Enamel Maturation as a Systems Physiology: Ion Transport and Pi Flux
Abstract
1. Introduction
2. Overview of Amelogenesis
2.1. Pre-Secretory Stage
2.2. Secretory Stage
2.3. Transition Stage
2.4. Maturation Stage
2.5. Molecular Regulation and Signaling
2.6. Clinical Implications and Enamel Defects
3. Ion Transport During Enamel Maturation
3.1. Integrated Regulation of Ion Transport During Enamel Maturation
3.2. Calcium Transport
3.3. Acid-Base Control: Bicarbonate and Chloride
3.4. Sodium and Potassium Handling
3.5. Magnesium and Trace Elements
3.6. Citrate Transport and Its Role in Enamel Mineralization
3.7. Phosphate Transport and Regulation During Enamel Maturation
3.7.1. Historical Insights into Phosphate Dynamics
3.7.2. Phosphate Transporters
- (a)
- SLC20 family: PiT1 (SLC20A1) and PiT2 (SLC20A2)PiT1 and PiT2 are type III Na+-phosphate symporters that import the monovalent phosphate species (H2PO4−) with an electrogenic 2 Na+:1 Pi stoichiometry, a property established by heterologous transport measurements and refined by recent structural work (Table 1) [237,286]. In vivo mapping across murine tooth germs shows Slc20a1/PiT1 is predominantly expressed in ameloblasts, with signal strongest postnatally and most evident in maturing cells, while odontoblasts are largely negative in these sections. By contrast, multiple dentin/odontoblast model systems and human pulp-derived odontoblasts do express SLC20A1 in vitro, indicating species-, stage-, and model-dependent differences. PiT2 shows a developmentally dynamic pattern that includes a transient but strong signal that appears in secretory ameloblasts, while high and persistent expression is found in the stratum intermedium and, later, the papillary and sub-odontoblastic layers as teeth mature [9,287]. More recent systematic in situ/LacZ analyses similarly localize Slc20a2 away from ameloblasts and into supporting layers [9,287]. Functionally, the SLC20 carriers are widely regarded as “housekeeping” phosphate importers that maintain intracellular Pi for ATP generation and biosynthesis in polarized epithelia. In enamel organs, this role aligns with the energy-intensive transitions from secretion into maturation [288,289]. Older foundational studies that first identified the PiT family as Na+-dependent phosphate symporters using viral receptor clones in oocytes remain key precedents for their transport identity [290].
- (b)
- SLC34 family: NaPi-IIb (SLC34A2)NaPi-IIb is the type II sodium-phosphate cotransporter identified in the enamel organ and operates as an electrogenic 3 Na+:1 HPO42− carrier, providing high-capacity Pi transport (Table 1) [237,291]. In rodents, NaPi-IIb expression is low in secretory ameloblasts and rises sharply in maturation, and immunolocalization shows intense signal over the apical plasma membrane of early and late maturation ameloblasts with only weak apical staining in secretory cells. Papillary cells also stain for NaPi-IIb. This stage- and domain-specific pattern aligns with the increased mineral demand and RA↔SA modulation that characterize maturation [1,10]. Beyond stage control, NaPi-IIb function and abundance are pH- and milieu-responsive in epithelia. In the intestine, it exhibits pH-dependent transport kinetics, and its brush-border abundance increases during metabolic acidosis, suggesting a general capacity for acid-linked up-regulation, although this has not yet been demonstrated directly in ameloblasts [292,293,294]. Reports of strong apical NaPi-IIb in late maturation indicate a potential role in the apical Pi uptake from the enamel space into the ameloblasts (Figure 3). Any apical efflux toward the matrix would require a bona fide exporter such as XPR1, but such an apical efflux role remains highly speculative for NaPi-IIb [10].
- (c)
- XPR1 (phosphate exporter)XPR1 is the only recognized inorganic phosphate exporter in mammalian cells, and structural and biochemical work now defines its Pi-export mechanism [11,295]. In teeth, XPR1 is expressed during postnatal stages when enamel mineralization accelerates, rising alongside other Pi transporters as ameloblasts shift from matrix secretion toward protein resorption and crystal deposition (Figure 3); this timing is compatible with an efflux role at the matrix-facing surface, although direct membrane-polarity mapping in ameloblasts remains limited. Taken together, the convergence of export mechanism (from other tissues) and developmental expression (in enamel organs) makes XPR1 the leading candidate for the apical efflux limb that complements SLC20/SLC34-mediated uptake (Table 1) [9,11].
3.7.3. Transport Mechanisms and Intracellular Handling of Phosphate During Enamel Maturation
3.7.4. Systemic Versus Local Regulation of Phosphate Availability (With Temporal Dynamics of Incorporation)
4. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Jayasudha; Baswaraj; HK, N.; KB, P. Enamel regeneration—Current progress and challenges. J. Clin. Diagn. Res. 2014, 8, ZE06–ZE09. [Google Scholar]
- Bartlett, J.D. Dental enamel development: Proteinases and their enamel matrix substrates. ISRN Dent. 2013, 2013, 684607. [Google Scholar] [CrossRef]
- Smith, C.E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998, 9, 128–161. [Google Scholar] [CrossRef]
- Lacruz, R.S. Enamel: Molecular identity of its transepithelial ion transport system. Cell Calcium 2017, 65, 1–7. [Google Scholar] [CrossRef]
- Robinson, C. Enamel maturation: A brief background with implications for some enamel dysplasias. Front. Physiol. 2014, 5, 388. [Google Scholar] [CrossRef]
- Bronckers, A.L. Ion Transport by Ameloblasts during Amelogenesis. J. Dent. Res. 2017, 96, 243–253. [Google Scholar]
- Wong, F.S.; Elliott, J.C.; Davis, G.R.; Anderson, P. X-ray microtomographic study of mineral distribution in enamel of mandibular rat incisors. J. Anat. 2000, 196 Pt 3, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Merametdjian, L.; David, A.; Bon, N.; Couasnay, G.; Guicheux, J.; Gaucher, C.; Beck-Cormier, S.; Beck, L. Expression of Phosphate Transporters during Dental Mineralization. J. Dent. Res. 2018, 97, 209–217. [Google Scholar] [PubMed]
- Bronckers, A.L.; Lyaruu, D.; Jalali, R.; Medina, J.F.; Zandieh-Doulabi, B.; DenBesten, P.K. Ameloblast Modulation and Transport of Cl−, Na+, and K+ during Amelogenesis. J. Dent. Res. 2015, 94, 1740–1747. [Google Scholar] [CrossRef]
- Yan, R.; Chen, H.; Liu, C.; Zhao, J.; Wu, D.; Jiang, J.; Gong, J.; Jiang, D. Human XPR1 structures reveal phosphate export mechanism. Nature 2024, 633, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Regnstrand, T.; Skott, P.; Mäkitie, O.; Björnsdottir, S.; Garming-Legert, K. Dental health of patients with X-linked hypophosphatemia: A controlled study. Front. Oral Health 2023, 4, 1087761. [Google Scholar] [CrossRef]
- Nanci, A.; Ten Cate, A.R. Ten Cate’s Oral Histology: Development, Structure, and Function, 8th ed.; Elsevier: St. Louis, MO, USA, 2013; p. 407. [Google Scholar]
- Klein, O.D.; Duverger, O.; Shaw, W.; Lacruz, R.S.; Joester, D.; Moradian-Oldak, J.; Pugach, M.K.; Wright, J.T.; Millar, S.E.; Kulkarni, A.B.; et al. Meeting report: A hard look at the state of enamel research. Int. J. Oral Sci. 2017, 9, e3. [Google Scholar] [CrossRef]
- Kegulian, N.C.; Visakan, G.; Bapat, R.A.; Moradian-Oldak, J. Ameloblastin and its multifunctionality in amelogenesis: A review. Matrix Biol. 2024, 131, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? Int. J. Mol. Sci. 2020, 21, 4031. [Google Scholar] [CrossRef]
- Balic, A. Concise Review: Cellular and Molecular Mechanisms Regulation of Tooth Initiation. Stem Cells 2019, 37, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Wang, J.; Okamoto, K.; Iwata, T. The next generation of regenerative dentistry: From tooth development biology to periodontal tissue, dental pulp, and whole tooth reconstruction in the clinical setting. Regen. Ther. 2025, 28, 333–344. [Google Scholar] [CrossRef]
- Biggs, L.C.; Mikkola, M.L. Early inductive events in ectodermal appendage morphogenesis. Semin. Cell Dev. Biol. 2014, 25–26, 11–21. [Google Scholar] [CrossRef]
- Jussila, M.; Thesleff, I. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb. Perspect. Biol. 2012, 4, a008425. [Google Scholar] [CrossRef]
- Novacescu, D.; Dumitru, C.S.; Zara, F.; Raica, M.; Suciu, C.S.; Barb, A.C.; Rakitovan, M.; Armega Anghelescu, A.; Cindrea, A.C.; Diana, S.; et al. The Morphogenesis, Pathogenesis, and Molecular Regulation of Human Tooth Development—A Histological Review. Int. J. Mol. Sci. 2025, 26, 6209. [Google Scholar] [CrossRef]
- Robinson, C.; Brookes, S.J.; Shore, R.C.; Kirkham, J. The developing enamel matrix: Nature and function. Eur. J. Oral Sci. 1998, 106 (Suppl. S1), 282–291. [Google Scholar] [CrossRef]
- Warshawsky, H. The fine structure of secretory ameloblasts in rat incisors. Anat. Rec. 1968, 161, 211–229. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Nanci, A.; Kurtz, I.; Wright, J.T.; Paine, M.L. Regulation of pH During Amelogenesis. Calcif. Tissue Int. 2010, 86, 91–103. [Google Scholar] [CrossRef]
- Smith, C.E.L.; Poulter, J.A.; Antanaviciute, A.; Kirkham, J.; Brookes, S.J.; Inglehearn, C.F.; Mighell, A.J. Amelogenesis Imperfecta; Genes, Proteins, and Pathways. Front. Physiol. 2017, 8, 435. [Google Scholar] [CrossRef]
- Warshawsky, H.; Smith, C.E. Morphological classification of rat incisor ameloblasts. Anat. Rec. 1974, 179, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Reith, E.J. The stages of amelogenesis as observed in molar teeth of young rats. J. Ultrastruct. Res. 1970, 30, 111–151. [Google Scholar] [CrossRef]
- Smith, C.E.; Nanci, A. Protein dynamics of amelogenesis. Anat. Rec. 1996, 245, 186–207. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Smith, C.E.; Kurtz, I.; Hubbard, M.J.; Paine, M.L. New paradigms on the transport functions of maturation-stage ameloblasts. J. Dent. Res. 2013, 92, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 9th ed.; Elsevier: St. Louis, MO, USA, 2023. [Google Scholar]
- Thesleff, I.; Hurmerinta, K. Tissue interactions in tooth development. Differentiation 1981, 18, 75–88. [Google Scholar] [CrossRef]
- Reibring, C.G.; El Shahawy, M.; Hallberg, K.; Harfe, B.D.; Linde, A.; Gritli-Linde, A. Loss of BMP2 and BMP4 Signaling in the Dental Epithelium Causes Defective Enamel Maturation and Aberrant Development of Ameloblasts. Int. J. Mol. Sci. 2022, 23, 6095. [Google Scholar] [CrossRef] [PubMed]
- Bei, M. Molecular genetics of ameloblast cell lineage. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312B, 437–444. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Kim, S.O.; Radlanski, R.J.; Butcher, K.; Schneider, R.A.; DenBesten, P.K. Ameloblast differentiation in the human developing tooth: Effects of extracellular matrices. Matrix Biol. 2010, 29, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Karcher-Djuricic, V.; Staubli, A.; Meyer, J.M.; Ruch, J.V. Acellular dental matrices promote functional differentiation of ameloblasts. Differentiation 1985, 29, 169–175. [Google Scholar] [CrossRef]
