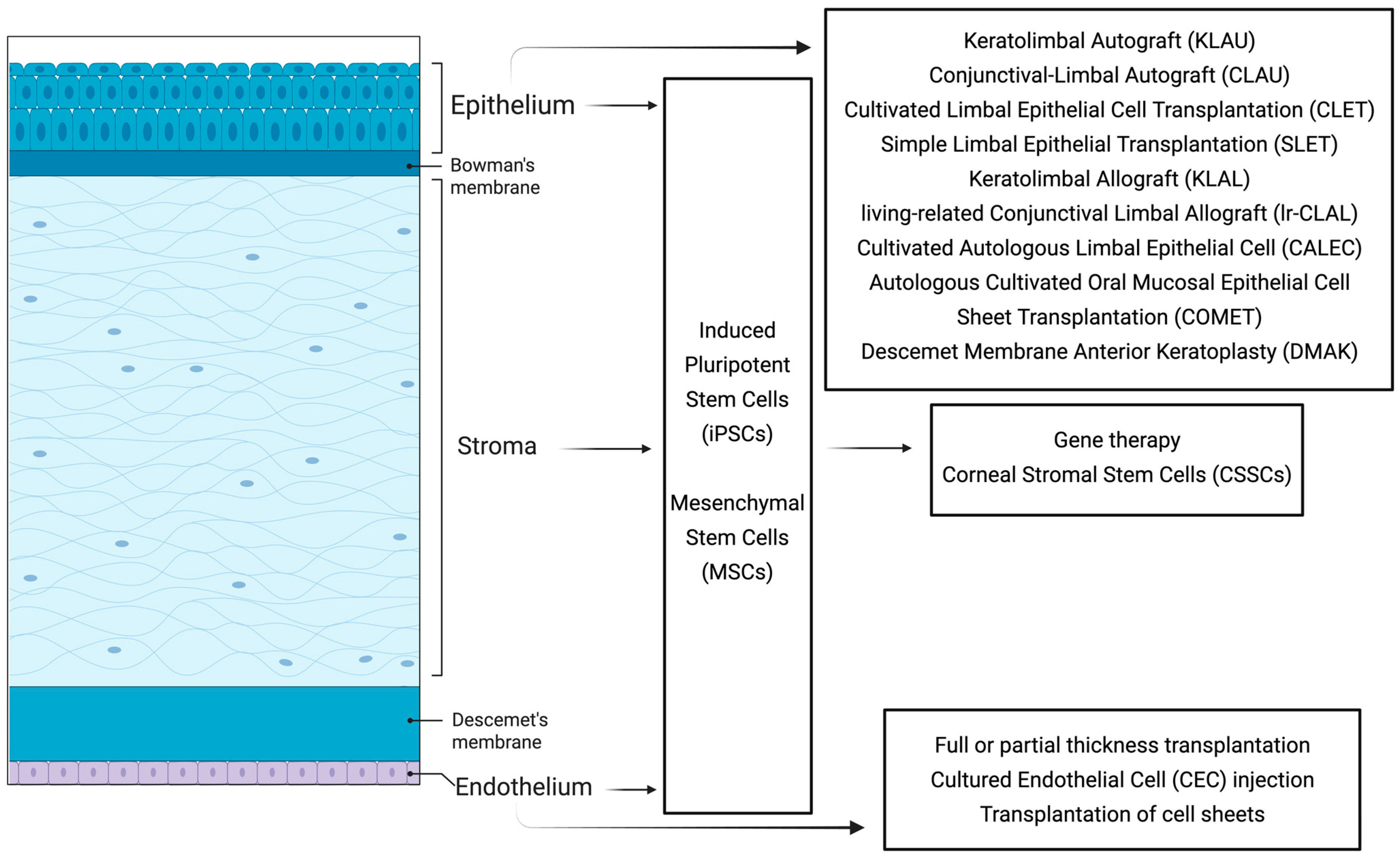
2. Corneal Anatomy
2.1. The Corneal Epithelium and the Bowman’s Membrane
2.2. The Corneal Stroma
2.3. Descemet’s Membrane and Corneal Endothelial Cell Layer
3. Corneal Epithelial Disorders
3.1. Conjunctival–Limbal Autograft (CLAU) and Keratolimbal Autograft (KLAU)
3.2. Ex Vivo-Cultivated Limbal Epithelial Cell Transplantation (CLET)
3.3. Simple Limbal Epithelial Transplantation (SLET)
3.4. Keratolimbal Allograft (KLAL) and Living-Related Conjunctival Limbal Allograft (lr-CLAL)
3.5. Cultivated Autologous Limbal Epithelial Cell (CALEC)
3.6. Autologous Cultivated Oral Mucosal Epithelial Cell Sheet Transplantation (COMET)
3.7. Induced Pluripotent Stem Cells (iPSCs)
3.8. Mesenchymal Stem Cells (MSCs)
3.9. Descemet Membrane Anterior Keratoplasty (DMAK)
4. Corneal Stromal Disorders
4.1. Corneal Stromal Stem Cells (CSSCs)
4.2. Gene Therapy
5. Corneal Endothelial Disorders
5.1. Full Thickness or Partial Corneal Transplantation
5.2. Cultured Endothelial Cell (CEC) Injection Therapy
5.3. Transplantation of Cell Sheets
5.4. Induced Pluripotent Stem Cell Therapy
6. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Report on Vision. Available online: https://www.who.int/publications/i/item/9789241516570 (accessed on 14 August 2025).
