Osteopontin Preconditioning Improves the Regenerative Effects of Mesenchymal Stem Cells In Vitro but Not Their Therapeutic Efficacy Following Hypoxia-Ischemia in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Mesenchymal Stem Cell Culture and Osteopontin Preconditioning
2.3. Membrane Protein Extraction from MSCs
2.4. Total Protein Extraction from MSCs
2.5. Western Blot
2.6. Transwell Migration Assay
2.7. MSC Gene Expression
2.8. TGF-β ELISA
2.9. Primary Microglia Culture
2.10. Non-Contact MSC/Microglia Co-Culture
2.11. TNF-α ELISA
2.12. Neural Stem Cell Culture
2.13. Non-Contact MSC/NSC Co-Culture
2.14. Immunocytochemistry
2.15. Animals and HI Injury Model in Neonatal Mice
2.16. Brain Gene Expression Profiling
2.17. Intranasal Mesenchymal Stem Cell Treatment
2.18. Histology, Image Acquisition, and Analysis of Brain Tissue
2.19. Statistical Analysis
3. Results
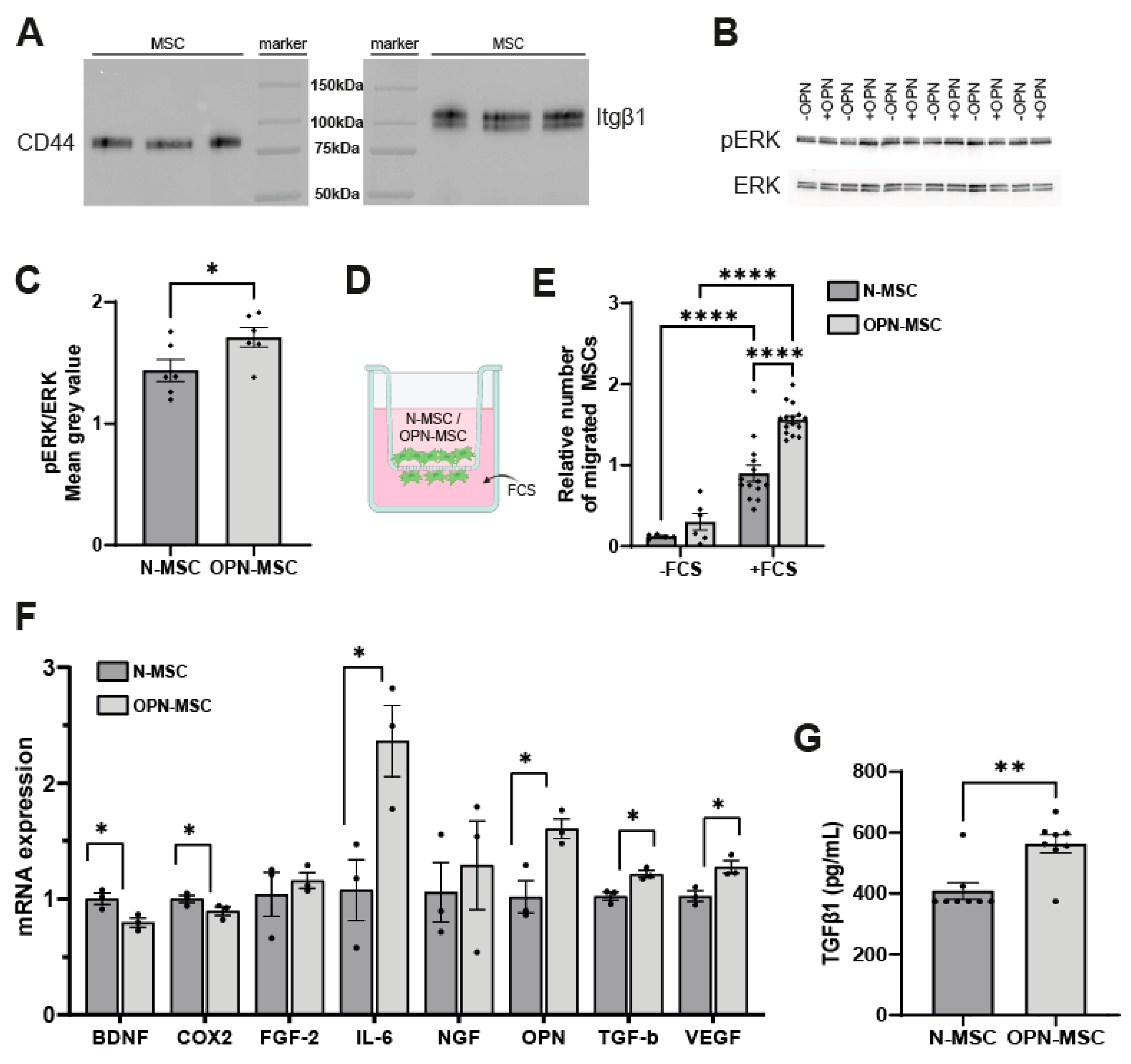
3.1. Osteopontin Preconditioning of MSCs Activates the Intracellular ERK Pathway, Enhances Their Migratory Capacity, and Changes MSC Gene Expression
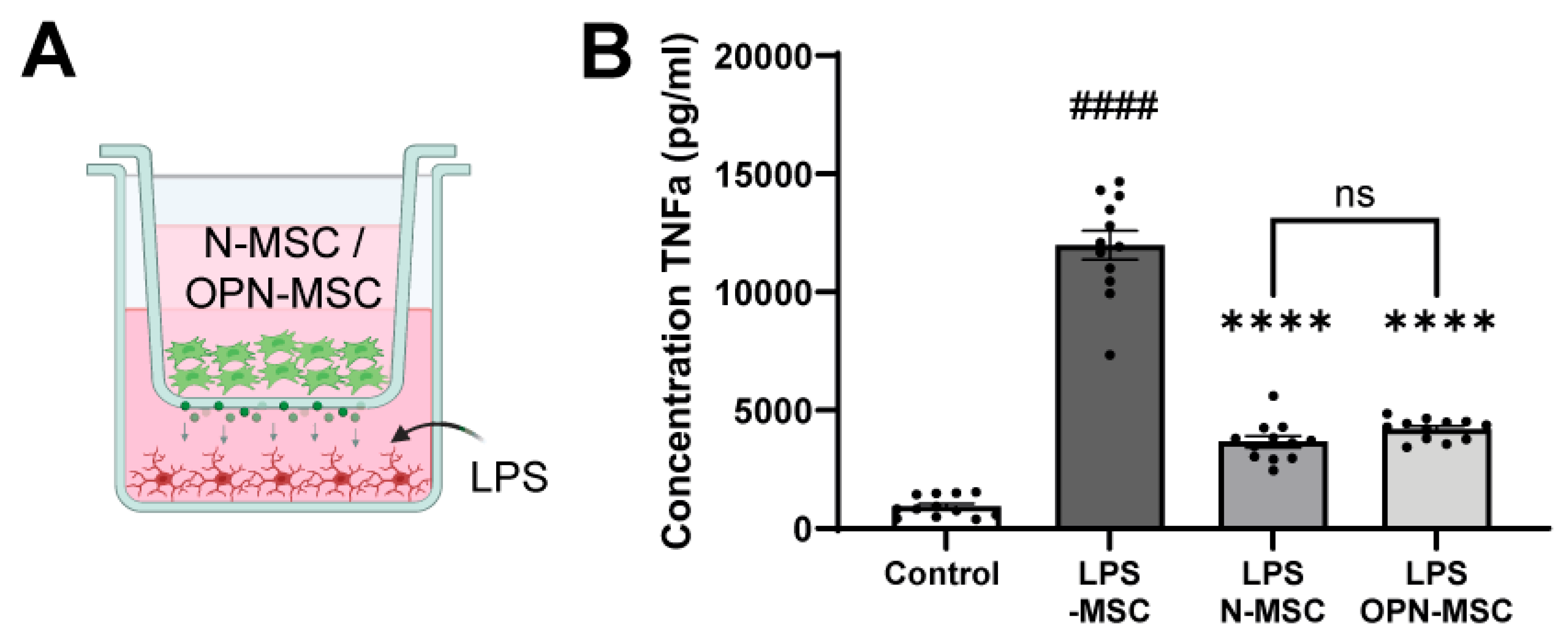
3.2. Osteopontin Preconditioning Does Not Impair Anti-Inflammatory Capacity of MSCs
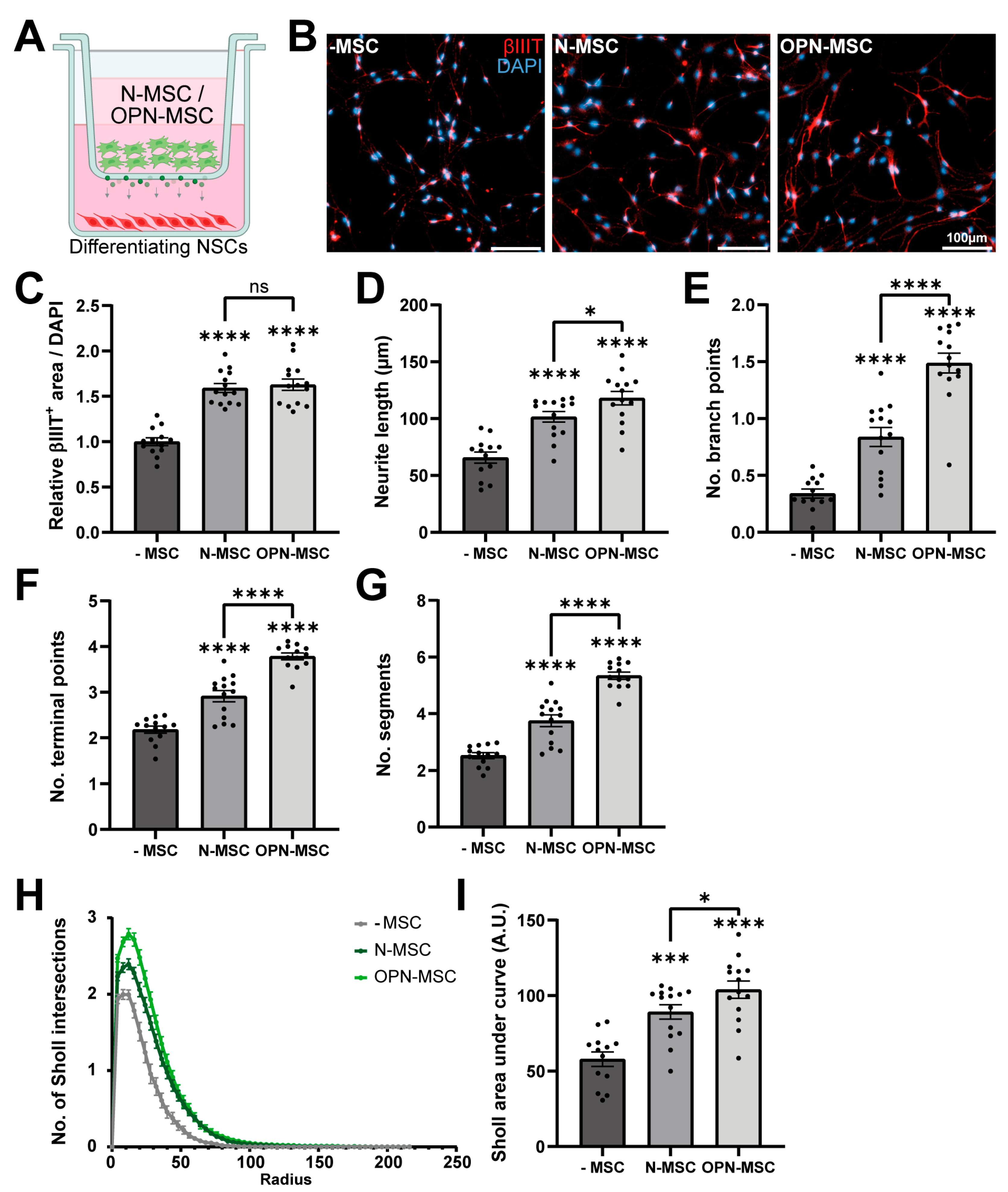
3.3. Osteopontin Preconditioning of MSCs Enhances Their Neurotrophic Potential
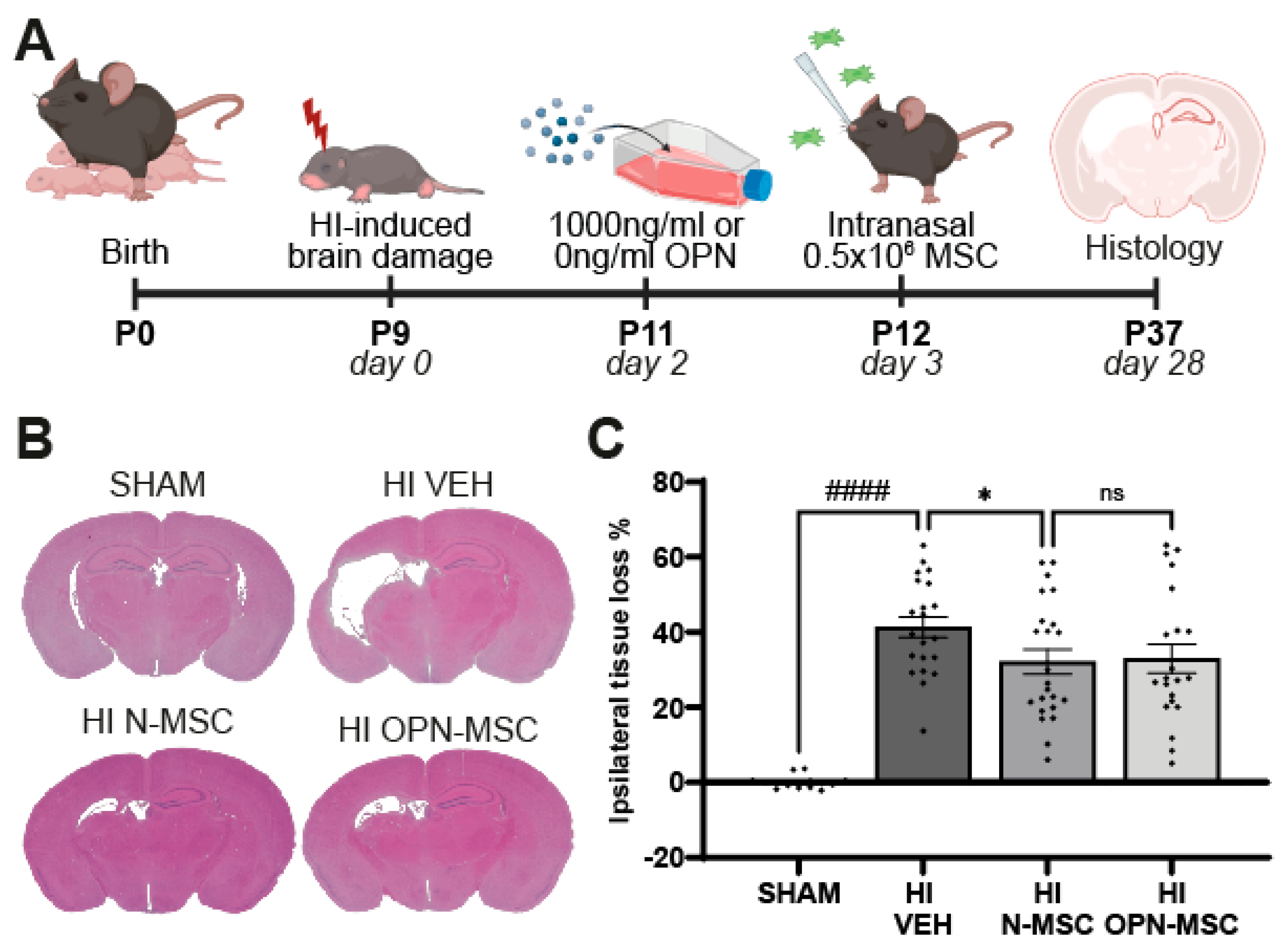
3.4. Osteopontin Preconditioning Does Not Enhance the Therapeutic Efficacy of Intranasal MSC Therapy After Neonatal HI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bdnf | brain-derived neurotrophic factor |
| Cox-2 | cycooxigenase-2 |
| ERK | extracellular-signal-regulated kinase |
| FCS | fetal calf serum |
| Fgf-2 | fibroblast growth factor 2 |
| HI | hypoxia-ischemia/hypoxic-ischemic |
| HIE | hypoxic-ischemic encephalopathy |
| Il-6 | interleukin-6 |
| MSCs | mesenchymal stem cells |
| NGF | nerve growth factor |
| N-MSCs | naïve (non-preconditioned) MSCs |
| NSCs | neural stem cells |
| Opn | osteopontin |
| OPN-MSCs | osteopontin-preconditioned MSCs |
| SEM | standard error of the mean |
| Spp1 | secreted phosphoprotein 1 |
| Tgf-β | transforming growth factor β |
| Tnf-α | tumor necrosis factor α |
| Vegf | vascular endothelial growth factor |
References
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-Ischemic Encephalopathy A Review for the Clinician. JAMA Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wassink, G.; Gunn, E.R.; Drury, P.P.; Bennet, L.; Gunn, A.J. The mechanisms and treatment of asphyxial encephalopathy. Front. Neurosci. 2014, 8, 40. [Google Scholar] [CrossRef]
