Impact of SARS-CoV-2 Infection and Vaccination on Pregnancy Outcome and Passive Neonatal Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Anti-SARS-CoV-2 NCP ELISA (IgG)
2.3. Anti-SARS-CoV-2 QuantiVac ELISA (IgG)
2.4. EUROLINE Anti-TO.R.C.H.Profile (IgG) and (IgM)
2.5. Statistics
3. Results
3.1. Patient Cohort
- (1)
- The control group comprised 17 women with no history of SARS-CoV-2 vaccination or confirmed infection.
- (2)
- The vaccinated group included 35 women who had received at least one dose of SARS-CoV-2 vaccine during pregnancy but had no documented history of infection.
- (3)
- Twenty-one patients with confirmed SARS-CoV-2 infection during pregnancy but without vaccination were considered the infected group.
- (4)
- Fifteen women who tested positive for SARS-CoV-2 by PCR at the time of the delivery were categorized as the acutely infected group (ten of them had been vaccinated).
- (5)
- The vaccinated and infected group included 47 women who had received at least one dose of vaccine and had a previous confirmed SARS-CoV-2 infection during pregnancy.
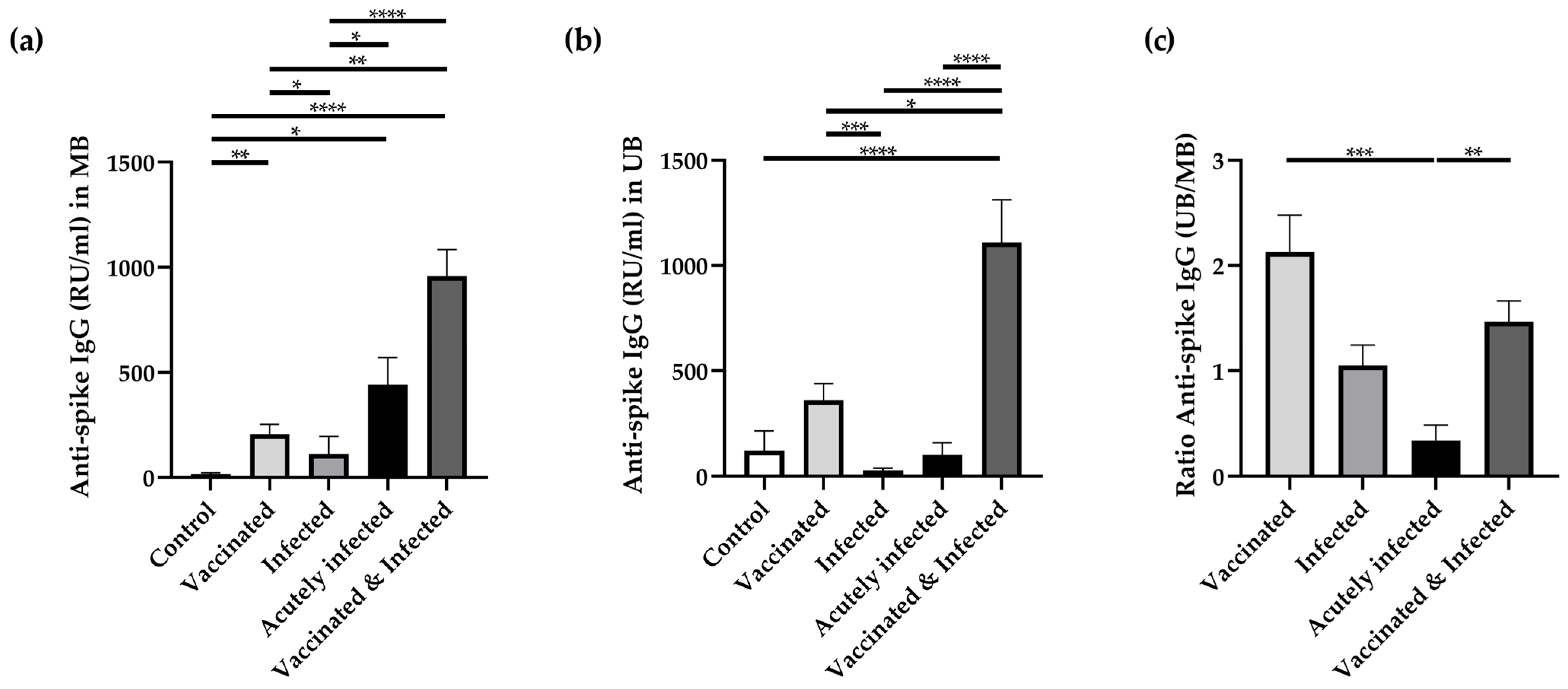
3.2. Anti-Spike Antibodies in Maternal and Umbilical Cord Blood
3.3. Anti-NCP Antibodies in Maternal and Cord Blood
3.4. Correlation Between Anti-NCP and Anti-Spike Protein IgG
3.5. Correlation Between Anti-NCP Antibody Presence and Anti-Spike Antibodies with the Presence of TORCH Antibodies in Maternal and Umbilical Cord Blood
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Kumari, S.; Pandey, B.; Mistry, H.; Bihani, S.C.; Das, A.; Prashar, V.; Gupta, G.D.; Panicker, L.; Kumar, M. Structural insights into SARS-CoV-2 proteins. J. Mol. Biol. 2021, 433, 166725. [Google Scholar] [CrossRef]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin. Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef] [PubMed]
- Marchand, G.; Patil, A.S.; Masoud, A.T.; Ware, K.; King, A.; Ruther, S.; Brazil, G.; Calteux, N.; Ulibarri, H.; Parise, J.; et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to June 3, 2021. AJOG Glob. Rep. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022, 226, 68–89.e63. [Google Scholar] [CrossRef]
- McClymont, E.; Albert, A.Y.; Alton, G.D.; Boucoiran, I.; Castillo, E.; Fell, D.B.; Kuret, V.; Poliquin, V.; Reeve, T.; Scott, H.; et al. Association of SARS-CoV-2 Infection During Pregnancy with Maternal and Perinatal Outcomes. JAMA 2022, 327, 1983–1991. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Pannaraj, P.S.; Boom, J.A.; Sahni, L.C.; Chiotos, K.; Cameron, M.A.; et al. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N. Engl. J. Med. 2022, 387, 109–119. [Google Scholar] [CrossRef]
