Knocking Out Rap1a Attenuates Cardiac Remodeling and Fibrosis in a Male Murine Model of Angiotensin II-Induced Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Angiotensin II (AngII) Delivery
2.2.1. Preparation of Osmotic Pumps
2.2.2. Pump Implantation Procedure
2.2.3. Study Completion
2.3. Echocardiographic Assessments of Left Ventricle
2.4. Collagen Visualization and Quantification
2.5. Statistical Analysis
3. Results
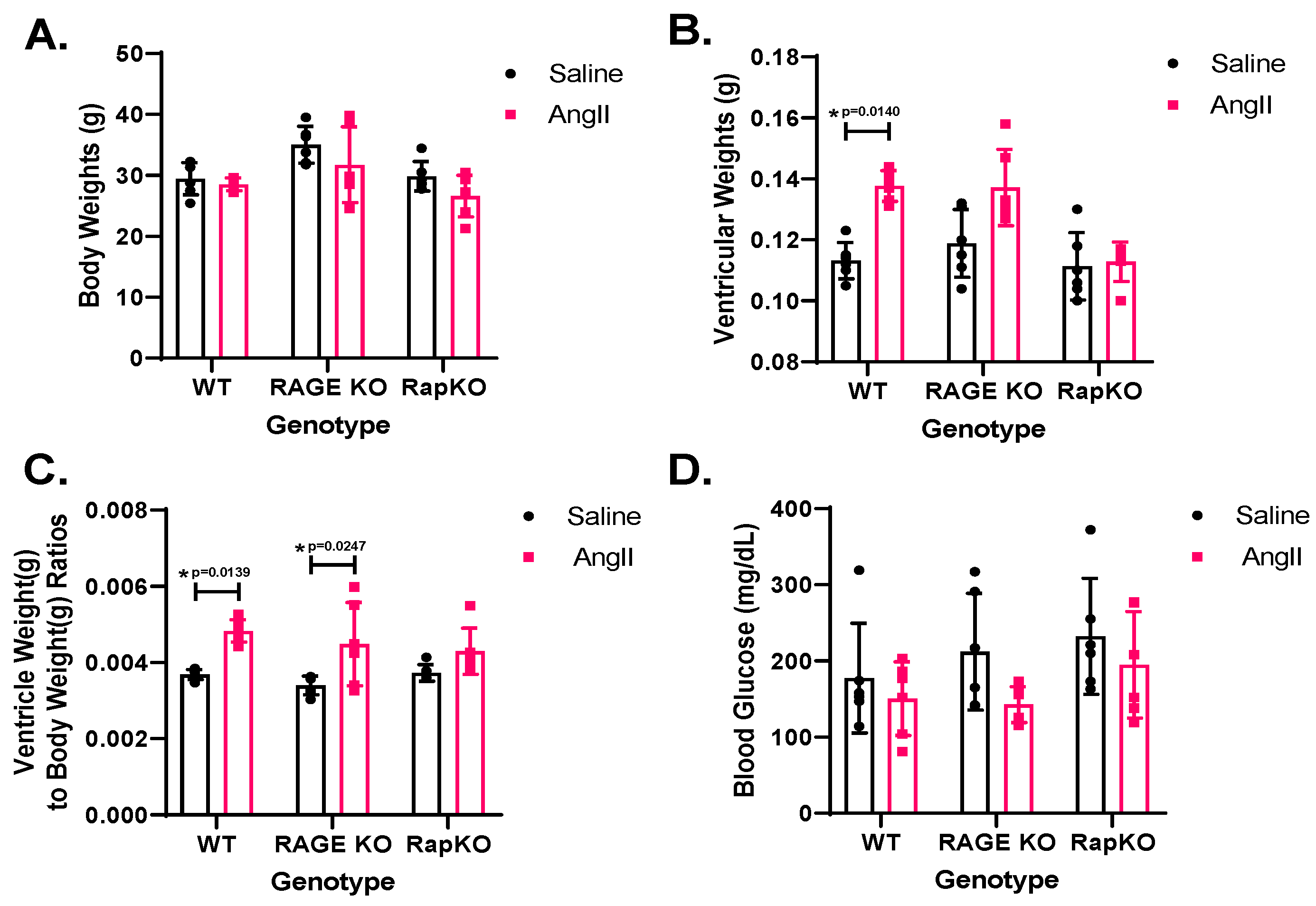
3.1. Morphometric Measurements
3.2. Heart Rate (HR)
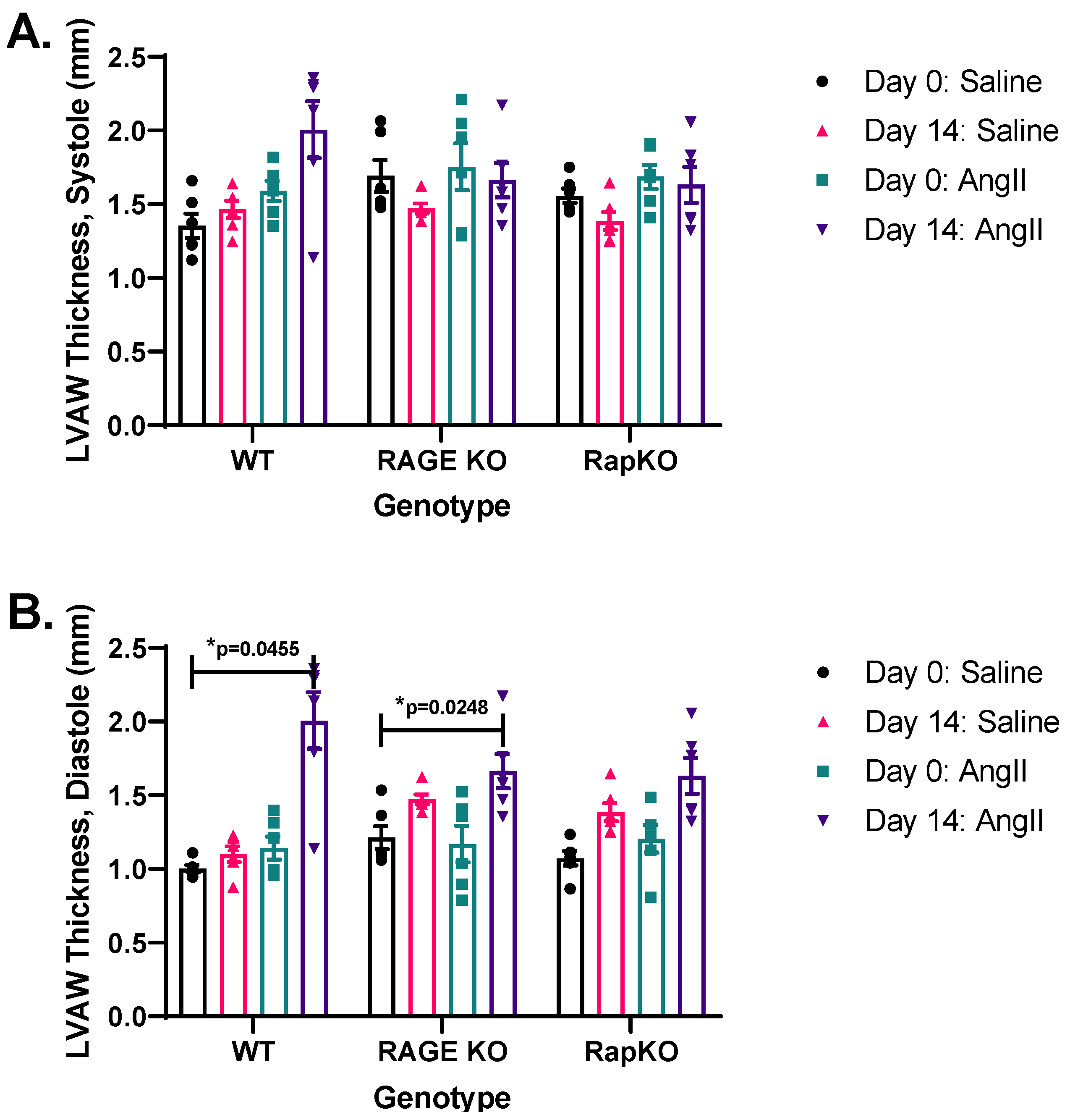
3.3. Systolic and Diastolic Left Ventricular Anterior Wall (LVAW) Thicknesses
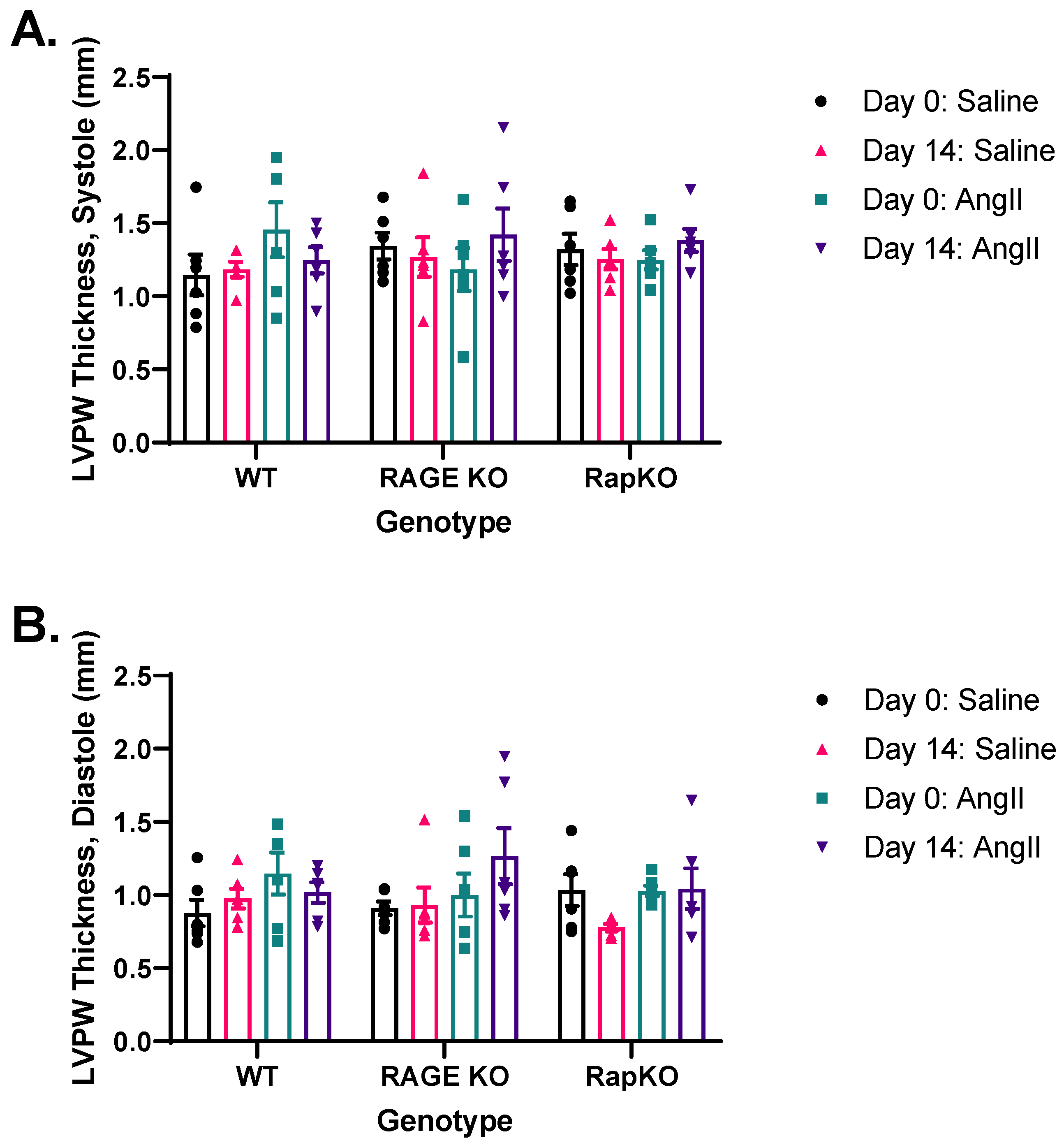
3.4. Systolic and Diastolic Left Ventricular Posterior Wall (LVPW) Thicknesses
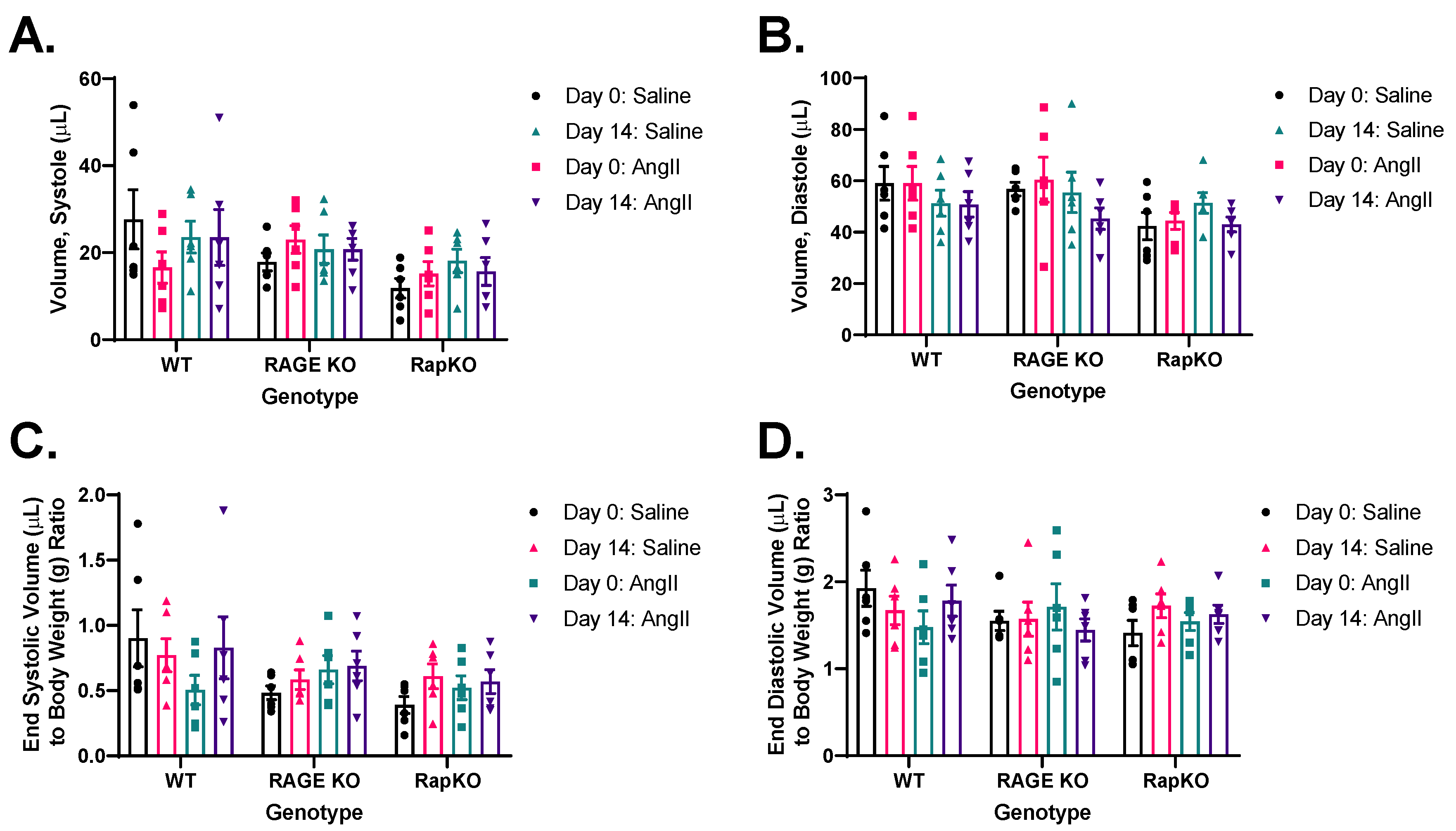
3.5. End-Systolic and End-Diastolic Left Ventricular (LV) Volumes
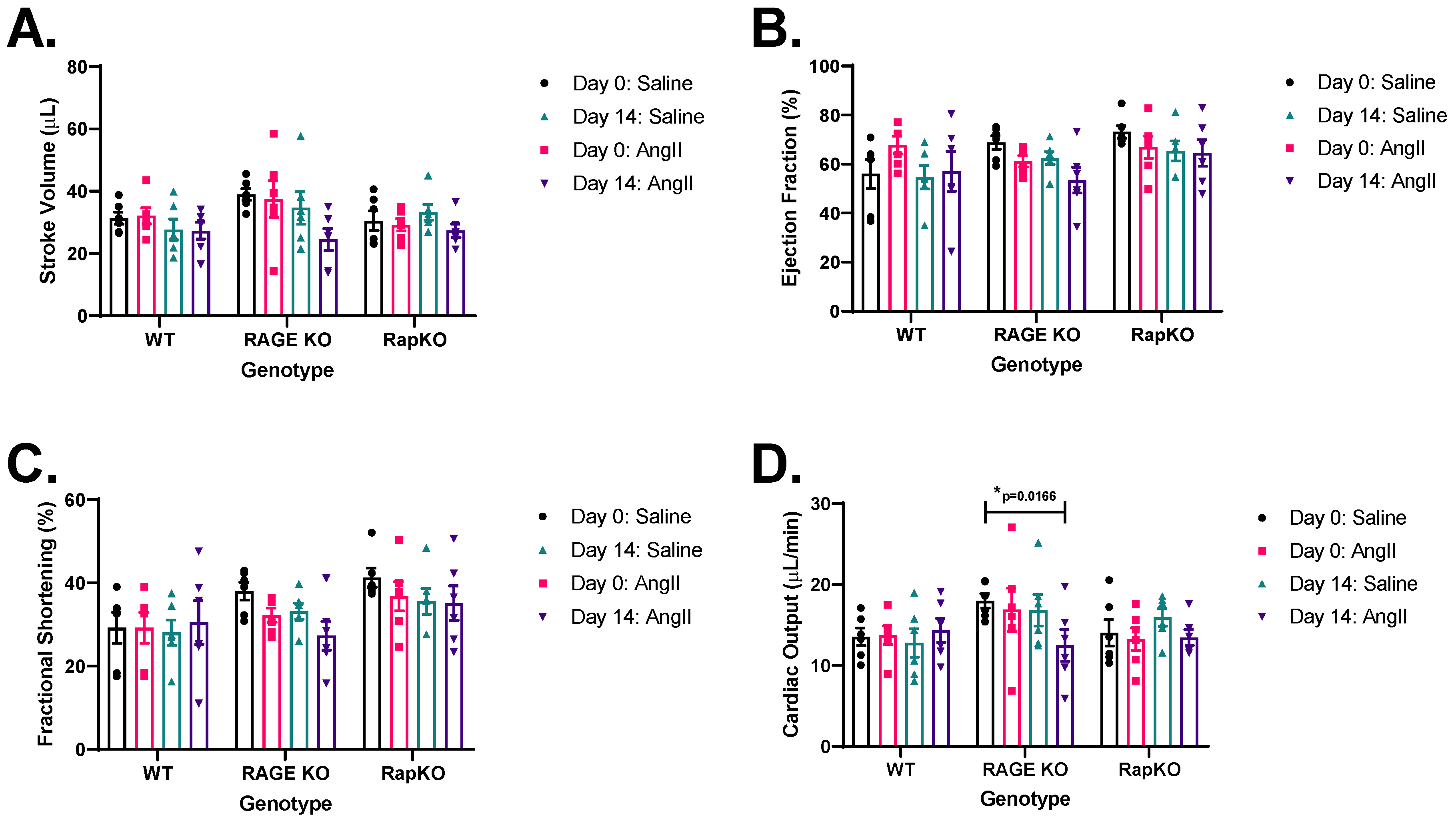
3.6. Left Ventricular (LV) Functional Analysis by Echocardiography
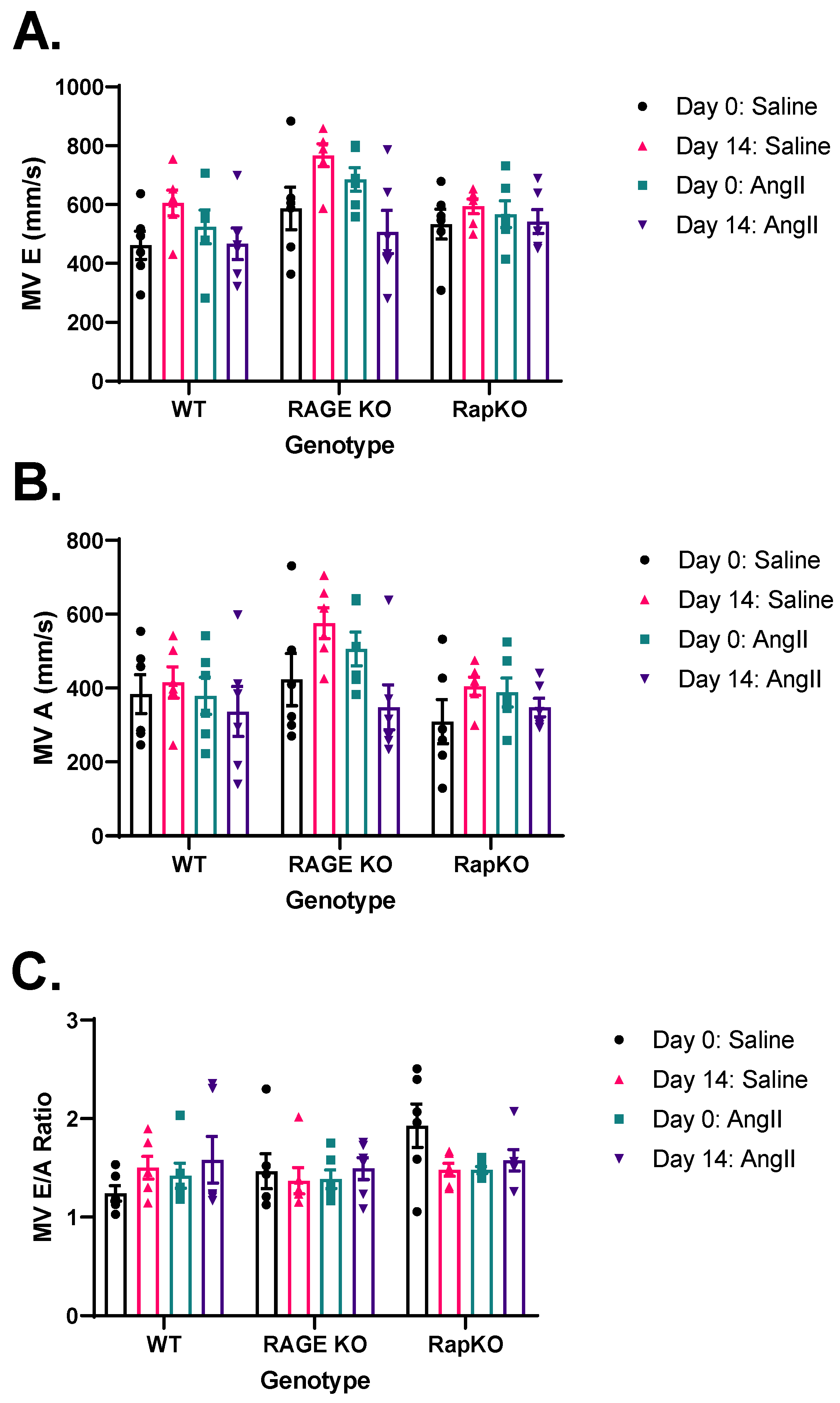
3.7. Mitral Valve (MV) Flow Parameters
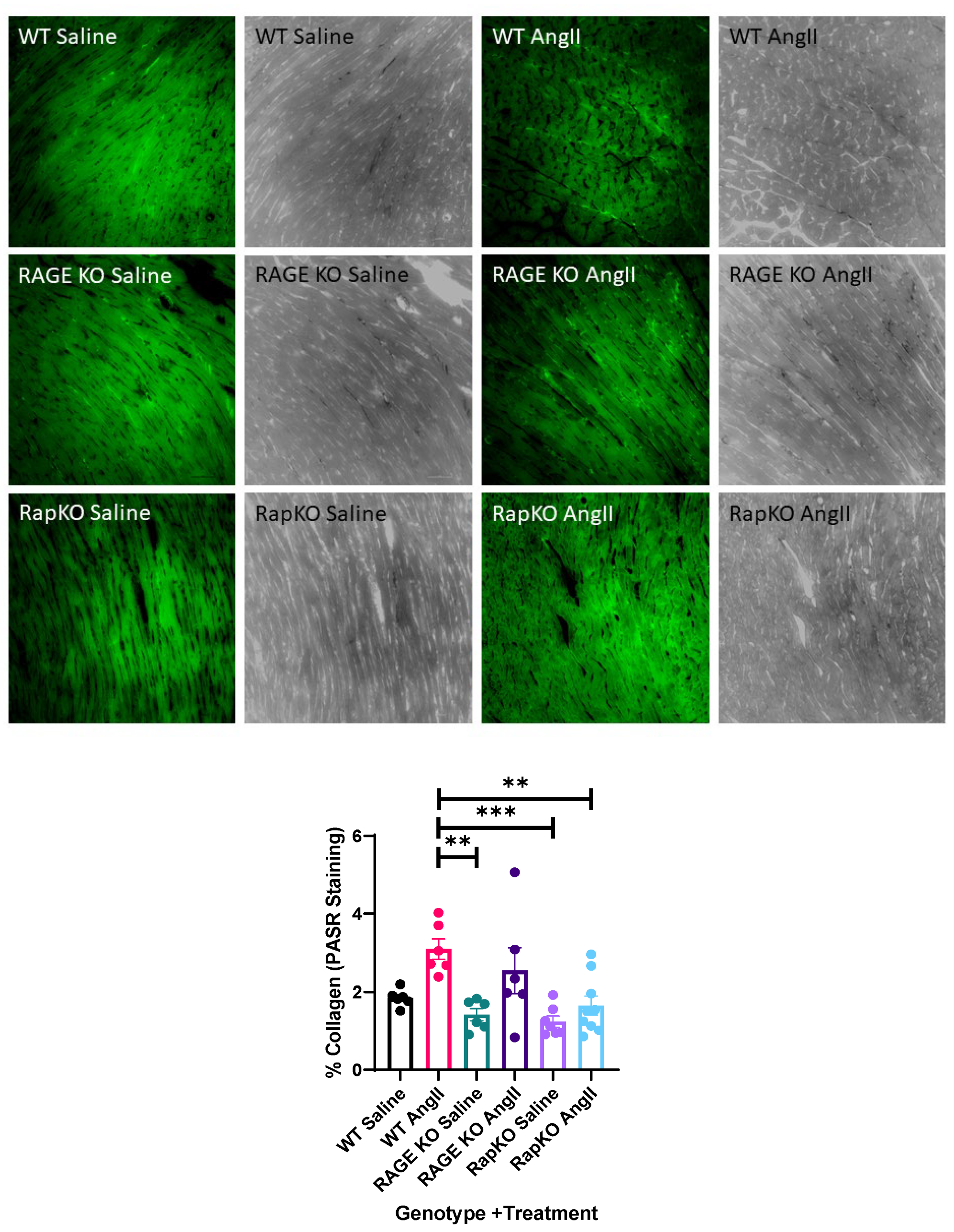
3.8. Myocardial Collagen Percentage
3.9. Summary of Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AngII | Angiotensin II |
| AT1R | Angiotensin II Type 1 Receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| PI3K | Phosphoinositide 3-Kinase |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RAGE | Receptor for Advanced Glycation End-products |
| Rap1a | RAS-related Protein 1A |
| Epac1 | Exchange Protein directly Activated by cAMP 1 |
| Epac2 | Exchange Protein directly Activated by cAMP 2 |
| WT | Wild-Type |
| RAGE KO | RAGE Knock-Out |
| RapKO | Rap1a Knock-Out |
| VW | Ventricular Weight |
| BW | Body Weight |
| LVAW | Left Ventricular Anterior Wall |
