Viewing Inflammation and Immunoregulation Under the Calpain System Lens
Abstract
1. Introduction
2. Calpain (Calpain-1 and -2) Expression and Actions in Different Immune (Innate and Adaptive) Cells
2.1. Epithelial Cells
Signaling Events That Stimulate Calpain Activation in Epithelial Cells to Induce Their Immunological Functions
2.2. Endothelial Cells
2.3. Calpains in Myeloid Innate Immune Cells (MICs)
2.3.1. Macrophages
2.3.2. Neutrophils
2.3.3. DCs
2.3.4. Mast Cells
3. Calpains in Innate Lymphoid Cells (ILCs)
4. Calpains in T Cells
5. Calpains in B Cells
6. Future Perspectives and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, V. Inflammation research sails through the sea of immunology to reach immunometabolism. Int. Immunopharmacol. 2019, 73, 128–145. [Google Scholar] [CrossRef]
- Kumar, V. Introductory Chapter: The Journey of Inflammation and Inflammatory Disease Research—Past, Present, and Future. In Inflammation in the 21st Century; Kumar, V., Aguilera, A., Athari, S.S., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Rao, K.V.S. Immunology in India: An emerging story. Nat. Immunol. 2008, 9, 1319–1322. [Google Scholar] [CrossRef]
- Rocha e Silva, M. A brief survey of the history of inflammation. Agents Actions 1994, 43, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. History of Inflammation Research. In Physiology of Inflammation; Ley, K., Ed.; Springer: New York, NY, USA, 2001; pp. 1–10. [Google Scholar]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Kumar, V.; Stewart, J.H.T. Immune Homeostasis: A Novel Example of Teamwork. Methods Mol. Biol. 2024, 2782, 1–24. [Google Scholar] [CrossRef]
- Nobs, S.P.; Kopf, M. Tissue-resident macrophages: Guardians of organ homeostasis. Trends Immunol. 2021, 42, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.I.; Farber, D.L. Tissue-Resident Immune Cells in Humans. Annu. Rev. Immunol. 2022, 40, 195–220. [Google Scholar] [CrossRef]
- Gebhardt, T.; Palendira, U.; Tscharke, D.C.; Bedoui, S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 2018, 283, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Wiedow, O.; Meyer-Hoffert, U. Neutrophil serine proteases: Potential key regulators of cell signalling during inflammation. J. Intern. Med. 2005, 257, 319–328. [Google Scholar] [CrossRef]
- Heutinck, K.M.; ten Berge, I.J.M.; Hack, C.E.; Hamann, J.; Rowshani, A.T. Serine proteases of the human immune system in health and disease. Mol. Immunol. 2010, 47, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.A.; Campbell, R.L.; Davies, P.L. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature 2008, 456, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Wendt, A.; Thompson, V.F.; Goll, D.E. Interaction of calpastatin with calpain: A review. Biol. Chem. 2004, 385, 465–472. [Google Scholar] [CrossRef]
- Glading, A.; Lauffenburger, D.A.; Wells, A. Cutting to the chase: Calpain proteases in cell motility. Trends Cell Biol. 2002, 12, 46–54. [Google Scholar] [CrossRef]
- Franco, S.J.; Huttenlocher, A. Regulating cell migration: Calpains make the cut. J. Cell Sci. 2005, 118, 3829–3838. [Google Scholar] [CrossRef]
- Abe, K.; Takeichi, M. NMDA-Receptor Activation Induces Calpain-Mediated β-Catenin Cleavages for Triggering Gene Expression. Neuron 2007, 53, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Bollino, D.; Balan, I.; Aurelian, L. Valproic acid induces neuronal cell death through a novel calpain-dependent necroptosis pathway. J. Neurochem. 2015, 133, 174–186. [Google Scholar] [CrossRef]
- Davis, M.A.; Fairgrieve, M.R.; Den Hartigh, A.; Yakovenko, O.; Duvvuri, B.; Lood, C.; Thomas, W.E.; Fink, S.L.; Gale, M. Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation. Proc. Natl. Acad. Sci. USA 2019, 116, 5061–5070. [Google Scholar] [CrossRef]
- Schleimer, R.P.; Kato, A.; Kern, R.; Kuperman, D.; Avila, P.C. Epithelium: At the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 2007, 120, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, I.; Ye, F.; McNally, B.; Willette, M.; Flaño, E. Toll-Like Receptor Expression and Induction of Type I and Type III Interferons in Primary Airway Epithelial Cells. J. Virol. 2013, 87, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, H. Mucosal epithelial cells: The initial sentinels and responders controlling and regulating immune responses to viral infections. Cell. Mol. Immunol. 2021, 18, 1628–1630. [Google Scholar] [CrossRef]