- Goldshtein, I.; Nevo, D.; Steinberg, D.M.; Rotem, R.S.; Gorfine, M.; Chodick, G.; Segal, Y. Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women. JAMA 2021, 326, 728–735. [Google Scholar] [CrossRef]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 5128. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Murphy, E.A.; Sukhu, A.C.; Yee, J.; Singh, S.; Eng, D.; Zhao, Z.; Riley, L.E.; Yang, Y.J. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet. Gynecol. 2021, 138, 278–280. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
- Burns, M.D.; Muir, C.; Atyeo, C.; Davis, J.P.; Demidkin, S.; Akinwunmi, B.; Fasano, A.; Gray, K.J.; Alter, G.; Shook, L.L.; et al. Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers. Vaccines 2022, 10, 1696. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E.; et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months—17 States, July 2021-January 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 264–270. [Google Scholar] [CrossRef]
- Espino, A.; El Costa, H.; Tabiasco, J.; Al-Daccak, R.; Jabrane-Ferrat, N. Innate Immune Response to Viral Infections at the Maternal-Fetal Interface in Human Pregnancy. Front. Med. 2021, 8, 674645. [Google Scholar] [CrossRef] [PubMed]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021, 224, 382.e1–382.e18. [Google Scholar] [CrossRef]
- Lange, B.; Jaeger, V.K.; Harries, M.; Rucker, V.; Streeck, H.; Blaschke, S.; Petersmann, A.; Toepfner, N.; Nauck, M.; Hassenstein, M.J.; et al. Estimates of protection levels against SARS-CoV-2 infection and severe COVID-19 in Germany before the 2022/2023 winter season: The IMMUNEBRIDGE project. Infection 2024, 52, 139–153. [Google Scholar] [CrossRef]
- Offergeld, R.; Preussel, K.; Zeiler, T.; Aurich, K.; Baumann-Baretti, B.I.; Ciesek, S.; Corman, V.M.; Dienst, V.; Drosten, C.; Gorg, S.; et al. Monitoring the SARS-CoV-2 Pandemic: Prevalence of Antibodies in a Large, Repetitive Cross-Sectional Study of Blood Donors in Germany—Results from the SeBluCo Study 2020–2022. Pathogens 2023, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Can. Med Assoc. J. 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.; Saade, G.R.; Grobman, W.A.; Manuck, T.A.; Miodovnik, M.; Sowles, A.; Clark, K.; et al. Disease Severity and Perinatal Outcomes of Pregnant Patients with Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2021, 137, 571–580. [Google Scholar] [CrossRef]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 Infection with Serious Maternal Morbidity and Mortality from Obstetric Complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babal, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental Tissue Destruction and Insufficiency from COVID-19 Causes Stillbirth and Neonatal Death from Hypoxic-Ischemic Injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Guida, J.P.; Cecatti, J.G.; Souza, R.T.; Pacagnella, R.C.; Ribeiro-do-Valle, C.C.; Luz, A.G.; Lajos, G.J.; Surita, F.G.; Nobrega, G.M.; Griggio, T.B.; et al. Preeclampsia among women with COVID-19 during pregnancy and its impact on maternal and perinatal outcomes: Results from a national multicenter study on COVID in Brazil, the REBRACO initiative. Pregnancy Hypertens. 2022, 28, 168–173. [Google Scholar] [CrossRef]
