The Role of Microbiota in Ovarian Cancer: Implications for Treatment Response and Therapeutic Strategies
Abstract
1. Introduction
2. Mechanistic Framework: Microbiota–Host Crosstalk in Ovarian Carcinogenesis
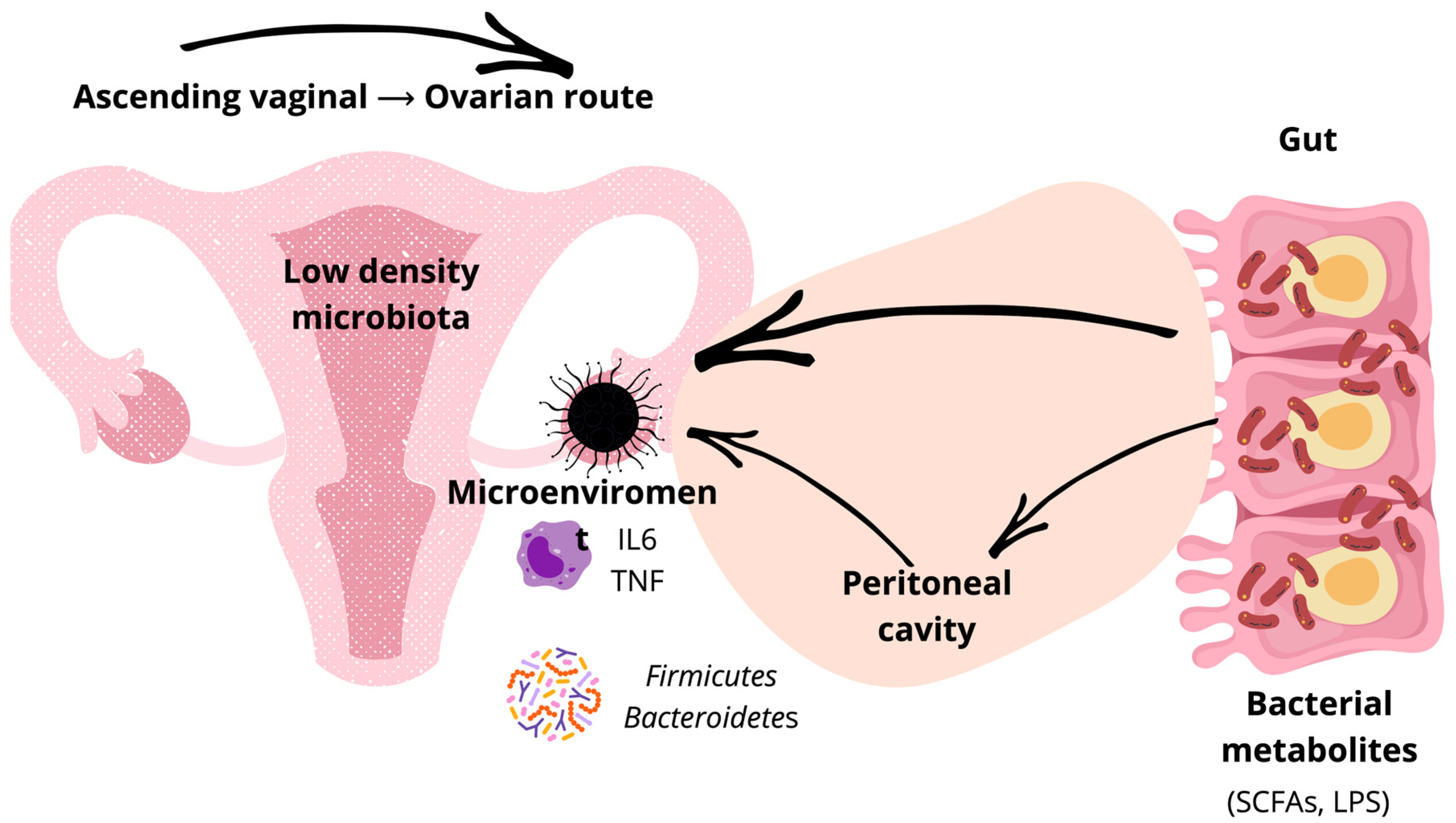
3. Microbial Niches and Routes of Dissemination in OC
3.1. Lower Reproductive Tract
3.2. Upper Reproductive Tract
3.3. Gut and Systemic Sources
4. Stage- and Site-Specific Microbiota Alterations in OC: Mechanisms, Biomarkers, and Therapeutic Implications