- Bals, R.; Hiemstra, P.S. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004, 23, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.; Hornef, M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 2012, 13, 684–698. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- de Vries, M.H.; Kuijk, E.W.; Nieuwenhuis, E.E.S. Innate immunity of the gut epithelium: Blowing in the WNT? Mucosal Immunol. 2025, 18, 1005–1012. [Google Scholar] [CrossRef]
- Constant, D.A.; Nice, T.J.; Rauch, I. Innate immune sensing by epithelial barriers. Curr. Opin. Immunol. 2021, 73, 1–8. [Google Scholar] [CrossRef]
- Johnston, S.L.; Goldblatt, D.L.; Evans, S.E.; Tuvim, M.J.; Dickey, B.F. Airway Epithelial Innate Immunity. Front. Physiol. 2021, 12, 749077. [Google Scholar] [CrossRef]
- Kato, A.; Schleimer, R.P. Beyond inflammation: Airway epithelial cells are at the interface of innate and adaptive immunity. Curr. Opin. Immunol. 2007, 19, 711–720. [Google Scholar] [CrossRef]
- Hui, C.C.; Yu, A.; Heroux, D.; Akhabir, L.; Sandford, A.J.; Neighbour, H.; Denburg, J.A. Thymic stromal lymphopoietin (TSLP) secretion from human nasal epithelium is a function of TSLP genotype. Mucosal Immunol. 2015, 8, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 2023, 23, 24–37. [Google Scholar] [CrossRef]
- Hatton, C.F.; Botting, R.A.; Dueñas, M.E.; Haq, I.J.; Verdon, B.; Thompson, B.J.; Spegarova, J.S.; Gothe, F.; Stephenson, E.; Gardner, A.I.; et al. Delayed induction of type I and III interferons mediates nasal epithelial cell permissiveness to SARS-CoV-2. Nat. Commun. 2021, 12, 7092. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, S.; Roy, S.; Varma, S.; Bose, M. Pulmonary epithelial cells are a source of interferon-gamma in response to Mycobacterium tuberculosis infection. Immunol. Cell Biol. 2007, 85, 229–237. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Elvington, M.L.; Perng, Y.-C.; Liszewski, M.K.; Byers, D.E.; Farkouh, C.; Yusen, R.D.; Lenschow, D.J.; Brody, S.L.; Atkinson, J.P. Intracellular C3 Protects Human Airway Epithelial Cells from Stress-associated Cell Death. Am. J. Respir. Cell Mol. Biol. 2019, 60, 144–157. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Starick, M.; Aponte Alburquerque, R.; Kulkarni, H.S. Local complement activation and modulation in mucosal immunity. Mucosal Immunol. 2024, 17, 739–751. [Google Scholar] [CrossRef]
- Chaudhary, N.; Jayaraman, A.; Reinhardt, C.; Campbell, J.D.; Bosmann, M. A single-cell lung atlas of complement genes identifies the mesothelium and epithelium as prominent sources of extrahepatic complement proteins. Mucosal Immunol. 2022, 15, 927–939. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Liszewski, M.K.; Brody, S.L.; Atkinson, J.P. The complement system in the airway epithelium: An overlooked host defense mechanism and therapeutic target? J. Allergy Clin. Immunol. 2018, 141, 1582–1586.e1. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K.; Ozantürk, A.N.; Kulkarni, D.H.; Ma, L.; Barve, R.A.; Dannull, L.; Lu, A.; Starick, M.; McPhatter, J.N.; Garnica, L.; et al. Lung epithelial cell–derived C3 protects against pneumonia-induced lung injury. Sci. Immunol. 2023, 8, eabp9547. [Google Scholar] [CrossRef]
- Bush, K.T.; Tsukamoto, T.; Nigam, S.K. Selective degradation of E-cadherin and dissolution of E-cadherin-catenin complexes in epithelial ischemia. Am. J. Physiol. Ren. Physiol. 2000, 278, F847–F852. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Shearer, T.R.; Azuma, M. Loss of Calpastatin Leads to Activation of Calpain in Human Lens Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5278–5283. [Google Scholar] [CrossRef] [PubMed]
- Rasl, J.; Caslavsky, J.; Grusanovic, J.; Chvalova, V.; Kosla, J.; Adamec, J.; Grousl, T.; Klimova, Z.; Vomastek, T. Depletion of calpain2 accelerates epithelial barrier establishment and reduces growth factor-induced cell scattering. Cell. Signal. 2024, 121, 111295. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, M.S.; Chung, S.K. Calpain and Caspase-12 Expression in Lens Epithelial Cells of Diabetic Cataracts. Am. J. Ophthalmol. 2016, 167, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Harris, F.; Dennison, S.; Singh, J.; Phoenix, D.A. Calpains: Targets of cataract prevention? Trends Mol. Med. 2004, 10, 78–84. [Google Scholar] [CrossRef]