- Van Schie, P.E.M.; Schijns, J.; Becher, J.G.; Barkhof, F.; Van Weissenbruch, M.M.; Vermeulen, R.J. Long-term motor and behavioral outcome after perinatal hypoxic-ischemic encephalopathy. Eur. J. Paediatr. Neurol. 2015, 19, 354–359. [Google Scholar] [CrossRef]
- Ferriero, D.M. Neonatal Brain Injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef]
- Pappas, A.; Shankaran, S.; McDonald, S.A.; Vohr, B.R.; Hintz, S.R.; Ehrenkranz, R.A.; Tyson, J.E.; Yolton, K.; Das, A.; Bara, R.; et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics 2015, 135, e624–e634. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Ritter, S.; Brotschi, B.; Werner, H.; Caflisch, J.; Martin, E.; Latal, B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 454–459. [Google Scholar] [CrossRef]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; Mcdonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-Body Hypothermia for Neonates with Hypoxic–Ischemic Encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef]
- Kariholu, U.; Montaldo, P.; Markati, T.; Lally, P.J.; Pryce, R.; Teiserskas, J.; Liow, N.; Oliveira, V.; Soe, A.; Shankaran, S.; et al. Therapeutic hypothermia for mild neonatal encephalopathy: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 225–228. [Google Scholar] [CrossRef]
- Donega, V.; Nijboer, C.H.; van Tilborg, G.; Dijkhuizen, R.M.; Kavelaars, A.; Heijnen, C.J. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 2014, 261, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Donega, V.; Nijboer, C.H.; Braccioli, L.; Slaper-Cortenbach, I.; Kavelaars, A.; Van Bel, F.; Heijnen, C.J. Intranasal administration of human MSC for ischemic brain injury in the mouse: In vitro and in vivo neuroregenerative functions. PLoS ONE 2014, 9, e112339. [Google Scholar] [CrossRef]
- Donega, V.; van Velthoven, C.T.J.; Nijboer, C.H.; van Bel, F.; Kas, M.J.H.; Kavelaars, A.; Heijnen, C.J. Intranasal Mesenchymal Stem Cell Treatment for Neonatal Brain Damage: Long-Term Cognitive and Sensorimotor Improvement. PLoS ONE 2013, 8, e51253. [Google Scholar] [CrossRef]
- Donega, V.; Nijboer, C.H.; Van Velthoven, C.T.J.; Youssef, S.A.; De Bruin, A.; Van Bel, F.; Kavelaars, A.; Heijnen, C.J. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr. Res. 2015, 78, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Donega, V.; Van Velthoven, C.T.J.; Nijboer, C.H.; Kavelaars, A.; Heijnen, C.J. The endogenous regenerative capacity of the damaged newborn brain: Boosting neurogenesis with mesenchymal stem cell treatment. J. Cereb. Blood Flow. Metab. 2013, 33, 625–634. [Google Scholar] [CrossRef]