- Pham, C.D.; Smith, C.E.; Hu, Y.; Hu, J.C.; Simmer, J.P.; Chun, Y.P. Endocytosis and Enamel Formation. Front. Physiol. 2017, 8, 529. [Google Scholar] [CrossRef]
- Begue-Kirn, C.; Krebsbach, P.H.; Bartlett, J.D.; Butler, W.T. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: Tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur. J. Oral Sci. 1998, 106, 963–970. [Google Scholar] [CrossRef]
- Visakan, G.; Su, J.; Moradian-Oldak, J. Ameloblastin promotes polarization of ameloblast cell lines in a 3-D cell culture system. Matrix Biol. 2022, 105, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Reith, E.J. The ultrastructure of ameloblasts during matrix formation and the maturation of enamel. J. Biophys. Biochem. Cytol. 1961, 9, 825–839. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Fukumoto, S.; Bikle, D.D.; Oda, Y. Transcriptional Regulation of Dental Epithelial Cell Fate. Int. J. Mol. Sci. 2020, 21, 8952. [Google Scholar] [CrossRef]
- Hu, J.C.; Hu, Y.; Smith, C.E.; McKee, M.D.; Wright, J.T.; Yamakoshi, Y.; Papagerakis, P.; Hunter, G.K.; Feng, J.Q.; Yamakoshi, F.; et al. Enamel defects and ameloblast-specific expression in Enam knock-out/lacz knock-in mice. J. Biol. Chem. 2008, 283, 10858–10871. [Google Scholar] [CrossRef]
- Nanci, A.; Zalzal, S.; Lavoie, P.; Kunikata, M.; Chen, W.; Krebsbach, P.H.; Yamada, Y.; Hammarström, L.; Simmer, J.P.; Fincham, A.G.; et al. Comparative immunochemical analyses of the developmental expression and distribution of ameloblastin and amelogenin in rat incisors. J. Histochem. Cytochem. 1998, 46, 911–934. [Google Scholar] [CrossRef] [PubMed]
- Landin, M.A.; Shabestari, M.; Babaie, E.; Reseland, J.E.; Osmundsen, H. Gene Expression Profiling during Murine Tooth Development. Front. Genet. 2012, 3, 139. [Google Scholar] [CrossRef]
- Vainio, S.; Karavanova, I.; Jowett, A.; Thesleff, I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 1993, 75, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, P.; Laurikkala, J.; Itäranta, P.; Vainio, S.; Itoh, N.; Thesleff, I. Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev. Dyn. 2000, 219, 322–332. [Google Scholar] [CrossRef]
- Dassule, H.R.; Lewis, P.; Bei, M.; Maas, R.; McMahon, A.P. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 2000, 127, 4775–4785. [Google Scholar] [CrossRef]
- Krebsbach, P.H.; Lee, S.K.; Matsuki, Y.; Kozak, C.A.; Yamada, K.M.; Yamada, Y. Full-length sequence, localization, and chromosomal mapping of ameloblastin. A novel tooth-specific gene. J. Biol. Chem. 1996, 271, 4431–4435. [Google Scholar] [CrossRef]
- Fukumoto, S.; Kiba, T.; Hall, B.; Iehara, N.; Nakamura, T.; Longenecker, G.; Krebsbach, P.H.; Nanci, A.; Kulkarni, A.B.; Yamada, Y. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J. Cell Biol. 2004, 167, 973–983. [Google Scholar] [CrossRef]
- Paine, M.L.; White, S.N.; Luo, W.; Fong, H.; Sarikaya, M.; Snead, M.L. Regulated gene expression dictates enamel structure and tooth function. Matrix Biol. 2001, 20, 273–292. [Google Scholar] [CrossRef]
- Deporter, D.A.; Ten Cate, A.R. Fine structural localization of alkaline phosphatase in relation to enamel formation in the mouse molar. Arch. Oral Biol. 1976, 21, 7–12. [Google Scholar] [CrossRef]
- Ten Cate, A.R. The distribution of alkaline phosphatase in the human tooth germ. Arch. Oral Biol. 1962, 7, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ida-Yonemochi, H.; Ohshiro, K.; Swelam, W.; Metwaly, H.; Saku, T. Perlecan, a basement membrane-type heparan sulfate proteoglycan, in the enamel organ: Its intraepithelial localization in the stellate reticulum. J. Histochem. Cytochem. 2005, 53, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Katchburian, E.; Holt, S.J. Studies on the development of ameloblasts. I. Fine structure. J. Cell Sci. 1972, 11, 415–447. [Google Scholar] [CrossRef]
- Simmer, J.P.; Richardson, A.S.; Hu, Y.Y.; Smith, C.E.; Ching-Chun Hu, J. A post-classical theory of enamel biomineralization... and why we need one. Int. J. Oral Sci. 2012, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Simmer, J.P. Kallikrein-related peptidase-4 (KLK4): Role in enamel formation and revelations from ablated mice. Front. Physiol. 2014, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.P.; Warshawsky, H. Dynamics of enamel formation in the rat incisor tooth. J. Dent. Res. 1979, 58, 950–975. [Google Scholar] [CrossRef] [PubMed]
- Fukae, M.; Tanabe, T.; Murakami, C.; Dohi, N.; Uchida, T.; Shimizu, M. Primary structure of the porcine 89-kDa enamelin. Adv. Dent. Res. 1996, 10, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.A.; Conway, J.F.; Margolis, H.C.; Simmer, J.P.; Beniash, E. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc. Natl. Acad. Sci. USA 2011, 108, 14097–14102. [Google Scholar] [CrossRef]
- Fincham, A.G.; Moradian-Oldak, J.; Diekwisch, T.G.; Lyaruu, D.M.; Wright, J.T.; Bringas, P., Jr.; Slavkin, H.C. Evidence for amelogenin “nanospheres” as functional components of secretory-stage enamel matrix. J. Struct. Biol. 1995, 115, 50–59. [Google Scholar] [CrossRef]
- Beniash, E.; Simmer, J.P.; Margolis, H.C. The effect of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J. Struct. Biol. 2005, 149, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Tran, B.; Beniash, E.; Kwak, S.Y.; Margolis, H.C. Proteolysis by MMP20 Prevents Aberrant Mineralization in Secretory Enamel. J. Dent. Res. 2019, 98, 468–475. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, T.; Zhu, W.; Pandya, M.; Gopinathan, G.; Allen, M.; Reed, D.; Keiderling, T.; Liao, X.; Diekwisch, T.G.H. Highly acidic pH facilitates enamel protein self-assembly, apatite crystal growth and enamel protein interactions in the early enamel matrix. Front. Physiol. 2022, 13, 1019364. [Google Scholar]
- Bartlett, J.D.; Jin, T.; Zhu, W.; Pandya, M.; Gopinathan, G.; Allen, M.; Reed, D.; Keiderling, T.; Liao, X.; Diekwisch, T.G.H. MMP20-generated amelogenin cleavage products prevent formation of fan-shaped enamel malformations. Sci. Rep. 2021, 11, 10570. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Hu, J.C.; Hu, Y.; Zhang, S.; Liang, T.; Wang, S.K.; Kim, J.W.; Yamakoshi, Y.; Chun, Y.H.; Bartlett, J.D.; et al. A genetic model for the secretory stage of dental enamel formation. J. Struct. Biol. 2021, 213, 107805. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Hu, Y.; Hu, J.C.; Simmer, J.P. Ultrastructure of early amelogenesis in wild-type, Amelx−/−, and Enam−/− mice: Enamel ribbon initiation on dentin mineral and ribbon orientation by ameloblasts. Mol. Genet. Genom. Med. 2016, 4, 662–683. [Google Scholar] [CrossRef]
- Stifler, C.A.; Yamazaki, H.; Gilbert, P.U.P.A.; Margolis, H.C.; Beniash, E. Loss of biological control of enamel mineralization in amelogenin-phosphorylation-deficient mice. J. Struct. Biol. 2022, 214, 107844. [Google Scholar] [CrossRef]
- Woltgens, J.H.; Lyaruu, D.M.; Bronckers, A.L.; Bervoets, T.J.; Van Duin, M. Biomineralization during early stages of the developing tooth in vitro with special reference to secretory stage of amelogenesis. Int. J. Dev. Biol. 1995, 39, 203–212. [Google Scholar]
- Al-Ansari, S.; Jalali, R.; Plotkin, L.I.; Bronckers, A.L.J.J.; DenBesten, P.; Zhang, Y.; Raber-Durlacher, J.E.; de Lange, J.; Rozema, F.R. The Importance of Connexin 43 in Enamel Development and Mineralization. Front. Physiol. 2018, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Antoine, D.; Hillson, S.; Dean, M.C. The developmental clock of dental enamel: A test for the periodicity of prism cross-striations in modern humans and an evaluation of the most likely sources of error in histological studies of this kind. J. Anat. 2009, 214, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.C.; Humphrey, L.; Groom, A.; Hassett, B. Variation in the timing of enamel formation in modern human deciduous canines. Arch. Oral Biol. 2020, 114, 104719. [Google Scholar] [CrossRef]
- McFarlane, G.; Loch, C.; Guatelli-Steinberg, D.; Bayle, P.; Le Luyer, M.; Sabel, N.; Nava, A.; Floyd, B.; Skinner, M.; White, S.; et al. Enamel daily secretion rates of deciduous molars from a global sample of children. Arch. Oral Biol. 2021, 132, 105290. [Google Scholar] [CrossRef]
- Reid, D.J.; Ferrell, R.J. The relationship between number of striae of Retzius and their periodicity in imbricational enamel formation. J. Hum. Evol. 2006, 50, 195–202. [Google Scholar] [CrossRef]
- Modesto-Mata, M.; Dean, M.C.; Lacruz, R.S.; Bromage, T.G.; García-Campos, C.; Martínez de Pinillos, M.; Martín-Francés, L.; Martinón-Torres, M.; Carbonell, E.; Arsuaga, J.L.; et al. Short and long period growth markers of enamel formation distinguish European Pleistocene hominins. Sci. Rep. 2020, 10, 4665. [Google Scholar] [CrossRef]
- Eli, I.; Sarnat, H.; Talmi, E. Effect of the birth process on the neonatal line in primary tooth enamel. Pediatr. Dent. 1989, 11, 220–223. [Google Scholar]
- Mountain, R.V.; Zhu, Y.; Pickett, O.R.; Lussier, A.A.; Goldstein, J.M.; Roffman, J.L.; Bidlack, F.B.; Dunn, E.C. Association of Maternal Stress and Social Support During Pregnancy with Growth Marks in Children’s Primary Tooth Enamel. JAMA Netw. Open 2021, 4, e2129129. [Google Scholar] [CrossRef]
- Harada, H.; Kettunen, P.; Jung, H.S.; Mustonen, T.; Wang, Y.A.; Thesleff, I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J. Cell Biol. 1999, 147, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Juuri, E.; Saito, K.; Ahtiainen, L.; Seidel, K.; Tummers, M.; Hochedlinger, K.; Klein, O.D.; Thesleff, I.; Michon, F. Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev. Cell 2012, 23, 317–328. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Liu, Y.; Ho, T.V.; Grimes, W.; Ho, H.A.; Park, S.; Wang, S.; Chai, Y. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev. Cell 2015, 33, 125–135. [Google Scholar] [CrossRef]
- Alghadeer, A.; Hanson-Drury, S.; Patni, A.P.; Ehnes, D.D.; Zhao, Y.T.; Li, Z.; Phal, A.; Vincent, T.; Lim, Y.C.; O’Day, D.; et al. Single-cell census of human tooth development enables generation of human enamel. Dev. Cell 2023, 58, 2163–2180e9. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Richardson, A.S.; Wang, S.K.; Reid, B.M.; Bai, Y.; Hu, Y.; Hu, J.C. Ameloblast transcriptome changes from secretory to maturation stages. Connect. Tissue Res. 2014, 55, 29–32. [Google Scholar] [CrossRef]
- Wang, S.; Choi, M.; Richardson, A.S.; Reid, B.M.; Seymen, F.; Yildirim, M.; Tuna, E.; Gençay, K.; Simmer, J.P.; Hu, J.C. STIM1 and SLC24A4 Are Critical for Enamel Maturation. J. Dent. Res. 2014, 93, 94S–100S. [Google Scholar] [CrossRef]
- Smith, C.E.; Issid, M.; Margolis, H.C.; Moreno, E.C. Developmental changes in the pH of enamel fluid and its effects on matrix-resident proteinases. Adv. Dent. Res. 1996, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, E. Fine structure of the stratum intermedium, stellate reticulum, and outer enamel epithelium in the enamel organ of the kitten. J. Anat. 1978, 126 Pt 2, 247–260. [Google Scholar]
- Kallenbach, E. Fine structure of rat incisor ameloblasts in transition between enamel secretion and maturation stages. Tissue Cell 1974, 6, 173–190. [Google Scholar] [CrossRef]
