Valeric Acid: A Gut-Derived Metabolite as a Potential Epigenetic Modulator of Neuroinflammation in the Gut–Brain Axis
Highlights
- Valeric Acid (VA), a gut-derived short-chain fatty acid (SCFA), acts as a selective inhibitor of Class I Histone Deacetylases (HDACs), particularly HDAC3.
- VA modulates neuroinflammation and promotes neuroprotection by both epigenetic and GABAergic mechanisms.
- VA offers a safer, physiological strategy to the non-selective pharmacological analogue, Valproic Acid (VPA), which is limited by significant systemic toxicity.
- Elucidating VA’s role might promote microbiome-derived compounds for targeted epigenetic modulation of neurodegenerative disorders.
Abstract
1. Introduction
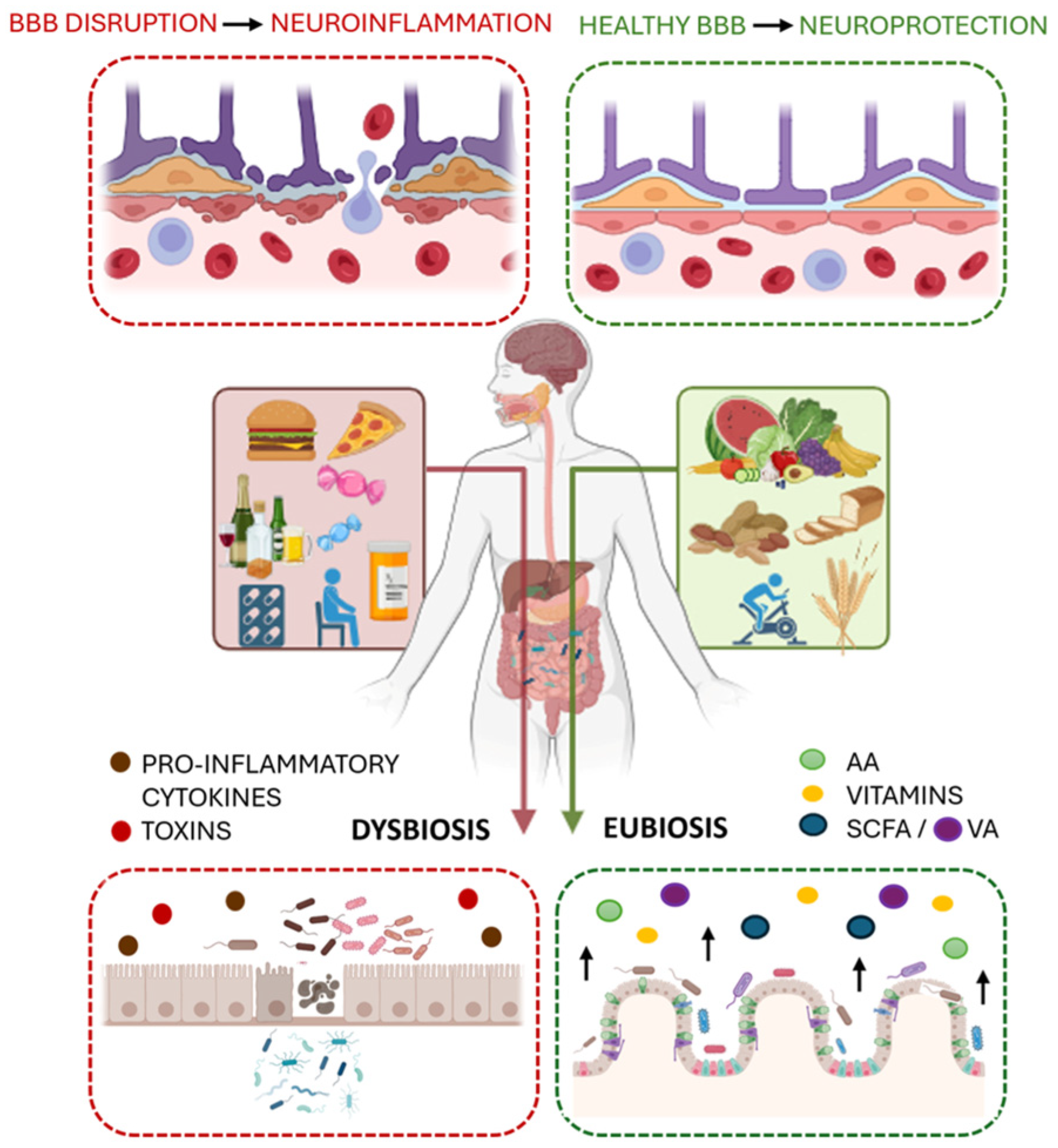
2. The Gut Microbiota: Eubiosis and Dysbiosis
3. The Role of Short-Chain Fatty Acids (SCFAs): Systemic Impact
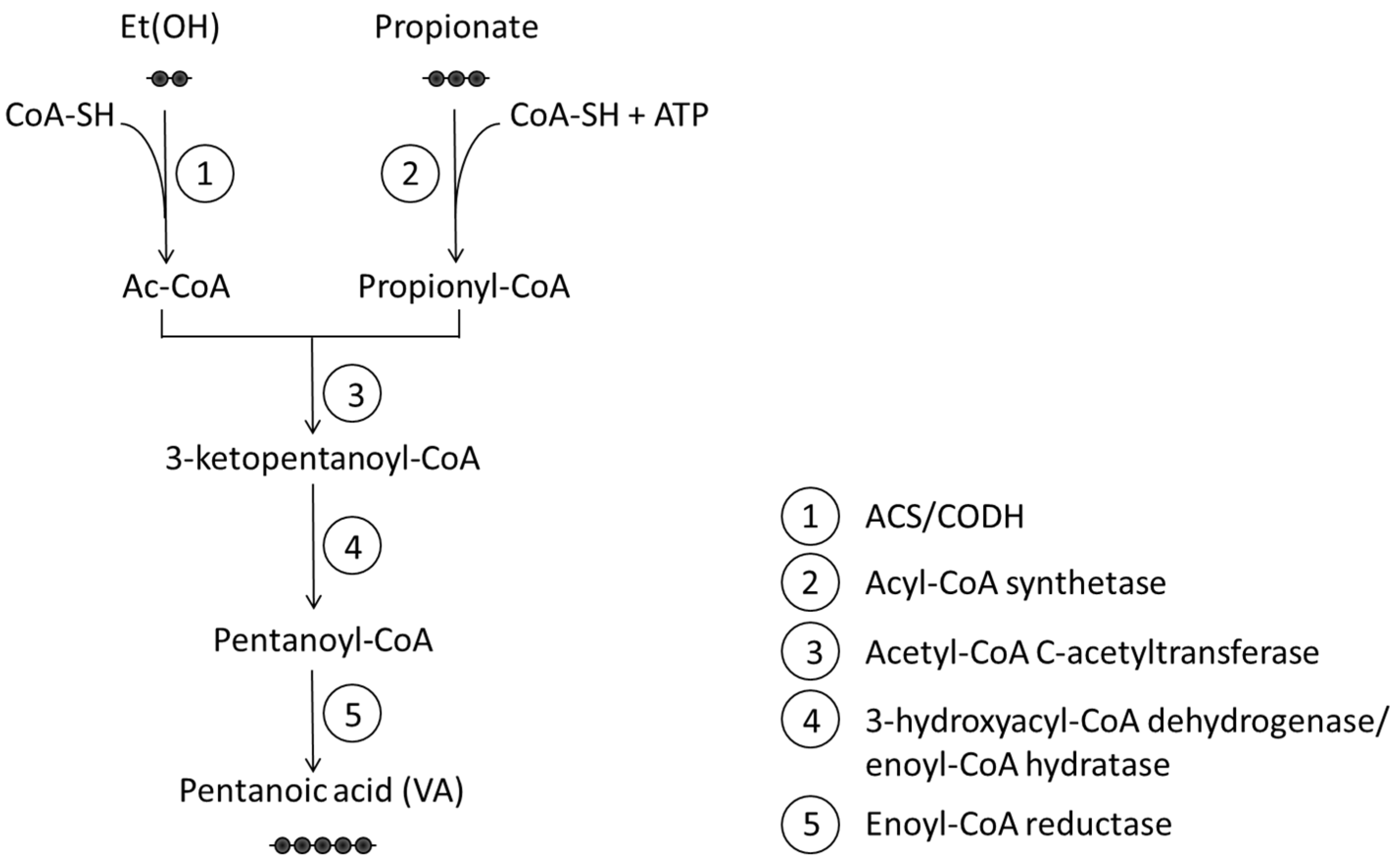
4. Valeric Acid: Biosynthesis, Metabolism, and Local Effects
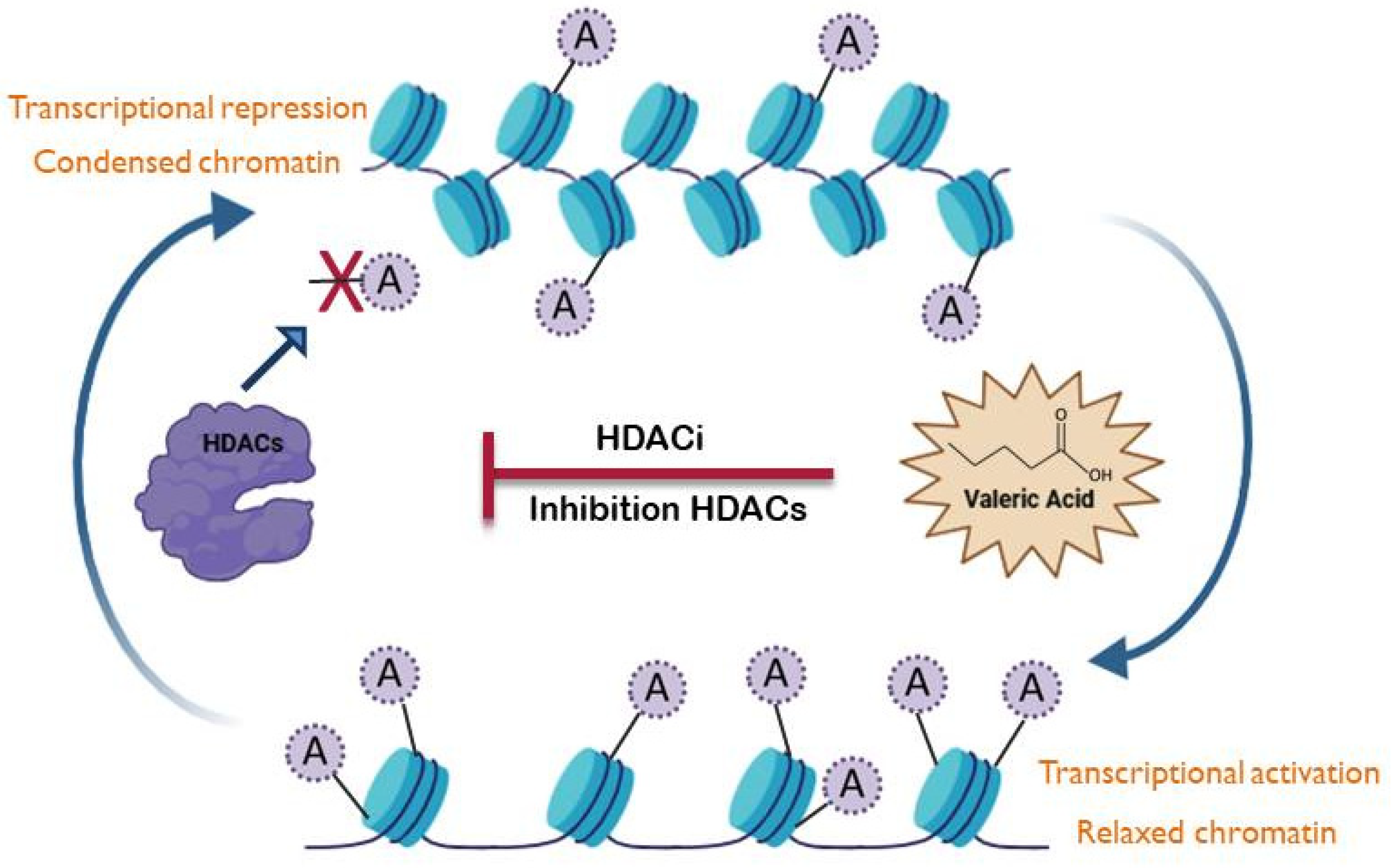
5. Valeric Acid as an Epigenetic Modulator
6. Valeric Acid: Systemic Effects and Neuroprotection
7. From a Pharmacological Drug to a Physiological Mediator: Valproic Acid and Valeric Acid in Epigenetics
8. Clinical and Epigenetic Challenges in VA Research
9. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBA | gut–brain axis |
| CNS | central nervous system |
| BBB | the blood-brain barrier |
| SCFA | short-chain fatty acids |
| VA | valeric acid |
| HDAC | histone deacetylases |
| HDACi | HDAC inhibitors |
| ROS | reactive oxygen species |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-1β, IL-6 | Interleukins |
| COX-2 | Cyclooxygenase-2 |
| VEGF-A | Vascular Endothelial Growth Factor-A |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MMP-9 | Matrix metalloproteinase-9 |
| NLRP3 | NOD-like receptor protein 3 |
| AD | Alzheimer’s Disease |
| PD | Parkinson’s Disease |
| MS | multiple sclerosis |
| FTD | frontotemporal dementia |
| ASD | autism spectrum disorder |
| GABA | gamma-aminobutyric acid |
| CAZymes | carbohydrate-active enzymes |
| MCTs | H+-coupled monocarboxylate transporters |
| SMCTs | sodium-coupled monocarboxylate transporters |
| Tregs | regulatory T cells |
| APCs | antigen-presenting cells |
| POCD | postoperative cognitive dysfunction |
| ICV-STZ | intracerebroventricular streptozotocin |
| PTZ | picrotoxin |
References
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Suganya, K.; Koo, B.S. Gut–brain axis: Role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Mayer, E. Advances in Brain–Gut–Microbiome Interactions: A Comprehensive Update on Signaling Mechanisms, Disorders, and Therapeutic Implications. Cell. Mol. Gastroenterol. Hepatol. 2024, 18, 1–13. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- García-Domínguez, M. Neuroinflammation: Mechanisms, Dual Roles, and Therapeutic Strategies in Neurological Disorders. Curr. Issues Mol. Biol. 2025, 47, 417. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S.; Müller, V. The dual nature of neuroin fl ammation in networked brain. Front. Immunol. 2025, 16, 1659947. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef]
- Appleton, J. The gut-brain axis: Influence of microbiota on mood and mental health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2021, 164, 105314. [Google Scholar] [CrossRef]
- Saji, N.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 19227. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef]
- Escalante, J.; Artaiz, O.; Diwakarla, S.; McQuade, R.M. Leaky gut in systemic inflammation: Exploring the link between gastrointestinal disorders and age-related diseases. GeroScience 2025, 47, 1–22. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Guan, N.L.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Nyström, E.E.L.; Sergi, D.; Gustafsson, J.K. Diet, microbiota, and the mucus layer: The guardians of our health. Front. Immunol. 2022, 13, 953196. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in neurological disorders: Pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; York, E.M.; MacVicar, B.A. Immunometabolism in the Brain: How Metabolism Shapes Microglial Function. Trends Neurosci. 2020, 43, 854–869. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, C.Y.; Lee, E.; Lee, C.; Lee, K.S.; Lee, J.; Park, H.; Choi, B.; Hwang, I.; Kim, J.; et al. Microglial NLRP3-gasdermin D activation impairs blood-brain barrier integrity through interleukin-1β-independent neutrophil chemotaxis upon peripheral inflammation in mice. Nat. Commun. 2025, 16, 699. [Google Scholar] [CrossRef]