- Avul, Z.; Güven, C.M.; Feyzioğlu, B.S.; Sancar, Ş. Evaluation of second trimester uterine artery doppler indices in women diagnosed with COVID-19 during the first trimester of their pregnancy. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5293–5300. [Google Scholar] [CrossRef]
- Anuk, A.T.; Tanacan, A.; Yetiskin, F.D.Y.; Buyuk, G.N.; Senel, S.A.; Keskin, H.L.; Moraloglu, O.; Uygur, D. Doppler assessment of the fetus in pregnant women recovered from COVID-19. J. Obstet. Gynaecol. Res. 2021, 47, 1757–1762. [Google Scholar] [CrossRef]
- Alouini, S.; Guinard, J.; Belin, O.; Mesnard, L.; Werner, E.; Prazuck, T.; Pichon, C. Maternal-Fetal Implications of SARS CoV-2 Infection during Pregnancy, Viral, Serological Analyses of Placenta and Cord Blood. Int. J. Environ. Res. Public Health 2022, 19, 2105. [Google Scholar] [CrossRef]
- Bahrami, R.; Schwartz, D.A.; Karimi-Zarchi, M.; Javaheri, A.; Dastgheib, S.A.; Ferdosian, F.; Noorishadkam, M.; Mirjalili, S.R.; Neamatzadeh, H. Meta-analysis of the frequency of intrauterine growth restriction and preterm premature rupture of the membranes in pregnant women with COVID-19. Turk. J. Obstet. Gynecol. 2021, 18, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Crispi, F.; Llurba, E.; Pascal, R.; Larroya, M.; Trilla, C.; Camacho, M.; Medina, C.; Dobano, C.; Gomez-Roig, M.D.; et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Infection on Pregnancy Outcomes: A Population-based Study. Clin. Infect. Dis. 2021, 73, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Narang, K.; Miller, M.; Trinidad, C.; Wick, M.; Theiler, R.; Weaver, A.L.; Mehta, R.A.; Schenone, M. Impact of asymptomatic and mild COVID-19 infection on fetal growth during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 281, 63–67. [Google Scholar] [CrossRef]
- Mitta, M.; Holt, L.; Chandrasekaran, S.; Dude, C. The association between parental SARS-CoV-2 infection in pregnancy and fetal growth restriction. J. Perinat. Med. 2024, 52, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.B.; Cavalcante, C.; Sarno, M.; Barini, R.; Kwak-Kim, J. Maternal immune responses and obstetrical outcomes of pregnant women with COVID-19 and possible health risks of offspring. J. Reprod. Immunol. 2021, 143, 103250. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, O.; Engjom, H.; Vousden, N.; Ramakrishnan, R.; Aabakke, A.J.M.; Äyräs, O.; Donati, S.; Jónasdóttir, E.; Knight, M.; Overtoom, E.M.; et al. Variations across Europe in hospitalization and management of pregnant women with SARS-CoV-2 during the initial phase of the pandemic: Multi-national population-based cohort study using the International Network of Obstetric Survey Systems (INOSS). Acta Obstet. Gynecol. Scand. 2023, 102, 1521–1530. [Google Scholar] [CrossRef]