| LVPW | Left Ventricular Posterior Wall |
| LVAW Diastolic | Left Ventricular Anterior Wall Diastolic Thickness |
| LVPW Diastolic | Left Ventricular Posterior Wall Diastolic Thickness |
| LV | Left Ventricle |
| SV | Stroke Volume |
| %EF | Percentage Ejection Fraction |
| %FS | Percentage Fractional Shortening |
| CO | Cardiac Output |
| MV E | Mitral Valve Early Filling Velocity |
| MV A | Mitral Valve Late Filling Velocity |
| MV E/A | Mitral Valve E/A Ratio |
| NHE3 | Sodium/Hydrogen Exchanger 3 |
| cAMP | Cyclic Adenosine Monophosphate |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| TNF-α | Tumor Necrosis Factor Alpha |
References
- Gao, Y.; Zhao, D.; Xie, W.-Z.; Meng, T.; Xu, C.; Liu, Y.; Zhang, P.; Bi, X.; Zhao, Z. Rap1GAP Mediates Angiotensin II-Induced Cardiomyocyte Hypertrophy by Inhibiting Autophagy and Increasing Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 7848027. [Google Scholar] [CrossRef]
- Ke, Y.; Karki, P.; Zhang, C.; Li, Y.; Nguyen, T.; Birukov, K.G.; Birukova, A.A. Mechanosensitive Rap1 Activation Promotes Barrier Function of Lung Vascular Endothelium under Cyclic Stretch. Mol. Biol. Cell 2019, 30, 959–974. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, J.; Ren, J.; Luo, Y.; Sun, X. Epac: A Promising Therapeutic Target for Vascular Diseases: A Review. Front. Pharmacol. 2022, 13, 929152. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and Pathological Cardiac Hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, Y. Molecular Mechanisms Underlying Vascular Remodeling in Hypertension. Rev. Cardiovasc. Med. 2024, 25, 72. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.K.; Dhalla, N.S. Angiotensin II-Induced Signal Transduction Mechanisms for Cardiac Hypertrophy. Cells 2022, 11, 3336. [Google Scholar] [CrossRef]