- Costiniti, V.; Bomfim, G.H.; Li, Y.; Mitaishvili, E.; Ye, Z.W.; Zhang, J.; Townsend, D.M.; Giacomello, M.; Lacruz, R.S. Mitochondrial Function in Enamel Development. Front. Physiol. 2020, 11, 538. [Google Scholar] [CrossRef]
- Simmer, J.P.; Papagerakis, P.; Smith, C.E.; Fisher, D.C.; Rountrey, A.N.; Zheng, L.; Hu, J.C. Regulation of dental enamel shape and hardness. J. Dent. Res. 2010, 89, 1024–1038. [Google Scholar] [CrossRef]
- Jalali, R.; Guo, J.; Zandieh-Doulabi, B.; Bervoets, T.J.; Paine, M.L.; Boron, W.F.; Parker, M.D.; Bijvelds, M.J.; Medina, J.F.; DenBesten, P.K.; et al. NBCe1 (SLC4A4) a potential pH regulator in enamel organ cells during enamel development in the mouse. Cell Tissue Res. 2014, 358, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Paine, M.L. Bicarbonate Transport During Enamel Maturation. Calcif. Tissue Int. 2017, 101, 457–464. [Google Scholar] [CrossRef]
- Skobe, Z. The vascular pattern in the papillary region of rat incisor and molar tooth enamel organ. J. Dent. Res. 1980, 59, 1457–1460. [Google Scholar] [CrossRef]
- Garant, P.R.; Nagy, A.R.; Cho, M.I. A freeze-fracture study of the papillary layer of the rat incisor enamel organ. Tissue Cell 1984, 16, 635–645. [Google Scholar] [CrossRef]
- Smith, C.E.L.; Kirkham, J.; Day, P.F.; Soldani, F.; McDerra, E.J.; Poulter, J.A.; Inglehearn, C.F.; Mighell, A.J.; Brookes, S.J. A Fourth KLK4 Mutation Is Associated with Enamel Hypomineralisation and Structural Abnormalities. Front. Physiol. 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Lyaruu, D.M.; Bronckers, A.L.; Mulder, L.; Mardones, P.; Medina, J.F.; Kellokumpu, S.; Oude Elferink, R.P.; Everts, V. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol. 2008, 27, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bronckers, A.; Kalogeraki, L.; Jorna, H.J.; Wilke, M.; Bervoets, T.J.; Lyaruu, D.M.; Zandieh-Doulabi, B.; Denbesten, P.; de Jonge, H. The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone 2010, 46, 1188–1196. [Google Scholar] [CrossRef]
- Lin, H.M.; Nakamura, H.; Noda, T.; Ozawa, H. Localization of H+-ATPase and carbonic anhydrase II in ameloblasts at maturation. Calcif. Tissue Int. 1994, 55, 38–45. [Google Scholar] [CrossRef]
- Krajewski, S.; Hugger, A.; Krajewska, M.; Reed, J.C.; Mai, J.K. Developmental expression patterns of Bcl-2, Bcl-x, Bax, and Bak in teeth. Cell Death Differ. 1998, 5, 408–415. [Google Scholar] [CrossRef]
- Kubota, K.; Lee, D.H.; Tsuchiya, M.; Young, C.S.; Everett, E.T.; Martinez-Mier, E.A.; Snead, M.L.; Nguyen, L.; Urano, F.; Bartlett, J.D. Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J. Biol. Chem. 2005, 280, 23194–23202. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Ma, L.; Gu, H.; Li, J.; Lei, S. Fluoride induced endoplasmic reticulum stress and calcium overload in ameloblasts. Arch. Oral Biol. 2016, 69, 95–101. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Sharma, R.; Tye, C.E.; Sugiyama, T.; Bartlett, J.D. Transforming growth factor-beta1 expression is up-regulated in maturation-stage enamel organ and may induce ameloblast apoptosis. Eur. J. Oral Sci. 2009, 117, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, Y.; Lin, Z.; Ji, M. The role of the TGF-beta1 signaling pathway in the process of amelogenesis. Front. Physiol. 2025, 16, 1586769. [Google Scholar] [CrossRef] [PubMed]
- Abramyan, J.; Geetha-Loganathan, P.; Šulcová, M.; Buchtová, M. Role of Cell Death in Cellular Processes During Odontogenesis. Front. Cell Dev. Biol. 2021, 9, 671475. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Vaeth, M.; Fornai, C.; Vinu, M.; Bromage, T.G.; Nurbaeva, M.K.; Sorge, J.L.; Coelho, P.G.; Idaghdour, Y.; Feske, S.; et al. Store-operated Ca2+ entry controls ameloblast cell function and enamel development. JCI Insight 2017, 2, e91166. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Hu, Y.; Kawasaki, K.; Zhang, H.; Zhang, C.; Saunders, T.L.; Simmer, J.P.; Hu, J.C. Odontogenesis-associated phosphoprotein truncation blocks ameloblast transition into maturation in OdaphC41*/C41* mice. Sci. Rep. 2021, 11, 1132. [Google Scholar] [CrossRef]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef]
- Free, R.; DeRocher, K.; Cooley, V.; Xu, R.; Stock, S.R.; Joester, D. Mesoscale structural gradients in human tooth enamel. Proc. Natl. Acad. Sci. USA 2022, 119, e2211285119. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Kirkham, J.; Hallsworth, A.S. Volume distribution and concentration of protein, mineral and water in developing bovine enamel. Arch. Oral Biol. 1988, 33, 159–162. [Google Scholar] [CrossRef]
- Weatherell, J.A.; Robinson, C.; Hallsworth, A.S. Variations in the chemical composition of human enamel. J. Dent. Res. 1974, 53, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Josephsen, K.; Takano, Y.; Zahn, D.; Fejerskov, O.; Frische, S. Fluctuations in surface pH of maturing rat incisor enamel are a result of cycles of H+-secretion by ameloblasts and variations in enamel buffer characteristics. Bone 2014, 60, 227–234. [Google Scholar] [CrossRef]
- Simmer, J.P.; Hu, Y.; Lertlam, R.; Yamakoshi, Y.; Hu, J.C. Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J. Biol. Chem. 2009, 284, 19110–19121. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Richardson, A.S.; Hu, Y.; Bartlett, J.D.; Hu, J.C.; Simmer, J.P. Effect of kallikrein 4 loss on enamel mineralization: Comparison with mice lacking matrix metalloproteinase 20. J. Biol. Chem. 2011, 286, 18149–18160. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Connell, S.; Brookes, S.J.; Kirkham, J.; Shore, R.C.; Smith, D.A. Surface chemistry of enamel apatite during maturation in relation to pH: Implications for protein removal and crystal growth. Arch. Oral Biol. 2005, 50, 267–270. [Google Scholar] [CrossRef]
- Liu, H.; Yan, X.; Pandya, M.; Luan, X.; Diekwisch, T.G. Daughters of the Enamel Organ: Development, Fate, and Function of the Stratum Intermedium, Stellate Reticulum, and Outer Enamel Epithelium. Stem Cells Dev. 2016, 25, 1580–1590. [Google Scholar] [CrossRef]
- Inubushi, T.; Nag, P.; Sasaki, J.I.; Shiraishi, Y.; Yamashiro, T. The significant role of glycosaminoglycans in tooth development. Glycobiology 2024, 34, cwae024. [Google Scholar] [CrossRef]
- Thesleff, I.; Sharpe, P. Signalling networks regulating dental development. Mech. Dev. 1997, 67, 111–123. [Google Scholar] [CrossRef]
- Liu, F.; Chu, E.Y.; Watt, B.; Zhang, Y.; Gallant, N.M.; Andl, T.; Yang, S.H.; Lu, M.M.; Piccolo, S.; Schmidt-Ullrich, R.; et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 2008, 313, 210–224. [Google Scholar] [CrossRef]
- Jarvinen, E.; Salazar-Ciudad, I.; Birchmeier, W.; Taketo, M.M.; Jernvall, J.; Thesleff, I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 18627–18632. [Google Scholar] [CrossRef]
- Guan, X.; Xu, M.; Millar, S.E.; Bartlett, J.D. Beta-catenin is essential for ameloblast movement during enamel development. Eur. J. Oral Sci. 2016, 124, 221–227. [Google Scholar] [CrossRef]
- Fausser, J.L.; Schlepp, O.; Aberdam, D.; Meneguzzi, G.; Ruch, J.V.; Lesot, H. Localization of antigens associated with adherens junctions, desmosomes, and hemidesmosomes during murine molar morphogenesis. Differentiation 1998, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, B.C.; Wang, M.Y.; Dobeck, J.M.; Albergo, K.L.; Skobe, Z. The cadherin-catenin complex is expressed alternately with the adenomatous polyposis coli protein during rat incisor amelogenesis. J. Histochem. Cytochem. 2000, 48, 397–406. [Google Scholar] [CrossRef]
- Cho, A.; Haruyama, N.; Hall, B.; Danton, M.J.; Zhang, L.; Arany, P.; Mooney, D.J.; Harichane, Y.; Goldberg, M.; Gibson, C.W.; et al. TGF-ss regulates enamel mineralization and maturation through KLK4 expression. PLoS ONE 2013, 8, e82267. [Google Scholar] [CrossRef]
- Yokozeki, M.; Afanador, E.; Nishi, M.; Kaneko, K.; Shimokawa, H.; Yokote, K.; Deng, C.; Tsuchida, K.; Sugino, H.; Moriyama, K. Smad3 is required for enamel biomineralization. Biochem. Biophys. Res. Commun. 2003, 305, 684–690. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Han, T.; Sun, Y.; Zhang, J. TGF-beta1 and TGFBR1 are expressed in ameloblasts and promote MMP20 expression. Anat. Rec. 2009, 292, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M.; Chiba, R.; Karakida, T.; Yamazaki, H.; Yamamoto, R.; Kobayashi, S.; Niwa, T.; Margolis, H.C.; Nagano, T.; Yamakoshi, Y.; et al. Potential function of TGF-beta isoforms in maturation-stage ameloblasts. J. Oral Biosci. 2019, 61, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.Y.; Mun, S.; Kang, K.J.; Lim, J.C.; Kim, S.Y.; Han, K.; Jang, Y.J. Amelogenic transcriptome profiling in ameloblast-like cells derived from adult gingival epithelial cells. Sci. Rep. 2019, 9, 3736. [Google Scholar] [CrossRef]
- Huang, X.; Xu, X.; Bringas, P., Jr.; Hung, Y.P.; Chai, Y. Smad4-Shh-Nfic signaling cascade-mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J. Bone Miner Res. 2010, 25, 1167–1178. [Google Scholar] [CrossRef]
- Takamori, K.; Hosokawa, R.; Xu, X.; Deng, X.; Bringas, P., Jr.; Chai, Y. Epithelial fibroblast growth factor receptor 1 regulates enamel formation. J. Dent. Res. 2008, 87, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Graf, D.; Luder, H.; Gridley, T.; Bluteau, G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development 2010, 137, 3025–3035. [Google Scholar] [CrossRef]
- Li, C.Y.; Prochazka, J.; Goodwin, A.F.; Klein, O.D. Fibroblast growth factor signaling in mammalian tooth development. Odontology 2014, 102, 1–13. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Kim, J.M.; Oh, H.; Park, K.H.; Choo, M.K.; Sano, Y.; Tye, C.E.; Skobe, Z.; Davis, R.J.; Park, J.M.; et al. p38alpha MAPK is required for tooth morphogenesis and enamel secretion. J. Biol. Chem. 2015, 290, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.F.; Tidyman, W.E.; Jheon, A.H.; Sharir, A.; Zheng, X.; Charles, C.; Fagin, J.A.; McMahon, M.; Diekwisch, T.G.; Ganss, B.; et al. Abnormal Ras signaling in Costello syndrome (CS) negatively regulates enamel formation. Hum. Mol. Genet. 2014, 23, 682–692. [Google Scholar] [CrossRef]
- Gritli-Linde, A.; Bei, M.; Maas, R.; Zhang, X.M.; Linde, A.; McMahon, A.P. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 2002, 129, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, A.; Shalehin, N.; Takebe, H.; Shimo, T.; Irie, K. Sonic Hedgehog Signaling and Tooth Development. Int. J. Mol. Sci. 2020, 21, 1587. [Google Scholar] [CrossRef]
- Yasukawa, M.; Ishida, K.; Yuge, Y.; Hanaoka, M.; Minami, Y.; Ogawa, M.; Sasaki, T.; Saito, M.; Tsuji, T. Dpysl4 is involved in tooth germ morphogenesis through growth regulation, polarization and differentiation of dental epithelial cells. Int. J. Biol. Sci. 2013, 9, 382–390. [Google Scholar] [CrossRef]