- Wagenaar, N.; Nijboer, C.H.; van Bel, F. Repair of neonatal brain injury: Bringing stem cell-based therapy into clinical practice. Dev. Med. Child. Neurol. 2017, 59, 997–1003. [Google Scholar] [CrossRef]
- Baak, L.M.; Wagenaar, N.; van der Aa, N.E.; Groenendaal, F.; Dudink, J.; Tataranno, M.L.; Mahamuud, U.; Verhage, C.H.; Eijsermans, R.M.J.C.; Smit, L.S.; et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in The Netherlands (PASSIoN): A first-in-human, open-label intervention study. Lancet Neurol. 2022, 21, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, N.; Baak, L.M.; van der Aa, N.E.; Groenendaal, F.; Dudink, J.; Tataranno, M.L.; Koopman, C.; Verhage, C.H.; Eijsermans, R.M.J.C.; van Teeseling, H.C.; et al. PASSIoN Trial (Perinatal Arterial Stroke Treated With Intranasal Stromal Cells): 2-Year Safety and Neurodevelopment. Stroke 2025, 56, 9. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- De Palma, S.T.; Hermans, E.C.; Shamorkina, T.M.; Trayford, C.; van Rijt, S.; Heck, A.J.R.; Nijboer, C.H.A.; de Theije, C.G.M. Hypoxic Preconditioning Enhances the Potential of Mesenchymal Stem Cells to Treat Neonatal Hypoxic-Ischemic Brain Injury. Stroke 2025, 56, 1872–1882. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Calligaris, M.; Zito, G.; Busà, R.; Bulati, M.; Iannolo, G.; Gallo, A.; Carreca, A.P.; Cuscino, N.; Castelbuono, S.; Carcione, C.; et al. Proteomic analysis and functional validation reveal distinct therapeutic capabilities related to priming of mesenchymal stromal/stem cells with IFN-γ and hypoxia: Potential implications for their clinical use. Front. Cell Dev. Biol. 2024, 12, 1385712. [Google Scholar] [CrossRef]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor. Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, H.; Bulbule, A.; Kundu, G.C. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol. 2006, 16, 79–87. [Google Scholar] [CrossRef]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef]
- Wang, X.; Louden, C.; Yue, T.L.; Ellison, J.A.; Barone, F.C.; Solleveld, H.A.; Feuerstein, G.Z. Delayed Expression of Osteopontin after Focal Stroke in the Rat. J. Neurosci. 1998, 18, 2075–2083. [Google Scholar] [CrossRef]