- Yokoyama, S.; Kawai, T.; Yamamoto, K.; Yibin, H.; Yamamoto, H.; Kakino, A.; Takeshita, H.; Nozato, Y.; Fujimoto, T.; Hongyo, K.; et al. RAGE Ligands Stimulate Angiotensin II Type I Receptor (AT1) via RAGE/AT1 Complex on the Cell Membrane. Sci. Rep. 2021, 11, 5759. [Google Scholar] [CrossRef]
- Shaw, S.S.; Schmidt, A.M.; Banes, A.K.; Wang, X.; Stern, D.M.; Marrero, M.B. S100B-RAGE-Mediated Augmentation of Angiotensin II-Induced Activation of JAK2 in Vascular Smooth Muscle Cells Is Dependent on PLD2. Diabetes 2003, 52, 2381–2388. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.; Coughlan, M.T.; Harcourt, B.E.; Kantharidis, P.; Thallas-Bonke, V.; Okuda, S.; Cooper, M.E.; Forbes, J.M. Ramipril Inhibits AGE-RAGE-Induced Matrix Metalloproteinase-2 Activation in Experimental Diabetic Nephropathy. Diabetol. Metab. Syndr. 2014, 6, 86. [Google Scholar] [CrossRef]
- Ihara, Y.; Egashira, K.; Nakano, K.; Ohtani, K.; Kubo, M.; Koga, J.; Iwai, M.; Horiuchi, M.; Gang, Z.; Yamagishi, S.; et al. Upregulation of the Ligand–RAGE Pathway via the Angiotensin II Type I Receptor Is Essential in the Pathogenesis of Diabetic Atherosclerosis. J. Mol. Cell. Cardiol. 2007, 43, 455–464. [Google Scholar] [CrossRef]
- Jeong, J.; Cho, S.; Seo, M.; Lee, B.-S.; Jang, Y.; Lim, S.; Park, S. Soluble RAGE Attenuates Ang II-Induced Arterial Calcification via Inhibiting AT1R-HMGB1-RAGE Axis. Atherosclerosis 2022, 346, 53–62. [Google Scholar] [CrossRef]
- Burr, S.D.; Stewart, J.A., Jr. Rap1a Regulates Cardiac Fibroblast Contraction of 3D Diabetic Collagen Matrices by Increased Activation of the AGE/RAGE Cascade. Cells 2021, 10, 1286. [Google Scholar] [CrossRef]