- Hermans, F.; Hemeryck, L.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Intertwined Signaling Pathways Governing Tooth Development: A Give-and-Take Between Canonical Wnt and Shh. Front. Cell Dev. Biol. 2021, 9, 758203. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Fukumoto, S.; Nakamura, T.; Haruyama, N.; Suzuki, S.; Hatakeyama, Y.; Shum, L.; Gibson, C.W.; Yamada, Y.; Kulkarni, A.B. Synergistic roles of amelogenin and ameloblastin. J. Dent. Res. 2009, 88, 318–322. [Google Scholar] [CrossRef]
- Nishikawa, S. Cytoskeleton, intercellular junctions, planar cell polarity, and cell movement in amelogenesis. J. Oral Biosci. 2017, 59, 197–204. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, D.S.; Ryoo, H.M.; Park, J.T.; Park, S.J.; Bae, H.S.; Cho, M.I.; Park, J.C. The odontogenic ameloblast-associated protein (ODAM) cooperates with RUNX2 and modulates enamel mineralization via regulation of MMP-20. J. Cell Biochem. 2010, 111, 755–767. [Google Scholar] [CrossRef]
- Chu, Q.; Gao, Y.; Gao, X.; Dong, Z.; Song, W.; Xu, Z.; Xiang, L.; Wang, Y.; Zhang, L.; Li, M.; et al. Ablation of Runx2 in Ameloblasts Suppresses Enamel Maturation in Tooth Development. Sci. Rep. 2018, 8, 9594. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, T.; Machado, M.; Rao, A.; Krishan, K.; Garg, A.K. Enamel hypoplasia and its role in identification of individuals: A review of literature. Indian J. Dent. 2015, 6, 99–102. [Google Scholar] [CrossRef]
- Modrić, V.E.; Verzak, Ž.; Karlović, Z. Developmental Defects of Enamel in Children with Intellectual Disability. Acta Stomatol. Croat. 2016, 50, 65–71. [Google Scholar]
- Kierdorf, U.; Death, C.; Hufschmid, J.; Witzel, C.; Kierdorf, H. Developmental and Post-Eruptive Defects in Molar Enamel of Free-Ranging Eastern Grey Kangaroos (Macropus giganteus) Exposed to High Environmental Levels of Fluoride. PLoS ONE 2016, 11, e0147427. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.J.; Mangum, J.E.; Perez, V.A.; Nervo, G.J.; Hall, R.K. Molar Hypomineralisation: A Call to Arms for Enamel Researchers. Front. Physiol. 2017, 8, 546. [Google Scholar] [CrossRef]
- Wang, S.K.; Zhang, H.; Lin, H.C.; Wang, Y.L.; Lin, S.C.; Seymen, F.; Koruyucu, M.; Simmer, J.P.; Hu, J.C. AMELX Mutations and Genotype-Phenotype Correlation in X-Linked Amelogenesis Imperfecta. Int. J. Mol. Sci. 2024, 25, 6132. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Yamakoshi, Y. Enamelin and autosomal-dominant amelogenesis imperfecta. Crit. Rev. Oral Biol. Med. 2003, 14, 387–398. [Google Scholar] [CrossRef]
- Hart, P.S.; Michalec, M.D.; Seow, W.K.; Hart, T.C.; Wright, J.T. Identification of the enamelin (g.8344delG) mutation in a new kindred and presentation of a standardized ENAM nomenclature. Arch. Oral Biol. 2003, 48, 589–596. [Google Scholar] [CrossRef]
- Wang, Y.L.; Lin, H.C.; Liang, T.; Lin, J.C.; Simmer, J.P.; Hu, J.C.; Wang, S.K. ENAM Mutations Can Cause Hypomaturation Amelogenesis Imperfecta. J. Dent. Res. 2024, 103, 662–671. [Google Scholar] [CrossRef]
- Ozdemir, D.; Hart, P.S.; Ryu, O.H.; Choi, S.J.; Ozdemir-Karatas, M.; Firatli, E.; Piesco, N.; Hart, T.C. MMP20 active-site mutation in hypomaturation amelogenesis imperfecta. J. Dent. Res. 2005, 84, 1031–1035. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, J.; Seymen, F.; Koruyucu, M.; Gencay, K.; Shin, T.J.; Hyun, H.K.; Lee, Z.H.; Hu, J.C.; Simmer, J.P.; et al. Analyses of MMP20 Missense Mutations in Two Families with Hypomaturation Amelogenesis Imperfecta. Front. Physiol. 2017, 8, 229. [Google Scholar] [CrossRef]
- Lee, Y.; Zhang, H.; Seymen, F.; Kim, Y.J.; Kasimoglu, Y.; Koruyucu, M.; Simmer, J.P.; Hu, J.C.; Kim, J.W. Novel KLK4 Mutations Cause Hypomaturation Amelogenesis Imperfecta. J. Pers. Med. 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Zhang, H.; Lee, Y.; Seymen, F.; Koruyucu, M.; Kasimoglu, Y.; Simmer, J.P.; Hu, J.C.; Kim, J.W. Novel WDR72 Mutations Causing Hypomaturation Amelogenesis Imperfecta. J. Pers. Med. 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Katsura, K.; Nakano, Y.; Zhang, Y.; Shemirani, R.; Li, W.; Den Besten, P. WDR72 regulates vesicle trafficking in ameloblasts. Sci. Rep. 2022, 12, 2820. [Google Scholar] [CrossRef]
- Parry, D.A.; Poulter, J.A.; Logan, C.V.; Brookes, S.J.; Jafri, H.; Ferguson, C.H.; Anwari, B.M.; Rashid, Y.; Zhao, H.; Johnson, C.A. Identification of mutations in SLC24A4, encoding a potassium-dependent sodium/calcium exchanger, as a cause of amelogenesis imperfecta. Am. J. Hum. Genet. 2013, 92, 307–312. [Google Scholar] [CrossRef]
- Wang, S.K.; Reid, B.M.; Dugan, S.L.; Roggenbuck, J.A.; Read, L.; Aref, P.; Taheri, A.P.; Yeganeh, M.Z.; Simmer, J.P.; Hu, J.C. FAM20A mutations associated with enamel renal syndrome. J. Dent. Res. 2014, 93, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bloch-Zupan, A.; Rey, T.; Jimenez-Armijo, A.; Kawczynski, M.; Kharouf, N.; O-Rare Consortium; Dure-Molla, M.; Noirrit, E.; Hernandez, M.; Joseph-Beaudin, C.; et al. Amelogenesis imperfecta: Next-generation sequencing sheds light on Witkop’s classification. Front. Physiol. 2023, 14, 1130175. [Google Scholar]
- DenBesten, P.; Li, W. Chronic fluoride toxicity: Dental fluorosis. Monogr. Oral Sci. 2011, 22, 81–96. [Google Scholar]
- Shahroom, N.S.B.; Mani, G.; Ramakrishnan, M. Interventions in management of dental fluorosis, an endemic disease: A systematic review. J. Family Med. Prim. Care 2019, 8, 3108–3113. [Google Scholar] [PubMed]
- Aoba, T.; Fejerskov, O. Dental fluorosis: Chemistry and biology. Crit. Rev. Oral Biol. Med. 2002, 13, 155–170. [Google Scholar] [CrossRef]
- Weerheijm, K.L. Molar incisor hypomineralisation (MIH). Eur. J. Paediatr. Dent. 2003, 4, 114–120. [Google Scholar]
- Silva, M.J.; Scurrah, K.J.; Craig, J.M.; Manton, D.J.; Kilpatrick, N. Etiology of molar incisor hypomineralization—A systematic review. Community Dent. Oral Epidemiol. 2016, 44, 342–353. [Google Scholar] [CrossRef]
- Goodman, A.H.; Rose, J.C. Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. Am. J. Phys. Anthropol. 1990, 33, 59–110. [Google Scholar] [CrossRef]
- Seow, W.K.; Humphrys, C.; Tudehope, D.I. Increased prevalence of developmental dental defects in low birth-weight, prematurely born children: A controlled study. Pediatr. Dent. 1987, 9, 221–225. [Google Scholar]
- Almuallem, Z.; Busuttil-Naudi, A. Molar incisor hypomineralisation (MIH)—An overview. Br. Dent. J. 2018, 225, 601–609. [Google Scholar] [CrossRef]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin hypersensitivity: Etiology, diagnosis and treatment; a literature review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- Lygidakis, N.A.; Garot, E.; Somani, C.; Taylor, G.D.; Rouas, P.; Wong, F.S.L. Best clinical practice guidance for clinicians dealing with children presenting with molar-incisor-hypomineralisation (MIH): An updated European Academy of Paediatric Dentistry policy document. Eur. Arch. Paediatr. Dent. 2022, 23, 3–21. [Google Scholar] [CrossRef]
- Lagarde, M.; Vennat, E.; Attal, J.P.; Dursun, E. Strategies to optimize bonding of adhesive materials to molar-incisor hypomineralization-affected enamel: A systematic review. Int. J. Paediatr. Dent. 2020, 30, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Pini, N.I.; Sundfeld-Neto, D.; Aguiar, F.H.; Sundfeld, R.H.; Martins, L.R.; Lovadino, J.R.; Lima, D.A. Enamel microabrasion: An overview of clinical and scientific considerations. World J. Clin. Cases 2015, 3, 34–41. [Google Scholar] [CrossRef]
- Chen, C.F.; Hu, J.C.; Bresciani, E.; Peters, M.C.; Estrella, M.R. Treatment considerations for patient with Amelogenesis Imperfecta: A review. Braz. Dent. Sci. 2013, 16, 7–18. [Google Scholar] [CrossRef]
- Pousette Lundgren, G.; Dahllof, G. Advances in clinical diagnosis and management of amelogenesis imperfecta in children and adolescents. J. Dent. 2024, 147, 105149. [Google Scholar] [CrossRef]
- Chan, B.; Cheng, I.C.; Rozita, J.; Gorshteyn, I.; Huang, Y.; Shaffer, I.; Chang, C.; Li, W.; Lytton, J.; Den Besten, P.; et al. Sodium/(calcium + potassium) exchanger NCKX4 optimizes KLK4 activity in the enamel matrix microenvironment to regulate ECM modeling. Front. Physiol. 2023, 14, 1116091. [Google Scholar] [CrossRef] [PubMed]
- Bronckers, A.L.; Lyaruu, D.M.; Guo, J.; Bijvelds, M.J.; Bervoets, T.J.; Zandieh-Doulabi, B.; Medina, J.F.; Li, Z.; Zhang, Y.; DenBesten, P.K. Composition of mineralizing incisor enamel in cystic fibrosis transmembrane conductance regulator-deficient mice. Eur. J. Oral Sci. 2015, 123, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ngu, J.; Bronckers, A.L.J.J.; Katsura, K.; Zhang, Y.; Den Besten, P.K. Na+ and K+ transport and maturation stage ameloblast modulation. Front. Physiol. 2023, 14, 1124444. [Google Scholar] [CrossRef]
- Josephsen, K.; Takano, Y.; Frische, S.; Praetorius, J.; Nielsen, S.; Aoba, T.; Fejerskov, O. Ion transporters in secretory and cyclically modulating ameloblasts: A new hypothesis for cellular control of preeruptive enamel maturation. Am. J. Physiol. Cell Physiol. 2010, 299, C1299–C1307. [Google Scholar] [CrossRef]
- Sasaki, S.; Takagi, T.; Suzuki, M. Cyclical changes in pH in bovine developing enamel as sequential bands. Arch. Oral Biol. 1991, 36, 227–231. [Google Scholar] [CrossRef]
- Nurbaeva, M.K.; Eckstein, M.; Concepcion, A.R.; Smith, C.E.; Srikanth, S.; Paine, M.L.; Gwack, Y.; Hubbard, M.J.; Feske, S.; Lacruz, R.S. Dental enamel cells express functional SOCE channels. Sci. Rep. 2015, 5, 15803. [Google Scholar] [CrossRef]
- Wen, X.; Lacruz, R.S.; Smith, C.E.; Paine, M.L. Gene-expression profile and localization of Na+/K+-ATPase in rat enamel organ cells. Eur. J. Oral Sci. 2014, 122, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Lodder, J.C.; Zandieh-Doulabi, B.; Micha, D.; Melvin, J.E.; Catalan, M.A.; Mansvelder, H.D.; DenBesten, P.; Bronckers, A. The Role of Na:K:2Cl Cotransporter 1 (NKCC1/SLC12A2) in Dental Epithelium during Enamel Formation in Mice. Front. Physiol. 2017, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Garant, P.R.; Sasaki, T.; Colflesh, P.E. Na-K-ATPase in the enamel organ: Localization and possible roles in enamel formation. Adv. Dent. Res. 1987, 1, 267–275. [Google Scholar] [CrossRef]
- Bori, E.; Guo, J.; Rácz, R.; Burghardt, B.; Földes, A.; Kerémi, B.; Harada, H.; Steward, M.C.; Den Besten, P.; Bronckers, A.L.; et al. Evidence for Bicarbonate Secretion by Ameloblasts in a Novel Cellular Model. J. Dent. Res. 2016, 95, 588–596. [Google Scholar] [CrossRef]
- Eckstein, M.; Vaeth, M.; Aulestia, F.J.; Costiniti, V.; Kassam, S.N.; Bromage, T.G.; Pedersen, P.; Issekutz, T.; Idaghdour, Y.; Moursi, A.M.; et al. Differential regulation of Ca2+ influx by ORAI channels mediates enamel mineralization. Sci. Signal 2019, 12, eaav4663. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Nanci, A.; White, S.N.; Wen, X.; Wang, H.; Zalzal, S.F.; Luong, V.Q.; Schuetter, V.L.; Conti, P.S.; Kurtz, I.; et al. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J. Biol. Chem. 2010, 285, 24432–24438. [Google Scholar] [CrossRef]
- Wen, X.; Kurtz, I.; Paine, M.L. Prevention of the disrupted enamel phenotype in Slc4a4-null mice using explant organ culture maintained in a living host kidney capsule. PLoS ONE 2014, 9, e97318. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Coffey, N.; Hayes, M.; Burke, F.; Harding, M.; Plant, B. The prevalence of developmental defects of enamel in people with cystic fibrosis: A systematic review. BMC Oral Health 2024, 24, 446. [Google Scholar] [CrossRef]