- Gupta, P.D.; Johar, K.; Vasavada, A. Causative and preventive action of calcium in cataractogenesis. Acta Pharmacol. Sin. 2004, 25, 1250–1256. [Google Scholar]
- Biswas, S.; Harris, F.; Singh, J.; Phoenix, D. Role of calpains in diabetes mellitus-induced cataractogenesis: A mini review. Mol. Cell. Biochem. 2004, 261, 151–159. [Google Scholar] [CrossRef]
- Chen, L.; Yao, K.; Fu, Q.L. Potential immune involvement in cataract: From mechanisms to future scope of therapies. Int. J. Ophthalmol. 2025, 18, 541–548. [Google Scholar] [CrossRef]
- Kumar, V. Toll-Like Receptors in Adaptive Immunity. Handb. Exp. Pharmacol. 2022, 276, 95–131. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Kumar, V.; Stewart, J.H., IV. Toll-Like Receptors in Immunity and Inflammation. In Thirty Years Since the Discovery of Toll-Like Receptors; Kumar, V., Ed.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Kumar, V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int. Immunopharmacol. 2020, 89, 107087. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Gribar, S.C.; Richardson, W.M.; Sodhi, C.P.; Hackam, D.J. No Longer an Innocent Bystander: Epithelial Toll-like Receptor Signaling in the Development of Mucosal Inflammation. Mol. Med. 2008, 14, 645–659. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Soranno, D.E.; Coopersmith, C.M.; Brinkworth, J.F.; Factora, F.N.F.; Muntean, J.H.; Mythen, M.G.; Raphael, J.; Shaw, A.D.; Vachharajani, V.; Messer, J.S. A review of gut failure as a cause and consequence of critical illness. Crit. Care 2025, 29, 91. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.; Truong-Tran, A.Q.; Plitt, J.R.; Beck, L.A.; Schleimer, R.P. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004, 31, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Tengroth, L.; Millrud, C.R.; Kvarnhammar, A.M.; Kumlien Georén, S.; Latif, L.; Cardell, L.-O. Functional Effects of Toll-Like Receptor (TLR)3, 7, 9, RIG-I and MDA-5 Stimulation in Nasal Epithelial Cells. PLoS ONE 2014, 9, e98239. [Google Scholar] [CrossRef]
- Invernizzi, R.; Lloyd, C.M.; Molyneaux, P.L. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology 2020, 160, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Eckert, R.L.; Rorke, E.A. Molecular biology of keratinocyte differentiation. Environ. Health Perspect. 1989, 80, 109–116. [Google Scholar] [CrossRef]
- Kumar, V. Going, Toll-like receptors in skin inflammation and inflammatory diseases. EXCLI J. 2021, 20, 52–79. [Google Scholar] [CrossRef]
- Cha, J.; Kim, T.-G.; Ryu, J.-H. Conversation between skin microbiota and the host: From early life to adulthood. Exp. Mol. Med. 2025, 57, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Tang, S.; Chen, T.; Yang, M.; Wang, L.; Yu, Z.; Xie, B.; Qian, C.; Xu, S.; Li, N.; Cao, X.; et al. Extracellular calcium elicits feedforward regulation of the Toll-like receptor-triggered innate immune response. Cell Mol. Immunol. 2017, 14, 180–191. [Google Scholar] [CrossRef]
- Birla, H.; Xia, J.; Gao, X.; Zhao, H.; Wang, F.; Patel, S.; Amponsah, A.; Bekker, A.; Tao, Y.-X.; Hu, H. Toll-like receptor 4 activation enhances Orai1-mediated calcium signal promoting cytokine production in spinal astrocytes. Cell Calcium 2022, 105, 102619. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, S.H.; Das, A.; Yang, S.-N.; Jung, K.H.; Kim, M.K.; Berggren, P.-O.; Lee, Y.; Chai, J.C.; Kim, H.J.; et al. TLR3-/4-Priming Differentially Promotes Ca2+ Signaling and Cytokine Expression and Ca2+-Dependently Augments Cytokine Release in hMSCs. Sci. Rep. 2016, 6, 23103. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zanoni, I.; Cullen, T.W.; Goodman, A.L.; Kagan, J.C. Mechanisms of Toll-like Receptor 4 Endocytosis Reveal a Common Immune-Evasion Strategy Used by Pathogenic and Commensal Bacteria. Immunity 2015, 43, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C.; Su, T.; Horng, T.; Chow, A.; Akira, S.; Medzhitov, R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 2008, 9, 361–368. [Google Scholar] [CrossRef]