- Ni, Y.; Teng, T.; Li, R.; Simonyi, A.; Sun, G.Y.; Lee, J.C. TNFα alters occludin and cerebral endothelial permeability: Role of p38MAPK. PLoS ONE 2017, 12, e0170346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Yi, M.; Wang, L.; Huang, Y. Role of gasdermin D in inflammatory diseases: From mechanism to therapeutics. Front. Immunol. 2024, 15, 1456244. [Google Scholar] [CrossRef]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Turner-Evans, D.B.; Jensen, K.T.; Ali, S.; Paterson, T.; Sheridan, A.; Ray, R.P.; Wolff, T.; Lauritzen, J.S.; Rubin, G.M.; Bock, D.D.; et al. The Neuroanatomical Ultrastructure and Function of a Biological Ring Attractor. Neuron 2020, 108, 145–163. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.C.; Liu, X.Y.; Deng, X.N.; Tian, Q.; Du, Y.J. Epigenetic Modulation of Microglia Function and Phenotypes in Neurodegenerative Diseases. Neural Plast. 2021, 2021, 9912686. [Google Scholar] [CrossRef]
- Ghiglieri, V.; Picconi, B.; Sgobio, C.; Bagetta, V.; Barone, I.; Paillè, V.; Di Filippo, M.; Polli, F.; Gardoni, F.; Altrock, W.; et al. Epilepsy-induced abnormal striatal plasticity in Bassoon mutant mice. Eur. J. Neurosci. 2009, 29, 1979–7993. [Google Scholar] [CrossRef]
- Giallongo, S.; Longhitano, L.; Denaro, S.; D’Aprile, S.; Torrisi, F.; La Spina, E.; Giallongo, C.; Mannino, G.; Lo Furno, D.; Zappalà, A.; et al. The Role of Epigenetics in Neuroinflammatory-Driven Diseases. Int. J. Mol. Sci. 2022, 23, 15218. [Google Scholar] [CrossRef] [PubMed]
- Kobow, K.; Blümcke, I. The methylation hypothesis: Do epigenetic chromatin modifications play a role in epileptogenesis? Epilepsia 2011, 52, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Leus, N.G.J.; Zwinderman, M.R.H.; Dekker, F.J. Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-κB-mediated inflammation. Curr. Opin. Chem. Biol. 2016, 33, 160–168. [Google Scholar] [CrossRef]
- Sullivan, K.E.; Reddy, A.B.M.; Dietzmann, K.; Suriano, A.R.; Kocieda, V.P.; Stewart, M.; Bhatia, M. Epigenetic Regulation of Tumor Necrosis Factor Alpha. Mol. Cell. Biol. 2007, 27, 5147–5160. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Cervellati, C.; Cordone, V.; Hayek, J.; Valacchi, G. Compromised immune/inflammatory responses in Rett syndrome. Free Radic. Biol. Med. 2020, 152, 100–106. [Google Scholar] [CrossRef]
- Siniscalco, D.; Cirillo, A.; Bradstreet, J.J.; Antonucci, N. Epigenetic findings in autism: New perspectives for therapy. Int. J. Environ. Res. Public Health 2013, 10, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, R.; Neumann, M.; Mackenzie, I.R. Correction: Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 2013, 9, 240. [Google Scholar] [CrossRef]
- Boylan, K.; DeJesus-Hernandez, M.; Rush, B.; Desaro, P.; Johnston, A.; Kryston, T.; Rutherford, N.; Baker, M.; Wszolek, Z.; Dickson, D.; et al. Phenotype of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia with ALS (FTD/ALS) associated with the GGGGCC repeat expansion in C9ORF72 (c9FTD/ALS). Neurology 2012, 78, S05.003. [Google Scholar]
- Oeckl, P.; Weydt, P.; Steinacker, P.; Anderl-Straub, S.; Nordin, F.; Volk, A.E.; Diehl-Schmid, J.; Andersen, P.M.; Kornhuber, J.; Danek, A.; et al. Different neuroinflammatory profile in amyotrophic lateral sclerosis and frontotemporal dementia is linked to the clinical phase. J. Neurol. Neurosurg. Psychiatry 2019, 90, 4–10. [Google Scholar] [CrossRef]
- Woollacott, I.O.C.; Nicholas, J.M.; Heslegrave, A.; Heller, C.; Foiani, M.S.; Dick, K.M.; Russell, L.L.; Paterson, R.W.; Keshavan, A.; Fox, N.C.; et al. Cerebrospinal fluid soluble TREM2 levels in frontotemporal dementia differ by genetic and pathological subgroup. Alzheimers Res. Ther. 2018, 10, 79. [Google Scholar] [CrossRef]
- Bright, F.; Werry, E.L.; Dobson-Stone, C.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kassiou, M.; et al. Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 2019, 15, 540–555. [Google Scholar] [CrossRef]
- Chu, M.; Wen, L.; Jiang, D.; Liu, L.; Nan, H.; Yue, A.; Wang, Y.; Wang, Y.; Qu, M.; Wang, N.; et al. Peripheral inflammation in behavioural variant frontotemporal dementia: Associations with central degeneration and clinical measures. J. Neuroinflamm. 2023, 20, 65. [Google Scholar] [CrossRef]
- Veerappan, C.S.; Sleiman, S.; Coppola, G. Epigenetics of Alzheimer’s Disease and Frontotemporal Dementia. Neurotherapeutics 2013, 10, 709–721. [Google Scholar] [CrossRef]
- Murthy, M.; Rizzu, P.; Heutink, P.; Mill, J.; Lashley, T.; Bettencourt, C. Epigenetic Age Acceleration in Frontotemporal Lobar Degeneration: A Comprehensive Analysis in the Blood and Brain. Cells 2023, 12, 1922. [Google Scholar] [CrossRef]
- Barros, S.P.; Offenbacher, S. Epigenetics: Connecting environment and genotype to phenotype and disease. J. Dent. Res. 2009, 88, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Bobetsis, Y.A.; Barros, S.P.; Lin, D.M.; Arce, R.M.; Offenbacher, S. Altered gene expression in murine placentas in an infection-induced intrauterine growth restriction model: A microarray analysis. J. Reprod. Immunol. 2010, 85, 140–148. [Google Scholar] [CrossRef]
- Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef]
- Doñas, C.; Loyola, A.; Rosemblatt, M. Exploring Epigenetic Drugs in the Regulation of Inflammatory Autoimmune Diseases. In Translational Studies on Inflammation; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Bonnaud, E.M.; Suberbielle, E.; Malnou, C.E. Histone acetylation in neuronal (dys)function. Biomol. Concepts 2016, 7, 103–116. [Google Scholar] [CrossRef]
- Pham, T.M.; Winblad, B.; Granholm, A.C.; Mohammed, A.H. Environmental influences on brain neurotrophins in rats. Pharmacol. Biochem. Behav. 2002, 73, 167–175. [Google Scholar] [CrossRef]
- Liyanage, V.R.B.; Rastegar, M. Rett syndrome and MeCP2. NeuroMolecular Med. 2014, 16, 231–264. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; D’Mello, S.R.; Narayanan, V. Epigenetics, Autism Spectrum, and Neurodevelopmental Disorders. Neurotherapeutics 2013, 10, 742–756. [Google Scholar] [CrossRef]
- Chiarini, A.; Gui, L.; Viviani, C.; Armato, U.; Dal Prà, I. NLRP3 Inflammasome’s Activation in Acute and Chronic Brain Diseases—An Update on Pathogenetic Mechanisms and Therapeutic Perspectives with Respect to Other Inflammasomes. Biomedicines 2023, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Biagi, E.; Turroni, S.; Maccaferri, S.; Figini, P.; Brigidi, P. Dynamic efficiency of the human intestinal microbiota. Crit. Rev. Microbiol. 2015, 41, 165–171. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Di Chiano, M.; Sallustio, F.; Fiocco, D.; Rocchetti, M.T.; Spano, G.; Pontrelli, P.; Moschetta, A.; Gesualdo, L.; Gadaleta, R.M.; Gallone, A. Psychobiotic Properties of Lactiplantibacillus plantarum in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 9489. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Acevedo-Román, A.; Pagán-Zayas, N.; Velázquez-Rivera, L.I.; Torres-Ventura, A.C.; Godoy-Vitorino, F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. Int. J. Mol. Sci. 2024, 25, 9715. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.T.; Di Chiano, M.; Elouardi, I.; Fiocco, D. Psychobiotic properties of probiotic lactic acid bacteria and bifidobacteria in paediatric neurological disorders. Glob. Pediatr. 2025, 13, 100266. [Google Scholar] [CrossRef]
- Zheng, Y.; Bonfili, L.; Wei, T.; Eleuteri, A.M. Understanding the Gut–Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders. Nutrients 2023, 15, 4631. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What defines a healthy gut microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Wang, K.; Wu, L.Y.; Dou, C.Z.; Guan, X.; Wu, H.G.; Liu, H.R. Research Advance in Intestinal Mucosal Barrier and Pathogenesis of Crohn’s Disease. Gastroenterol. Res. Pract. 2016, 2016, 9686238. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Itoh, T.; Kuwahara, T.; Oshima, K.; Toh, H.; Toyoda, A.; Takami, H.; Morita, H.; Sharma, V.K.; Srivastava, T.P.; et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007, 14, 169–181. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Nielsen, D.S.G.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Ghanim, H.; Batra, M.; Abuaysheh, S.; Green, K.; Makdissi, A.; Kuhadiya, N.D.; Chaudhuri, A.; Dandona, P. Antiinflammatory and ROS Suppressive Effects of the Addition of Fiber to a High-Fat High-Calorie Meal. J. Clin. Endocrinol. Metab. 2017, 102, 858–869. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrné, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, e80476. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D. Diet and inflammation: A link to metabolic and cardiovascular diseases. Eur. Heart J. 2006, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, H.W.J.; Naylor, C.P.E.; MacFarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, Å.M.; Björck, I.M.E.; Nyman, E.M.G.L. Combinations of indigestible carbohydrates affect short-chain fatty acid formation in the hindgut of rats. J. Nutr. 2002, 132, 3098–3104. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Richards, L.B.; Li, M.; van Esch, B.C.A.M.; Garssen, J.; Folkerts, G. The effects of short-chain fatty acids on the cardiovascular system. PharmaNutrition 2016, 4, 68–111. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Akimova, T.; Beier, U.H.; Liu, Y.; Wang, L.; Hancock, W.W. Histone/protein deacetylases and T-cell immune responses. Blood 2012, 119, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.; Rokem, J.S. Organic and Fatty Acid Production, Microbial. In Encyclopedia of Microbiology, 3rd ed.; Academic Press: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Khan, A.; Akram, M.; Thiruvengadam, M.; Daniyal, M.; Zakki, S.A.; Munir, N.; Zainab, R.; Heydari, M.; Mosavat, S.H.; Rebezov, M.; et al. Anti-anxiety Properties of Selected Medicinal Plants. Curr. Pharm. Biotechnol. 