- Chang, E.H.; Lacruz, R.S.; Bromage, T.G.; Bringas, P., Jr.; Welsh, M.J.; Zabner, J.; Paine, M.L. Enamel pathology resulting from loss of function in the cystic fibrosis transmembrane conductance regulator in a porcine animal model. Cells Tissues Organs 2011, 194, 249–254. [Google Scholar] [CrossRef]
- Luder, H.U.; Gerth-Kahlert, C.; Ostertag-Benzinger, S.; Schorderet, D.F. Dental phenotype in Jalili syndrome due to a c.1312 dupC homozygous mutation in the CNNM4 gene. PLoS ONE 2013, 8, e78529. [Google Scholar] [CrossRef]
- Yamazaki, D.; Funato, Y.; Miura, J.; Sato, S.; Toyosawa, S.; Furutani, K.; Kurachi, Y.; Omori, Y.; Furukawa, T.; Tsuda, T.; et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: A mouse model. PLoS Genet. 2013, 9, e1003983. [Google Scholar] [CrossRef]
- Ogata, K.; Tsumuraya, T.; Oka, K.; Shin, M.; Okamoto, F.; Kajiya, H.; Katagiri, C.; Ozaki, M.; Matsushita, M.; Okabe, K. The crucial role of the TRPM7 kinase domain in the early stage of amelogenesis. Sci. Rep. 2017, 7, 18099. [Google Scholar] [CrossRef]
- Nurbaeva, M.K.; Eckstein, M.; Feske, S.; Lacruz, R.S. Ca2+ transport and signalling in enamel cells. J. Physiol. 2017, 595, 3015–3039. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zinn, V.; Lefkelidou, A.; Taqi, N.; Chatzistavrou, X.; Balam, T.; Nervina, J.; Papagerakis, S.; Papagerakis, P. Orai1 expression pattern in tooth and craniofacial ectodermal tissues and potential functions during ameloblast differentiation. Dev. Dyn. 2015, 244, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Berdal, A.; Nanci, A.; Smith, C.E.; Ahluwalia, J.P.; Thomasset, M.; Cuisinier-Gleizes, P.; Mathieu, H. Differential expression of calbindin-D 28 kDa in rat incisor ameloblasts throughout enamel development. Anat. Rec. 1991, 230, 149–163. [Google Scholar] [CrossRef]
- Bailleul-Forestier, I.; Davideau, J.L.; Papagerakis, P.; Noble, I.; Nessmann, C.; Peuchmaur, M.; Berdal, A. Immunolocalization of vitamin D receptor and calbindin-D28k in human tooth germ. Pediatr. Res. 1996, 39 Pt 1, 636–642. [Google Scholar] [CrossRef]
- Sasaki, T.; Garant, P.R. Calmodulin in rat incisor secretory ameloblasts as revealed by protein A-gold immunocytochemistry. Calcif. Tissue Int. 1987, 40, 294–297. [Google Scholar] [CrossRef]
- Davideau, J.L.; Celio, M.R.; Hotton, D.; Berdal, A. Developmental pattern and subcellular localization of parvalbumin in the rat tooth germ. Arch. Oral Biol. 1993, 38, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Ooshima, T.; Sobue, S.; Tabata, M.J.; Kurisu, K.; Wakisaka, S. Calbindin D28k-like immunoreactivity during the formation of the enamel-free area in the rat molar teeth. Anat. Rec. 2000, 258, 384–390. [Google Scholar] [CrossRef]
- Bawden, J.W.; Wennberg, A. In vitro study of cellular influence on 45Ca uptake in developing rat enamel. J. Dent. Res. 1977, 56, 313–319. [Google Scholar] [CrossRef]
- Wennberg, A.; Bawden, J.W. Comparison of 33P with 45Ca distribution in developing rat molar enamel in vivo and in vitro. J. Dent. Res. 1978, 57, 111–117. [Google Scholar] [CrossRef]
- Kawamoto, T.; Shimizu, M. Pathway and speed of calcium movement from blood to mineralizing enamel. J. Histochem. Cytochem. 1997, 45, 213–230. [Google Scholar] [CrossRef]
- Souza Bomfim, G.H.; Mitaishvili, E.; Schnetkamp, P.P.M.; Lacruz, R.S. Na+/Ca2+ exchange in enamel cells is dominated by the K+-dependent NCKX exchanger. J. Gen. Physiol. 2024, 156. [Google Scholar] [CrossRef]
- Okumura, R.; Shibukawa, Y.; Muramatsu, T.; Hashimoto, S.; Nakagawa, K.; Tazaki, M.; Shimono, M. Sodium-calcium exchangers in rat ameloblasts. J. Pharmacol. Sci. 2010, 112, 223–230. [Google Scholar] [CrossRef]
- Robertson, S.Y.T.; Wen, X.; Yin, K.; Chen, J.; Smith, C.E.; Paine, M.L. Multiple Calcium Export Exchangers and Pumps Are a Prominent Feature of Enamel Organ Cells. Front. Physiol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Stafford, N.; Wilson, C.; Oceandy, D.; Neyses, L.; Cartwright, E.J. The Plasma Membrane Calcium ATPases and Their Role as Major New Players in Human Disease. Physiol. Rev. 2017, 97, 1089–1125. [Google Scholar] [CrossRef]
- Hu, P.; Lacruz, R.S.; Smith, C.E.; Smith, S.M.; Kurtz, I.; Paine, M.L. Expression of the sodium/calcium/potassium exchanger, NCKX4, in ameloblasts. Cells Tissues Organs 2012, 196, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Bronckers, A.L.; Jalali, R.; Lytton, J. Reduced Protein Expression of the Na+/Ca2++K+-Exchanger (SLC24A4) in Apical Plasma Membranes of Maturation Ameloblasts of Fluorotic Mice. Calcif. Tissue Int. 2017, 100, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Said, R.; Lobanova, L.; Papagerakis, S.; Papagerakis, P. Calcium Sets the Clock in Ameloblasts. Front. Physiol. 2020, 11, 920. [Google Scholar] [CrossRef]
- Rui, H.; Das, A.; Nakamoto, R.; Roux, B. Proton Countertransport and Coupled Gating in the Sarcoplasmic Reticulum Calcium Pump. J. Mol. Biol. 2018, 430, 5050–5065. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.J.; Lyons, I.; Hoogendoorn, B.; Burge, S.; Kwok, P.Y.; O’Donovan, M.C.; Craddock, N.; Owen, M.J. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Hum. Mol. Genet. 1999, 8, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Hovnanian, A. Darier’s disease: From dyskeratosis to endoplasmic reticulum calcium ATPase deficiency. Biochem. Biophys. Res. Commun. 2004, 322, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zeng, W.; Han, Y.; John, S.; Ottolia, M.; Jiang, Y. Structural mechanisms of the human cardiac sodium-calcium exchanger NCX1. Nat. Commun. 2023, 14, 6181. [Google Scholar] [CrossRef]
- Paine, M.L.; Snead, M.L.; Wang, H.J.; Abuladze, N.; Pushkin, A.; Liu, W.; Kao, L.Y.; Wall, S.M.; Kim, Y.H.; Kurtz, I. Role of NBCe1 and AE2 in secretory ameloblasts. J. Dent. Res. 2008, 87, 391–395. [Google Scholar] [CrossRef]
- Chen, L.M.; Liu, Y.; Boron, W.F. Role of an extracellular loop in determining the stoichiometry of Na+-HCO3− cotransporters. J. Physiol. 2011, 589 Pt 4, 877–890. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Hilvo, M.; Kurtz, I.; Paine, M.L. A survey of carbonic anhydrase mRNA expression in enamel cells. Biochem. Biophys. Res. Commun. 2010, 393, 883–887. [Google Scholar] [CrossRef]
- Reibring, C.G.; El Shahawy, M.; Hallberg, K.; Kannius-Janson, M.; Nilsson, J.; Parkkila, S.; Sly, W.S.; Waheed, A.; Linde, A.; Gritli-Linde, A. Expression patterns and subcellular localization of carbonic anhydrases are developmentally regulated during tooth formation. PLoS ONE 2014, 9, e96007. [Google Scholar] [CrossRef]
- Leite, L.D.R.; Resende, K.K.M.; Rosa, L.D.S.; Mazzeu, J.F.; de Oliveira, L.C.; Scher, M.D.C.S.D.; Acevedo, A.C.; Yamaguti, P.M. Carbonic anhydrase II deficiency syndrome with amelogenesis imperfecta linked to a homozygous CA2 deletion. Intractable Rare Dis. Res. 2023, 12, 202–205. [Google Scholar] [CrossRef]
- Racz, R.; Földes, A.; Bori, E.; Zsembery, Á.; Harada, H.; Steward, M.C.; DenBesten, P.; Bronckers, A.L.J.J.; Gerber, G.; Varga, G. No Change in Bicarbonate Transport but Tight-Junction Formation Is Delayed by Fluoride in a Novel Ameloblast Model. Front. Physiol. 2017, 8, 940. [Google Scholar] [CrossRef]
- Wright, J.T.; Kiefer, C.L.; Hall, K.I.; Grubb, B.R. Abnormal enamel development in a cystic fibrosis transgenic mouse model. J. Dent. Res. 1996, 75, 966–973. [Google Scholar] [CrossRef]
- Jalali, R.; Zandieh-Doulabi, B.; DenBesten, P.K.; Seidler, U.; Riederer, B.; Wedenoja, S.; Micha, D.; Bronckers, A.L. Slc26a3/Dra and Slc26a6 in Murine Ameloblasts. J. Dent. Res. 2015, 94, 1732–1739. [Google Scholar] [CrossRef]
- Bronckers, A.L.; Guo, J.; Zandieh-Doulabi, B.; Bervoets, T.J.; Lyaruu, D.M.; Li, X.; Wangemann, P.; DenBesten, P. Developmental expression of solute carrier family 26A member 4 (SLC26A4/pendrin) during amelogenesis in developing rodent teeth. Eur. J. Oral Sci. 2011, 119 (Suppl. S1), 185–192. [Google Scholar] [CrossRef]
- Ko, S.B.; Zeng, W.; Dorwart, M.R.; Luo, X.; Kim, K.H.; Millen, L.; Goto, H.; Naruse, S.; Soyombo, A.; Thomas, P.J.; et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat. Cell Biol. 2004, 6, 343–350. [Google Scholar] [CrossRef]
- Yin, K.; Guo, J.; Lin, W.; Robertson, S.Y.T.; Soleimani, M.; Paine, M.L. Deletion of Slc26a1 and Slc26a7 Delays Enamel Mineralization in Mice. Front. Physiol. 2017, 8, 307. [Google Scholar] [CrossRef]
- Sarkar, J.; Wen, X.; Simanian, E.J.; Paine, M.L. V-type ATPase proton pump expression during enamel formation. Matrix Biol. 2016, 52–54, 234–245. [Google Scholar] [CrossRef]
- Varga, G.; DenBesten, P.; Rácz, R.; Zsembery, Á. Importance of bicarbonate transport in pH control during amelogenesis—Need for functional studies. Oral Dis. 2018, 24, 879–890. [Google Scholar] [CrossRef]
- Johnson, L.; Ganss, B.; Wang, A.; Zirngibl, R.A.; Johnson, D.E.; Owen, C.; Bradley, G.; Voronov, I. V-ATPases Containing a3 Subunit Play a Direct Role in Enamel Development in Mice. J. Cell. Biochem. 2017, 118, 3328–3340. [Google Scholar] [CrossRef] [PubMed]
- Foldes, A.; Sang-Ngoen, T.; Kádár, K.; Rácz, R.; Zsembery, Á.; DenBesten, P.; Steward, M.C.; Varga, G. Three-Dimensional Culture of Ameloblast-Originated HAT-7 Cells for Functional Modeling of Defective Tooth Enamel Formation. Front. Pharmacol. 2021, 12, 682654. [Google Scholar] [CrossRef] [PubMed]
- Slepkov, E.R.; Rainey, J.K.; Sykes, B.D.; Fliegel, L. Structural and functional analysis of the Na+/H+ exchanger. Biochem. J. 2007, 401, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.A.; Mighell, A.J.; El-Sayed, W.; Shore, R.C.; Jalili, I.K.; Dollfus, H.; Bloch-Zupan, A.; Carlos, R.; Carr, I.M.; Downey, L.M. Mutations in CNNM4 cause Jalili syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am. J. Hum. Genet. 2009, 84, 266–273. [Google Scholar] [CrossRef]
- Gunther, T.; Vormann, J.; Hollriegl, V. Characterization of Na+-dependent Mg2+ efflux from Mg2+-loaded rat erythrocytes. Biochim. Biophys. Acta 1990, 1023, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kadar, K.; Juhász, V.; Földes, A.; Rácz, R.; Zhang, Y.; Löchli, H.; Kató, E.; Köles, L.; Steward, M.C.; DenBesten, P.; et al. TRPM7-Mediated Calcium Transport in HAT-7 Ameloblasts. Int. J. Mol. Sci. 2021, 22, 3992. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Matsushima, A.; Kajiya, H.; Okamoto, F.; Ogata, K.; Oka, K.; Ohshima, H.; Bartlett, J.D.; Okabe, K. Conditional knockout of transient receptor potential melastatin 7 in the enamel epithelium: Effects on enamel formation. Eur. J. Oral Sci. 2023, 131, e12920. [Google Scholar] [CrossRef]
- Nakano, Y.; Le, M.H.; Abduweli, D.; Ho, S.P.; Ryazanova, L.V.; Hu, Z.; Ryazanov, A.G.; Den Besten, P.K.; Zhang, Y. A Critical Role of TRPM7 As an Ion Channel Protein in Mediating the Mineralization of the Craniofacial Hard Tissues. Front. Physiol. 2016, 7, 258. [Google Scholar] [CrossRef]