5. Intratumoral Microbiota in OC: Distinct Signatures and Oncogenic Pathways
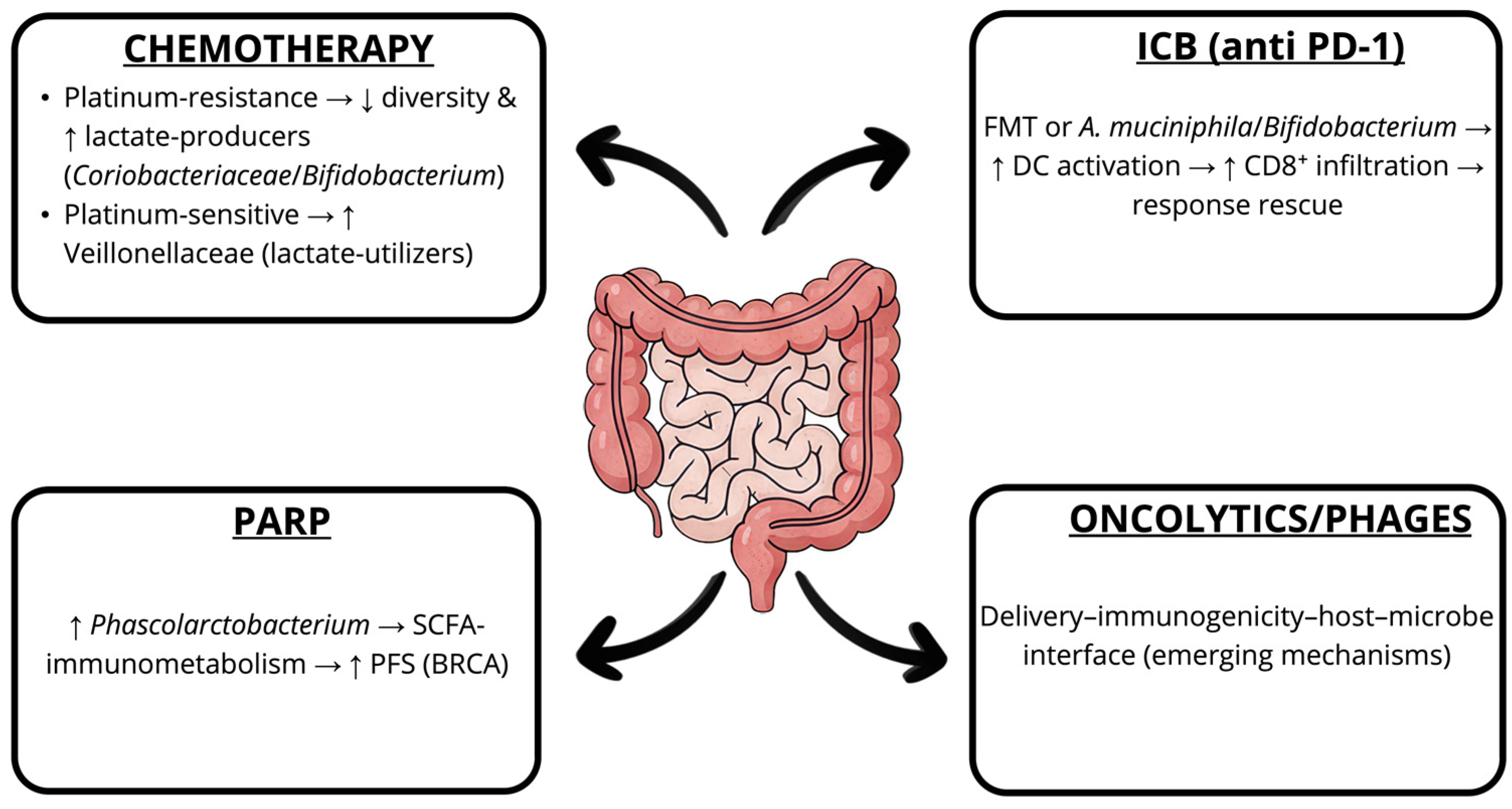
6. Antibiotics, Dysbiosis, and Therapy Resistance
7. Microbiota and Treatment Response in OC
8. Therapeutic Potential of the Microbiome Modulation
9. Challenges, Methodological Constraints, and Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/en (accessed on 30 June 2025).