2021, 23, 1041–1060. [Google Scholar] [CrossRef]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Goyal, R.; Gupta, V.; Dhar, K.L. GABAergic effect of valeric acid from Valeriana wallichii in amelioration of ICV STZ induced dementia in rats. Rev. Bras. Farmacogn. 2016, 26, 484–489. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.S.; Sang, B.I. An Efficient New Process for the Selective Production of Odd-Chain Carboxylic Acids by Simple Carbon Elongation Using Megasphaera hexanoica. Sci. Rep. 2019, 9, 11999. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Arboleya, S.; González, S.; Salazar, N. Diet and Microbiome in Health and Aging. Nutrients 2022, 14, 3250. [Google Scholar] [CrossRef]
- Moles, L.; Otaegui, D. The impact of diet on microbiota evolution and human health. Is diet an adequate tool for microbiota modulation? Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone deacetylases (HDACs): Evolution, specificity, role in transcriptional complexes, and pharmacological actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Li, Y.; Ling, C.; Lu, L. Valeric acid acts as a novel HDAC3 inhibitor against prostate cancer. Med. Oncol. 2022, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A.; Rifkind, R.A.; Richon, V.M.; Breslow, R.; Miller, T.; Kelly, W.K. Histone deacetylases and cancer: Causes and therapies. Nat. Rev. Cancer 2001, 1, 194–202. [Google Scholar] [CrossRef]
- Kumari, B.; Kumari, U.; Singh, D.K.; Husain, G.M.; Patel, D.K.; Shakya, A.; Singh, R.B.; Modi, G.P.; Singh, G.K. Molecular Targets of Valeric Acid: A Bioactive Natural Product for Endocrine, Metabolic, and Immunological Disorders. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 1506–1517. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Lai, Z.; Shan, W.; Li, J.; Min, J.; Zeng, X.; Zuo, Z. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol. Psychiatry 2021, 26, 7167–7187. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102. [Google Scholar] [CrossRef]
- Benke, D.; Barberis, A.; Kopp, S.; Altmann, K.H.; Schubiger, M.; Vogt, K.E.; Rudolph, U.; Möhler, H. GABAA receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology 2009, 56, 174–181. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch. Eur. J. Physiol. 2019, 471, 1441–1453. [Google Scholar] [CrossRef]
- Edvinsson, L.; Krause, D.N. Pharmacological characterization of GABA receptors mediating vasodilation of cerebral arteries in vitro. Brain Res. 1979, 173, 89–97. [Google Scholar] [CrossRef]
- Lloyd, K.G.; Dreksler, S. An analysis of [3H]gamma-aminobutyric acid (GABA) binding in the human brain. Brain Res. 1979, 163, 77–87. [Google Scholar] [CrossRef]
- Nouri, M.H.K.; Abad, A.N.A. Gabaergic system role in aqueous extract of Valeriana officinalis L. root on PTZ-induced clonic seizure threshold in mice. Afr. J. Pharm. Pharmacol. 2011, 5, 1212–1217. [Google Scholar] [CrossRef]
- Mulyawan, E.; Ahmad, M.R.; Islam, A.A.; Massi, M.N.; Hatta, M.; Arif, S.K. Analysis of GABRB3 gene mRNA expression and motor coordination after administration of valerian extracts (Valeriana officinalis) in BALB/c mice. F1000Research 2020, 9, 670. [Google Scholar] [CrossRef]
- Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, A.S.K.; Wang, L.M.; Alabdei, H.H.; Liloglou, T. Effect of valproic acid on histone deacetylase expression in oral cancer (Review). Oncol. Lett. 2024, 27, 197. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Mazzocchetti, P.; D’Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Curr. Neuropharmacol. 2018, 17, 926–946. [Google Scholar] [CrossRef] [PubMed]
- Curcio, A.; Rocca, R.; Alcaro, S.; Artese, A. The Histone Deacetylase Family: Structural Features and Application of Combined Computational Methods. Pharmaceuticals 2024, 17, 620, Correction in Pharmaceuticals 2024, 17, 1520. https://doi.org/10.3390/ph17111520. [Google Scholar] [CrossRef]
- Gurvich, N.; Tsygankova, O.M.; Meinkoth, J.L.; Klein, P.S. Histone Deacetylase Is a Target of Valproic Acid-Mediated Cellular Differentiation. Cancer Res. 2004, 64, 1079–7086. [Google Scholar] [CrossRef]
- Milutinovic, S.; Detich, N.; Szyf, M. Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis 2007, 28, 560–571. [Google Scholar] [CrossRef]