- Schultz, T.E.; Mathmann, C.D.; Domínguez Cadena, L.C.; Muusse, T.W.; Kim, H.; Wells, J.W.; Ulett, G.C.; Hamerman, J.A.; Brooks, A.J.; Kobe, B.; et al. TLR4 endocytosis and endosomal TLR4 signaling are distinct and independent outcomes of TLR4 activation. EMBO Rep. 2025, 26, 2740–2766. [Google Scholar] [CrossRef]
- Stack, J.; Doyle, S.L.; Connolly, D.J.; Reinert, L.S.; O’Keeffe, K.M.; McLoughlin, R.M.; Paludan, S.R.; Bowie, A.G. TRAM is required for TLR2 endosomal signaling to type I IFN induction. J. Immunol. 2014, 193, 6090–6102. [Google Scholar] [CrossRef]
- De Dios, R.; Nguyen, L.; Ghosh, S.; McKenna, S.; Wright, C.J. CpG-ODN-mediated TLR9 innate immune signalling and calcium dyshomeostasis converge on the NFκB inhibitory protein IκBβ to drive IL1α and IL1β expression. Immunology 2020, 160, 64–77. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; Lapointe, T.K.; Jackson, S.; Beck, P.L.; Jones, N.L.; Buret, A.G. Helicobacter pylori Activates Calpain via Toll-Like Receptor 2 To Disrupt Adherens Junctions in Human Gastric Epithelial Cells. Infect. Immun. 2011, 79, 3887–3894. [Google Scholar] [CrossRef]
- Kumar, V.; Ahmad, A. Targeting calpains: A novel immunomodulatory approach for microbial infections. Eur. J. Pharmacol. 2017, 814, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Bergounioux, J.; Elisee, R.; Prunier, A.-L.; Donnadieu, F.; Sperandio, B.; Sansonetti, P.; Arbibe, L. Calpain Activation by the Shigella flexneri Effector VirA Regulates Key Steps in the Formation and Life of the Bacterium’s Epithelial Niche. Cell Host Microbe 2012, 11, 240–252. [Google Scholar] [CrossRef]
- Lapaquette, P.; Fritah, S.; Lhocine, N.; Andrieux, A.; Nigro, G.; Mounier, J.; Sansonetti, P.; Dejean, A. Shigella entry unveils a calcium/calpain-dependent mechanism for inhibiting sumoylation. eLife 2017, 6, e27444. [Google Scholar] [CrossRef]
- Fritah, S.; Lhocine, N.; Golebiowski, F.; Mounier, J.; Andrieux, A.; Jouvion, G.; Hay, R.T.; Sansonetti, P.; Dejean, A. Sumoylation controls host anti-bacterial response to the gut invasive pathogen Shigella flexneri. EMBO Rep. 2014, 15, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Karhausen, J.; Ulloa, L.; Yang, W. SUMOylation Connects Cell Stress Responses and Inflammatory Control: Lessons From the Gut as a Model Organ. Front. Immunol. 2021, 12, 646633. [Google Scholar] [CrossRef]
- Eislmayr, K.D.; Nichols, C.A.; Liu, F.L.; Yuvaraj, S.; Babirye, J.P.; Roncaioli, J.L.; Vickery, J.; Barton, G.M.; Lesser, C.F.; Vance, R.E. Macrophages orchestrate elimination of Shigella from the intestinal epithelial cell niche via TLR-induced IL-12 and IFN-γ. Cell Host Microbe 2025, 33, 1535–1549.e7. [Google Scholar] [CrossRef]
- Pore, D.; Mahata, N.; Pal, A.; Chakrabarti, M.K. 34 kDa MOMP of Shigella flexneri promotes TLR2 mediated macrophage activation with the engagement of NF-kappaB and p38 MAP kinase signaling. Mol. Immunol. 2010, 47, 1739–1746. [Google Scholar] [CrossRef]
- Schuhmann, D.; Godoy, P.; Weiss, C.; Gerloff, A.; Singer, M.V.; Dooley, S.; Böcker, U. Interfering with interferon-γ signalling in intestinal epithelial cells: Selective inhibition of apoptosis-maintained secretion of anti-inflammatory interleukin-18 binding protein. Clin. Exp. Immunol. 2011, 163, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lebrusant-Fernandez, M.; Ap Rees, T.; Jimeno, R.; Angelis, N.; Ng, J.C.; Fraternali, F.; Li, V.S.W.; Barral, P. IFN-γ-dependent regulation of intestinal epithelial homeostasis by NKT cells. Cell Rep. 2024, 43, 114948. [Google Scholar] [CrossRef]
- Wells, A.; Huttenlocher, A.; Lauffenburger, D.A. Calpain proteases in cell adhesion and motility. Int. Rev. Cytol. 2005, 245, 1–16. [Google Scholar] [CrossRef]
- Chun, J.; Prince, A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 2009, 5, 47–58. [Google Scholar] [CrossRef]
- Chun, J.; Prince, A. Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. J. Leukoc. Biol. 2009, 86, 1135–1144. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-Like Receptor 2 Controls Mucosal Inflammation by Regulating Epithelial Barrier Function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Kumar, V.; Everingham, S.; Hall, C.; Greer, P.A.; Craig, A.W.B. Calpains promote neutrophil recruitment and bacterial clearance in an acute bacterial peritonitis model. Eur. J. Immunol. 2014, 44, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Soong, G.; Chun, J.; Parker, D.; Prince, A. Staphylococcus aureus Activation of Caspase 1/Calpain Signaling Mediates Invasion Through Human Keratinocytes. J. Infect. Dis. 2012, 205, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef]