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Weroha, S.J.; Cliby, W. Ovarian Cancer: A Review. JAMA 2025, 334, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Yan, M.; Xiang, Z. Gut and oral microbiota in gynecological cancers: Interaction, mechanism, and therapeutic value. NPJ Biofilms Microbiomes 2024, 10, 104. [Google Scholar] [CrossRef]
- Cani, P.D.; van Hul, M. Gut microbiota in overweight and obesity: Crosstalk with adipose tissue. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 164–183. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Joos, R.; Boucher, K.; Lavelle, A.; Arumugam, M.; Blaser, M.J.; Claesson, M.J.; Clarke, G.; Cotter, P.D.; De Sordi, L.; Dominguez-Bello, M.G.; et al. Examining the healthy human microbiome concept. Nat. Rev. Microbiol. 2025, 23, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Ray, D.; Kidane, D. Gut Microbiota Imbalance and Base Excision Repair Dynamics in Colon Cancer. J. Cancer 2016, 7, 1421–1430. [Google Scholar] [CrossRef]
- Klimesova, K.; Kverka, M.; Zakostelska, Z.; Hudcovic, T.; Hrncir, T.; Stepankova, R.; Rossmann, P.; Ridl, J.; Kostovcik, M.; Mrazek, J.; et al. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflamm. Bowel Dis. 2013, 19, 1266–1277. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
- Chalif, J.; Wang, H.; Spakowicz, D.; Quick, A.; Arthur, E.K.; O’Malley, D.; Chambers, L.M. The microbiome and gynecologic cancer: Cellular mechanisms and clinical applications. Int. J. Gynecol. Cancer 2023, 34, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, M.; Ding, Y.; Chen, J. Microbial infection, inflammation and epithelial ovarian cancer. Oncol. Lett. 2017, 14, 1911–1919. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, L.; Leiliang, X.; Qu, C.; Wu, W.; Wen, R.; Huang, N.; He, Q.; Cheng, Q.; Liu, G.; et al. Beyond the Gut: The intratumoral microbiome’s influence on tumorigenesis and treatment response. Cancer Commun. 2024, 44, 1130–1167. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Liu, C.W.; Chi, L.; Yang, Y.; Lu, K. Effects of Gut Microbiome on Carcinogenic DNA Damage. Chem. Res. Toxicol. 2020, 33, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, L.; Zhang, Y.; Ye, K. Lipopolysaccharide-Educated Cancer-Associated Fibroblasts Facilitate Malignant Progression of Ovarian Cancer Cells via the NF-kB/IL-6/JAK2 Signal Transduction. Mol. Biotechnol. 2024, 67, 317–328. [Google Scholar] [CrossRef]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Szajnik, M.; Szczepanski, M.J.; Czystowska, M.; Elishaev, E.; Mandapathil, M.; Nowak-Markwitz, E.; Spaczynski, M.; Whiteside, T.L. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene 2009, 28, 4353–4363. [Google Scholar] [CrossRef] [PubMed]
- Park, G.B.; Kim, D. TLR5/7-mediated PI3K activation triggers epithelial-mesenchymal transition of ovarian cancer cells through WAVE3-dependent mesothelin or OCT4/SOX2 expression. Oncol. Rep. 2017, 38, 3167–3176. [Google Scholar] [CrossRef]
- Park, G.B.; Chung, Y.H.; Kim, D. Induction of galectin-1 by TLR-dependent PI3K activation enhances epithelial-mesenchymal transition of metastatic ovarian cancer cells. Oncol. Rep. 2017, 37, 3137–3145. [Google Scholar] [CrossRef]
- Lin, H.; Zeng, Z.; Zhang, H.; Jia, Y.; Pang, J.; Chen, J.; Zhang, H. Gut-Vaginal Microbiome Crosstalk in Ovarian Cancer: Implications for Early Diagnosis. Pathogens 2025, 14, 635. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Mert, I.; Walther-Antonio, M.; Mariani, A. Case for a role of the microbiome in gynecologic cancers: Clinician’s perspective. J. Obstet. Gynaecol. Res. 2018, 44, 1693–1704. [Google Scholar] [CrossRef]
- Collaborative Group On Epidemiological Studies Of Ovarian, C.; Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Kim, N. Roles of Sex Hormones and Gender in the Gut Microbiota. J. Neurogastroenterol. Motil. 2021, 27, 314–325. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Coburn, S.B.; Falk, R.T.; Manson, J.E.; Brinton, L.A.; Gass, M.L.; Kuller, L.H.; Rohan, T.E.; Pfeiffer, R.M.; Qi, L.; et al. Circulating estrogens and postmenopausal ovarian and endometrial cancer risk among current hormone users in the Women’s Health Initiative Observational Study. Cancer Causes Control 2019, 30, 1201–1211. [Google Scholar] [CrossRef]