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Wiley, L.; SundarRaj, N.; Sun, T.T.; Thoft, R.A. Regional heterogeneity in human corneal and limbal epithelia: An immunohistochemical evaluation. Investig. Ophthalmol. Vis. Sci. 1991, 32, 594–602. [Google Scholar]
- Hanna, C.; Bicknell, D.S.; O’Brien, J.E. Cell turnover in the adult human eye. Arch. Ophthalmol. 1961, 65, 695–698. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Tuft, S.J.; Coster, D.J. The corneal endothelium. Eye 1990, 4 Pt 3, 389–424. [Google Scholar] [CrossRef]
- Zavala, J.; Lopez Jaime, G.R.; Rodriguez Barrientos, C.A.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef]
- Basu, S.; Sureka, S.P.; Shanbhag, S.S.; Kethiri, A.R.; Singh, V.; Sangwan, V.S. Simple Limbal Epithelial Transplantation: Long-Term Clinical Outcomes in 125 Cases of Unilateral Chronic Ocular Surface Burns. Ophthalmology 2016, 123, 1000–1010. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Holland, E.J. Keratolimbal allograft. Curr. Opin. Ophthalmol. 2017, 28, 377–381. [Google Scholar] [CrossRef]
- Chen, K.; Soleimani, M.; Koganti, R.; Cheraqpour, K.; Habeel, S.; Djalilian, A.R. Cell-based therapies for limbal stem cell deficiency: A literature review. Ann. Eye Sci. 2023, 8, 6. [Google Scholar] [CrossRef]
- Daya, S.M. Conjunctival-limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Jurkunas, U.; Johns, L.; Armant, M. Cultivated Autologous Limbal Epithelial Cell Transplantation: New Frontier in the Treatment of Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2022, 239, 244–268. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.B.; Waszczykowska, A. Gene therapy strategies in ophthalmology-an overview of current developments and future prospects. J. Appl. Genet. 2025. [Google Scholar] [CrossRef]
- Nuzzi, A.; Pozzo Giuffrida, F.; Luccarelli, S.; Nucci, P. Corneal Epithelial Regeneration: Old and New Perspectives. Int. J. Mol. Sci. 2022, 23, 13114. [Google Scholar] [CrossRef]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Di Girolamo, N. Moving epithelia: Tracking the fate of mammalian limbal epithelial stem cells. Prog. Retin. Eye Res. 2015, 48, 203–225. [Google Scholar] [CrossRef]
- Dora, N.J.; Hill, R.E.; Collinson, J.M.; West, J.D. Lineage tracing in the adult mouse corneal epithelium supports the limbal epithelial stem cell hypothesis with intermittent periods of stem cell quiescence. Stem Cell Res. 2015, 15, 665–677. [Google Scholar] [CrossRef]
- Mort, R.L.; Douvaras, P.; Morley, S.D.; Dora, N.; Hill, R.E.; Collinson, J.M.; West, J.D. Stem cells and corneal epithelial maintenance: Insights from the mouse and other animal models. Results Probl. Cell Differ. 2012, 55, 357–394. [Google Scholar] [CrossRef]
- Majo, F.; Rochat, A.; Nicolas, M.; Jaoude, G.A.; Barrandon, Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008, 456, 250–254. [Google Scholar] [CrossRef]
- Chang, C.Y.; Green, C.R.; McGhee, C.N.; Sherwin, T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Miri, A.; Alomar, T.; Yeung, A.M.; Said, D.G. The role of limbal stem cells in corneal epithelial maintenance: Testing the dogma. Ophthalmology 2009, 116, 856–863. [Google Scholar] [CrossRef]
- Hernandez-Bogantes, E.; Amescua, G.; Navas, A.; Garfias, Y.; Ramirez-Miranda, A.; Lichtinger, A.; Graue-Hernandez, E.O. Minor ipsilateral simple limbal epithelial transplantation (mini-SLET) for pterygium treatment. Br. J. Ophthalmol. 2015, 99, 1598–1600. [Google Scholar] [CrossRef]
- Daya, S.M.; Chan, C.C.; Holland, E.J.; on Behalf of the Members of The Cornea Society Ocular Surface Procedures Nomenclature Committee. Cornea Society nomenclature for ocular surface rehabilitative procedures. Cornea 2011, 30, 1115–1119. [Google Scholar] [CrossRef]
- Yao, T.Y.; Wang, J.S.; Geng, W.; Xie, H.T.; Zhang, M.C. Conjunctival Limbal Autograft Combined with Amnion-Assisted Conjunctival Epithelial Redirection for Unilateral Total Limbal Stem Cell Deficiency after Severe Chemical Burn. J. Clin. Med. 2023, 12, 6235. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Nikpoor, N.; Rao Donthineni, P.; Singh, V.; Chodosh, J.; Basu, S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2020, 104, 247–253. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Tran, J.A.; Dohlman, T.H.; Zhang, L.J.; Lorch, A.; Elze, T.; Miller, J.W.; Yin, J.; Oke, I.; Dana, R. Visual Outcomes of Limbal Stem Cell Transplantation in the IRIS(R) Registry. Ophthalmology 2025, 132, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: A review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef]
- Ng, X.Y.; Peh, G.S.L.; Yam, G.H.; Tay, H.G.; Mehta, J.S. Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy. Int. J. Mol. Sci. 2023, 24, 12433. [Google Scholar] [CrossRef]
- Surico, P.L.; Barone, V.; Singh, R.B.; Coassin, M.; Blanco, T.; Dohlman, T.H.; Basu, S.; Chauhan, S.K.; Dana, R.; Di Zazzo, A. Potential applications of mesenchymal stem cells in ocular surface immune-mediated disorders. Surv. Ophthalmol. 2025, 70, 467–479. [Google Scholar] [CrossRef]
- Burman, S.; Sangwan, V. Cultivated limbal stem cell transplantation for ocular surface reconstruction. Clin. Ophthalmol. 2008, 2, 489–502. [Google Scholar]