- Trimm, E.; Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 2023, 20, 197–210. [Google Scholar] [CrossRef]
- Mai, J.; Virtue, A.; Shen, J.; Wang, H.; Yang, X.-F. An evolving new paradigm: Endothelial cells—Conditional innate immune cells. J. Hematol. Oncol. 2013, 6, 61. [Google Scholar] [CrossRef]
- Einhorn, S.; Eldor, A.; Vlodavsky, I.; Fuks, Z.; Panet, A. Production and characterization of interferon from endothelial cells. J. Cell Physiol. 1985, 122, 200–204. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Xu, K.; Shao, Y.; Saaoud, F.; Snyder, N.W.; Yang, L.; Yu, J.; Wu, S.; Hu, W.; et al. Editorial: Endothelial cells as innate immune cells. Front. Immunol. 2022, 13, 1035497. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.T.; Fisher, M.J.; Sumbria, R.K. Brain endothelial cells as phagocytes: Mechanisms and implications. Fluids Barriers CNS 2025, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by endothelial cells—Partnering up with the immune system? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef]
- Shao, Y.; Saredy, J.; Yang, W.Y.; Sun, Y.; Lu, Y.; Saaoud, F.; Drummer, C.; Johnson, C.; Xu, K.; Jiang, X.; et al. Vascular Endothelial Cells and Innate Immunity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e138–e152. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. A systems view of the vascular endothelium in health and disease. Cell 2024, 187, 4833–4858. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N.M.; Wang, Y.; Youn, J.Y.; Cai, H. Endothelial cell calpain as a critical modulator of angiogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1326–1335. [Google Scholar] [CrossRef]
- Miyazaki, T.; Akasu, R.; Miyazaki, A. Calpain proteolytic systems counteract endothelial cell adaptation to inflammatory environments. Inflamm. Regen. 2020, 40, 5. [Google Scholar] [CrossRef]
- Youn, J.-Y.; Wang, T.; Cai, H. An Ezrin/Calpain/PI3K/AMPK/eNOSs1179 Signaling Cascade Mediating VEGF-Dependent Endothelial Nitric Oxide Production. Circ. Res. 2009, 104, 50–59. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Yates, C.C.; Wells, A. IP-10 Blocks Vascular Endothelial Growth Factor-Induced Endothelial Cell Motility and Tube Formation via Inhibition of Calpain. Circ. Res. 2006, 98, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cui, Z.; Li, Z.; Block, E.R. Calpain-2 regulation of VEGF-mediated angiogenesis. FASEB J. 2006, 20, 1443–1451. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Youn, J.Y.; Cai, H. Protein Phosphotyrosine Phosphatase 1B (PTP1B) in Calpain-dependent Feedback Regulation of Vascular Endothelial Growth Factor Receptor (VEGFR2) in Endothelial Cells: Implications in VEGF-Dependent Angiogenesis and Diabetic Wound Healing. J. Biol. Chem. 2017, 292, 407–416. [Google Scholar] [CrossRef]
- Liu, W.; Ma, K.; Kwon, S.H.; Garg, R.; Patta, Y.R.; Fujiwara, T.; Gurtner, G.C. The Abnormal Architecture of Healed Diabetic Ulcers Is the Result of FAK Degradation by Calpain 1. J. Investig. Dermatol. 2017, 137, 1155–1165. [Google Scholar] [CrossRef]
- Yi, C.; Wu, W.; Zheng, D.; Peng, G.; Huang, H.; Shen, Z.; Teng, X. Targeted inhibition of endothelial calpain delays wound healing by reducing inflammation and angiogenesis. Cell Death Dis. 2020, 11, 533. [Google Scholar] [CrossRef]
- Rembe, J.-D.; Garabet, W.; Augustin, M.; Dissemond, J.; Ibing, W.; Schelzig, H.; Stuermer, E.K. Immunomarker profiling in human chronic wound swabs reveals IL-1 beta/IL-1RA and CXCL8/CXCL10 ratios as potential biomarkers for wound healing, infection status and regenerative stage. J. Transl. Med. 2025, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.I.; Van Raemdonck, K.; Mohammad, G.; Kangave, D.; Van Damme, J.; Abu El-Asrar, A.M.; Struyf, S. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp. Eye Res. 2013, 109, 67–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Thai, K.; Kepecs, D.M.; Winer, D.; Gilbert, R.E. Reversing CXCL10 Deficiency Ameliorates Kidney Disease in Diabetic Mice. Am. J. Pathol. 2018, 188, 2763–2773. [Google Scholar] [CrossRef]