- Leao, L.; Esmail, G.A.; Miri, S.; Mottawea, W.; Hammami, R. In vitro modeling of the female gut microbiome: Effects of sex hormones and psychotropic drugs. Microbiol. Spectr. 2025, e0235025. [Google Scholar] [CrossRef]
- Sipos, A.; Ujlaki, G.; Mikó, E.; Maka, E.; Szabó, J.; Uray, K.; Krasznai, Z.; Bai, P. The role of the microbiome in ovarian cancer: Mechanistic insights into oncobiosis and to bacterial metabolite signaling. Mol. Med. 2021, 27, 33. [Google Scholar] [CrossRef]
- Yang, R.; Qian, L. Research on Gut Microbiota-Derived Secondary Bile Acids in Cancer Progression. Integr. Cancer Ther. 2022, 21, 15347354221114100. [Google Scholar] [CrossRef]
- Kovacs, T.; Miko, E.; Vida, A.; Sebo, E.; Toth, J.; Csonka, T.; Boratko, A.; Ujlaki, G.; Lente, G.; Kovacs, P.; et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci. Rep. 2019, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.; Zaineddin, A.K.; Vrieling, A.; Heinz, J.; Linseisen, J.; Flesch-Janys, D.; Chang-Claude, J. Estimated enterolignans, lignan-rich foods, and fibre in relation to survival after postmenopausal breast cancer. Br. J. Cancer 2011, 105, 1151–1157. [Google Scholar] [CrossRef]
- Kim, N.; Yang, C. Butyrate as a Potential Modulator in Gynecological Disease Progression. Nutrients 2024, 16, 4196. [Google Scholar] [CrossRef]
- Nobels, A.; van Marcke, C.; Jordan, B.F.; Van Hul, M.; Cani, P.D. The gut microbiome and cancer: From tumorigenesis to therapy. Nat. Metab. 2025, 7, 895–917. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, A. Contributions of colonic short-chain Fatty Acid receptors in energy homeostasis. Front. Endocrinol. 2014, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy 2011, 2011, 869647. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Qu, D.; Sakamoto, T.; Fukasawa, I.; Hayashi, M.; Inaba, N. Telomerase expression and cell proliferation in ovarian cancer cells induced by histone deacetylase inhibitors. Arch. Gynecol. Obstet. 2008, 277, 15–19. [Google Scholar] [CrossRef]
- Chao, K.C.; Chang, C.C.; Yen, M.S.; Wang, P.H. Anti-tumor activity of histone deacetylase inhibitors and the effect on ATP-binding cassette in ovarian carcinoma cells. Eur. J. Gynaecol. Oncol. 2010, 31, 402–410. [Google Scholar]
- Wu, Z.; Pfeiffer, R.M.; Byrd, D.A.; Wan, Y.; Ansong, D.; Clegg-Lamptey, J.N.; Wiafe-Addai, B.; Edusei, L.; Adjei, E.; Titiloye, N.; et al. Associations of Circulating Estrogens and Estrogen Metabolites with Fecal and Oral Microbiome in Postmenopausal Women in the Ghana Breast Health Study. Microbiol. Spectr. 2023, 11, e0157223. [Google Scholar] [CrossRef]
- Xie, Y.; Xie, F.; Zhou, X.; Zhang, L.; Yang, B.; Huang, J.; Wang, F.; Yan, H.; Zeng, L.; Zhang, L.; et al. Microbiota in Tumors: From Understanding to Application. Adv. Sci. 2022, 9, e2200470. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Lee, E.J.; Lee, M. Association between pelvic inflammatory disease and risk of ovarian cancer: An updated meta-analysis. Gynecol. Oncol. 2020, 157, 542–548. [Google Scholar] [CrossRef]
- Lo, H.W.; Weng, S.F.; Chen, H.S.; Tsai, E.M. Pelvic inflammatory disease is associated with ovarian cancer development in women with endometriosis: A cohort study in Taiwan. Int. J. Gynaecol. Obstet. 2022, 158, 145–152. [Google Scholar] [CrossRef]
- Nene, N.R.; Reisel, D.; Leimbach, A.; Franchi, D.; Jones, A.; Evans, I.; Knapp, S.; Ryan, A.; Ghazali, S.; Timms, J.F.; et al. Association between the cervicovaginal microbiome, BRCA1 mutation status, and risk of ovarian cancer: A case-control study. Lancet Oncol. 2019, 20, 1171–1182. [Google Scholar] [CrossRef]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.B.; Kyrgiou, M. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, A.; Kawabata, A.; Shirahige, K.; Akiyama, T.; Okamoto, A.; Sutani, T. Altered cervicovaginal microbiota in premenopausal ovarian cancer patients. Gene 2022, 811, 146083. [Google Scholar] [CrossRef]
- Hemalatha, R.; Mastromarino, P.; Ramalaxmi, B.A.; Balakrishna, N.V.; Sesikeran, B. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: A randomized, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3097–3105. [Google Scholar] [CrossRef]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef]