- Hurley, E.; Geisler, B.P.; Lupattelli, A.; Poblador-Plou, B.; Lassalle, R.; Jové, J.; Bernard, M.-A.; Sakr, D.; Sanfélix-Gimeno, G.; Sánchez-Saez, F.; et al. COVID-19 and pregnancy: A European study on pre- and post-infection medication use. Eur. J. Clin. Pharmacol. 2024, 80, 707–716. [Google Scholar] [CrossRef]
- Bruno, A.M.; Zang, C.; Xu, Z.; Wang, F.; Weiner, M.G.; Guthe, N.; Fitzgerald, M.; Kaushal, R.; Carton, T.W.; Metz, T.D. Association between acquiring SARS-CoV-2 during pregnancy and post-acute sequelae of SARS-CoV-2 infection: RECOVER electronic health record cohort analysis. eClinicalMedicine 2024, 73, 102654. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Jarlhelt, I.; Hansen, C.B.; Perez-Alos, L.; Weihe, P.; Petersen, M.S.; Garred, P. SARS-CoV-2 anti-RBD and anti-N protein responses are differentially regulated between mother-child pairs: Insight from a national study cohort at the Faroe Islands. Front. Immunol. 2024, 15, 1418678. [Google Scholar] [CrossRef]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Nikoloudis, D.; Saldari, C.; Konstantinakou, K.E.; Vasileiou, I.V.; Tsakris, A. Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies. Pathogens 2025, 14, 415. [Google Scholar] [CrossRef]
- Murphy, E.A.; Guzman-Cardozo, C.; Sukhu, A.C.; Parks, D.J.; Prabhu, M.; Mohammed, I.; Jurkiewicz, M.; Ketas, T.J.; Singh, S.; Canis, M.; et al. SARS-CoV-2 vaccination, booster, and infection in pregnant population enhances passive immunity in neonates. Nat. Commun. 2023, 14, 4598. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Carreno, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e4. [Google Scholar] [CrossRef]
- Sturrock, S.; Cavell, B.; Alexander, F.; Apostolakis, K.; Barro, C.; Daniel, O.; Dixon, L.; Halkerston, R.; Hall, T.; Hesp, J.R.; et al. Maternal and Placental Antibody Responses in SARS-CoV-2 Vaccination and Natural Infection During Pregnancy. Pediatr. Infect. Dis. J. 2025, 44, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, C.; Visco, V.; Biselli, R.; Cattaruzza, M.S.; Carreras, G.; Salerno, G.; Lista, F.; Capobianchi, M.R.; Castilletti, C.; Lapa, D.; et al. Safety of Multiple Vaccinations and Durability of Vaccine-Induced Antibodies in an Italian Military Cohort 5 Years after Immunization. Biomedicines 2021, 10, 6. [Google Scholar] [CrossRef]
- Masry, A.; Bayoumi, M.A.A.; Chandra, P.; Abukhadijah, H.J.; Olukade, T.; Abdelhady, I.; Thazhe, S.; Paramban, R.; Sudarsanan, A.; Abraham, J.; et al. Impact of pregnant mothers’ previous COVID-19 infection and vaccination on newborns’ serological profiling. Front. Immunol. 2025, 16, 1526264. [Google Scholar] [CrossRef]