- Millan, J.L.; Whyte, M.P. Alkaline Phosphatase and Hypophosphatasia. Calcif. Tissue Int. 2016, 98, 398–416. [Google Scholar] [CrossRef]
- Irizarry, A.R.; Yan, G.; Zeng, Q.; Lucchesi, J.; Hamang, M.J.; Ma, Y.L.; Rong, J.X. Defective enamel and bone development in sodium-dependent citrate transporter (NaCT) Slc13a5 deficient mice. PLoS ONE 2017, 12, e0175465. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Kopel, J.J.; Lawrence, J.J.; Neugebauer, V.; Ganapathy, V. Plasma Membrane Na+-Coupled Citrate Transporter (SLC13A5) and Neonatal Epileptic Encephalopathy. Molecules 2017, 22, 378. [Google Scholar] [CrossRef]
- Hu, J.C.; Liang, T.; Zhang, H.; Hu, Y.; Yamakoshi, Y.; Yamamoto, R.; Zhang, C.; Li, H.; Smith, C.E.; Simmer, J.P. Citrate Transporter Expression and Localization: The Slc13a5(Flag) Mouse Model. Int. J. Mol. Sci. 2025, 26, 6707. [Google Scholar] [CrossRef]
- Yadav, M.C.; de Oliveira, R.C.; Foster, B.L.; Fong, H.; Cory, E.; Narisawa, S.; Sah, R.L.; Somerman, M.; Whyte, M.P.; Millán, J.L. Enzyme replacement prevents enamel defects in hypophosphatasia mice. J. Bone Miner Res. 2012, 27, 1722–1734. [Google Scholar] [CrossRef]
- Whyte, M.P. Hypophosphatasia: An overview for 2017. Bone 2017, 102, 15–25. [Google Scholar] [CrossRef]
- Giovannini, D.; Touhami, J.; Charnet, P.; Sitbon, M.; Battini, J.L. Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep. 2013, 3, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Legati, A.; Giovannini, D.; Nicolas, G.; López-Sánchez, U.; Quintáns, B.; Oliveira, J.R.; Sears, R.L.; Ramos, E.M.; Spiteri, E.; Sobrido, M.J.; et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 2015, 47, 579–581. [Google Scholar] [CrossRef]
- Beck-Cormier, S.; Lelliott, C.J.; Logan, J.G.; Lafont, D.T.; Merametdjian, L.; Leitch, V.D.; Butterfield, N.C.; Protheroe, H.J.; Croucher, P.I.; Baldock, P.A.; et al. Slc20a2, Encoding the Phosphate Transporter PiT2, Is an Important Genetic Determinant of Bone Quality and Strength. J. Bone Miner. Res. 2019, 34, 1101–1114. [Google Scholar] [CrossRef]
- Tsai, J.Y.; Chu, C.H.; Lin, M.G.; Chou, Y.H.; Hong, R.Y.; Yen, C.Y.; Hsiao, C.D.; Sun, Y.J. Structure of the sodium-dependent phosphate transporter reveals insights into human solute carrier SLC20. Sci. Adv. 2020, 6, eabb4024. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Ravera, S.; Sorribas, V.; Stange, G.; Levi, M.; Murer, H.; Biber, J.; Forster, I.C. The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am. J. Physiol. Ren. Physiol. 2009, 296, F691–F699. [Google Scholar] [CrossRef]
- Jonsson, A.L.M.; Hernando, N.; Knöpfel, T.; Mogensen, S.; Bendstrup, E.; Hilberg, O.; Christensen, J.H.; Simonsen, U.; Wagner, C.A. Impaired phosphate transport in SLC34A2 variants in patients with pulmonary alveolar microlithiasis. Hum. Genom. 2022, 16, 13. [Google Scholar] [CrossRef]
- Jonsson, A.L.M.; Hilberg, O.; Simonsen, U.; Christensen, J.H.; Bendstrup, E. New insights in the genetic variant spectrum of SLC34A2 in pulmonary alveolar microlithiasis; a systematic review. Orphanet. J. Rare Dis. 2023, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Sakai, H.; Shibata, Y.; Shibata, M.; Mataki, S.; Kato, Y. Expression and localization of ferritin mRNA in ameloblasts of rat incisor. Arch. Oral Biol. 1998, 43, 367–378. [Google Scholar] [CrossRef]
- Wen, X.; Paine, M.L. Iron deposition and ferritin heavy chain (Fth) localization in rodent teeth. BMC Res. Notes 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Ozawa, H. Cytochemical studies on the ferritin-containing vesicles of the rat incisor ameloblasts with special reference to the acid phosphatase activity. Calcif. Tissue Int. 1981, 33, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Sukseree, S.; Schwarze, U.Y.; Gruber, R.; Gruber, F.; Quiles Del Rey, M.; Mancias, J.D.; Bartlett, J.D.; Tschachler, E.; Eckhart, L. ATG7 is essential for secretion of iron from ameloblasts and normal growth of murine incisors during aging. Autophagy 2020, 16, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.T.; Tintos-Hernández, J.A.; Murali, C.N.; Penon-Portmann, M.; Flores-Mendez, M.; Santana, A.; Bulos, J.A.; Du, K.; Dupuis, L.; Damseh, N.; et al. Heterozygous nonsense variants in the ferritin heavy-chain gene FTH1 cause a neuroferritinopathy. HGG Adv. 2023, 4, 100236. [Google Scholar] [PubMed]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Hardy, N.C.; Chinoy, A.F.; Bartlett, J.D.; Hu, J.C. How Fluoride Protects Dental Enamel from Demineralization. J. Int. Soc. Prev. Community Dent. 2020, 10, 134–141. [Google Scholar] [CrossRef]
- Leroy, N.; Bres, E. Structure and substitutions in fluorapatite. Eur. Cell Mater. 2001, 2, 36–48. [Google Scholar] [CrossRef]
- Weatherell, J.A.; Robinson, C.; Hallsworth, A.S. Changes in the fluoride concentration of the labial enamel surface with age. Caries Res. 1972, 6, 312–324. [Google Scholar] [CrossRef]
- Kyllonen, M.S.; Parkkila, S.; Rajaniemi, H.; Waheed, A.; Grubb, J.H.; Shah, G.N.; Sly, W.S.; Kaunisto, K. Localization of carbonic anhydrase XII to the basolateral membrane of H+-secreting cells of mouse and rat kidney. J. Histochem. Cytochem. 2003, 51, 1217–1224. [Google Scholar] [CrossRef]
- Yin, K.; Lei, Y.; Wen, X.; Lacruz, R.S.; Soleimani, M.; Kurtz, I.; Snead, M.L.; White, S.N.; Paine, M.L. SLC26A Gene Family Participate in pH Regulation during Enamel Maturation. PLoS ONE 2015, 10, e0144703. [Google Scholar] [CrossRef] [PubMed]
- Bronckers, A.L.; Lyaruu, D.M.; Bervoets, T.J.; Medina, J.F.; DenBesten, P.; Richter, J.; Everts, V. Murine ameloblasts are immunonegative for Tcirg1, the v-H-ATPase subunit essential for the osteoclast plasma proton pump. Bone 2012, 50, 901–908. [Google Scholar] [CrossRef]
- Le, M.H.; Nakano, Y.; Abduweli Uyghurturk, D.; Zhu, L.; Den Besten, P.K. Fluoride Alters Klk4 Expression in Maturation Ameloblasts through Androgen and Progesterone Receptor Signaling. Front. Physiol. 2017, 8, 925. [Google Scholar] [CrossRef]
- Ji, M.; Xiao, L.; Xu, L.; Huang, S.; Zhang, D. How pH is regulated during amelogenesis in dental fluorosis. Exp. Ther. Med. 2018, 16, 3759–3765. [Google Scholar] [CrossRef]
- Schnetkamp, P.P. Na-Ca or Na-Ca-K exchange in rod photoreceptors. Prog. Biophys. Mol. Biol. 1989, 54, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Szerencsei, R.T.; Prinsen, C.F.; Schnetkamp, P.P. Stoichiometry of the retinal cone Na/Ca-K exchanger heterologously expressed in insect cells: Comparison with the bovine heart Na/Ca exchanger. Biochemistry 2001, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.M.; Cohen, M.J.; MacRenaris, K.W.; Pasteris, J.D.; Seda, T.; Joester, D. Dental materials. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 2015, 347, 746–750. [Google Scholar] [CrossRef]
- Hoylaerts, M.F.; Van Kerckhoven, S.; Kiffer-Moreira, T.; Sheen, C.; Narisawa, S.; Millán, J.L. Functional significance of calcium binding to tissue-nonspecific alkaline phosphatase. PLoS ONE 2015, 10, e0119874. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.T. The influence of diets low in magnesium upon the histological appearance of the incisor tooth of the rat. J. Physiol. 1940, 99, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Ozawa, H.; Crenshaw, M.A. Ca-ATPase and ALPase activities at the initial calcification sites of dentin and enamel in the rat incisor. Cell Tissue Res. 1986, 243, 91–99. [Google Scholar] [CrossRef]
- Klimuszko, E.; Orywal, K.; Sierpinska, T.; Sidun, J.; Golebiewska, M. Evaluation of calcium and magnesium contents in tooth enamel without any pathological changes: In vitro preliminary study. Odontology 2018, 106, 369–376. [Google Scholar] [CrossRef]
- Robinson, C.; Weatherell, J.A.; Hallsworth, A.S. Distribution of magnesium in mature human enamel. Caries Res. 1981, 15, 70–77. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Paramonova, E.V.; Avakyan, L.A.; Eremina, N.V.; Makarova, S.V.; Bulina, N.V. Effect of Magnesium Substitution on Structural Features and Properties of Hydroxyapatite. Materials 2023, 16, 5945. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.M.; Joester, D. Mapping residual organics and carbonate at grain boundaries and the amorphous interphase in mouse incisor enamel. Front. Physiol. 2015, 6, 57. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.; Gilbert, P.U. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef]
- Xu, J.; Shi, H.; Luo, J.; Yao, H.; Wang, P.; Li, Z.; Wei, J. Advanced materials for enamel remineralization. Front. Bioeng. Biotechnol. 2022, 10, 985881. [Google Scholar] [CrossRef]
- Lale, S.; Solak, H.; Hınçal, E.; Vahdettin, L. In Vitro Comparison of Fluoride, Magnesium, and Calcium Phosphate Materials on Prevention of White Spot Lesions around Orthodontic Brackets. Biomed. Res. Int. 2020, 2020, 1989817. [Google Scholar] [CrossRef]
- McKee, M.D.; Zerounian, C.; Martineau-Doizé, B.; Warshawsky, H. Specific binding sites for transferrin on ameloblasts of the enamel maturation zone in the rat incisor. Anat. Rec. 1987, 218, 123–127. [Google Scholar] [CrossRef]
- Stein, G.; Boyle, P.E. Pigmentation of the enamel of albino rat incisor teeth. Arch. Oral Biol. 1959, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, T.; Itoh, K.; Uwayama, J.; Shibata, Y.; Yamaguchi, A.; Sano, T.; Ishii, T.; Yoshida, H.; Yamamoto, M. Nrf2 deficiency causes tooth decolourization due to iron transport disorder in enamel organ. Genes Cells 2004, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Srot, V.; Houari, S.; Kapun, G.; Bussmann, B.; Predel, F.; Pokorny, B.; Bužan, E.; Salzberger, U.; Fenk, B.; Kelsch, M.; et al. Ingenious Architecture and Coloration Generation in Enamel of Rodent Teeth. ACS Nano 2024, 18, 11270–11283. [Google Scholar] [CrossRef]
- Smith, T.; Codrea, V. Red Iron-Pigmented Tooth Enamel in a Multituberculate Mammal from the Late Cretaceous Transylvanian “Hateg Island”. PLoS ONE 2015, 10, e0132550. [Google Scholar] [CrossRef]
- Arosio, P.; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 2017, 69, 414–422. [Google Scholar] [CrossRef]
- Imhof, T.; Rosenblatt, K.; Pryymachuk, G.; Weiland, D.; Noetzel, N.; Deschner, J.; Baris, O.R.; Kimoloi, S.; Koch, M.; Wiesner, R.J.; et al. Epithelial loss of mitochondrial oxidative phosphorylation leads to disturbed enamel and impaired dentin matrix formation in postnatal developed mouse incisor. Sci. Rep. 2020, 10, 22037. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, N.; Zhang, Q.; Chu, E.Y.; Tower, R.J.; Li, Z.; Guo, S.; Yuan, S.; Khare, P.A.; Zhang, C.; Verardo, A.; et al. A specialized metabolic pathway partitions citrate in hydroxyapatite to impact mineralization of bones and teeth. Proc. Natl. Acad. Sci. USA 2022, 119, e2212178119. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Agudo, E.; Ruiz-Agudo, C.; Di Lorenzo, F.; Alvarez-Lloret, P.; Ibañez-Velasco, A.; Rodriguez-Navarro, C. Citrate Stabilizes Hydroxylapatite Precursors: Implications for Bone Mineralization. ACS Biomater. Sci. Eng. 2021, 7, 2346–2357. [Google Scholar] [CrossRef]