- Tschernichovsky, R.; Goodman, A. Risk-Reducing Strategies for Ovarian Cancer in BRCA Mutation Carriers: A Balancing Act. Oncologist 2017, 22, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Pellecchia, G.; Arcieri, M.; Pericolini, E.; Bogani, G.; Poli, A.; Paparcura, F.; Pregnolato, S.; Armenise, D.; Frossi, B.; et al. The Relationship Between the Vaginal Microbiota and the Ovarian Cancer Microenvironment: A Journey from Ideas to Insights. Cells 2025, 14, 1590. [Google Scholar] [CrossRef]
- Sieh, W.; Kobel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Hogdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Tone, A.A.; Begley, H.; Sharma, M.; Murphy, J.; Rosen, B.; Brown, T.J.; Shaw, P.A. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin. Cancer Res. 2008, 14, 4067–4078. [Google Scholar] [CrossRef]
- Nguyen, H.; Syed, V. Progesterone inhibits growth and induces apoptosis in cancer cells through modulation of reactive oxygen species. Gynecol. Endocrinol. 2011, 27, 830–836. [Google Scholar] [CrossRef]
- Kumar, L.; Dwivedi, M.; Jain, N.; Shete, P.; Solanki, S.; Gupta, R.; Jain, A. The Female Reproductive Tract Microbiota: Friends and Foe. Life 2023, 13, 1313. [Google Scholar] [CrossRef] [PubMed]
- Brewster, W.R.; Burkett, W.C.; Ko, E.M.; Bae-Jump, V.; Nicole McCoy, A.; Keku, T.O. An evaluation of the microbiota of the upper reproductive tract of women with and without epithelial ovarian cancer. Gynecol. Oncol. Rep. 2022, 42, 101017. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Badger, T.C.; Groesch, K.; Diaz-Sylvester, P.L.; Wilson, T.; Ghareeb, A.; Martin, J.A.; Cregger, M.; Welge, M.; Bushell, C.; et al. Assessment of peritoneal microbial features and tumor marker levels as potential diagnostic tools for ovarian cancer. PLoS ONE 2020, 15, e0227707. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, C.; Huang, J.; Xia, M.; Guo, E.; Li, N.; Lu, H.; Shan, W.; Wu, Y.; Li, Y.; et al. The biodiversity Composition of Microbiome in Ovarian Carcinoma Patients. Sci. Rep. 2019, 9, 1691. [Google Scholar] [CrossRef] [PubMed]
- Wetendorf, M.; Li, R.; Wu, S.P.; Liu, J.; Creighton, C.J.; Wang, T.; Janardhan, K.S.; Willson, C.J.; Lanz, R.B.; Murphy, B.D.; et al. Constitutive expression of progesterone receptor isoforms promotes the development of hormone-dependent ovarian neoplasms. Sci. Signal 2020, 13, eaaz9646. [Google Scholar] [CrossRef]
- Zakarya, R.; Howell, V.M.; Colvin, E.K. Modelling Epithelial Ovarian Cancer in Mice: Classical and Emerging Approaches. Int. J. Mol. Sci. 2020, 21, 4806. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, L.; Han, L.; Fu, G.; Tuo, X.; Ma, S.; Li, Q.; Wang, Y.; Liang, D.; Tang, M.; et al. The differential distribution of bacteria between cancerous and noncancerous ovarian tissues in situ. J. Ovarian Res. 2020, 13, 8. [Google Scholar] [CrossRef]
- Choi, S.Y.; Choi, J.H. Ovarian Cancer and the Microbiome: Connecting the Dots for Early Diagnosis and Therapeutic Innovations—A Review. Medicina 2024, 60, 516. [Google Scholar] [CrossRef]
- Mauro, L.J.; Spartz, A.; Austin, J.R.; Lange, C.A. Reevaluating the Role of Progesterone in Ovarian Cancer: Is Progesterone Always Protective? Endocr. Rev. 2023, 44, 1029–1046. [Google Scholar] [CrossRef]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha, N.; Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- D’Antonio, D.L.; Marchetti, S.; Pignatelli, P.; Piattelli, A.; Curia, M.C. The Oncobiome in Gastroenteric and Genitourinary Cancers. Int. J. Mol. Sci. 2022, 23, 9664. [Google Scholar] [CrossRef] [PubMed]
- Asangba, A.E.; Chen, J.; Goergen, K.M.; Larson, M.C.; Oberg, A.L.; Casarin, J.; Multinu, F.; Kaufmann, S.H.; Mariani, A.; Chia, N.; et al. Diagnostic and prognostic potential of the microbiome in ovarian cancer treatment response. Sci. Rep. 2023, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xu, B.; Wang, X.; Wan, W.H.; Lu, J.; Kong, D.; Jin, Y.; You, W.; Sun, H.; Mu, X.; et al. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology 2023, 77, 48–64. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Z.; Lv, M.; Chen, Y.; Liu, Y. Intestinal dysbiosis promotes epithelial-mesenchymal transition by activating tumor-associated macrophages in ovarian cancer. Pathog. Dis. 2019, 77, ftz019. [Google Scholar] [CrossRef]
- Mahoney, D.E.; Chalise, P.; Rahman, F.; Pierce, J.D. Influences of Gastrointestinal Microbiota Dysbiosis on Serum Proinflammatory Markers in Epithelial Ovarian Cancer Development and Progression. Cancers 2022, 14, 3022. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, Y.; Yang, C.; Wang, D.; Zhang, D.; Luo, X.; Zhang, H.; Huang, H.; Zhang, H.; Jiang, Y.; et al. Association between vaginal microbiota and the progression of ovarian cancer. J. Med. Virol. 2023, 95, e28898. [Google Scholar] [CrossRef]