- da Silva, M.C.; Diniz, G.T.N.; Correia, M.; da Silva, N.C.H.; Barbosa, C.R.M.; Ferreira, A.; de Melo, M.I.B.; de Magalhaes, J.J.F.; Donadi, E.A.; Souza, A.I.; et al. Autoantibody production in pregnancy: Relationship with mRNA BNT162b2 immunization, active COVID-19, and pre-eclampsia. Front. Immunol. 2025, 16, 1613088. [Google Scholar] [CrossRef]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642.e10. [Google Scholar] [CrossRef]
- Clements, T.; Rice, T.F.; Vamvakas, G.; Barnett, S.; Barnes, M.; Donaldson, B.; Jones, C.E.; Kampmann, B.; Holder, B. Update on Transplacental Transfer of IgG Subclasses: Impact of Maternal and Fetal Factors. Front. Immunol. 2020, 11, 1920. [Google Scholar] [CrossRef] [PubMed]
- Mahant, A.M.; Trejo, F.E.; Aguilan, J.T.; Sidoli, S.; Permar, S.R.; Herold, B.C. Antibody attributes, Fc receptor expression, gestation and maternal SARS-CoV-2 infection modulate HSV IgG placental transfer. iScience 2023, 26, 107648. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.E.; Edwards, E.S.J.; Aui, P.M.; Varese, N.; Stojanovic, S.; McMahon, J.; Peleg, A.Y.; Boo, I.; Drummer, H.E.; Hogarth, P.M.; et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci. Immunol. 2020, 5, eabf8891. [Google Scholar] [CrossRef]
- St Clair, L.A.; Eldesouki, R.E.; Sachithanandham, J.; Yin, A.; Fall, A.; Morris, C.P.; Norton, J.M.; Forman, M.; Abdullah, O.; Dhakal, S.; et al. Reduced control of SARS-CoV-2 infection is associated with lower mucosal antibody responses in pregnant women. medRxiv 2023. [Google Scholar] [CrossRef]
- Blaszczuk, A.; Michalski, A.; Malm, M.; Drop, B.; Polz-Dacewicz, M. Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers. Vaccines 2022, 10, 1169. [Google Scholar] [CrossRef]
- Dobano, C.; Santano, R.; Jimenez, A.; Vidal, M.; Chi, J.; Rodrigo Melero, N.; Popovic, M.; Lopez-Aladid, R.; Fernandez-Barat, L.; Tortajada, M.; et al. Immunogenicity and crossreactivity of antibodies to the nucleocapsid protein of SARS-CoV-2: Utility and limitations in seroprevalence and immunity studies. Transl. Res. 2021, 232, 60–74. [Google Scholar] [CrossRef]
- Muthiah, N.; Galagoda, G.; Handunnetti, S.; Peiris, S.; Pathirana, S. Dynamics of maternally transferred antibodies against measles, mumps, and rubella in infants in Sri Lanka. Int. J. Infect. Dis. 2021, 107, 129–134. [Google Scholar] [CrossRef]