- Mello, M.L.S. Sodium Valproate-Induced Chromatin Remodeling. Front. Cell Dev. Biol. 2021, 9, 645518. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Pillai, R.B.; Shekar, K.V.; Lane, J.B.; Motil, K.J.; Skinner, S.A.; Tarquinio, D.C.; Glaze, D.G.; McGwin, G.; Kaufmann, W.E.; et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in rett syndrome. J. Med. Genet. 2014, 51, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Huppke, P.; Köhler, K.; Brockmann, K.; Stettner, G.M.; Gärtner, J. Treatment of epilepsy in Rett syndrome. Eur. J. Paediatr. Neurol. 2007, 11, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.P.K.; Mellios, N.; Sur, M. Rett syndrome: Insights into genetic, molecular and circuit mechanisms. Nat. Rev. Neurosci. 2018, 19, 368–382. [Google Scholar] [CrossRef]
- Guo, W.; Tsujimura, K.; Otsuka, I.M.; Irie, K.; Igarashi, K.; Nakashima, K.; Zhao, X. VPA alleviates neurological deficits and restores gene expression in a mouse model of rett syndrome. PLoS ONE 2014, 9, e100215. [Google Scholar] [CrossRef]
- Landrieu, P.; Baets, J.; De Jonghe, P. Hereditary motor-sensory, motor, and sensory neuropathies in childhood. In Handbook of Clinical Neurology; North-Holland Publishing Company: New York, NY, USA, 2013; Volume 113. [Google Scholar] [CrossRef]
- Aicardi, J.; Goutières, F. A Progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 1984, 15, 49–54. [Google Scholar] [CrossRef]
- Lebon, P.; Badoual, J.; Ponsot, G.; Goutières, F.; Hémeury-Cukier, F.; Aicardi, J. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J. Neurol. Sci. 1988, 84, 201–208. [Google Scholar] [CrossRef]
- Crow, Y.J.; Chase, D.S.; Lowenstein Schmidt, J.; Szynkiewicz, M.; Forte, G.M.A.; Gornall, H.L.; Oojageer, A.; Anderson, B.; Pizzino, A.; Helman, G.; et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am. J. Med. Genet. Part A 2015, 167, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Livingston, J.H.; Crow, Y.J. Neurologic phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi-Goutières syndrome and beyond. Neuropediatrics 2016, 47, 355–360. [Google Scholar] [CrossRef]
- Uggenti, C.; Lepelley, A.; Depp, M.; Badrock, A.P.; Rodero, M.P.; El-Daher, M.T.; Rice, G.I.; Dhir, S.; Wheeler, A.P.; Dhir, A.; et al. cGAS-mediated induction of type I interferon due to inborn errors of histone pre-mRNA processing. Nat. Genet. 2020, 52, 1364–1372. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- von Stülpnagel, C.; van Baalen, A.; Borggraefe, I.; Eschermann, K.; Hartlieb, T.; Kiwull, L.; Pringsheim, M.; Wolff, M.; Kudernatsch, M.; Wiegand, G.; et al. Network for Therapy in Rare Epilepsies (NETRE): Lessons from the Past 15 Years. Front. Neurol. 2021, 11, 322510. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, F.; Cross, Z.M.; Woidill, S.; McMann, J.M.; Rand, E.B.; Takanohashi, A.; Ulrick, N.; Shults, J.; Vanderver, A.L.; Adang, L. Hepatic Involvement in Aicardi-Goutières Syndrome. Neuropediatrics 2021, 52, 441–447. [Google Scholar] [CrossRef]
- Lim, H.K.; Yoon, J.H.; Song, M. Autism Spectrum Disorder Genes: Disease-Related Networks and Compensatory Strategies. Front. Mol. Neurosci. 2022, 15, 922840. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, N. A Double-Blind Placebo-Controlled Trial of Acediprol (Valproate Sodium) for Global Severity in Child Autism Spectrum Disorders. Online J. Neurol. Brain Disord. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Wiśniowiecka-Kowalnik, B.; Nowakowska, B.A. Genetics and epigenetics of autism spectrum disorder—Current evidence in the field. J. Appl. Genet. 2019, 60, 37–47. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes 2023, 14, 677. [Google Scholar] [CrossRef]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int. J. Dev. Neurosci. 2015, 43, 70–77. [Google Scholar] [CrossRef]
- Li, B.; Xiong, Y.; Li, Y. The Impact of Valproic Acid on Microbiota in a Mouse Model of Autism Spectrum Disorder. Psychiatry Clin. Psychopharmacol. 2025, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; El Kouhen, N.; Bennani, S.; Getachew, B.; Aschner, M.; Tizabi, Y. Roles of Epigenetics and Glial Cells in Drug-Induced Autism Spectrum Disorder. Biomolecules 2024, 14, 437. [Google Scholar] [CrossRef]
- Fukuchi, M.; Nii, T.; Ishimaru, N.; Minamino, A.; Hara, D.; Takasaki, I.; Tabuchi, A.; Tsuda, M. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci. Res. 2009, 65, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kawanai, T.; Ago, Y.; Watanabe, R.; Inoue, A.; Taruta, A.; Onaka, Y.; Hasebe, S.; Hashimoto, H.; Matsuda, T.; Takuma, K. Prenatal Exposure to Histone Deacetylase Inhibitors Affects Gene Expression of Autism-Related Molecules and Delays Neuronal Maturation. Neurochem. Res. 2016, 41, 2574–2584. [Google Scholar] [CrossRef]
- Gazzina, S.; Manes, M.A.; Padovani, A.; Borroni, B. Clinical and biological phenotypes of frontotemporal dementia: Perspectives for disease modifying therapies. Eur. J. Pharmacol. 2017, 817, 76–85. [Google Scholar] [CrossRef]
- Bottero, V.; Alrafati, F.; Santiago, J.A.; Potashkin, J.A. Transcriptomic and Network Meta-Analysis of Frontotemporal Dementias. Front. Mol. Neurosci. 2021, 14, 747798. [Google Scholar] [CrossRef]