- Hu, X.; Xu, X.; Zeng, X.; Jin, R.; Wang, S.; Jiang, H.; Tang, Y.; Chen, G.; Wei, J.; Chen, T.; et al. Gut microbiota dysbiosis promotes the development of epithelial ovarian cancer via regulating Hedgehog signaling pathway. Gut Microbes 2023, 15, 2221093. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, X.; Hu, D.; Huang, J.; Guo, E.; Xiao, R.; Li, W.; Sun, C.; Chen, G. Akkermansia supplementation reverses the tumor-promoting effect of the fecal microbiota transplantation in ovarian cancer. Cell Rep. 2022, 41, 111890. [Google Scholar] [CrossRef]
- Ye, Y.; Gao, Y.; Fang, Y.; Xu, L.; He, F. Anticancer Effect of Puerarin on Ovarian Cancer Progression Contributes to the Tumor Suppressor Gene Expression and Gut Microbiota Modulation. J. Immunol. Res. 2022, 2022, 4472509. [Google Scholar] [CrossRef]
- D’Amico, F.; Perrone, A.M.; Rampelli, S.; Coluccelli, S.; Barone, M.; Ravegnini, G.; Fabbrini, M.; Brigidi, P.; De Iaco, P.; Turroni, S. Gut Microbiota Dynamics during Chemotherapy in Epithelial Ovarian Cancer Patients Are Related to Therapeutic Outcome. Cancers 2021, 13, 3999. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, Y.; He, Q.; Huang, J.; Song, Y.; Wan, X.; Li, Y. Integrated Analysis of Microbiome and Transcriptome Data Reveals the Interplay Between Commensal Bacteria and Fibrin Degradation in Endometrial Cancer. Front. Cell Infect. Microbiol. 2021, 11, 748558. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, G.; Kong, F.; Wang, P.; Han, C.; Ding, Q.; Jiang, H.; Deng, S. Unraveling the role of tissue colonized microbiome in ovarian cancer progression. Comput. Biol. Med. 2024, 177, 108641. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Ma, J. Causal relationships of gut microbiota and blood metabolites with ovarian cancer and endometrial cancer: A Mendelian randomization study. J. Ovarian Res. 2025, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, C.; Li, H.; Ding, C. Exploring the causal associations of the gut microbiota and plasma metabolites with ovarian cancer: An approach of mendelian randomization analysis combined with network pharmacology and molecular docking. J. Ovarian Res. 2025, 18, 27. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, S.; Wang, X.; Qie, R. Associations between gut microbiota and gynecological cancers: A bi-directional two-sample Mendelian randomization study. Medicine 2024, 103, e37628. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Liu, Y.; Li, Y.; Yao, H.; Han, Y.; Liu, X. Association between gut microbiota, plasma metabolites, and ovarian cancer: A Mendelian randomization study. Medicine 2024, 103, e40479. [Google Scholar] [CrossRef]
- Gong, W.; Jin, G.; Bao, Y.; Liu, Q.; Ni, M.; Wang, J.; Mao, S.; Zhang, Y.; Zheng, Z. Characteristics and potential diagnostic value of gut microbiota in ovarian tumor patients. Sci. Rep. 2025, 15, 16504. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, W.J.; Wu, F.; Zhang, X.Y.; Li, X.; Wei, J.; Chen, T.T.; Liu, Z.X. Individuality and generality of intratumoral microbiome in the three most prevalent gynecological malignancies: An observational study. Microbiol. Spectr. 2024, 12, e0100424. [Google Scholar] [CrossRef]
- Huang, Q.; Wei, X.; Li, W.; Ma, Y.; Chen, G.; Zhao, L.; Jiang, Y.; Xie, S.; Chen, Q.; Chen, T. Endogenous Propionibacterium acnes Promotes Ovarian Cancer Progression via Regulating Hedgehog Signalling Pathway. Cancers 2022, 14, 5178. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, S.; Fu, S.; Zhou, X.; Min, Y.; Lang, J.; Chen, M. Intratumoral microbiota composition in women’s cancers: A systematic review and meta-analysis. Front. Oncol. 2025, 15, 1544786. [Google Scholar] [CrossRef]