- Carmona, G.; Göttig, S.; Orlandi, A.; Scheele, J.; Bäuerle, T.; Jugold, M.; Kiessling, F.; Henschler, R.; Zeiher, A.M.; Dimmeler, S.; et al. Role of the Small GTPase Rap1 for Integrin Activity Regulation in Endothelial Cells and Angiogenesis. Blood 2009, 113, 488–497. [Google Scholar] [CrossRef]
- Qrareya, A.N.; Wise, N.S.; Hodges, E.R.; Mahdi, F.; Stewart, J.A., Jr.; Paris, J.J. HIV-1 Tat Upregulates the Receptor for Advanced Glycation End Products and Superoxide Dismutase-2 in the Heart of Transgenic Mice. Viruses 2022, 14, 2191. [Google Scholar] [CrossRef]
- Tan, Y.-Q.; Li, J.; Chen, H.-W. Epac, a Positive or Negative Signaling Molecule in Cardiovascular Diseases. Biomed. Pharmacother. 2022, 148, 112726. [Google Scholar] [CrossRef] [PubMed]
- Roberts, O.L.; Dart, C. CAMP Signalling in the Vasculature: The Role of Epac (Exchange Protein Directly Activated by CAMP). Biochem. Soc. Trans. 2014, 42, 89–97. [Google Scholar] [CrossRef]
- Cullere, X.; Shaw, S.K.; Andersson, L.; Hirahashi, J.; Luscinskas, F.W.; Mayadas, T.N. Regulation of Vascular Endothelial Barrier Function by Epac, a CAMP-Activated Exchange Factor for Rap GTPase. Blood 2005, 105, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Flentje, A.; Kalsi, R.; Monahan, T.S. Small GTPases and Their Role in Vascular Disease. Int. J. Mol. Sci. 2019, 20, 917. [Google Scholar] [CrossRef] [PubMed]
- Spindler, V.; Schlegel, N.; Waschke, J. Role of GTPases in Control of Microvascular Permeability. Cardiovasc. Res. 2010, 87, 243–253. [Google Scholar] [CrossRef]
- Wang, X.; Nie, X.; Wang, H.; Ren, Z. Roles of Small GTPases in Cardiac Hypertrophy (Review). Mol. Med. Rep. 2024, 30, 208. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Joladarashi, D.; Dudeja, P.; Sun, L.; Kanwar, Y.S. Modulation of Angiotensin II-Induced Inflammatory Cytokines by the Epac1-Rap1A-NHE3 Pathway: Implications in Renal Tubular Pathobiology. Am. J. Physiol.-Ren. Physiol. 2014, 306, F1260–F1274. [Google Scholar] [CrossRef]