- Hedtjarn, M.; Mallard, C.; Hagberg, H. Inflammatory Gene Profiling in the Developing Mouse Brain after Hypoxia-Ischemia. J. Cereb. Blood Flow. Metab. 2004, 24, 1333–1351. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.J.; Heijnen, C.J.; Van Bel, F.; Kavelaars, A. Osteopontin enhances endogenous repair after neonatal hypoxic-ischemic brain injury. Stroke 2011, 42, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Matusan-Ilijas, K.; Behrem, S.; Jonjic, N.; Zarkovic, K.; Lucin, K. Osteopontin expression correlates with angiogenesis and survival in malignant astrocytoma. Pathol. Oncol. Res. 2008, 14, 293–298. [Google Scholar] [CrossRef]
- Chabas, D.; Baranzini, S.E.; Mitchell, D.; Bernard, C.C.A.; Rittling, S.R.; Denhardt, D.T.; Sobel, R.A.; Lock, C.; Karpuj, M.; Pedotti, R.; et al. The Influence of the Proinflammatory Cytokine, Osteopontin, on Autoimmune Demyelinating Disease. Science 2001, 294, 1731–1735. [Google Scholar] [CrossRef]
- Maetzler, W.; Berg, D.; Schalamberidze, N.; Melms, A.; Schott, K.; Mueller, J.C.; Liaw, L.; Gasser, T.; Nitsch, C. Osteopontin is elevated in Parkinson’s disease and its absence leads to reduced neurodegeneration in the MPTP model. Neurobiol. Dis. 2007, 25, 473–482. [Google Scholar] [CrossRef]
- Yan, Y.P.; Lang, B.T.; Vemuganti, R.; Dempsey, R.J. Osteopontin is a mediator of the lateral migration of neuroblasts from the subventricular zone after focal cerebral ischemia. Neurochem. Int. 2009, 55, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.S.G.; Dempsey, R.J. Osteopontin increases the proliferation of neural progenitor cells. Int. J. Dev. Neurosci. 2012, 30, 359–362. [Google Scholar] [CrossRef]
- Rabenstein, M.; Hucklenbroich, J.; Willuweit, A.; Ladwig, A.; Fink, G.R.; Schroeter, M.; Langen, K.J.; Rueger, M.A. Osteopontin mediates survival, proliferation and migration of neural stem cells through the chemokine receptor CXCR4. Stem Cell Res. Ther. 2015, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Meller, R.; Stevens, S.L.; Minami, M.; Cameron, J.A.; King, S.; Rosenzweig, H.; Doyle, K.; Lessov, N.S.; Simon, R.P.; Stenzel-Poore, M.P. Neuroprotection by osteopontin in stroke. J. Cereb. Blood Flow. Metab. 2005, 25, 217–225. [Google Scholar] [CrossRef]
- Suzuki, H.; Hasegawa, Y.; Kanamaru, K.; Zhang, J.H. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 2010, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, Q.; Suzuki, H.; Hartman, R.; Tang, J.; Zhang, J.H. Osteopontin reduced hypoxia-ischemia neonatal brain injury by suppression of apoptosis in a rat pup model. Stroke 2011, 42, 764–769. [Google Scholar] [CrossRef]
- Suzuki, H.; Ayer, R.; Sugawara, T.; Chen, W.; Sozen, T.; Hasegawa, Y.; Kanamaru, K.; Zhang, J.H. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit. Care Med. 2010, 38, 612–618. [Google Scholar] [CrossRef]