- Chen, J.; Xian, G.; Xiao, Z.; Ge, F.; Yuan, S.; Li, B.; Liang, X.; Cai, Z.; Zhang, N.; Zhang, L.; et al. Biomineralization-inspired scaffolds using citrate-based polymers to stabilize amorphous calcium phosphate promote osteogenesis and angiogenesis for bone defect repair. Bioact. Mater. 2025, 56, 260–276. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Warshawsky, H.; Nanci, A. Cyclical incorporation of 33P into rat incisor enamel in vivo as visualized by whole-mount radioautography. Arch. Oral Biol. 1989, 34, 989–993. [Google Scholar] [CrossRef]
- Smith, C.E.; McKee, M.D.; Nanci, A. Cyclic induction and rapid movement of sequential waves of new smooth-ended ameloblast modulation bands in rat incisors as visualized by polychrome fluorescent labeling and GBHA-staining of maturing enamel. Adv. Dent. Res. 1987, 1, 162–175. [Google Scholar] [CrossRef]
- McKee, M.D. Use of backscattered electron imaging on developed radioautographic emulsions: Application to viewing rat incisor enamel maturation pattern following 45calcium injection. J. Electron. Microsc. Tech. 1987, 5, 357–365. [Google Scholar] [CrossRef]
- Robinson, C.; Hiller, C.R.; Weatherell, J.A. Uptake of 32P-labelled phosphate into developing rat incisor enamel. Calcif. Tissue Res. 1974, 15, 143–152. [Google Scholar] [CrossRef]
- Hiller, C.R.; Robinson, C.; Weatherell, J.A. Variations in the composition of developing rat incisor enamel. Calcif. Tissue Res. 1975, 18, 1–12. [Google Scholar] [CrossRef]
- Crouthamel, M.H.; Lau, W.L.; Leaf, E.M.; Chavkin, N.W.; Wallingford, M.C.; Peterson, D.F.; Li, X.; Liu, Y.; Chin, M.T.; Levi, M.; et al. Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: Redundant roles for PiT-1 and PiT-2. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Bottger, P.; Hede, S.E.; Grunnet, M.; Høyer, B.; Klaerke, D.A.; Pedersen, L. Characterization of transport mechanisms and determinants critical for Na+-dependent Pi symport of the PiT family paralogs human PiT1 and PiT2. Am. J. Physiol. Cell Physiol. 2006, 291, C1377–C1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, J.; Wang, M.; She, J. Structure and function of human XPR1 in phosphate export. Nat. Commun. 2025, 16, 2983. [Google Scholar] [CrossRef]
- Ravera, S.; Virkki, L.V.; Murer, H.; Forster, I.C. Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am. J. Physiol. Cell Physiol. 2007, 293, C606–C620. [Google Scholar] [CrossRef]
- Zhao, D.; Vaziri Sani, F.; Nilsson, J.; Rodenburg, M.; Stocking, C.; Linde, A.; Gritli-Linde, A. Expression of Pit2 sodium-phosphate cotransporter during murine odontogenesis is developmentally regulated. Eur. J. Oral Sci. 2006, 114, 517–523. [Google Scholar] [CrossRef]
- Virkki, L.V.; Biber, J.; Murer, H.; Forster, I.C. Phosphate transporters: A tale of two solute carrier families. Am. J. Physiol. Renal. Physiol. 2007, 293, F643–F654. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A. Pharmacology of Mammalian Na+-Dependent Transporters of Inorganic Phosphate. Handb. Exp. Pharmacol. 2024, 283, 285–317. [Google Scholar]
- Kavanaugh, M.P.; Miller, D.G.; Zhang, W.; Law, W.; Kozak, S.L.; Kabat, D.; Miller, A.D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA 1994, 91, 7071–7075. [Google Scholar] [CrossRef]
- Marks, J.; Debnam, E.S.; Unwin, R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. Renal. Physiol. 2010, 299, F285–F296. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Xiang, Z.; Cao, M.; He, J. Different phosphate transport in the duodenum and jejunum of chicken response to dietary phosphate adaptation. Asian-Australas J. Anim. Sci. 2012, 25, 1457–1465. [Google Scholar] [CrossRef]
- Graham, C.; Nalbant, P.; Schölermann, B.; Hentschel, H.; Kinne, R.K.; Werner, A. Characterization of a type IIb sodium-phosphate cotransporter from zebrafish (Danio rerio) kidney. Am. J. Physiol. Renal. Physiol. 2003, 284, F727–F736. [Google Scholar] [CrossRef]
- Stauber, A.; Radanovic, T.; Stange, G.; Murer, H.; Wagner, C.A.; Biber, J. Regulation of intestinal phosphate transport. II. Metabolic acidosis stimulates Na+-dependent phosphate absorption and expression of the Na+-P(i) cotransporter NaPi-IIb in small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G501–G506. [Google Scholar] [CrossRef]
- Zuo, P.; Wang, W.; Dai, Z.; Zheng, J.; Yu, S.; Wang, G.; Yin, Y.; Liang, L.; Yin, Y. Synergistic activation of the human phosphate exporter XPR1 by KIDINS220 and inositol pyrophosphate. Nat. Commun. 2025, 16, 2879. [Google Scholar] [CrossRef] [PubMed]
- Knopfel, T.; Pastor-Arroyo, E.M.; Schnitzbauer, U.; Kratschmar, D.V.; Odermatt, A.; Pellegrini, G.; Hernando, N.; Wagner, C.A. The intestinal phosphate transporter NaPi-IIb (Slc34a2) is required to protect bone during dietary phosphate restriction. Sci. Rep. 2017, 7, 11018. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Z.; Ackerman, L.; Zhang, Y.; Bonde, J.; Li, W.; Cheng, Y.; Habelitz, S. Protein nanoribbons template enamel mineralization. Proc. Natl. Acad. Sci. USA 2020, 117, 19201–19208. [Google Scholar] [CrossRef] [PubMed]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef]
- McIntyre, B.; Solesio, M.E. Mitochondrial inorganic polyphosphate (polyP): The missing link of mammalian bioenergetics. Neural Regen. Res. 2021, 16, 2227–2228. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Moreno, S.N. Acidocalcisomes. Cell Calcium 2011, 50, 113–119. [Google Scholar] [CrossRef]
- Da Costa, R.T.; Urquiza, P.; Perez, M.M.; Du, Y.; Khong, M.L.; Zheng, H.; Guitart-Mampel, M.; Elustondo, P.A.; Scoma, E.R.; Hambardikar, V.; et al. Mitochondrial inorganic polyphosphate is required to maintain proteostasis within the organelle. Front. Cell Dev. Biol. 2024, 12, 1423208. [Google Scholar] [CrossRef]
- Muller, W.E.G.; Schroder, H.C.; Wang, X. Inorganic Polyphosphates As Storage for and Generator of Metabolic Energy in the Extracellular Matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef]
- Omelon, S.J.; Grynpas, M.D. Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem. Rev. 2008, 108, 4694–4715. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.E.G.; Ackermann, M.; Al-Nawas, B.; Righesso, L.A.R.; Muñoz-Espí, R.; Tolba, E.; Neufurth, M.; Schröder, H.C.; Wang, X. Amplified morphogenetic and bone forming activity of amorphous versus crystalline calcium phosphate/polyphosphate. Acta Biomater. 2020, 118, 233–247. [Google Scholar] [CrossRef]
- McGaughey, C. Binding of polyphosphates and phosphonates to hydroxyapatite, subsequent hydrolysis, phosphate exchange and effects on demineralization, mineralization and microcrystal aggregation. Caries Res. 1983, 17, 229–241. [Google Scholar] [CrossRef]
- McGaughey, C.; Stowell, E.C. Effects of polyphosphates on the solubility and mineralization of HA: Relevance to a rationale for anticaries activity. J. Dent. Res. 1977, 56, 579–587. [Google Scholar] [CrossRef]
- Hotton, D.; Mauro, N.; Lézot, F.; Forest, N.; Berdal, A. Differential expression and activity of tissue-nonspecific alkaline phosphatase (TNAP) in rat odontogenic cells in vivo. J. Histochem. Cytochem. 1999, 47, 1541–1552. [Google Scholar] [CrossRef]
- Lorenz, B.; Schroder, H.C. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim. Biophys. Acta 2001, 1547, 254–261. [Google Scholar] [CrossRef]
- Millan, J.L. Alkaline Phosphatases: Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal 2006, 2, 335–341. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millan, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef]
- Lowe, D.; Sanvictores, T.; Zubair, M.; John, S. Alkaline Phosphatase; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Sipe, J.B.; Hessle, L.; Dhanyamraju, R.; Atti, E.; Camacho, N.P.; Millán, J.L.; Dhamyamraju, R. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am. J. Pathol. 2004, 164, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Hoac, B.; Kiffer-Moreira, T.; Millán, J.L.; McKee, M.D. Polyphosphates inhibit extracellular matrix mineralization in MC3T3-E1 osteoblast cultures. Bone 2013, 53, 478–486. [Google Scholar] [CrossRef]
- Da Costa, R.T.; Nichenko, A.; Perez, M.M.; Tokarska-Schlattner, M.; Kavehmoghaddam, S.; Hambardikar, V.; Scoma, E.R.; Seifert, E.L.; Schlattner, U.; Drake, J.C.; et al. Mammalian mitochondrial inorganic polyphosphate (polyP) and cell signaling: Crosstalk between polyP and the activity of AMPK. Mol. Metab. 2025, 91, 102077. [Google Scholar] [CrossRef]
- Schoeppe, R.; Waldmann, M.; Jessen, H.J.; Renné, T. An Update on Polyphosphate In Vivo Activities. Biomolecules 2024, 14, 937. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Yoshino, H.; Haraguchi-Kitakamae, M.; Ishizu, H.; Shimizu, T.; Iwasaki, N.; Amizuka, N. Matrix Vesicle-Mediated Mineralization and Osteocytic Regulation of Bone Mineralization. Int. J. Mol. Sci. 2022, 23, 9941. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Khong, M.L.; Lui, E.L.H.; Mebarek, S.; Magne, D.; Buchet, R.; Tanner, J.A. Long-chain polyphosphate in osteoblast matrix vesicles: Enrichment and inhibition of mineralization. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 199–209. [Google Scholar] [CrossRef]
- Kus, F.; Smolenski, R.T.; Tomczyk, M. Inorganic Polyphosphate-Regulator of Cellular Metabolism in Homeostasis and Disease. Biomedicines 2022, 10, 913. [Google Scholar] [CrossRef]
- Millan, J.L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef]
- Walker, V. The Intricacies of Renal Phosphate Reabsorption—An Overview. Int. J. Mol. Sci. 2024, 25, 4684. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Ruminska, J.; Kaufmann, M.; Dursun, I.; Patti, M.; Kranz, B.; Pronicka, E.; Ciara, E.; Akcay, T.; Bulus, D.; et al. Autosomal-Recessive Mutations in SLC34A1 Encoding Sodium-Phosphate Cotransporter 2A Cause Idiopathic Infantile Hypercalcemia. J. Am. Soc. Nephrol. 2016, 27, 604–614. [Google Scholar] [PubMed]
- Yu, Y.; Sanderson, S.R.; Reyes, M.; Sharma, A.; Dunbar, N.; Srivastava, T.; Jüppner, H.; Bergwitz, C. Novel NaPi-IIc mutations causing HHRH and idiopathic hypercalciuria in several unrelated families: Long-term follow-up in one kindred. Bone 2012, 50, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.; Chavez, M.B.; Tan, M.H.; Kolli, T.N.; Giovani, P.A.; Hammersmith, K.J.; Bowden, S.A.; Foster, B.L. Mineralization Defects in the Primary Dentition Associated With X-Linked Hypophosphatemic Rickets. JBMR Plus 2021, 5, e10463. [Google Scholar]
- Arhar, A.; Pavlic, A.; Hocevar, L. Characteristics of oral health of patients with X-linked hypophosphatemia: Case reports and literature review. BDJ Open 2024, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Wuersching, S.N.; Kollmuss, M.; Poxleitner, P.; Dewenter, I.; Brandenburg, L.S.; Steybe, D.; Fegg, F.N.; Smolka, W.; Otto, S.; et al. X-Linked Hypophosphatemia: Does Targeted Therapy Modify Dental Impairment? J. Clin. Med. 2023, 12, 7546. [Google Scholar] [CrossRef]
- Segawa, H.; Onitsuka, A.; Furutani, J.; Kaneko, I.; Aranami, F.; Matsumoto, N.; Tomoe, Y.; Kuwahata, M.; Ito, M.; Matsumoto, M.; et al. Npt2a and Npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development. Am. J. Physiol. Renal Physiol. 2009, 297, F671–F678. [Google Scholar] [CrossRef]