- Chen, J.; Hu, L.; Wu, M.; Zhong, T.; Zhou, Y.H.; Hu, Y. Kinetics of IgG antibody to cytomegalovirus (CMV) after birth and seroprevalence of anti-CMV IgG in Chinese children. Virol. J. 2012, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Chasqueira, M.J.; Marques, A.; Rodrigues, L.; Marcal, M.; Tuna, M.; Braz, M.C.; Neto, A.S.; Mendes, C.; Lito, D.; et al. Lower prevalence of congenital cytomegalovirus infection in Portugal: Possible impact of COVID-19 lockdown? Eur. J. Pediatr. 2022, 181, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.V.; Fernicola, F.; Arienti, F.; Carli, A.; Colciago, E.; Locatelli, A.; Trotta, M.; Procopio, A.; Zammarchi, L.; Ornaghi, S. Indirect impact of SARS-CoV-2 pandemic on incidence of maternal primary cytomegalovirus and Toxoplasma gondii infection in pregnancy. Int. J. Gynaecol. Obstet. 2024, 166, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.R.; Holder, B.; Jones, C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front. Immunol. 2017, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 17) | Vaccinated (n = 35) | Infected (n = 21) | Acutely Infected (n = 15) | Vaccinated and Infected (n = 47) | p Value | |
|---|---|---|---|---|---|---|
| Maternal age, years | 30.5 ± 7.4 | 31.2 ± 5.4 | 29.3 ± 6.1 | 31.2 ± 4.8 | 32.1 ± 6.2 | 0.4975 |
| Gestational age, weeks | 38.5 ± 1.5 | 39.4 ± 1.6 | 38.3 ± 1.5 | 39.3 ± 1.2 | 38.9 ± 1.7 | 0.0503 |
| Twin pregnancies % (N) | 11.8% (2) | 0 | 19.0% (4) | 0 | 2.1% (1) | |
| Gravidity | 2.9 ± 2.0 | 2.1 ± 1.5 | 3.0 ± 1.9 | 2.3 ± 1.5 | 2.4 ± 1.6 | 0.2397 |
| Parity | 2.5 ± 1.9 | 1.7 ± 1 | 2.2 ± 1.3 | 1.9 ± 1.2 | 1.8 ± 1 | 0.3866 |
| Abnormal fetal ultrasound, % (N) | 11.8% (2) | 8.6% (3) | 19.0% (4) | 20.0% (3) | 17.0% (8) | 0.6672 |
| Cesarean section, % (N) | 41.2% (7) | 37.1% (13) | 42.9% (9) | 46.7% (7) | 55.3% (26) | 0.6208 |
| Blood loss, mL | 364 ± 122 | 334 ± 119 | 376 ± 175 | 457 ± 312 | 393 ± 138 | 0.3935 |
| Obstetric complications, % (N) | 23.5% (4) | 62.9% (22) | 42.9% (9) | 60.0% (9) | 40.0% (19) | 0.0546 |
| Neonatal complications, % (N) | 29.4% (5) | 42.9% (15) | 28.6% (6) | 40.0% (4) | 36.2% (17) | 0.5611 |
| Birth weight, g | 3321 ± 609 | 3465 ± 447 | 3202 ± 533 | 3576 ± 625 | 3544 ± 523 | 0.0684 |
| Neonatal weight at discharge, g | 3075 ± 517 | 3279 ± 403 | 2942 ± 495 | 3375 ± 567 | 3337 ± 444 | 0.0078 |
| Birth length, cm | 50.3 ± 3 | 51.4 ± 2.2 | 50.4 ± 2.7 | 52.7 ± 2.2 | 51.2 ± 3.8 | 0.0483 |
| UB * pH | 7.31 ± 0.09 | 7.27 ± 0.09 | 7.30 ± 0.06 | 7.25 ± 0.07 | 7.26 ± 0.09 | 0.0793 |
| UB * Base excess | −1.2 ± 4.1 | −1.4 ± 4.5 | −1.2 ± 2.5 | −2.6 ± 3.3 | −2.2 ± 3.6 | 0.5527 |
| APGAR 1 min | 9.2 | 9 | 8.2 | 9.2 | 8.6 | 0.9707 |
| APGAR 5 min | 9.7 | 9.3 | 9.6 | 9.9 | 9.4 | 0.9029 |
| APGAR 10 min | 9.9 | 9.7 | 9.9 | 10 | 9.7 | 0.3808 |
| Control | Vaccinated | Infected | Acutely Infected | Vaccinated and Infected | |
|---|---|---|---|---|---|
| Positive anti-NCP IgG in MB (%; N) | 12.5% (2/15) | 12.5% (4/32) | 44.4% (8/18) | 50% (7/14) | 40% (16/40) |
| Positive anti-NCP IgG in UB (%; N) | 33.3% (3/9) | 22.2% (6/27) | 47.4% (9/19) | 36.4% (4/11) | 60% (21/35) |