- Meallet, M.A.; Espana, E.M.; Grueterich, M.; Ti, S.E.; Goto, E.; Tseng, S.C. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology 2003, 110, 1585–1592. [Google Scholar] [CrossRef]
- Pietryga, K.; Jesse, K.; Drzyzga, R.; Konka, A.; Zembala-John, J.; Kowalik, A.; Kielbowicz, Z.; Cwirko, M.; Buldak, R.J.; Dobrowolski, D.; et al. Bio-printing method as a novel approach to obtain a fibrin scaffold settled by limbal epithelial cells for corneal regeneration. Sci. Rep. 2024, 14, 23352. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal tissue engineering: Recent advances and future perspectives. Tissue Eng. Part. B Rev. 2015, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Tidu, A.; Ghoubay-Benallaoua, D.; Lynch, B.; Haye, B.; Illoul, C.; Allain, J.M.; Borderie, V.M.; Mosser, G. Development of human corneal epithelium on organized fibrillated transparent collagen matrices synthesized at high concentration. Acta Biomater. 2015, 22, 50–58. [Google Scholar] [CrossRef]
- Polisetti, N.; Roschinski, B.; Schlotzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int. J. Mol. Sci. 2021, 22, 10067. [Google Scholar] [CrossRef]
- Bobba, S.; Chow, S.; Watson, S.; Di Girolamo, N. Clinical outcomes of xeno-free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: A prospective case series. Stem Cell Res. Ther. 2015, 6, 23. [Google Scholar] [CrossRef]
- Gonzalez, S.; Chen, L.; Deng, S.X. Comparative Study of Xenobiotic-Free Media for the Cultivation of Human Limbal Epithelial Stem/Progenitor Cells. Tissue Eng. Part C Methods 2017, 23, 219–227. [Google Scholar] [CrossRef]
- Brown, K.D.; Low, S.; Mariappan, I.; Abberton, K.M.; Short, R.; Zhang, H.; Maddileti, S.; Sangwan, V.; Steele, D.; Daniell, M. Plasma polymer-coated contact lenses for the culture and transfer of corneal epithelial cells in the treatment of limbal stem cell deficiency. Tissue Eng. Part A 2014, 20, 646–655. [Google Scholar] [CrossRef]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; De Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef]
- Sharma, S.M.; Fuchsluger, T.; Ahmad, S.; Katikireddy, K.R.; Armant, M.; Dana, R.; Jurkunas, U.V. Comparative analysis of human-derived feeder layers with 3T3 fibroblasts for the ex vivo expansion of human limbal and oral epithelium. Stem Cell Rev. Rep. 2012, 8, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Omoto, M.; Miyashita, H.; Shimmura, S.; Higa, K.; Kawakita, T.; Yoshida, S.; McGrogan, M.; Shimazaki, J.; Tsubota, K. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Jurkunas, U.V. Cultivated Autologous Limbal Epithelial Cell Transplantation: A Review of Clinical Trials and Applications. Cornea 2025, 44, 1071–1077. [Google Scholar] [CrossRef]
- Wu, M.F.; Stachon, T.; Seitz, B.; Langenbucher, A.; Szentmary, N. Effect of human autologous serum and fetal bovine serum on human corneal epithelial cell viability, migration and proliferation in vitro. Int. J. Ophthalmol. 2017, 10, 908–913. [Google Scholar] [CrossRef]
- Niruthisard, D.; Bonnet, C.; Tanasugarn, L.; Le, B.; Deng, S.X. Autologous Serum Eye Drops in the Management of Limbal Stem Cell Deficiency Associated With Glaucoma Surgery. Eye Contact Lens 2023, 49, 19–24. [Google Scholar] [CrossRef]
- Kenyon, K.R.; Tseng, S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–722; discussion 722–723. [Google Scholar] [CrossRef]
- Le, Q.; Chauhan, T.; Yung, M.; Tseng, C.H.; Deng, S.X. Outcomes of Limbal Stem Cell Transplant: A Meta-analysis. JAMA Ophthalmol. 2020, 138, 660–670. [Google Scholar] [CrossRef]
- Eslani, M.; Cheung, A.Y.; Kurji, K.; Pierson, K.; Sarnicola, E.; Holland, E.J. Long-term outcomes of conjunctival limbal autograft in patients with unilateral total limbal stem cell deficiency. Ocul. Surf. 2019, 17, 670–674. [Google Scholar] [CrossRef]
- Kreimei, M.; Sorkin, N.; Einan-Lifshitz, A.; Rootman, D.S.; Chan, C.C. Long-term outcomes of donor eyes after conjunctival limbal autograft and allograft harvesting. Can. J. Ophthalmol. 2019, 54, 565–569. [Google Scholar] [CrossRef]
- Cauchi, P.A.; Ang, G.S.; Azuara-Blanco, A.; Burr, J.M. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am. J. Ophthalmol. 2008, 146, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Bienzobas, V.; Nava-Castaneda, A.; Jimenez-Corona, A.; Kahuam-Lopez, N.; Ramirez-Miranda, A.; Navas, A.; Graue-Hernandez, E.O. Comparison of mini-simple limbal epithelial transplantation and conjunctival-limbal autograft for the treatment of primary pterygium: A randomised controlled trial. Br. J. Ophthalmol. 2023, 107, 1776–1781. [Google Scholar] [CrossRef]
- Tan, D.T.; Ficker, L.A.; Buckley, R.J. Limbal transplantation. Ophthalmology 1996, 103, 29–36. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; Vemuganti, G.K.; Sejpal, K.; Subramaniam, S.V.; Bandyopadhyay, S.; Krishnaiah, S.; Gaddipati, S.; Tiwari, S.; Balasubramanian, D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br. J. Ophthalmol. 2011, 95, 1525–1529. [Google Scholar] [CrossRef]
- Ramachandran, C.; Basu, S.; Sangwan, V.S.; Balasubramanian, D. Concise review: The coming of age of stem cell treatment for corneal surface damage. Stem Cells Transl. Med. 2014, 3, 1160–1168. [Google Scholar] [CrossRef]