- Potz, B.A.; Sabe, A.A.; Sabe, S.A.; Lawandy, I.J.; Abid, M.R.; Clements, R.T.; Sellke, F.W. Calpain inhibition decreases myocardial fibrosis in chronically ischemic hypercholesterolemic swine. J. Thorac. Cardiovasc. Surg. 2022, 163, e11–e27. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Taketomi, Y.; Saito, Y.; Hosono, T.; Lei, X.-F.; Kim-Kaneyama, J.-r.; Arata, S.; Takahashi, H.; Murakami, M.; Miyazaki, A. Calpastatin Counteracts Pathological Angiogenesis by Inhibiting Suppressor of Cytokine Signaling 3 Degradation in Vascular Endothelial Cells. Circ. Res. 2015, 116, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Gariano, R.F.; Gardner, T.W. Retinal angiogenesis in development and disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Zhang, J.; Patel, J.M.; Block, E.R. Hypoxia-specific upregulation of calpain activity and gene expression in pulmonary artery endothelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1998, 275, L461–L468. [Google Scholar] [CrossRef]
- Aono, Y.; Ariyoshi, H.; Tsuji, Y.; Ueda, A.; Tokunaga, M.; Sakon, M.; Monden, M. Localized Activation of m-Calpain in Human Umbilical Vein Endothelial Cells Upon Hypoxia. Thromb. Res. 2001, 102, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.-G.; Chen, Q.-W.; Li, X.-S.; Zheng, M.-M.; Ke, D.-Z.; Deng, W.; Li, G.-Q.; Jiang, J.; Wu, Z.-Q.; Wang, L.; et al. Suppression of NHE1 by small interfering RNA inhibits HIF-1α-induced angiogenesis in vitro via modulation of calpain activity. Microvasc. Res. 2011, 81, 160–168. [Google Scholar] [CrossRef]
- Deng, H.; Tian, X.; Sun, H.; Liu, H.; Lu, M.; Wang, H. Calpain-1 mediates vascular remodelling and fibrosis via HIF-1α in hypoxia-induced pulmonary hypertension. J. Cell Mol. Med. 2022, 26, 2819–2830. [Google Scholar] [CrossRef]
- Sanson, M.; Ingueneau, C.; Vindis, C.; Thiers, J.C.; Glock, Y.; Rousseau, H.; Sawa, Y.; Bando, Y.; Mallat, Z.; Salvayre, R.; et al. Oxygen-regulated protein-150 prevents calcium homeostasis deregulation and apoptosis induced by oxidized LDL in vascular cells. Cell Death Differ. 2008, 15, 1255–1265. [Google Scholar] [CrossRef]
- Tsimikas, S.; Witztum, J.L. Oxidized phospholipids in cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Vindis, C.; Elbaz, M.; Escargueil-Blanc, I.; Augé, N.; Heniquez, A.; Thiers, J.-C.; Nègre-Salvayre, A.; Salvayre, R. Two Distinct Calcium-Dependent Mitochondrial Pathways Are Involved in Oxidized LDL-Induced Apoptosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 639–645. [Google Scholar] [CrossRef]
- Gonçalves, I.; Nitulescu, M.; Saido, T.C.; Dias, N.; Pedro, L.M.; e Fernandes, J.F.; Ares, M.P.S.; Pörn-Ares, I. Activation of calpain-1 in human carotid artery atherosclerotic lesions. BMC Cardiovasc. Disord. 2009, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, Q.; Cao, L.; Wang, Y.; Chen, Y.; Zhang, X.; Xiao, F.; Yang, Y.; Hayden, M.R.; Liu, Y.; et al. Toll-Like Receptor 4 Mediated Oxidized Low-Density Lipoprotein-Induced Foam Cell Formation in Vascular Smooth Muscle Cells via Src and Sirt1/3 Pathway. Mediat. Inflamm. 2021, 2021, 6639252. [Google Scholar] [CrossRef]
- Miyazaki, T.; Taketomi, Y.; Takimoto, M.; Lei, X.-F.; Arita, S.; Kim-Kaneyama, J.-r.; Arata, S.; Ohata, H.; Ota, H.; Murakami, M.; et al. m-Calpain Induction in Vascular Endothelial Cells on Human and Mouse Atheromas and Its Roles in VE-Cadherin Disorganization and Atherosclerosis. Circulation 2011, 124, 2522–2532. [Google Scholar] [CrossRef]
- Jiang, J.; Hiron, T.K.; Chalisey, A.; Malhotra, Y.; Agbaedeng, T.; O’Callaghan, C.A. Ox-LDL induces a non-inflammatory response enriched for coronary artery disease risk in human endothelial cells. Sci. Rep. 2025, 15, 21877. [Google Scholar] [CrossRef]
- Miyazaki, T.; Taketomi, Y.; Higashi, T.; Ohtaki, H.; Takaki, T.; Ohnishi, K.; Hosonuma, M.; Kono, N.; Akasu, R.; Haraguchi, S.; et al. Hypercholesterolemic Dysregulation of Calpain in Lymphatic Endothelial Cells Interferes With Regulatory T-Cell Stability and Trafficking. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e66–e82. [Google Scholar] [CrossRef]