- Blauwendraat, C.; Wilke, C.; Simón-Sánchez, J.; Jansen, I.E.; Reifschneider, A.; Capell, A.; Haass, C.; Castillo-Lizardo, M.; Biskup, S.; Maetzler, W.; et al. The wide genetic landscape of clinical frontotemporal dementia: Systematic combined sequencing of 121 consecutive subjects. Genet. Med. 2018, 20, 240–249. [Google Scholar] [CrossRef]
- Ribeiro, A.C.R.; Jahr, F.M.; Hawkins, E.; Kronfol, M.M.; Younis, R.M.; McClay, J.L.; Deshpande, L.S. Epigenetic histone acetylation and Bdnf dysregulation in the hippocampus of rats exposed to repeated, low-dose diisopropylfluorophosphate. Life Sci. 2021, 281, 119765. [Google Scholar] [CrossRef]
- Khan, M.S.; Nasiripour, S.; Bopassa, J.C. Parkinson Disease Signaling Pathways, Molecular Mechanisms, and Potential Therapeutic Strategies: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 6416. [Google Scholar] [CrossRef]
- Larsson, P.; Ulfhammer, E.; Magnusson, M.; Bergh, N.; Lunke, S.; El-Osta, A.; Medcalf, R.L.; Svensson, P.A.; Karlsson, L.; Jern, S. Role of histone acetylation in the stimulatory effect of valproic acid on vascular endothelial tissue-type plasminogen activator expression. PLoS ONE 2012, 7, e31573. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhen, Y.; Wang, G.; Liu, B. Deconvoluting the Complexity of Reactive Oxygen Species (ROS) in Neurodegenerative Diseases. Front. Neuroanat. 2022, 16, 910427. [Google Scholar] [CrossRef]
- Yang, H.S.; Onos, K.D.; Choi, K.; Keezer, K.J.; Skelly, D.A.; Carter, G.W.; Howell, G.R. Natural genetic variation determines microglia heterogeneity in wild-derived mouse models of Alzheimer’s disease. Cell Rep. 2021, 34, 108739. [Google Scholar] [CrossRef]
- Frontiers Production Office. Erratum: Autophagy and neurodegeneration: Unraveling the role of C9ORF72 in the regulation of autophagy and its relationship to ALS-FTD pathology. Front. Cell. Neurosci. 2023, 17, 1225439. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Nagoor Meeran, M.F.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Valeric acid protects dopaminergic neurons by suppressing oxidative stress, neuroinflammation and modulating autophagy pathways. Int. J. Mol. Sci. 2020, 21, 7670. [Google Scholar] [CrossRef]
- Nijs, M.; Van Damme, P. The genetics of amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2024, 37, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.A.; Tanaz, R.; Cobos, S.N.; Torrente, M.P. Epigenetics in amyotrophic lateral sclerosis: A role for histone post-translational modifications in neurodegenerative disease. Transl. Res. 2019, 204, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.; Schmalbach, S.; Boeselt, S.; Sarlette, A.; Dengler, R.; Petri, S. Differential histone deacetylase mRNA expression patterns in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2010, 69, 573–581. [Google Scholar] [CrossRef]
- Boutillier, A.L.; Tzeplaeff, L.; Dupuis, L. The dark side of HDAC inhibition in ALS. EBioMedicine 2019, 41, 38–39. [Google Scholar] [CrossRef]
- Pigna, E.; Simonazzi, E.; Sanna, K.; Bernadzki, K.M.; Proszynski, T.; Heil, C.; Palacios, D.; Adamo, S.; Moresi, V. Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine 2019, 40, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Renzini, A.; Pigna, E.; Rocchi, M.; Cedola, A.; Gigli, G.; Moresi, V.; Coletti, D. Sex and HDAC4 Differently Affect the Pathophysiology of Amyotrophic Lateral Sclerosis in SOD1-G93A Mice. Int. J. Mol. Sci. 2023, 24, 98. [Google Scholar] [CrossRef]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal muscular atrophy: Mutations, testing, and clinical relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef]
- Le, T.T.; Pham, L.T.; Butchbach, M.E.R.; Zhang, H.L.; Monani, U.R.; Coovert, D.D.; Gavrilina, T.O.; Xing, L.; Bassell, G.J.; Burghes, A.H.M. SMNΔ7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 2005, 14, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Butchbach, M.E.R. Copy number variations in the survival motor neuron genes: Implications for spinal muscular atrophy and other neurodegenerative diseases. Front. Mol. Biosci. 2016, 3, 7. [Google Scholar] [CrossRef]
- Monani, U.R.; Sendtner, M.; Coovert, D.D.; Parsons, D.W.; Andreassi, C.; Le, T.T.; Jablonka, S.; Schrank, B.; Rossol, W.; Prior, T.W.; et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(-/-) mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000, 9, 333–339. [Google Scholar] [CrossRef]