- Rossen, J.; Amram, A.; Milani, B.; Park, D.; Harthan, J.; Joslin, C.; McMahon, T.; Djalilian, A. Contact Lens-induced Limbal Stem Cell Deficiency. Ocul. Surf. 2016, 14, 419–434. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Sarnicola, E.; Kurji, K.H.; Govil, A.; Mogilishetty, G.; Eslani, M.; Wright, E.; Brailey, P.; Holland, E.J. Cincinnati Protocol for Preoperative Screening and Donor Selection for Ocular Surface Stem Cell Transplantation. Cornea 2018, 37, 1192–1197. [Google Scholar] [CrossRef]
- Fallah, M.R.; Golabdar, M.R.; Amozadeh, J.; Zare, M.A.; Moghimi, S.; Fakhraee, G. Transplantation of conjunctival limbal autograft and amniotic membrane vs mitomycin C and amniotic membrane in treatment of recurrent pterygium. Eye 2008, 22, 420–424. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial Stem Cells-Physiology, Pathophysiology and Therapeutic Options. Cells 2021, 10, 2302. [Google Scholar] [CrossRef]
- Wong, H.; Wang, J.S.; Du, Y.L.; Xie, H.T.; Zhang, M.C. Sandwich (Amnion/Conjunctival-Limbal Autograft/Amnion) Transplantation for Recurrent Pterygium with Restrictive Strabismus. J. Clin. Med. 2022, 11, 7193. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Asl, N.S.; Ebrahimi, M.; Jabbehdari, S.; Bamdad, S.; Roshandel, D.; Eslani, M.; Momeni, M. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul. Surf. 2018, 16, 146–153. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Heidari-Keshel, S.; Behnaz, N.; Alemzadeh-Ansari, M.; Feizi, S.; Hassanpour, K.; Sadoughi, M.M.; Filutowski, O.; Ghahari, M. Mini-Conjunctival Limbal Autograft (Mini-CLAU) Using Platelet-Rich Plasma Eye Drops (E-PRP): A Case Series. Cornea 2023, 42, 1116–1123. [Google Scholar] [CrossRef]
- Murri, M.S.; Moshirfar, M.; Birdsong, O.C.; Ronquillo, Y.C.; Ding, Y.; Hoopes, P.C. Amniotic membrane extract and eye drops: A review of literature and clinical application. Clin. Ophthalmol. 2018, 12, 1105–1112. [Google Scholar] [CrossRef]
- Ghareeb, A.E.; Lako, M.; Figueiredo, F.C. Recent Advances in Stem Cell Therapy for Limbal Stem Cell Deficiency: A Narrative Review. Ophthalmol. Ther. 2020, 9, 809–831. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Kolli, S.; Lako, M.; Figueiredo, F.; Mudhar, H.; Ahmad, S. Loss of corneal epithelial stem cell properties in outgrowths from human limbal explants cultured on intact amniotic membrane. Regen. Med. 2008, 3, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Ardigo, D.; Milazzo, G.; Iotti, G.; Guatelli, P.; Pelosi, D.; De Luca, M. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl. Med. 2018, 7, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Thokala, P.; Singh, A.; Singh, V.K.; Rathi, V.M.; Basu, S.; Singh, V.; MacNeil, S.; Sangwan, V.S. Economic, clinical and social impact of simple limbal epithelial transplantation for limbal stem cell deficiency. Br. J. Ophthalmol. 2022, 106, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.S.; Kate, A.; Ganguly, S.; Jakati, S.; Deshmukh, R.; Basu, S. Five- to Twelve-Year Outcomes of Autologous Simple Limbal Epithelial Transplantation: Long-Term Corneal Epithelial Imaging and Phenotypic Analysis. Am. J. Ophthalmol. 2025, 273, 107–118. [Google Scholar] [CrossRef]
- Arora, R.; Dokania, P.; Manudhane, A.; Goyal, J.L. Preliminary results from the comparison of simple limbal epithelial transplantation with conjunctival limbal autologous transplantation in severe unilateral chronic ocular burns. Indian J. Ophthalmol. 2017, 65, 35–40. [Google Scholar] [CrossRef]
- Vazirani, J.; Ali, M.H.; Sharma, N.; Gupta, N.; Mittal, V.; Atallah, M.; Amescua, G.; Chowdhury, T.; Abdala-Figuerola, A.; Ramirez-Miranda, A.; et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: Multicentre results. Br. J. Ophthalmol. 2016, 100, 1416–1420. [Google Scholar] [CrossRef]
- Jain, N.; Mittal, V.; Sanandiya, D. Outcomes of Simple Limbal Epithelial Transplantation Without Amniotic Membrane Grafting in Unilateral Limbal Stem Cell Deficiency: A Case Series of 6 Patients. Cornea 2025, 44, 80–85. [Google Scholar] [CrossRef]
- Garg, A.; Goel, K.; Gour, A.; Sapra, M.; Sangwan, V.S.; Tripathi, R.; Tiwari, A. Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET). Cells 2025, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.; Sati, A.; Mishra, S.K.; Kumar, S.; Dhar, S. Innovative technique of mini-simple limbal epithelial transplantation in pediatric patients. Indian J. Ophthalmol. 2021, 69, 2222–2224. [Google Scholar] [CrossRef]
- Malyugin, B.E.; Gerasimov, M.Y.; Borzenok, S.A. Glueless Simple Limbal Epithelial Transplantation: The Report of the First 2 Cases. Cornea 2020, 39, 1588–1591. [Google Scholar] [CrossRef]
- Malyugin, B.E.; Kalinnikova, S.Y.; Knyazer, B.; Gerasimov, M.Y. Midterm Outcomes of Autologous Glueless Simple Limbal Epithelial Transplantation for Unilateral Limbal Stem Cell Deficiency. Cornea 2024, 43, 45–51. [Google Scholar] [CrossRef]
- Malyugin, B.; Kalinnikova, S.; Nefedova, O.; Gerasimov, M.; Muller, F.; Bernau, W. Autologous Glueless Simple Limbal Epithelial Transplantation for Unilateral Stem Cell Deficiency Using Femtosecond Laser-Assisted Limbal Stem Cell Harvesting: The Report of the First 3 Clinical Cases. Cornea 2025, 44, 1058–1069. [Google Scholar] [CrossRef]