- Yang, W.-X.; Liu, Y.; Zhang, S.-M.; Wang, H.-F.; Liu, Y.-F.; Liu, J.-L.; Li, X.-H.; Zeng, M.-R.; Han, Y.-Z.; Liu, F.-Y.; et al. Epac Activation Ameliorates Tubulointerstitial Inflammation in Diabetic Nephropathy. Acta Pharmacol. Sin. 2022, 43, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Lezoualc’h, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP Sensor EPAC Proteins and Their Role in Cardiovascular Function and Disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef]
- Grandoch, M.; Roscioni, S.S.; Schmidt, M. The Role of Epac Proteins, Novel CAMP Mediators, in the Regulation of Immune, Lung and Neuronal Function. Br. J. Pharmacol. 2010, 159, 265–284. [Google Scholar] [CrossRef]
- Gloerich, M.; Bos, J.L. Epac: Defining a New Mechanism for CAMP Action. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Fujita, T.; Cai, W.; Jin, M.; Namekata, I.; Mototani, Y.; Jin, H.; Ohnuki, Y.; Tsuneoka, Y.; Kurotani, R.; et al. Epac1-Dependent Phospholamban Phosphorylation Mediates the Cardiac Response to Stresses. J. Clin. Investig. 2014, 124, 2785–2801. [Google Scholar] [CrossRef]
- Laudette, M.; Coluccia, A.; Sainte-Marie, Y.; Solari, A.; Fazal, L.; Sicard, P.; Silvestri, R.; Mialet-Perez, J.; Pons, S.; Ghaleh, B.; et al. Identification of a Pharmacological Inhibitor of Epac1 That Protects the Heart against Acute and Chronic Models of Cardiac Stress. Cardiovasc. Res. 2019, 115, 1766–1777. [Google Scholar] [CrossRef]
- Laudette, M.; Zuo, H.; Lezoualc’h, F.; Schmidt, M. Epac Function and CAMP Scaffolds in the Heart and Lung. J. Cardiovasc. Dev. Dis. 2018, 5, 9. [Google Scholar] [CrossRef]