- Doyle, K.P.; Yang, T.; Lessov, N.S.; Ciesielski, T.M.; Stevens, S.L.; Simon, R.P.; King, J.S.; Stenzel-Poore, M.P. Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke. J. Cereb. Blood Flow. Metab. 2008, 28, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Synergistic effect of extracellularly supplemented osteopontin and osteocalcin on stem cell proliferation, osteogenic differentiation, and angiogenic properties. J. Cell Biochem. 2019, 120, 6555–6569. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef]
- Chakraborty, G.; Jain, S.; Behera, R.; Ahmed, M.; Sharma, P.; Kumar, V.; Kundu, G.C. The Multifaceted Roles of Osteopontin in Cell Signaling, Tumor Progression and Angiogenesis. Curr. Mol. Med. 2006, 6, 819–830. [Google Scholar] [CrossRef]
- O’Regan, A.; Berman, J.S. Osteopontin: A key cytokine in cell-mediated and granulomatous inflammation. Ganulomatous Dis. Rev. 2000, 81, 373–390. [Google Scholar] [CrossRef]
- Mazzali, M.; Kipari, T.; Ophascharoensuk, V.; Wesson, J.A.; Johnson, R.; Hughes, J. Osteopontin-a molecule for all seasons. Q. J. Med. 2002, 95, 3–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Lesani, P.; Lu, Z.; Zreiqat, H. Osteopontin Rejuvenates Senescent Adipose-Derived Stem Cells and Restores their Bone Tissue Regenerative Function. Stem Cell Rev. Rep. 2024, 20, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Luo, Q.; Qin, J.; Shi, Y.; Yang, L.; Ju, B.; Song, G. Osteopontin Promotes Mesenchymal Stem Cell Migration and Lessens Cell Stiffness via Integrin β1, FAK, and ERK Pathways. Cell Biochem. Biophys. 2013, 65, 455–462. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Q.; Sun, J.; Wang, A.; Shi, Y.; Ju, Y.; Morita, Y.; Song, G. Decreased nuclear stiffness via FAK-ERK1/2 signaling is necessary for osteopontin-promoted migration of bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 2017, 355, 172–181. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Q.; Sun, J.; Ju, Y.; Morita, Y.; Song, G. Chromatin organization regulated by EZH2-mediated H3K27me3 is required for OPN-induced migration of bone marrow-derived mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2018, 96, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Song, G.; Luo, Q.; Yuan, L.; Yang, L. Mesenchymal stem cells require integrin β1 for directed migration induced by osteopontin in vitro. Vitr. Cell Dev. Biol. Anim. 2011, 47, 241–250. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Q.; Sun, J.; Song, G. Cytoskeletal control of nuclear morphology and stiffness are required for OPN-induced bone marrow-derived mesenchymal stem cell migration. Biochem. Cell Biol. 2019, 97, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Wang, Z.; Wang, W.; Li, W.; Wu, Q.; Lei, X.; Ouyang, X.; Liang, Z. Effect of osteopontin in regulating bone marrow mesenchymal stem cell treatment of skin wounds in diabetic mice. Diabetes Metab. Res. Rev. 2014, 30, 457–466. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.; Li, W.; Jiang, J.; Cui, Y.; Li, S.; Wang, Z. Osteopontin activates mesenchymal stem cells to repair skin wound. PLoS ONE 2017, 12, e0185346. [Google Scholar] [CrossRef]
- Lee, M.N.; Hwang, H.S.; Oh, S.H.; Roshanzadeh, A.; Kim, J.W.; Song, J.H.; Kim, E.S.; Koh, J.T. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.N.; Song, J.H.; Oh, S.H.; Tham, N.T.; Kim, J.W.; Yang, J.W.; Kim, E.S.; Koh, J.T. The primary cilium directs osteopontin-induced migration of mesenchymal stem cells by regulating CD44 signaling and Cdc42 activation. Stem Cell Res. 2020, 45, 101799. [Google Scholar] [CrossRef]
- Vaes, J.E.G.; van Kammen, C.M.; Trayford, C.; van der Toorn, A.; Ruhwedel, T.; Benders, M.J.N.L.; Dijkhuizen, R.M.; Möbius, W.; van Rijt, S.H.; Nijboer, C.H. Intranasal mesenchymal stem cell therapy to boost myelination after encephalopathy of prematurity. Glia 2021, 69, 655–680. [Google Scholar] [CrossRef]
- Spittau, B.; Dokalis, N.; Prinz, M. The Role of TGFβ Signaling in Microglia Maturation and Activation. Trends Immunol. 2020, 41, 836–848. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Nesic-Taylor, O.; Qiu, J.; Rea, H.C.; Fabian, R.; Rassin, D.K.; Perez-Polo, J.R. Activation of nuclear factor-κB signaling pathway by interleukin-1 after hypoxia/ischemia in neonatal rat hippocampus and cortex. J. Neurochem. 2005, 93, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Poligone, B.; Baldwin, A.S. Positive and negative regulation of NF-κB by COX-2. Roles of different prostaglandins. J. Biol. Chem. 2001, 276, 38658–38664. [Google Scholar] [CrossRef]
- Kucherova, K.S.; Koroleva, E.S.; Alifirova, V.M. The Role of VEGF in Angiogenesis and Motor Recovery after Ischemic Stroke. Neurochem. J. 2023, 17, 528–533. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Investig. 2003, 111, 1843–1851. [Google Scholar] [CrossRef]
- Dobolyi, A.; Vincze, C.; Pál, G.; Lovas, G. The neuroprotective functions of transforming growth factor beta proteins. Int. J. Mol. Sci. 2012, 13, 8219–8258. [Google Scholar] [CrossRef]