- Ilari, L.; Daya, S.M. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology 2002, 109, 1278–1284. [Google Scholar] [CrossRef]
- Liang, L.; Sheha, H.; Tseng, S.C. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Arch. Ophthalmol. 2009, 127, 1428–1434. [Google Scholar] [CrossRef]
- Li, J.Y.; Cortina, M.S.; Greiner, M.A.; Kuo, A.N.; Miller, D.D.; Shtein, R.M.; Veldman, P.B.; Yin, J.; Kim, S.J.; Shen, J.F. Outcomes and Complications of Limbal Stem Cell Allograft Transplantation: A Report by the American Academy of Ophthalmology. Ophthalmology 2024, 131, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J. Epithelial transplantation for the management of severe ocular surface disease. Trans. Am. Ophthalmol. Soc. 1996, 94, 677–743. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J.; Mogilishetty, G.; Skeens, H.M.; Hair, D.B.; Neff, K.D.; Biber, J.M.; Chan, C.C. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10-year experience. Cornea 2012, 31, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Jurkunas, U.V.; Kaufman, A.R.; Yin, J.; Ayala, A.; Maguire, M.; Samarakoon, L.; Johns, L.K.; Parekh, M.; Li, S.; Gauthier, A.; et al. Cultivated autologous limbal epithelial cell (CALEC) transplantation for limbal tem cell deficiency: A phase I/II clinical trial of the first xenobiotic-free, serum-free, antibiotic-free manufacturing protocol developed in the US. Nat. Commun. 2025, 16, 1607. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Yin, J.; Johns, L.K.; Li, S.; Negre, H.; Shaw, K.L.; Samarakoon, L.; Ayala, A.R.; Kheirkhah, A.; Katikireddy, K.; et al. Cultivated autologous limbal epithelial cell (CALEC) transplantation: Development of manufacturing process and clinical evaluation of feasibility and safety. Sci. Adv. 2023, 9, eadg6470. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Ueta, M.; Imai, K.; Fukuoka, H.; Komai, S.; Ishida, G.; Kitazawa, K.; Yokoi, N.; et al. Oral Mucosal Epithelial Transplantation and Limbal-Rigid Contact Lens: A Therapeutic Modality for the Treatment of Severe Ocular Surface Disorders. Cornea 2020, 39 (Suppl. S1), S19–S27. [Google Scholar] [CrossRef]
- Venugopal, R.; Nagpal, R.; Mohanty, S.; Sen, S.; Kashyap, S.; Agarwal, T.; Maharana, P.K.; Vajpayee, R.B.; Sharma, N. Outcomes of Cultivated Oral Mucosal Epithelial Transplantation in Eyes With Chronic Stevens-Johnson Syndrome Sequelae. Am. J. Ophthalmol. 2021, 222, 82–91. [Google Scholar] [CrossRef]
- Ma, D.H.; Kuo, M.T.; Tsai, Y.J.; Chen, H.C.; Chen, X.L.; Wang, S.F.; Li, L.; Hsiao, C.H.; Lin, K.K. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye 2009, 23, 1442–1450. [Google Scholar] [CrossRef]
- Toshida, H.; Kasahara, T.; Kiriyama, M.; Iwasaki, Y.; Sugita, J.; Ichikawa, K.; Ohta, T.; Miyahara, K. Early Clinical Outcomes of the First Commercialized Human Autologous Ex Vivo Cultivated Oral Mucosal Epithelial Cell Transplantation for Limbal Stem Cell Deficiency: Two Case Reports and Literature Review. Int. J. Mol. Sci. 2023, 24, 8926. [Google Scholar] [CrossRef]
- Tandon, R.; Pandey, P.K.; Khan, T.A.; Das, A.K.; Kalaivani, M.; Majood, M.; Kashyap, S.; Sen, S.; Lomi, N.; Gupta, N.; et al. Comparative evaluation of autologous tissue-engineered ocular and oral mucosal tissue grafts- a prospective randomized controlled trial. BMC Biotechnol. 2024, 24, 82. [Google Scholar] [CrossRef]
- Chen, H.C.; Yeh, L.K.; Tsai, Y.J.; Lai, C.H.; Chen, C.C.; Lai, J.Y.; Sun, C.C.; Chang, G.; Hwang, T.L.; Chen, J.K.; et al. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5615–5623. [Google Scholar] [CrossRef]
- Komai, S.; Inatomi, T.; Nakamura, T.; Ueta, M.; Horiguchi, G.; Teramukai, S.; Kimura, Y.; Kagimura, T.; Fukushima, M.; Kinoshita, S.; et al. Long-term outcome of cultivated oral mucosal epithelial transplantation for fornix reconstruction in chronic cicatrising diseases. Br. J. Ophthalmol. 2022, 106, 1355–1362. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K.; et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef]
- Ahmad, S.; Figueiredo, F.; Lako, M. Corneal epithelial stem cells: Characterization, culture and transplantation. Regen. Med. 2006, 1, 29–44. [Google Scholar] [CrossRef]
- Brzeszczynska, J.; Samuel, K.; Greenhough, S.; Ramaesh, K.; Dhillon, B.; Hay, D.C.; Ross, J.A. Differentiation and molecular profiling of human embryonic stem cell-derived corneal epithelial cells. Int. J. Mol. Med. 2014, 33, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Shalom-Feuerstein, R.; Serror, L.; De La Forest Divonne, S.; Petit, I.; Aberdam, E.; Camargo, L.; Damour, O.; Vigouroux, C.; Solomon, A.; Gaggioli, C.; et al. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells 2012, 30, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS ONE 2012, 7, e45435. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Oie, Y.; Takayanagi, H.; Matsubara, S.; Yamada, T.; Nomura, M.; Yoshinaga, Y.; Maruyama, K.; Watanabe, A.; Takashima, K.; et al. Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: A single-arm, open-label, first-in-human interventional study in Japan. Lancet 2024, 404, 1929–1939. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Bains, K.K.; Fukuoka, H.; Hammond, G.M.; Sotozono, C.; Quantock, A.J. Recovering vision in corneal epithelial stem cell deficient eyes. Cont. Lens Anterior Eye 2019, 42, 350–358. [Google Scholar] [CrossRef]