- Kernochan, L.E.; Russo, M.L.; Woodling, N.S.; Huynh, T.N.; Avila, A.M.; Fischbeck, K.H.; Sumner, C.J. The role of histone acetylation in SMN gene expression. Hum. Mol. Genet. 2005, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Brichta, L.; Hofmann, Y.; Hahnen, E.; Siebzehnrubi, F.A.; Raschke, H.; Blumcke, I.; Eyupoglu, I.Y.; Wirth, B. Valproic acid increases the SMN2 protein level: A well-known drug as a potential therapy for spinal muscular atrophy. Hum. Mol. Genet. 2003, 12, 2481–2489. [Google Scholar] [CrossRef]
- Marasco, L.E.; Dujardin, G.; Sousa-Luís, R.; Liu, Y.H.; Stigliano, J.N.; Nomakuchi, T.; Proudfoot, N.J.; Krainer, A.R.; Kornblihtt, A.R. Counteracting chromatin effects of a splicing-correcting antisense oligonucleotide improves its therapeutic efficacy in spinal muscular atrophy. Cell 2022, 185, 2057–2070. [Google Scholar] [CrossRef]
- Swoboda, K.J.; Scott, C.B.; Reyna, S.P.; Prior, T.W.; LaSalle, B.; Sorenson, S.L.; Wood, J.; Acsadi, G.; Crawford, T.O.; Kissel, J.T.; et al. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS ONE 2009, 4, e5268. [Google Scholar] [CrossRef]
- Saito, T.; Nurputra, D.K.; Harahap, N.I.F.; Harahap, I.S.K.; Yamamoto, H.; Muneshige, E.; Nishizono, H.; Matsumura, T.; Fujimura, H.; Sakoda, S.; et al. A Study of valproic acid for patients with spinal muscular atrophy. Neurol. Clin. Neurosci. 2015, 3, 49–57. [Google Scholar] [CrossRef]
- Merchut, M.P. Multiple Sclerosis; Loyola Univeristy of Chicago: Chicago, IL, USA, 1999; Volume 359, pp. 169–180. [Google Scholar]
- Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.A.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; Freeman, C.; Hunt, S.E.; et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef]
- Guan, Y.; Jakimovski, D.; Ramanathan, M.; Weinstock-Guttman, B.; Zivadinov, R. The role of Epstein-Barr virus in multiple sclerosis: From molecular pathophysiology to in vivo imaging. Neural Regen. Res. 2019, 14, 373–386. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.Y.; Wu, Y.; Schluesener, H.J. Valproic acid ameliorates inflammation in experimental autoimmune encephalomyelitis rats. Neuroscience 2012, 221, 140–150. [Google Scholar] [CrossRef]
- Chen, P.S.; Wang, C.C.; Bortner, C.D.; Peng, G.S.; Wu, X.; Pang, H.; Lu, R.B.; Gean, P.W.; Chuang, D.M.; Hong, J.S. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 2007, 149, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Du, C.; Wei, W.; Wu, Z.; Zhao, G.; Li, Z.; Xie, X. The antiepileptic drug valproic acid restores T cell homeostasis and ameliorates pathogenesis of experimental autoimmune encephalomyelitis. J. Biol. Chem. 2012, 287, 28656–28665. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Svanström, H.; Stenager, E.; Magyari, M.; Koch-Henriksen, N.; Pasternak, B.; Hviid, A. The use of valproic acid and multiple sclerosis. Pharmacoepidemiol. Drug Saf. 2015, 24, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.E.; Dreifuss, F.E. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology 1996, 46, 465–469. [Google Scholar] [CrossRef]
- Lloyd, K.A. A scientific review: Mechanisms of valproate-mediated teratogenesis. Biosci. Horiz. 2013, 6, hzt003. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, M.; Mazzoldi, E.L.; Ferraro, R.M.; Pinelli, M.; Parigi, M.; Aghel, S.A.M.; Bugatti, M.; Collo, G.; Stocco, G.; Vermi, W.; et al. Progesterone receptor is constitutively expressed in induced Pluripotent Stem Cells (iPSCs). Stem Cell Rev. Rep. 2024, 20, 2303–2317. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, R.M.; Ginestra, P.S.; Seiti, M.; Bugatti, M.; Benini, G.; Ottelli, L.; Vermi, W.; Poliani, P.L.; Ceretti, E.; Giliani, S. Three-Dimensional-Bioprinted Embedded-Based Cerebral Organoids: An Alternative Approach for Mini-Brain In Vitro Modeling Beyond Conventional Generation Methods. Gels 2025, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhou, Y.; Gao, H. Prodrug strategy for enhanced therapy of central nervous system disease. Chem. Commun. 2021, 57, 8842–8855. [Google Scholar] [CrossRef] [PubMed]
- Powell-Jackson, P.R.; Tredger, J.M.; Williams, R. Hepatotoxicity to sodium valproate: A review. Gut 1984, 25, 673–681. [Google Scholar] [CrossRef]
| Disorder | Genetic Cause | Epigenetic Mechanism & Rationale | Preclinical/Clinical Evidence (VPA) | Valeric Acid (VA) | References |
|---|---|---|---|---|---|
| Rett Syndrome | MECP2 mutations | VPA, as an HDACi, is theoretically relevant to counteract MECP2 dysfunction. | Limited clinical evidence. Used for seizure management, not a disease-modifying therapy due to severe adverse effects. | N/A | [138,139,140,141] |
| Aicardi-Goutières Syndrome (AGS) | Mutations in genes like TREX1, SAMHD1. | Chronic activation of the cGAS-STING pathway and Type I interferon production leads to neuroinflammation. VPA might downregulate IFN-stimulated genes (ISGs) and pro-inflammatory cytokines. | Primarily preclinical research. Epigenetic modulation remains a theoretical approach. | N/A | [142,143,144,145,146,147,148,149,150] |