- Bouvet, M.; Blondeau, J.-P.; Lezoualc’h, F. The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology. Cells 2019, 8, 1543. [Google Scholar] [CrossRef]
- Burr, S.D.; Harmon, M.B.; Stewart, J.A., Jr. The Impact of Diabetic Conditions and AGE/RAGE Signaling on Cardiac Fibroblast Migration. Front. Cell Dev. Biol. 2020, 8, 112. [Google Scholar] [CrossRef]
- Burr, S.D.; Stewart, J.A. Extracellular Matrix Components Isolated from Diabetic Mice Alter Cardiac Fibroblast Function through the AGE/RAGE Signaling Cascade. Life Sci. 2020, 250, 117569. [Google Scholar] [CrossRef]
- Burr, S.D.; Stewart, J.A. Rap1a Overlaps the AGE/RAGE Signaling Cascade to Alter Expression of α-SMA, p-NF-ΚB, and p-PKC-ζ in Cardiac Fibroblasts Isolated from Type 2 Diabetic Mice. Cells 2021, 10, 557. [Google Scholar] [CrossRef]
- Matsiukevich, D.; Kovacs, A.; Li, T.; Kokkonen-Simon, K.; Matkovich, S.J.; Oladipupo, S.S.; Ornitz, D.M. Characterization of a Robust Mouse Model of Heart Failure with Preserved Ejection Fraction. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H203–H231. [Google Scholar] [CrossRef]
- Pickering, R.J.; Tikellis, C.; Rosado, C.J.; Tsorotes, D.; Dimitropoulos, A.; Smith, M.; Huet, O.; Seeber, R.M.; Abhayawardana, R.; Johnstone, E.K.; et al. Transactivation of RAGE Mediates Angiotensin-Induced Inflammation and Atherogenesis. J. Clin. Investig. 2019, 129, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Kassiri, Z.; Virag, J.A.I.; de Castro Brás, L.E.; Scherrer-Crosbie, M. Guidelines for Measuring Cardiac Physiology in Mice. Am. J. Physiol. Circ. Physiol. 2018, 314, H733–H752. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Marcantoni, A.; Gastineau, M.; Birkedal, R.; Rochais, F.; Garnier, A.; Lompré, A.M.; Vandecasteele, G.; Lezoualc’h, F. CAMP-Binding Protein Epac Induces Cardiomyocyte Hypertrophy. Circ. Res. 2005, 97, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Umemura, M.; Yokoyama, U.; Okumura, S.; Ishikawa, Y. The Role of Epac in the Heart. Cell. Mol. Life Sci. 2017, 74, 591–606. [Google Scholar] [CrossRef]
- Davis, P.A.; Pagnin, E.; Semplicini, A.; Avogaro, A.; Calò, L.A. Insulin Signaling, Glucose Metabolism, and the Angiotensin II Signaling System: Studies in Bartter’s/Gitelman’s Syndromes. Diabetes Care 2006, 29, 469–471. [Google Scholar] [CrossRef]
- Jonk, A.M.; Houben, A.J.H.M.; Schaper, N.C.; de Leeuw, P.W.; Serné, E.H.; Smulders, Y.M.; Stehouwer, C.D.A. Angiotensin II Enhances Insulin-Stimulated Whole-Body Glucose Disposal but Impairs Insulin-Induced Capillary Recruitment in Healthy Volunteers. J. Clin. Endocrinol. Metab. 2010, 95, 3901–3908. [Google Scholar] [CrossRef]
- Ciccarelli, L. Angiotensin II Receptor Blockers and Insulin Resistance. Hypertens. Res. 2010, 33, 779. [Google Scholar] [CrossRef]
- Fliser, D.; Schaefer, F.; Schmid, D.; Veldhuis, J.D.; Ritz, E. Angiotensin II Affects Basal, Pulsatile, and Glucose-Stimulated Insulin Secretion in Humans. Hypertension 1997, 30, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D.; Gurley, S.B.; Herrera, M.J.; Ruiz, P.; Griffiths, R.; Kumar, A.P.; Kim, H.-S.; Smithies, O.; Le, T.H.; Coffman, T.M. Angiotensin II Causes Hypertension and Cardiac Hypertrophy through Its Receptors in the Kidney. Proc. Natl. Acad. Sci. USA 2006, 103, 17985–17990. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II Cell Signaling: Physiological and Pathological Effects in the Cardiovascular System. Am. J. Physiol.-Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A.; Cashatt, D.O.; Borck, A.C.; Brown, J.E.; Carver, W.E. 17β-Estradiol Modulation of Angiotensin II-Stimulated Response in Cardiac Fibroblasts. J. Mol. Cell. Cardiol. 2006, 41, 97–107. [Google Scholar] [CrossRef]