- Shi, Y.; Gu, W.; Wei, Y.; Li, S.; Zhang, S.; Jiang, Y.; Chen, C.; Liu, T.; Shuai, L.; Zhou, X.; et al. Deficiency of Calpain-1 attenuates atherosclerotic plaque and calcification and improves vasomotor dysfunction in Apolipoprotein E knockout mice through inhibiting inflammation. Biochem. Biophys. Res. Commun. 2025, 749, 151369. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, J.; Zheng, D.; Su, L.; Peng, T.; Tang, J. Protective role of endothelial calpain knockout in lipopolysaccharide-induced acute kidney injury via attenuation of the p38-iNOS pathway and NO/ROS production. Exp. Mol. Med. 2020, 52, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Alluri, H.; Grimsley, M.; Anasooya Shaji, C.; Varghese, K.P.; Zhang, S.L.; Peddaboina, C.; Robinson, B.; Beeram, M.R.; Huang, J.H.; Tharakan, B. Attenuation of Blood-Brain Barrier Breakdown and Hyperpermeability by Calpain Inhibition. J. Biol. Chem. 2016, 291, 26958–26969. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Biering, S.B.; Castillo-Rojas, B.; DiBiasio-White, M.J.; Lo, N.T.; Espinosa, D.A.; Warnes, C.M.; Wang, C.; Cao, T.; Glasner, D.R.; et al. Flavivirus NS1-triggered endothelial dysfunction promotes virus dissemination. bioRxiv 2024. [Google Scholar] [CrossRef]
- Li, J.; Zheng, K.; Shen, H.; Wu, H.; Wan, C.; Zhang, R.; Liu, Z. Calpain-2 protein influences chikungunya virus replication and regulates vimentin rearrangement caused by chikungunya virus infection. Front. Microbiol. 2023, 14, 1229576. [Google Scholar] [CrossRef]
- Mackow, E.R.; Gorbunova, E.E.; Gavrilovskaya, I.N. Endothelial cell dysfunction in viral hemorrhage and edema. Front. Microbiol. 2014, 5, 733. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Roy, S.; Sadigh, B.; Datan, E.; Lockshin, R.A.; Zakeri, Z. Regulation of cell survival and death during Flavivirus infections. World J. Biol. Chem. 2014, 5, 93–105. [Google Scholar] [CrossRef]
- Hussain, A.M.; Zhang, Q.X.; Murray, A.G. Endothelial Cell Calpain Activity Facilitates Lymphocyte Diapedesis. Am. J. Transplant. 2005, 5, 2640–2648. [Google Scholar] [CrossRef]
- Filippi, M.D. Mechanism of Diapedesis: Importance of the Transcellular Route. Adv. Immunol. 2016, 129, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Mechanisms of Transendothelial Migration of Leukocytes. Circ. Res. 2009, 105, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Etwebi, Z.; Landesberg, G.; Preston, K.; Eguchi, S.; Scalia, R. Mechanistic Role of the Calcium-Dependent Protease Calpain in the Endothelial Dysfunction Induced by MPO (Myeloperoxidase). Hypertension 2018, 71, 761–770. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kalafati, L.; Hajishengallis, G.; Chavakis, T. Myelopoiesis in the Context of Innate Immunity. J. Innate Immun. 2018, 10, 365–372. [Google Scholar] [CrossRef]
- Schultze, J.L.; Mass, E.; Schlitzer, A. Emerging Principles in Myelopoiesis at Homeostasis and during Infection and Inflammation. Immunity 2019, 50, 288–301. [Google Scholar] [CrossRef]
- Chiba, Y.; Mizoguchi, I.; Hasegawa, H.; Ohashi, M.; Orii, N.; Nagai, T.; Sugahara, M.; Miyamoto, Y.; Xu, M.; Owaki, T.; et al. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell Mol. Life Sci. 2018, 75, 1363–1376. [Google Scholar] [CrossRef]
- Jost, P.J.; Höckendorf, U. Necroinflammation emerges as a key regulator of hematopoiesis in health and disease. Cell Death Differ. 2019, 26, 53–67. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kalafati, L.; Bornhäuser, M.; Hajishengallis, G.; Chavakis, T. Regulation of the Bone Marrow Niche by Inflammation. Front. Immunol. 2020, 11, 1540. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A. Mast cells: Emerging sentinel innate immune cells with diverse role in immunity. Mol. Immunol. 2010, 48, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A. Neutrophils: Cinderella of innate immune system. Int. Immunopharmacol. 2010, 10, 1325–1334. [Google Scholar] [CrossRef]
- Kumar, V. Dendritic cells in sepsis: Potential immunoregulatory cells with therapeutic potential. Mol. Immunol. 2018, 101, 615–626. [Google Scholar] [CrossRef]
- McDaniel, M.M.; Meibers, H.E.; Pasare, C. Innate control of adaptive immunity and adaptive instruction of innate immunity: Bi-directional flow of information. Curr. Opin. Immunol. 2021, 73, 25–33. [Google Scholar] [CrossRef]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; et al. The interaction of innate immune and adaptive immune system. MedComm 2024, 5, e714. [Google Scholar] [CrossRef]