- Ghannam, S.; Pene, J.; Moquet-Torcy, G.; Jorgensen, C.; Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef]
- Holan, V.; Hermankova, B.; Bohacova, P.; Kossl, J.; Chudickova, M.; Hajkova, M.; Krulova, M.; Zajicova, A.; Javorkova, E. Distinct Immunoregulatory Mechanisms in Mesenchymal Stem Cells: Role of the Cytokine Environment. Stem Cell Rev. Rep. 2016, 12, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Kossl, J.; Bohacova, P.; Hermankova, B.; Javorkova, E.; Zajicova, A.; Holan, V. Antiapoptotic Properties of Mesenchymal Stem Cells in a Mouse Model of Corneal Inflammation. Stem Cells Dev. 2021, 30, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Ko, A.Y.; Ko, J.H.; Lee, H.J.; Kim, M.K.; Wee, W.R.; Khwarg, S.I.; Oh, J.Y. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 2015, 23, 139–146. [Google Scholar] [CrossRef]
- Jia, Z.; Li, F.; Zeng, X.; Lv, Y.; Zhao, S. The effects of local administration of mesenchymal stem cells on rat corneal allograft rejection. BMC Ophthalmol. 2018, 18, 139. [Google Scholar] [CrossRef]
- Jia, Z.; Lv, Y.; Zhang, W.; Zhang, X.; Li, F.; Lu, X.; Zhao, S. Mesenchymal stem cell derived exosomes-based immunological signature in a rat model of corneal allograft rejection therapy. Front. Biosci. (Landmark Ed.) 2022, 27, 86. [Google Scholar] [CrossRef]
- Bonnet, C.; Gonzalez, S.; Deng, S.X. Limbal stem cell therapy. Curr. Opin. Ophthalmol. 2024, 35, 309–314. [Google Scholar] [CrossRef]
- Calonge, M.; Perez, I.; Galindo, S.; Nieto-Miguel, T.; Lopez-Paniagua, M.; Fernandez, I.; Alberca, M.; Garcia-Sancho, J.; Sanchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Boto de Los Bueis, A.; Vidal Arranz, C.; Del Hierro-Zarzuelo, A.; Diaz Valle, D.; Mendez Fernandez, R.; Gabarron Hermosilla, M.I.; Benitez Del Castillo, J.M.; Garcia-Arranz, M. Long-Term Effects of Adipose-Derived Stem Cells for the Treatment of Bilateral Limbal Stem Cell Deficiency. Curr. Eye Res. 2024, 49, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Perez, I.; Galindo, S.; Lopez-Miguel, A.; Nieto-Miguel, T.; de la Mata, A.; Lopez-Paniagua, M.; Alberca, M.; Herreras, J.M.; Calonge, M. In Vivo Confocal Microscopy in Limbal Stem Cell Deficiency After Mesenchymal Stem Cell Transplantation: A Sub-analysis from a Phase I-II Clinical Trial. Ophthalmol. Ther. 2023, 12, 3251–3262. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, J.; Saeed, Y.; Kao, W.W. Trends in using mesenchymal stromal/stem cells (MSCs) in treating corneal diseases. Ocul. Surf. 2022, 26, 255–267. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Reinisch, C.B.; Hou, J.H. Decellularized Descemet Membrane Anterior Keratoplasty With Allogeneic Simple Limbal Epithelial Transplantation for Partial Limbal Stem Cell Deficiency Following Partial Keratolimbal Allograft Failure. Cornea 2025, 44, 108–112. [Google Scholar] [CrossRef]
- Weiss, J.S.; Moller, H.U.; Aldave, A.J.; Seitz, B.; Bredrup, C.; Kivela, T.; Munier, F.L.; Rapuano, C.J.; Nischal, K.K.; Kim, E.K.; et al. IC3D classification of corneal dystrophies--edition 2. Cornea 2015, 34, 117–159. [Google Scholar] [CrossRef]
- Ashena, Z.; Niestrata, M.; Tavassoli, S. Management of Stromal Corneal Dystrophies; Review of the Literature with a Focus on Phototherapeutic Keratectomy and Keratoplasty. Vision 2023, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- El Zarif, M.; Alio Del Barrio, J.L.; Arnalich-Montiel, F.; De Miguel, M.P.; Makdissy, N.; Alio, J.L. Corneal Stroma Regeneration: New Approach for the Treatment of Cornea Disease. Asia Pac. J. Ophthalmol. 2020, 9, 571–579. [Google Scholar] [CrossRef]
- Suanno, G.; Genna, V.G.; Maurizi, E.; Dieh, A.A.; Griffith, M.; Ferrari, G. Cell therapy in the cornea: The emerging role of microenvironment. Prog. Retin. Eye Res. 2024, 102, 101275. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The Corneal Basement Membranes and Stromal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef]
- Volatier, T.; Cursiefen, C.; Notara, M. Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications. Cells 2024, 13, 163. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Carlson, E.C.; Funderburgh, M.L.; Birk, D.E.; Pearlman, E.; Guo, N.; Kao, W.W.; Funderburgh, J.L. Stem cell therapy restores transparency to defective murine corneas. Stem Cells 2009, 27, 1635–1642. [Google Scholar] [CrossRef]
- Harkin, D.G.; Foyn, L.; Bray, L.J.; Sutherland, A.J.; Li, F.J.; Cronin, B.G. Concise reviews: Can mesenchymal stromal cells differentiate into corneal cells? A systematic review of published data. Stem Cells 2015, 33, 785–791. [Google Scholar] [CrossRef]
- Khandaker, I.; Funderburgh, J.L.; Geary, M.L.; Funderburgh, M.L.; Jhanji, V.; Du, Y.; Hin-Fai Yam, G. A novel transgenic mouse model for corneal scar visualization. Exp. Eye Res. 2020, 200, 108270. [Google Scholar] [CrossRef]
- Ghoubay, D.; Borderie, M.; Grieve, K.; Martos, R.; Bocheux, R.; Nguyen, T.M.; Callard, P.; Chedotal, A.; Borderie, V.M. Corneal stromal stem cells restore transparency after N(2) injury in mice. Stem Cells Transl. Med. 2020, 9, 917–935. [Google Scholar] [CrossRef]
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E.; et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, 266ra172. [Google Scholar] [CrossRef]