- Knöferle, J.; Ramljak, S.; Koch, J.C.; Tönges, L.; Asif, A.R.; Michel, U.; Wouters, F.S.; Heermann, S.; Krieglstein, K.; Zerr, I.; et al. TGF-β 1 enhances neurite outgrowth via regulation of proteasome function and EFABP. Neurobiol. Dis. 2010, 38, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef] [PubMed]
- Plantman, S. Osteopontin is upregulated after mechanical brain injury and stimulates neurite growth from hippocampal neurons through β1 integrin and CD44. Neuroreport 2012, 23, 647–652. [Google Scholar] [CrossRef]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Liu, Q.F.; Liu, G.N. Positive regulation of the Egr-1/osteopontin positive feedback loop in rat vascular smooth muscle cells by TGF-β, ERK, JNK, and p38 MAPK signaling. Biochem. Biophys. Res. Commun. 2010, 396, 451–456. [Google Scholar] [CrossRef]
- Sondell, M.; Ran Lundborg, G.; Kanje, M. Vascular Endothelial Growth Factor Has Neurotrophic Activity and Stimulates Axonal Outgrowth, Enhancing Cell Survival and Schwann Cell Proliferation in the Peripheral Nervous System. J. Neurosci. 1999, 19, 5731–5740. [Google Scholar] [CrossRef]
- Schäfer, K.H.; Mestres, P.; März, P.; Rose-John, S.N. The IL-6/sIL-6R Fusion Protein Hyper-IL-6 Promotes Neurite Outgrowth and Neuron Survival in Cultured Enteric Neurons. J. Interferon Cytokin Res. 1999, 19, 527–532. [Google Scholar] [CrossRef]
- Albertsson, A.M.; Zhang, X.; Leavenworth, J.; Bi, D.; Nair, S.; Qiao, L.; Hagberg, H.; Mallard, C.; Cantor, H.; Wang, X. The effect of osteopontin and osteopontin-derived peptides on preterm brain injury. J. Neuroinflamm. 2014, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.S.; Merrill, G.F.; Shinohara, M.L.; Denhardt, D.T. Osteopontin expression during early cerebral ischemia-reperfusion in rats: Enhanced expression in the right cortex is suppressed by acetaminophen. PLoS ONE 2011, 6, e14568. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, H.Y.; Cha, J.H.; Choi, J.Y.; Lee, M.Y. Transient microglial and prolonged astroglial upregulation of osteopontin following transient forebrain ischemia in rats. Brain Res. 2007, 1151, 195–202. [Google Scholar] [CrossRef]
- Ellison, J.A.; Velier, J.J.; Spera, P.; Jonak, Z.L.; Wang, X.; Barone, F.C.; Feuerstein, G.Z. Osteopontin and its integrin receptor alpha(v)beta3 are upregulated during formation of the glial scar after focal stroke. Stroke 1998, 29, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dammer, E.B.; Brotzge, X.Z.; Chen, S.; Duong, D.M.; Seyfried, N.T.; Kuan, C.Y.; Sun, Y.Y. Osteopontin Is a Blood Biomarker for Microglial Activation and Brain Injury in Experimental Hypoxic-Ischemic Encephalopathy. eNeuro 2017, 4, ENEURO.0253-16.2016. [Google Scholar] [CrossRef]