| Correlation MB/UB Spearman r | 0.7698 | 0.6633 | 0.4893 | 1.000 | 0.5372 |
| Correlation MB/UB p value | 0.0545 | 0.0001 | 0.0335 | 0.0030 | 0.0005 |
| Confirmed SARS-CoV-2 Infection | Number of Vaccine Doses | |||
|---|---|---|---|---|
| r | p | r | p | |
| MB anti-NCP IgG | 0.3048 | 0.0010 | −0.1472 | 0.0986 |
| MB anti-spike IgG | 0.2107 | 0.0279 | 0.6273 | <0.0001 |
| UB anti-NCP IgG | 0.1538 | 0.1389 | −0.1327 | 0.1772 |
| UB anti-spike IgG | −0.05537 | 0.6105 | 0.6504 | <0.0001 |
| TORCH | MB Anti-NCP IgG+ | MB Anti-Spike IgG+ | MB Anti-Spike IgG Level | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| MB Toxoplasma gondii IgG | 0.1515 | 0.0879 | 0.04043 | 0.6597 | 0.05624 | 0.5401 |
| MB Rubella virus IgG | 0.01501 | 0.8696 | 0.06571 | 0.4834 | 0.09489 | 0.3110 |
| MB Cytomegalovirus IgG | −0.1062 | 0.2424 | −0.1242 | 0.1822 | −0.2647 | 0.0039 |
| MB Herpes simplex virus I IgG | −0.01627 | 0.8554 | −0.004460 | 0.9613 | −0.1602 | 0.0792 |
| MB Herpes simplex virus II IgG | 0.2043 | 0.0218 | −0.08373 | 0.3653 | −0.09920 | 0.2831 |
| UB Toxoplasma gondii IgG | 0.2316 | 0.0218 | 0.1104 | 0.2920 | 0.05221 | 0.6192 |
| UB Rubella virus IgG | - | - | - | - | - | - |
| UB Cytomegalovirus IgG | −0.1277 | 0.2200 | −0.1592 | 0.1361 | −0.2883 | 0.0062 |
| UB Herpes simplex virus I IgG | 0.02185 | 0.8318 | −0.01850 | 0.0582 | −0.09920 | 0.2831 |
| UB Herpes simplex virus II IgG | 0.3379 | 0.0007 | −0.1055 | 0.3169 | −0.07874 | 0.4556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehre, G.M.; Grabar, V.; Grage-Griebenow, E.; Klemens, O.; Scholz, L.; Hoymann, N.; Alboradi, S.; Ignatov, A.; Tchaikovski, S.; Busse, M. Impact of SARS-CoV-2 Infection and Vaccination on Pregnancy Outcome and Passive Neonatal Immunity. Cells 2025, 14, 1812. https://doi.org/10.3390/cells14221812
Uehre GM, Grabar V, Grage-Griebenow E, Klemens O, Scholz L, Hoymann N, Alboradi S, Ignatov A, Tchaikovski S, Busse M. Impact of SARS-CoV-2 Infection and Vaccination on Pregnancy Outcome and Passive Neonatal Immunity. Cells. 2025; 14(22):1812. https://doi.org/10.3390/cells14221812
Chicago/Turabian StyleUehre, Gina Marie, Valeriia Grabar, Evelin Grage-Griebenow, Oliver Klemens, Laura Scholz, Nils Hoymann, Suzan Alboradi, Atanas Ignatov, Svetlana Tchaikovski, and Mandy Busse. 2025. "Impact of SARS-CoV-2 Infection and Vaccination on Pregnancy Outcome and Passive Neonatal Immunity" Cells 14, no. 22: 1812. https://doi.org/10.3390/cells14221812
APA StyleUehre, G. M., Grabar, V., Grage-Griebenow, E., Klemens, O., Scholz, L., Hoymann, N., Alboradi, S., Ignatov, A., Tchaikovski, S., & Busse, M. (2025). Impact of SARS-CoV-2 Infection and Vaccination on Pregnancy Outcome and Passive Neonatal Immunity. Cells, 14(22), 1812. https://doi.org/10.3390/cells14221812