- Jhanji, V.; Santra, M.; Riau, A.K.; Geary, M.L.; Yang, T.; Rubin, E.; Yusoff, N.; Dhaliwal, D.K.; Mehta, J.S.; Yam, G.H. Combined Therapy Using Human Corneal Stromal Stem Cells and Quiescent Keratocytes to Prevent Corneal Scarring after Injury. Int. J. Mol. Sci. 2022, 23, 6980. [Google Scholar] [CrossRef] [PubMed]
- Alio Del Barrio, J.L.; El Zarif, M.; de Miguel, M.P.; Azaar, A.; Makdissy, N.; Harb, W.; El Achkar, I.; Arnalich-Montiel, F.; Alio, J.L. Cellular Therapy With Human Autologous Adipose-Derived Adult Stem Cells for Advanced Keratoconus. Cornea 2017, 36, 952–960. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Yam, G.H.; Yang, T.; Geary, M.L.; Santra, M.; Funderburgh, M.; Rubin, E.; Du, Y.; Sahel, J.A.; Jhanji, V.; Funderburgh, J.L. Human corneal stromal stem cells express anti-fibrotic microRNA-29a and 381-5p—A robust cell selection tool for stem cell therapy of corneal scarring. J. Adv. Res. 2023, 45, 141–155. [Google Scholar] [CrossRef]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Santra, M.; Geary, M.L.; Rubin, E.; Hsu, M.Y.S.; Funderburgh, M.L.; Chandran, C.; Du, Y.; Dhaliwal, D.K.; Jhanji, V.; Yam, G.H. Good manufacturing practice production of human corneal limbus-derived stromal stem cells and in vitro quality screening for therapeutic inhibition of corneal scarring. Stem Cell Res. Ther. 2024, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Panikker, P.; D’Souza, S.; Shetty, R.; Mohan, R.R.; Ghosh, A. Corneal Regeneration Using Gene Therapy Approaches. Cells 2023, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, Y.; Ma, L.; Yao, Y.; Ge, S.; Yang, Z.; Fan, X. AAV for Gene Therapy in Ocular Diseases: Progress and Prospects. Research 2023, 6, 0291. [Google Scholar] [CrossRef]
- Gupta, S.; Rodier, J.T.; Sharma, A.; Giuliano, E.A.; Sinha, P.R.; Hesemann, N.P.; Ghosh, A.; Mohan, R.R. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS ONE 2017, 12, e0172928. [Google Scholar] [CrossRef]
- Mohan, R.R.; Gupta, S.; Kumar, R.; Sinha, N.R.; Landreneau, J.; Sinha, P.R.; Tandon, A.; Chaurasia, S.S.; Hesemann, N.P. Tissue-targeted and localized AAV5-DCN and AAV5-PEDF combination gene therapy abrogates corneal fibrosis and concurrent neovascularization in rabbit eyes in vivo. Ocul. Surf. 2024, 32, 13–25. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, X.T.; Yu, Y.; Zhu, J.Y.; Dai, J.H.; Qu, X.M.; Le, Q.H.; Chu, R.Y. Inhibition of corneal fibrosis by Smad7 in rats after photorefractive keratectomy. Chin. Med. J. 2013, 126, 1445–1450. [Google Scholar] [CrossRef]
- Pastak, M.; Kleff, V.; Saban, D.R.; Czugala, M.; Steuhl, K.P.; Ergun, S.; Singer, B.B.; Fuchsluger, T.A. Gene Therapy for Modulation of T-Cell-Mediated Immune Response Provoked by Corneal Transplantation. Hum. Gene Ther. 2018, 29, 467–479. [Google Scholar] [CrossRef]
- Nguyen, P.; Yiu, S.C. Strategies for local gene therapy of corneal allograft rejection. Middle East. Afr. J. Ophthalmol. 2013, 20, 11–25. [Google Scholar] [CrossRef]
- Saika, S.; Ikeda, K.; Yamanaka, O.; Miyamoto, T.; Ohnishi, Y.; Sato, M.; Muragaki, Y.; Ooshima, A.; Nakajima, Y.; Kao, W.W.; et al. Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am. J. Pathol. 2005, 166, 1405–1418. [Google Scholar] [CrossRef]
- Saika, S.; Ikeda, K.; Yamanaka, O.; Flanders, K.C.; Nakajima, Y.; Miyamoto, T.; Ohnishi, Y.; Kao, W.W.; Muragaki, Y.; Ooshima, A. Therapeutic effects of adenoviral gene transfer of bone morphogenic protein-7 on a corneal alkali injury model in mice. Lab. Investig. 2005, 85, 474–486. [Google Scholar] [CrossRef]
- Parker, D.G.; Coster, D.J.; Brereton, H.M.; Hart, P.H.; Koldej, R.; Anson, D.S.; Williams, K.A. Lentivirus-mediated gene transfer of interleukin 10 to the ovine and human cornea. Clin. Exp. Ophthalmol. 2010, 38, 405–413. [Google Scholar] [CrossRef]
- Amador, C.; Shah, R.; Ghiam, S.; Kramerov, A.A.; Ljubimov, A.V. Gene Therapy in the Anterior Eye Segment. Curr. Gene Ther. 2022, 22, 104–131. [Google Scholar] [CrossRef]
- Gilger, B.C.; Hasegawa, T.; Sutton, R.B.; Bower, J.J.; Li, C.; Hirsch, M.L. A chimeric anti-vascularization immunomodulator prevents high-risk corneal transplantation rejection via ex vivo gene therapy. Mol. Ther. 2024, 32, 4006–4020. [Google Scholar] [CrossRef]
- Murphy, C.; Alvarado, J.; Juster, R.; Maglio, M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Investig. Ophthalmol. Vis. Sci. 1984, 25, 312–322. [Google Scholar]
- Edelhauser, H.F. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea 2000, 19, 263–273. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Amano, S. Corneal endothelial regeneration and tissue engineering. Prog. Retin. Eye Res. 2013, 35, 1–17. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Tanaka, K.; Hattori, S.; Irie, S.; Miyata, K.; Araie, M.; Amano, S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2992–2997. [Google Scholar] [CrossRef]
- Schmedt, T.; Silva, M.M.; Ziaei, A.; Jurkunas, U. Molecular bases of corneal endothelial dystrophies. Exp. Eye Res. 2012, 95, 24–34. [Google Scholar] [CrossRef]