| Genotype | Day 0: Saline Mean (SEM) | Day 0: AngII Mean (SEM) | Day 14: Saline Mean (SEM) | Day 14: AngII Mean (SEM) |
|---|---|---|---|---|
| WT | 430.43 (16.91) | 429.30 (19.82) | 459.79 (28.48) | 530.69 (30.36) |
| RAGE KO | 463.76 (20.41) | 454.99 (18.70) | 498.08 (23.26) | 517.49 (46.64) |
| RapKO | 460.68 (23.61) | 447.39 (22.06) | 483.61 (25.52) | 494.93 (17.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porter, C.S.; Brown, L.T.; Lacey, C.; Hickel, M.T.; Stewart, J.A., Jr. Knocking Out Rap1a Attenuates Cardiac Remodeling and Fibrosis in a Male Murine Model of Angiotensin II-Induced Hypertension. Cells 2025, 14, 1834. https://doi.org/10.3390/cells14221834
Porter CS, Brown LT, Lacey C, Hickel MT, Stewart JA Jr. Knocking Out Rap1a Attenuates Cardiac Remodeling and Fibrosis in a Male Murine Model of Angiotensin II-Induced Hypertension. Cells. 2025; 14(22):1834. https://doi.org/10.3390/cells14221834
Chicago/Turabian StylePorter, Cody S., Larissa T. Brown, Can’Torrius Lacey, Mason T. Hickel, and James A. Stewart, Jr. 2025. "Knocking Out Rap1a Attenuates Cardiac Remodeling and Fibrosis in a Male Murine Model of Angiotensin II-Induced Hypertension" Cells 14, no. 22: 1834. https://doi.org/10.3390/cells14221834
APA StylePorter, C. S., Brown, L. T., Lacey, C., Hickel, M. T., & Stewart, J. A., Jr. (2025). Knocking Out Rap1a Attenuates Cardiac Remodeling and Fibrosis in a Male Murine Model of Angiotensin II-Induced Hypertension. Cells, 14(22), 1834. https://doi.org/10.3390/cells14221834