- Domzaridou, E.; Van Staa, T.; Renehan, A.G.; Cook, N.; Welfare, W.; Ashcroft, D.M.; Palin, V. The Impact of Oral Antibiotics Prior to Cancer Diagnosis on Overall Patient Survival: Findings from an English Population-Based Cohort Study. Curr. Oncol. 2023, 30, 8434–8443. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Kuznicki, M.; Yao, M.; Chichura, A.; Gruner, M.; Reizes, O.; Debernardo, R.; Rose, P.G.; Michener, C.; Vargas, R. Impact of antibiotic treatment during platinum chemotherapy on survival and recurrence in women with advanced epithelial ovarian cancer. Gynecol. Oncol. 2020, 159, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zuo, Q.; Huang, G.; Qi, Z.; Wang, Y.; Zhu, S.; Zhong, Y.; Xiong, Y.; Chen, T.; Tan, B. Tripterygium glycosides sensitizes cisplatin chemotherapeutic potency by modulating gut microbiota in epithelial ovarian cancer. Front. Cell Infect. Microbiol. 2023, 13, 1236272. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Esakov Rhoades, E.L.; Bharti, R.; Braley, C.; Tewari, S.; Trestan, L.; Alali, Z.; Bayik, D.; Lathia, J.D.; Sangwan, N.; et al. Disruption of the Gut Microbiota Confers Cisplatin Resistance in Epithelial Ovarian Cancer. Cancer Res. 2022, 82, 4654–4669. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhang, X.; Fan, Y.; Chen, L.; Ma, X.; Yu, H.; Li, J.; Guan, X.; Zhao, P.; Yang, J. Changes of Intestinal Microbiota in Ovarian Cancer Patients Treated with Surgery and Chemotherapy. Cancer Manag. Res. 2020, 12, 8125–8135. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Jacobson, D.; Moore, K.; Gunderson, C.; Rowland, M.; Austin, R.; Honap, T.P.; Xu, J.; Warinner, C.; Sankaranarayanan, K.; Lewis, C.M., Jr. Shifts in gut and vaginal microbiomes are associated with cancer recurrence time in women with ovarian cancer. PeerJ 2021, 9, e11574. [Google Scholar] [CrossRef] [PubMed]
- Rosario, S.R.; Long, M.D.; Chilakapati, S.; Gomez, E.C.; Battaglia, S.; Singh, P.K.; Wang, J.; Wang, K.; Attwood, K.; Hess, S.M.; et al. Integrative multi-omics analysis uncovers tumor-immune-gut axis influencing immunotherapy outcomes in ovarian cancer. Nat. Commun. 2024, 15, 10609. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Okazawa-Sakai, M.; Sakai, S.A.; Hyodo, I.; Horasawa, S.; Sawada, K.; Fujisawa, T.; Yamamoto, Y.; Boku, S.; Hayasaki, Y.; Isobe, M.; et al. Gut microbiome associated with PARP inhibitor efficacy in patients with ovarian cancer. J. Gynecol. Oncol. 2025, 36, e38. [Google Scholar] [CrossRef]
- Islam, M.S.; Fan, J.; Pan, F. The power of phages: Revolutionizing cancer treatment. Front. Oncol. 2023, 13, 1290296. [Google Scholar] [CrossRef]
- Karimi, M.; Mirshekari, H.; Moosavi Basri, S.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Jewell, C.M. Phage display as a tool for vaccine and immunotherapy development. Bioeng. Transl. Med. 2020, 5, e10142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Luo, J.; Wu, W.; Luo, H.; Wei, W.; Lyu, H.; Wang, Y.; Yi, H.; Zhang, Y.; et al. Lactobacillus acidophilus potentiates oncolytic virotherapy through modulating gut microbiota homeostasis in hepatocellular carcinoma. Nat. Commun. 2025, 16, 3315. [Google Scholar] [CrossRef]
- Doestzada, M.; Vila, A.V.; Zhernakova, A.; Koonen, D.P.Y.; Weersma, R.K.; Touw, D.J.; Kuipers, F.; Wijmenga, C.; Fu, J. Pharmacomicrobiomics: A novel route towards personalized medicine? Protein Cell 2018, 9, 432–445. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Z.-q.; Miao, L.-L.; Xiang, X. Pharmacogenomics in Precision Medicine: From a Perspective of Ethnic Differences. In Pharmacogenomics in Precision Medicine; Springer: Singapore, 2020. [Google Scholar]
- Le Ngoc, K.; Pham, T.T.H.; Nguyen, T.K.; Huong, P.T. Pharmacomicrobiomics in precision cancer therapy: Bench to bedside. Front. Immunol. 2024, 15, 1428420. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Venkidesh, B.S.; Shankar, S.R.; Narasimhamurthy, R.K.; Rao, S.B.S.; Mumbrekar, K.D. Radioprotective potential of probiotics against gastrointestinal and neuronal toxicity: A preclinical study. Clin. Transl. Oncol. 2023, 25, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhong, Y.S.; Li, X.J.; Kang, Y.K.; Peng, Q.Y.; Ying, H.Z. Postbiotics in colorectal cancer: Intervention mechanisms and perspectives. Front. Microbiol. 2024, 15, 1360225. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Kim, J.H. Postbiotics: Functional Food Materials and Therapeutic Agents for Cancer, Diabetes, and Inflammatory Diseases. Foods 2023, 13, 89. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Cao, A.; Esserman, D.A.; Cartmel, B.; Irwin, M.L.; Ferrucci, L.M. Association between diet quality and ovarian cancer risk and survival. J. Natl. Cancer Inst. 2024, 116, 1095–1104. [Google Scholar] [CrossRef]