| Name | Forward Sequence (5′ ⟶ 3′) | Reverse Sequence (3′ ⟶ 5′) |
|---|---|---|
| Bdnf | CACATTACCTTCCAGCATCTGTTG | ACCATAGTAAGGAAAAGGATGGTCAT |
| Cox-2 | GGTCTGGTGCCTGGTCTG | CTCTCCTATGAGTATGAGTCTGC |
| Fgf-2 | GCGAGAAGAGCGACCCACAC | GAAGCCAGCAGCCGTCCATC |
| Il-6 | TCTAATTCATATCTTCAACCAAGAGG | TGGTCCTTAGCCACTCCTTC |
| Opn | TGGACTGAGGTCAAAGTCTAGGA | CCGCTCTTCATGTGAGAGGTGA |
| Ngf | ACGGGCAGCATGGTGGAG | TGTAGAACAACATGGACATTACGC |
| Tgf-β1 | GTGACAGCAAAGATAACAAAC | CTGAAGCAATAGTTGGTATCC |
| Vegf-b | GATCCTCTGCCCGCCTTG | CCCGTGGAGTCTGGAAAGC |
| β-actin | AGAGGGAAATCGTGCGTGAC | CAATAGTGATGACCTGGCCGT |
| Experimental Group | Number of Animals Gene Expression | Number of Animals Histological Outcome | ||||
|---|---|---|---|---|---|---|
| Total | Females | Males | Total | Females | Males | |
| SHAM | 7 | 3 | 4 | 13 | 6 | 7 |
| HI VEH | 9 | 4 | 5 | 23 | 10 | 13 |
| HI N-MSC | N.A. | 24 | 11 | 13 | ||
| HI OPN-MSC | N.A. | 22 | 10 | 12 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Palma, S.T.; van Wijk-Eeftink, C.N.; Baak, L.M.; Nijboer, C.H.A.; de Theije, C.G.M. Osteopontin Preconditioning Improves the Regenerative Effects of Mesenchymal Stem Cells In Vitro but Not Their Therapeutic Efficacy Following Hypoxia-Ischemia in Mice. Cells 2025, 14, 1824. https://doi.org/10.3390/cells14221824
De Palma ST, van Wijk-Eeftink CN, Baak LM, Nijboer CHA, de Theije CGM. Osteopontin Preconditioning Improves the Regenerative Effects of Mesenchymal Stem Cells In Vitro but Not Their Therapeutic Efficacy Following Hypoxia-Ischemia in Mice. Cells. 2025; 14(22):1824. https://doi.org/10.3390/cells14221824
Chicago/Turabian StyleDe Palma, Sara T., Celine N. van Wijk-Eeftink, Lisanne M. Baak, Cora H. A. Nijboer, and Caroline G. M. de Theije. 2025. "Osteopontin Preconditioning Improves the Regenerative Effects of Mesenchymal Stem Cells In Vitro but Not Their Therapeutic Efficacy Following Hypoxia-Ischemia in Mice" Cells 14, no. 22: 1824. https://doi.org/10.3390/cells14221824
APA StyleDe Palma, S. T., van Wijk-Eeftink, C. N., Baak, L. M., Nijboer, C. H. A., & de Theije, C. G. M. (2025). Osteopontin Preconditioning Improves the Regenerative Effects of Mesenchymal Stem Cells In Vitro but Not Their Therapeutic Efficacy Following Hypoxia-Ischemia in Mice. Cells, 14(22), 1824. https://doi.org/10.3390/cells14221824