| Autism Spectrum Disorder (ASD) | Multifactorial, including de novo mutations (e.g., ADNP, MECP2, SHANK3) and polygenic risk. | Environmental factors and genetic variants shape the epigenome. VPA’s epigenetic action and impact on gut microbiota could modulate ASD-like behaviors. | Mixed clinical evidence. Prenatal VPA exposure is a known risk factor for ASD. Therapeutic use in established ASD is limited, though some small trials have shown a reduction in repetitive behaviors. | VA as a potential pathological mediator/marker. | [121,151,152,153,154,155,156,157,158,159,160] |
| Genetic FTD, AD, PD | Mutations in GRN, C9orf72 (FTD), APP, PSEN1 (AD), LRRK2 (PD). | Epigenetic dysregulations contribute to pathogenesis, including protein aggregation (TDP-43, alpha-synuclein), mitochondrial dysfunction, and oxidative stress. VPA could shift glial cells from a pro-inflammatory to a neuroprotective phenotype. | Variable preclinical results. Use in human studies is restricted by safety concerns, but preclinical models have explored its potential. | Protection of dopaminergic neurons by reducing oxidative stress and modulating autophagy. | [161,162,163,164,165,166,167,168,169,170] |
| Amyotrophic Lateral Sclerosis (ALS) | Mutations in SOD1, C9orf72, FUS, TDP-43. | Epigenetic dysregulation, particularly involving HDACs. HDAC2 levels are increased in some ALS samples. However, the role of specific HDACs like HDAC4 is complex and context-dependent. | Complexity in preclinical findings. Studies on HDAC4 inhibition in mouse models have shown detrimental effects, highlighting the nuanced and sometimes contradictory role of individual HDAC isoforms. | N/A | [171,172,173,174,175,176] |
| Spinal Muscular Atrophy (SMA) | Mutations in SMN1/2 genes. | VPA, as an HDACi, can enhance SMN2 expression at RNA and protein levels. This effect is more pronounced in patients with a higher SMN2 copy number. | Mixed clinical results as a monotherapy. VPA was shown to increase SMN levels but provided limited benefits for motor deficits. Recent studies suggest VPA may act synergistically with other treatments like nusinersen. | N/A | [177,178,179,180,181,182,183,184,185] |
| Multiple Sclerosis (MS) | Multifactorial; genetic variants in HLA-DRB1 and other immune genes. | Genetic and environmental factors shape the epigenome. VPA modulates immune responses, promotes oligodendrocyte differentiation, and increases myelination gene expression. | Promising preclinical evidence in EAE models, showing reduced disease severity, inflammation, and demyelination. Clinical research is ongoing, but therapeutic use is not yet standardized due to safety concerns. | N/A | [186,187,188,189,190,191,192,193] |
| Comparative Features | Valeric Acid (VA) | Valproic Acid (VPA) |
|---|---|---|
| Origin | Gut Microbiota (Endogenous Metabolite) | Synthetic Drug (Pharmaceutical) |
| HDAC Targets | Selective, primarily Class I (HDAC3) | Non-selective, Class I & Class IIa |
| Effective Concentration (EC50) | Physiological Circulating levels = 0.18 µM); HDAC3 IC50 (in vitro) = 16.6 µM | 50–125 μg/mL (mM range) |
| Toxicity/Side Effects | Low/Minimal (Physiological) | High/Severe (Hepatotoxicity, Teratogenicity) |
| Primary Role | Physiological Epigenetic Mediator | Established Anticonvulsant/Mood Stabilizer |
| Brain Bioavailability | Effective permeability | Effective permeability |
| Clinical Status | Preclinical/Early-stage research | Established Drug |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paciolla, C.; Manganelli, M.; Di Chiano, M.; Montenegro, F.; Gallone, A.; Sallustio, F.; Guida, G. Valeric Acid: A Gut-Derived Metabolite as a Potential Epigenetic Modulator of Neuroinflammation in the Gut–Brain Axis. Cells 2025, 14, 1823. https://doi.org/10.3390/cells14221823
Paciolla C, Manganelli M, Di Chiano M, Montenegro F, Gallone A, Sallustio F, Guida G. Valeric Acid: A Gut-Derived Metabolite as a Potential Epigenetic Modulator of Neuroinflammation in the Gut–Brain Axis. Cells. 2025; 14(22):1823. https://doi.org/10.3390/cells14221823
Chicago/Turabian StylePaciolla, Chiara, Michele Manganelli, Mariagiovanna Di Chiano, Francesca Montenegro, Anna Gallone, Fabio Sallustio, and Gabriella Guida. 2025. "Valeric Acid: A Gut-Derived Metabolite as a Potential Epigenetic Modulator of Neuroinflammation in the Gut–Brain Axis" Cells 14, no. 22: 1823. https://doi.org/10.3390/cells14221823
APA StylePaciolla, C., Manganelli, M., Di Chiano, M., Montenegro, F., Gallone, A., Sallustio, F., & Guida, G. (2025). Valeric Acid: A Gut-Derived Metabolite as a Potential Epigenetic Modulator of Neuroinflammation in the Gut–Brain Axis. Cells, 14(22), 1823. https://doi.org/10.3390/cells14221823