- Chen, Y.H.; Bao, R.H.; Liu, J.C.; Liu, J.X.; Sun, J.N.; Wu, L.; Huang, D.H.; Li, X.Y.; Xiao, Q.; Ni, S.; et al. Association between pre-diagnosis and post-diagnosis Alternate Mediterranean Diet and ovarian cancer survival: Evidence from a prospective cohort study. J. Transl. Med. 2024, 22, 860. [Google Scholar] [CrossRef]
- Udumula, M.P.; Singh, H.; Faraz, R.; Poisson, L.; Tiwari, N.; Dimitrova, I.; Hijaz, M.; Gogoi, R.; Swenor, M.; Munkarah, A.; et al. Intermittent Fasting induced ketogenesis inhibits mouse epithelial ovarian tumors by promoting anti-tumor T cell response. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Perales-Puchalt, A.; Perez-Sanz, J.; Payne, K.K.; Svoronos, N.; Allegrezza, M.J.; Chaurio, R.A.; Anadon, C.; Calmette, J.; Biswas, S.; Mine, J.A.; et al. Frontline Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 2018, 103, 799–805. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Cohen, C.R.; Wierzbicki, M.R.; French, A.L.; Morris, S.; Newmann, S.; Reno, H.; Green, L.; Miller, S.; Powell, J.; Parks, T.; et al. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N. Engl. J. Med. 2020, 382, 1906–1915. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, C.; Sun, W.; Hou, G.; Yang, G.; Yuan, L. Lactobacillus supplementation prevents cisplatin-induced cardiotoxicity possibly by inflammation inhibition. Cancer Chemother. Pharmacol. 2018, 82, 999–1008. [Google Scholar] [CrossRef]
- Chambers, L.M.; Michener, C.M.; Rose, P.G.; Reizes, O.; Yao, M.; Vargas, R. Impact of antibiotic treatment on immunotherapy response in women with recurrent gynecologic cancer. Gynecol. Oncol. 2021, 161, 211–220. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, B.; Tang, T.; Xiao, Z.; Ye, F.; Li, X.; Wu, S.; Huang, J.G.; Jiang, S. Gut microbiota and risk of five common cancers: A univariable and multivariable Mendelian randomization study. Cancer Med. 2023, 12, 10393–10405. [Google Scholar] [CrossRef]
- Zitvogel, L.; Derosa, L.; Routy, B.; Loibl, S.; Heinzerling, L.; de Vries, I.J.M.; Engstrand, L.; Network, O.; Segata, N.; Kroemer, G. Impact of the ONCOBIOME network in cancer microbiome research. Nat. Med. 2025, 31, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhou, J.; Wang, Z.; Feng, C.; Fan, J.; Huang, J.; Hu, D.; Baban, B.; Wang, S.; Ma, D.; et al. Metagenomic analysis of the microbiome of the upper reproductive tract: Combating ovarian cancer through predictive, preventive, and personalized medicine. EPMA J. 2022, 13, 487–498. [Google Scholar] [CrossRef]
- Ingerslev, K.; Hogdall, E.; Skovrider-Ruminski, W.; Schnack, T.H.; Lidang, M.; Hogdall, C.; Blaakaer, J. The prevalence of EBV and CMV DNA in epithelial ovarian cancer. Infect. Agent. Cancer 2019, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Jablonska, A.; Studzinska, M.; Wilczynski, M.; Wilczynski, J.R. Detection and genotyping of CMV and HPV in tumors and fallopian tubes from epithelial ovarian cancer patients. Sci. Rep. 2019, 9, 19935. [Google Scholar] [CrossRef] [PubMed]
- Shanmughapriya, S.; Senthilkumar, G.; Vinodhini, K.; Das, B.C.; Vasanthi, N.; Natarajaseenivasan, K. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2311–2317. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Alwine, J.C.; Coukos, G.; Robertson, E.S. The ovarian cancer oncobiome. Oncotarget 2017, 8, 36225–36245. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, J.-R.; del Campo, R.; Avendaño-Ortiz, J.; Laguna-Olmos, M.; Carnero, A. The Role of Microbiota in Ovarian Cancer: Implications for Treatment Response and Therapeutic Strategies. Cells 2025, 14, 1813. https://doi.org/10.3390/cells14221813
Blanco J-R, del Campo R, Avendaño-Ortiz J, Laguna-Olmos M, Carnero A. The Role of Microbiota in Ovarian Cancer: Implications for Treatment Response and Therapeutic Strategies. Cells. 2025; 14(22):1813. https://doi.org/10.3390/cells14221813
Chicago/Turabian StyleBlanco, Jose-Ramon, Rosa del Campo, José Avendaño-Ortiz, Mariano Laguna-Olmos, and Amancio Carnero. 2025. "The Role of Microbiota in Ovarian Cancer: Implications for Treatment Response and Therapeutic Strategies" Cells 14, no. 22: 1813. https://doi.org/10.3390/cells14221813
APA StyleBlanco, J.-R., del Campo, R., Avendaño-Ortiz, J., Laguna-Olmos, M., & Carnero, A. (2025). The Role of Microbiota in Ovarian Cancer: Implications for Treatment Response and Therapeutic Strategies. Cells, 14(22), 1813. https://doi.org/10.3390/cells14221813