- Law, K.T.; Lee, C.K.; King, N.M.; Rabie, A.B. The relationship between eruption and length of mandibular incisors in young rats. Med. Sci. Monit. 2003, 9, BR47–BR53. [Google Scholar]
- McKee, M.D.; Martin, J.R.; Landis, W.J. Biophysical analyses of sequential bands of enamel related to ruffle-ended and smooth-ended maturation ameloblasts. J. Dent. Res. 1989, 68, 101–106. [Google Scholar] [CrossRef] [PubMed]
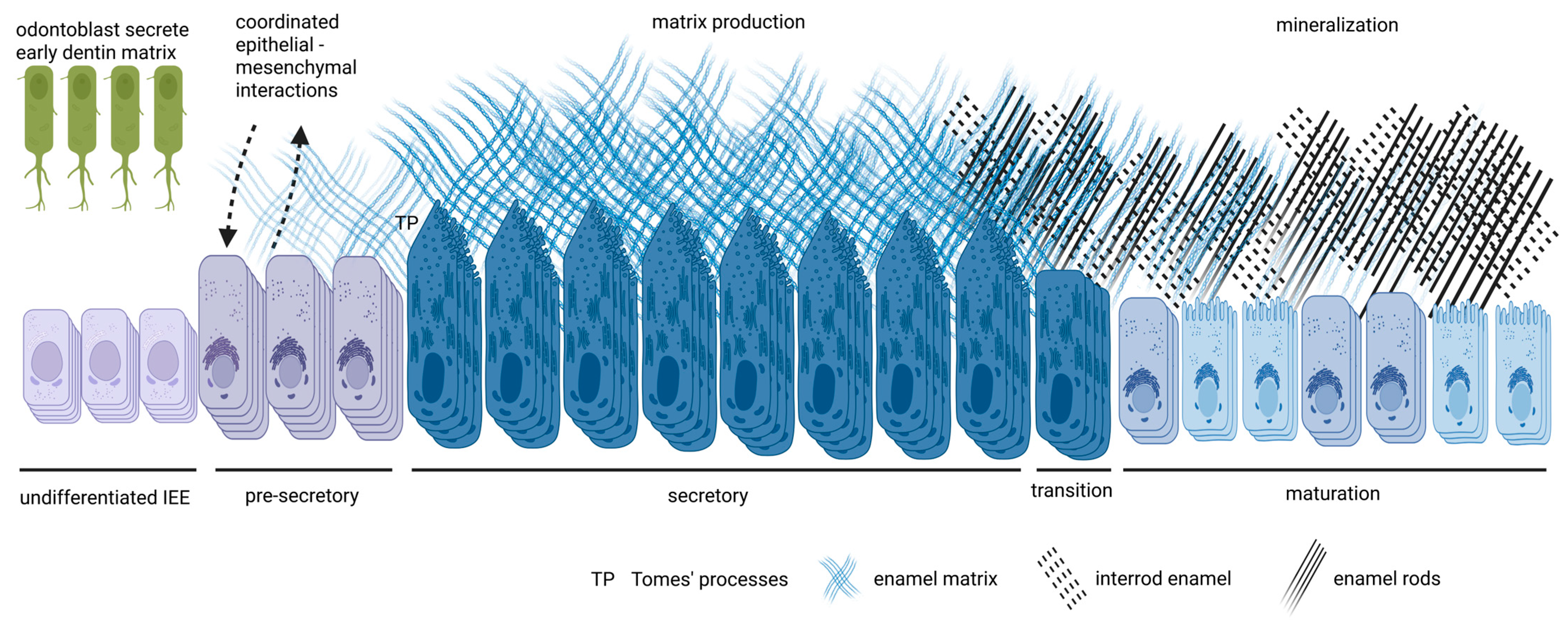
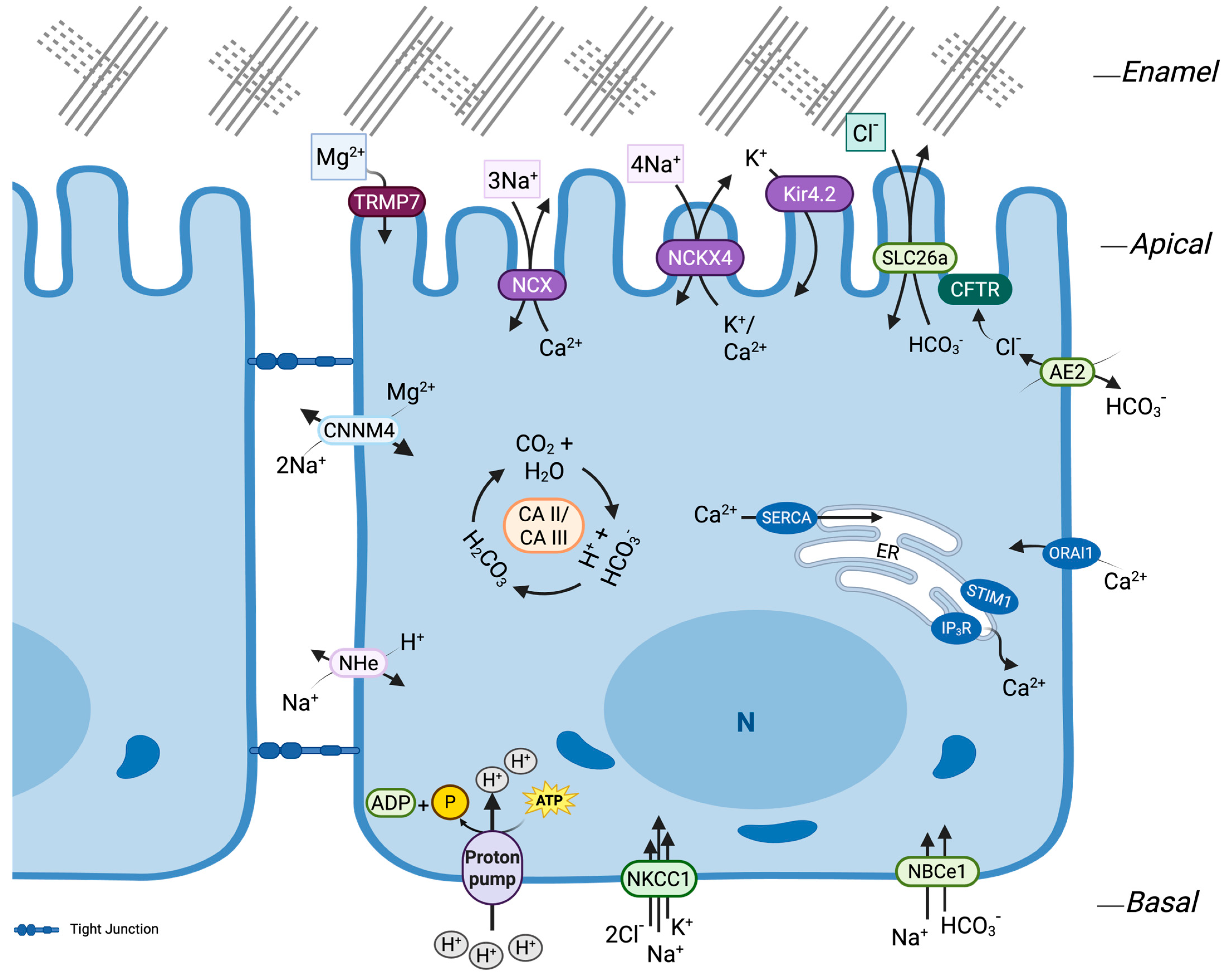
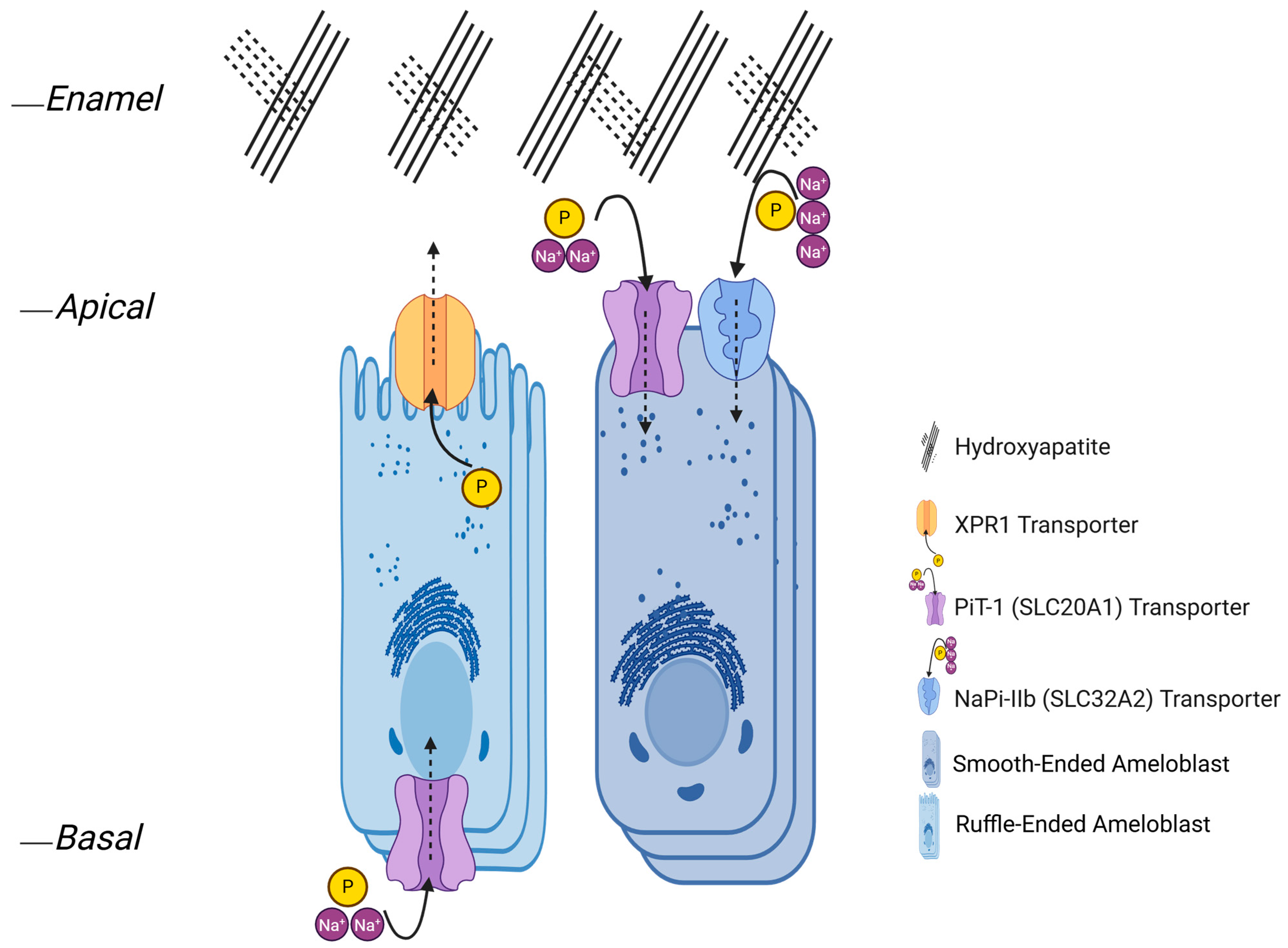
| Ion(s) | Gene/Protein | Ions Transported | Function in Ameloblasts | Location | Stoichiometry | Disease Associations | Key References |
|---|---|---|---|---|---|---|---|
| Ca2+ | STIM1/STIM2 + ORAI1 (SOCE/CRAC) | Ca2+ influx (store-operated) | Basolateral SOCE that replenishes cytosolic and ER Ca2+ to support maturation-stage transport and apical efflux | ORAI1 at basolateral plasma membrane; STIM1/2 in ER | Channel-mediated, non-stoichiometric | Loss/impairment → hypomineralized, AI-like enamel (Stim1/2 cKO; ORAI perturbation); STIM1 mutations linked to AI in humans | [101,173,186,202] |
| Ca2+ | ATP2A (SERCA) | Ca2+ into ER (H+ countertransport) | Refills ER Ca2+ stores; buffers cytosolic Ca2+ and terminates SOCE | ER membranes | 2 Ca2+ in:2–3 H+ out per ATP | ATP2A2 → Darier disease (skin); General Ca2+ imbalance; not AI-specific | [101,173,203,204,205] |
| Ca2+ | SLC24A4 (NCKX4) | Ca2+ efflux, Na+ in, K+ out | Major Ca2+ exporter during maturation; supports matrix mineralization and optimizes KLK4-mediated protein clearance | Apical membrane of ruffle-ended ameloblasts | Electrogenic 4 Na+ in:1 Ca2+ + 1 K+ out | Biallelic SLC24A4 variants → autosomal-recessive hypomaturation AI | [81,101,151,168,196,201] |
| Ca2+ | SLC8A1/3 (NCX1/3) | Ca2+ efflux, Na+ influx | Supplementary Ca2+ extrusion during maturation (minor vs. NCKX4) | Apical (Tomes’ process); remains detectable during the maturation stage | 3 Na+ in:1 Ca2+ out (electrogenic) | Not directly linked to AI | [101,196,197,198,206] |
| Ca2+ | ATP2B1/4 (PMCA1/4) | Ca2+ efflux, H+ counter-transport | ATP-driven clearance of cytosolic Ca2+; supports acid-base balance; complements NCKX4 during maturation | Basolateral, with occasional reports of apical/apicolateral labeling | 1 Ca2+ out:2 H+ in per ATP (overall electroneutral) | No direct AI; perturbation associates with hypomineralized enamel (e.g., Atp2b1a knockdown impairs tooth mineralization; PMCA4 reduced in Mmp20−/− enamel) | [198,199] |
| HCO3− (with Na+) | SLC4A4 (NBCe1) | Na+ + HCO3− influx | Basolateral bicarbonate supply | Basolateral | 1 Na+ + 2–3 HCO3− in | Mutations → enamel hypoplasia | [87,179,180,207,208] |
| HCO3−/H+ | Carbonic anhydrases: CA2/CA6/CA12 (CA II/VI/XII) | CO2 hydration → H+ + HCO3− | Local HCO3− generation to support apical HCO3− secretion and matrix pH control during maturation | CA II cytosol (ameloblasts and papillary layer); CA VI secreted → enamel fluid; CA XII membrane/apical | Enzymatic (reversible; no fixed stoichiometry) | CA II deficiency → enamel defects/hypoplasia | [24,209,210,211] |
| HCO3−/Cl− | SLC4A2 (AE2) | Cl−/HCO3− exchange | Basolateral HCO3− extrusion and Cl− uptake to support pH homeostasis and apical HCO3− secretion | Apical (secretory)/basolateral (maturation) | 1:1 (electroneutral) | Slc4a2/Ae2 knockout → hypomineralized enamel, failed maturation | [92,207,212] |
| HCO3−/Cl− | CFTR (CFTR) | Cl− and HCO3− (HCO3−-permeable anion channel) | Apical anion conductance that permits Cl− efflux and supports HCO3− secretion to neutralize protons during maturation; provides Cl− recycling for apical Cl−/HCO3− exchangers (e.g., SLC26 family) | Apical membrane of maturation-stage ameloblasts | Channel; electrodiffusive (no fixed stoichiometry) | Loss-of-function (cystic fibrosis) → hypomineralized, acidic maturation bands; altered Cl−/Ca2+ content in Cftr-null enamel | [1,169,177,213] |
| HCO3−/Cl− | SLC26A1 (Sat1)/A3 (Dra)/A4 (pendrin)/A6 (Pat1)/A7 (Sut2) | Cl−/HCO3− exchange (CFTR-coupled) | Apical HCO3− secretion for matrix neutralization and protein clearance (maturation) | Apical (A1, A3, A4 and A6); apical and supranuclear (A4); apical, partly subapical, cytoplasmic (A7) | Mostly 1:1; A6 often 1 Cl−:2 HCO3− | No direct enamel disorder; A4→Pendred (systemic); Slc26a7 deletion delays enamel (rat) | [214,215,216,217] |
| H+ | ATP6V (V-ATPase; e.g., ATP6V1B1/ATP6V0A4) | H+ efflux (proton pump) | Apical acidification during ruffle-ended phases; endo-lysosomal acidification supporting matrix protein resorption; a3 disruption impacts ameloblasts/enamel (mouse) | Apical membrane of ruffle-ended ameloblasts; endosomal/lysosomal membranes | ATP-driven (H+/ATP ≈ 2–4; electrogenic) | autosomal recessive distal renal tubular acidosis (dRTA) from ATP6V1B1/ATP6V0A4 mutations; impaired acidification can disrupt matrix processing | [4,36,218,219,220] |
| H+/Na+ | SLC9A1 (NHE1) | Na+/H+ exchange (H+ efflux, Na+ influx) | Basolateral pHi recovery/stabilization after acid loads; supports vectorial HCO3− secretion (via CA-generated HCO3− + H+ extrusion) | Basolateral | ~1 Na+:1 H+ (electroneutral) | Not directly linked to AI | [1,177,212,221,222] |
| Na+/K+ | ATP1A1/ATP1B1 (Na+/K+-ATPase α1/β1) | Na+ out, K+ in | Maintains the Na+ gradient that powers secondary transport (NBCe1, NCKX/NCX, NHE1; supports CFTR/SLC26 circuits and cell-volume control) | Cytoplasmic and Basolateral membrane of secretory and maturation ameloblasts (also papillary layer) | 3 Na+ out:2 K+ in (ATP) | Essential for epithelial transport; no enamel-specific AI link | [1,170,174] |
| Na+/K+/Cl− | SLC12A2 (NKCC1) | Na+, K+, 2 Cl− in | Osmolyte control and Cl− supply from support layers to sustain ameloblast ion transport; regulatory volume function | Papillary layer and outer enamel epithelium (non-ameloblast support cells; SI/SR in earlier stage context) | 1 Na+ + 1 K+ + 2 Cl− in | Nkcc1−/−: late-maturation ameloblasts disorganized/shorter and ~10% lower enamel mineral density; ↑ Connexin 43 (Cx43)/NBCe1/SLC26A3/A6 compensation (hypomineralization) | [1,68,175] |
| K+ | KCNJ15 (Kir4.2) | K+ recycling | K+ uptake from enamel fluid; helps stabilize apical membrane potential during ruffle-ended phases; coordinates with NCKX4/Na+ handling | Apical border of ruffle-ended ameloblasts and cytosol of SA (reduced in smooth-ended; mislocalized with fluorosis/Wdr72 loss) | Channel (rectifier; non-stoichiometric) | No direct AI link; apical localization is reduced in fluorosis | [168,170] |
| Mg2+ | CNNM4 | Mg2+ efflux (Na+-linked) | Prevents intracellular Mg2+ buildup; supports proper crystal chemistry and maturation | Basolateral membrane of maturation ameloblasts | Na+-linked; stoichiometry not established in ameloblasts (2 Na+:1 Mg2+ reported in other cells) | Biallelic CNNM4 Mutations → Jalili syndrome (cone-rod dystrophy + AI) | [1,184,223,224] |
| Mg2+ | TRPM7 | Divalent-permeable channel (Mg2+ influx; also Ca2+) | Maintains Mg2+ homeostasis; supplies Mg2+ needed for TNAP/ALPL activity; also contributes to/positively modulates Ca2+ entry | Plasma membrane of maturation ameloblasts (evident in HAT-7 and mouse incisor; basolateral enrichment proposed) | Channel (non-stoichiometric) | TRPM7 kinase-dead or enamel-epithelium cKO → hypomineralized/hypoplastic enamel; reduced ALPase activity partly rescued by Mg2+ | [185,225,226,227,228] |
| Citrate (Cit3−) | SLC13A5 (NaCT) | Na+-coupled citrate influx | Supplies intracellular citrate for metabolism and incorporation at the mineral front; modulates crystal surface chemistry/toughness and transiently chelates Ca2+ during maturation | Basolateral (ameloblasts); papillary layer/support cells | Electrogenic ~3–4 Na+:1 citrate (pH-dependent) | Biallelic SLC13A5 variants → developmental and epileptic encephalopathy (DEE25); reported enamel hypoplasia/thin enamel | [229,230,231] |
| PO43− | ALPL (TNAP) | Liberates PO43− by hydrolyzing PPi/other phosphomonoesters | Provides local orthophosphate for enamel mineral growth; supports matrix pH control | Stratum intermedium (high); also, maturation-stage ameloblasts | Enzyme (no fixed stoichiometry) | Hypophosphatasia (ALPL) → enamel defects/hypomineralization | [1,67,232,233] |
| PO43− | SLC53A1 (XPR1) | Pi efflux (non–Na+-coupled, IP6/InsP7-regulated) | Exports intracellular Pi toward the enamel space; coordinates with TNAP (PPi→Pi) and NaPi-IIb uptake to maintain matrix Pi/PPi balance | Apical (Tomes’ process, putative) | Not fully established; electrogenic (non-stoichiometric) | Biallelic XPR1 variants → primary familial brain calcification (PFBC/IBGC6); no enamel-specific phenotype | [11,233,234,235] |
| PO43− | SLC20A1/2 (PiT1/2) | Na+-coupled Pi influx | Pi import into ameloblasts (PiT1); PiT2 prominent in support layers → paracrine Pi supply | PiT1: ameloblasts (likely basolateral); PiT2: stratum intermedium/sub-odontoblastic layer | 2 Na+:1 Pi (in) | No firm AI link; Slc20a2−/− → dentin defects, enamel largely preserved | [1,9,236,237,238] |
| HPO42− | SLC34A2 (NaPi-IIb) | Na+-coupled Pi influx | Pi import to support mineral growth | Apical and nuclear in secretory; apical, and cytoplasmic in maturation | 3 Na+:1 HPO42− (in) | Variants → pulmonary alveolar microlithiasis (PAM); no direct AI link | [9,10,29,239,240] |
| Fe | Ferritin (FTH1) | Iron storage (Fe3+ mineral core) | Sequesters Fe to limit oxidative stress; transient reservoir for Fe later deposited into enamel surface (rodent incisors) | Cytosol and ferritin-containing vesicles of late-maturation ameloblasts; papillary layer | Nanocage; up to ~4500 Fe atoms | Disrupted iron handling (e.g., ATG7 loss) → removes the iron deposit that normally pigments rodent incisors and can impair enamel, not classified as AI (hereditary) | [241,242,243,244,245] |
| F− | (Fluoride incorporation) | F− substitution for OH− in apatite | Forms fluorapatite; lowers crystal solubility and increases acid resistance | Enamel mineral lattice (surface-enriched) | 1 F−:1 OH− (Ca10(PO4)6(OH)2 → Ca10(PO4)6F2) | Excess during development → fluorosis; low exposure → ↑ caries risk | [154,246,247,248,249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarinfar, M.; Aghazadeh, M.; Bapat, R.A.; Ji, Y.; Paine, M.L. Enamel Maturation as a Systems Physiology: Ion Transport and Pi Flux. Cells 2025, 14, 1821. https://doi.org/10.3390/cells14221821
Zarinfar M, Aghazadeh M, Bapat RA, Ji Y, Paine ML. Enamel Maturation as a Systems Physiology: Ion Transport and Pi Flux. Cells. 2025; 14(22):1821. https://doi.org/10.3390/cells14221821
Chicago/Turabian StyleZarinfar, Mehrnaz, Marziyeh Aghazadeh, Rucha Arun Bapat, Yanbin Ji, and Michael L. Paine. 2025. "Enamel Maturation as a Systems Physiology: Ion Transport and Pi Flux" Cells 14, no. 22: 1821. https://doi.org/10.3390/cells14221821
APA StyleZarinfar, M., Aghazadeh, M., Bapat, R. A., Ji, Y., & Paine, M. L. (2025). Enamel Maturation as a Systems Physiology: Ion Transport and Pi Flux. Cells, 14(22), 1821. https://doi.org/10.3390/cells14221821