- Eveleth, D.; Pizzuto, S.; Weant, J.; Jenkins-Eveleth, J.; Bradshaw, R.A. Proliferation of Human Corneal Endothelia in Organ Culture Stimulated by Wounding and the Engineered Human Fibroblast Growth Factor 1 Derivative TTHX1114. J. Ocul. Pharmacol. Ther. 2020, 36, 686–696. [Google Scholar] [CrossRef]
- Bartakova, A.; Kuzmenko, O.; Alvarez-Delfin, K.; Kunzevitzky, N.J.; Goldberg, J.L. A Cell Culture Approach to Optimized Human Corneal Endothelial Cell Function. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1617–1629. [Google Scholar] [CrossRef]
- Engelmann, K.; Friedl, P. Growth of human corneal endothelial cells in a serum-reduced medium. Cornea 1995, 14, 62–70. [Google Scholar] [CrossRef]
- Zhu, C.; Joyce, N.C. Proliferative response of corneal endothelial cells from young and older donors. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Bandeira, F.; Grottone, G.T.; Covre, J.L.; Cristovam, P.C.; Loureiro, R.R.; Pinheiro, F.I.; Casaroli-Marano, R.P.; Donato, W.; Gomes, J.A.P. A Framework for Human Corneal Endothelial Cell Culture and Preliminary Wound Model Experiments with a New Cell Tracking Approach. Int. J. Mol. Sci. 2023, 24, 2982. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Shima, N.; Kimoto, M.; Ebihara, N.; Murakami, A.; Yamagami, S. Optimization of Cultured Human Corneal Endothelial Cell Sheet Transplantation and Post-Operative Sheet Evaluation in a Rabbit Model. Curr. Eye Res. 2016, 41, 1178–1184. [Google Scholar] [CrossRef]
- Lai, J.Y.; Chen, K.H.; Hsiue, G.H. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 2007, 84, 1222–1232. [Google Scholar] [CrossRef]
- Niu, G.; Choi, J.S.; Wang, Z.; Skardal, A.; Giegengack, M.; Soker, S. Heparin-modified gelatin scaffolds for human corneal endothelial cell transplantation. Biomaterials 2014, 35, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Mimura, T.; Usui, T.; Amano, S. Descemet stripping automated endothelial keratoplasty using cultured corneal endothelial cells in a rabbit model. Arch. Ophthalmol. 2009, 127, 1321–1326. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ang, H.P.; Lwin, C.N.; Adnan, K.; George, B.L.; Seah, X.Y.; Lin, S.J.; Bhogal, M.; Liu, Y.C.; Tan, D.T.; et al. Regulatory Compliant Tissue-Engineered Human Corneal Endothelial Grafts Restore Corneal Function of Rabbits with Bullous Keratopathy. Sci. Rep. 2017, 7, 14149. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ong, H.S.; Adnan, K.; Ang, H.P.; Lwin, C.N.; Seah, X.Y.; Lin, S.J.; Mehta, J.S. Functional Evaluation of Two Corneal Endothelial Cell-Based Therapies: Tissue-Engineered Construct and Cell Injection. Sci. Rep. 2019, 9, 6087. [Google Scholar] [CrossRef]
- Levis, H.J.; Kureshi, A.K.; Massie, I.; Morgan, L.; Vernon, A.J.; Daniels, J.T. Tissue Engineering the Cornea: The Evolution of RAFT. J. Funct. Biomater. 2015, 6, 50–65. [Google Scholar] [CrossRef]
- Tsai, R.J.; Li, L.; Chen, J. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells(1). Am. J. Ophthalmol. 2000, 130, 543. [Google Scholar] [CrossRef]
- Tsai, M.C.; Kureshi, A.; Daniels, J.T. Tissue engineered corneal endothelium transplantation in an ex vivo human cornea organ culture model. Sci. Rep. 2025, 15, 12571. [Google Scholar] [CrossRef] [PubMed]
- Hatou, S.; Shimmura, S. Review: Corneal endothelial cell derivation methods from ES/iPS cells. Inflamm. Regen. 2019, 39, 19. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kato, T.M.; Sato, Y.; Umekage, M.; Ichisaka, T.; Tsukahara, M.; Takasu, N.; Yamanaka, S. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 2023, 4, 51–66.e10. [Google Scholar] [CrossRef]
- Hirayama, M.; Hatou, S.; Nomura, M.; Hokama, R.; Hirayama, O.I.; Inagaki, E.; Aso, K.; Sayano, T.; Dohi, H.; Hanatani, T.; et al. A first-in-human clinical study of an allogenic iPSC-derived corneal endothelial cell substitute transplantation for bullous keratopathy. Cell Rep. Med. 2025, 6, 101847. [Google Scholar] [CrossRef]
| Variables to Consider for Stem Cell-Based Therapies | |
|---|---|
| Indication | Unilateral vs. bilateral limbal stem cell deficiency, primary pterygium, recurrent pterygium |
| Autologous vs. allogenic | Autologous—from contralateral eye; Allogenic—from cadaveric tissue or living-related relative |
| Tissue source | Limbal stem cells, iPSCs, mesenchymal stem cells, oral mucosa |
| Substrate | Human amniotic membrane, fibrin, collagen, contact lenses, hydrogels |
| Feeder cells | Mouse 3T3 fibroblasts, human dermal fibroblasts, bone marrow-derived mesenchymal stem cells, xenobiotic free systems |
| Culture medium supplementation | Fetal calf serum, autologous serum, serum-free |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, E.; Henick, D.; Tahvildari, M. Advancements in Cellular Therapeutics in Corneal Diseases. Cells 2025, 14, 1838. https://doi.org/10.3390/cells14231838
Woo E, Henick D, Tahvildari M. Advancements in Cellular Therapeutics in Corneal Diseases. Cells. 2025; 14(23):1838. https://doi.org/10.3390/cells14231838
Chicago/Turabian StyleWoo, Elizabeth, Daniel Henick, and Maryam Tahvildari. 2025. "Advancements in Cellular Therapeutics in Corneal Diseases" Cells 14, no. 23: 1838. https://doi.org/10.3390/cells14231838
APA StyleWoo, E., Henick, D., & Tahvildari, M. (2025). Advancements in Cellular Therapeutics in Corneal Diseases. Cells, 14(23), 1838. https://doi.org/10.3390/cells14231838