- Kumar, V.; Stewart, J.H.T. cGLRs Join Their Cousins of Pattern Recognition Receptor Family to Regulate Immune Homeostasis. Int. J. Mol. Sci. 2024, 25, 1828. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bauer, C.; Stewart, J.H.t. Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME. J. Biomed. Sci. 2023, 30, 48. [Google Scholar] [CrossRef]
- Kumar, V.; Stewart Iv, J.H. Pattern-Recognition Receptors and Immunometabolic Reprogramming: What We Know and What to Explore. J. Innate Immun. 2024, 16, 295–323. [Google Scholar] [CrossRef] [PubMed]
- Takano, E.; Park, Y.H.; Kitahara, A.; Yamagata, Y.; Kannagi, R.; Murachi, T. Distribution of calpains and calpastatin in human blood cells. Biochem. Int. 1988, 16, 391–395. [Google Scholar]
- Huang, Z.; Hoffmann, F.W.; Norton, R.L.; Hashimoto, A.C.; Hoffmann, P.R. Selenoprotein K Is a Novel Target of m-Calpain, and Cleavage Is Regulated by Toll-like Receptor-induced Calpastatin in Macrophages. J. Biol. Chem. 2011, 286, 34830–34838. [Google Scholar] [CrossRef]
- Ma, J.; Kummarapurugu, A.B.; Zheng, S.; Ghio, A.J.; Deshpande, L.S.; Voynow, J.A. Neutrophil elastase activates macrophage calpain as a mechanism for phagocytic failure. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2025, 328, L93–L104. [Google Scholar] [CrossRef]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-specific macrophages: How they develop and choreograph tissue biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef]
- Vijayan, V.; Pradhan, P.; Braud, L.; Fuchs, H.R.; Gueler, F.; Motterlini, R.; Foresti, R.; Immenschuh, S. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide—A divergent role for glycolysis. Redox Biol. 2019, 22, 101147. [Google Scholar] [CrossRef]
- Schneemann, M.; Schoedon, G. Species differences in macrophage NO production are important. Nat. Immunol. 2002, 3, 102. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue macrophages: Origin, heterogenity, biological functions, diseases and therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Bruscia, E.M.; Zhang, P.X.; Satoh, A.; Caputo, C.; Medzhitov, R.; Shenoy, A.; Egan, M.E.; Krause, D.S. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J. Immunol. 2011, 186, 6990–6998. [Google Scholar] [CrossRef]
- Hofer, T.P.; Frankenberger, M.; Heimbeck, I.; Burggraf, D.; Wjst, M.; Wright, A.K.; Kerscher, M.; Nährig, S.; Huber, R.M.; Fischer, R.; et al. Decreased expression of HLA-DQ and HLA-DR on cells of the monocytic lineage in cystic fibrosis. J. Mol. Med. 2014, 92, 1293–1304. [Google Scholar] [CrossRef]
- Alexis, N.E.; Muhlebach, M.S.; Peden, D.B.; Noah, T.L. Attenuation of host defense function of lung phagocytes in young cystic fibrosis patients. J. Cyst. Fibros. 2006, 5, 17–25. [Google Scholar] [CrossRef]
- Jaganathan, D.; Bruscia, E.M.; Kopp, B.T. Emerging Concepts in Defective Macrophage Phagocytosis in Cystic Fibrosis. Int. J. Mol. Sci. 2022, 23, 7750. [Google Scholar] [CrossRef] [PubMed]
- Fettucciari, K.; Quotadamo, F.; Noce, R.; Palumbo, C.; Modesti, A.; Rosati, E.; Mannucci, R.; Bartoli, A.; Marconi, P. Group B Streptococcus (GBS) disrupts by calpain activation the actin and microtubule cytoskeleton of macrophages. Cell Microbiol. 2011, 13, 859–884. [Google Scholar] [CrossRef] [PubMed]
- Fettucciari, K.; Fetriconi, I.; Mannucci, R.; Nicoletti, I.; Bartoli, A.; Coaccioli, S.; Marconi, P. Group B Streptococcus induces macrophage apoptosis by calpain activation. J. Immunol. 2006, 176, 7542–7556. [Google Scholar] [CrossRef]
- Liu, X.; Van Vleet, T.; Schnellmann, R.G. The role of calpain in oncotic cell death. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 349–370. [Google Scholar] [CrossRef]
- Goldmann, O.; Sastalla, I.; Wos-Oxley, M.; Rohde, M.; Medina, E. Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell Microbiol. 2009, 11, 138–155. [Google Scholar] [CrossRef]
- Ulett, G.C.; Maclean, K.H.; Nekkalapu, S.; Cleveland, J.L.; Adderson, E.E. Mechanisms of group B streptococcal-induced apoptosis of murine macrophages. J. Immunol. 2005, 175, 2555–2562. [Google Scholar] [CrossRef]
- De-Leon-Lopez, Y.S.; Thompson, M.E.; Kean, J.J.; Flaherty, R.A. The PI3K-Akt pathway is a multifaceted regulator of the macrophage response to diverse group B Streptococcus isolates. Front. Cell Infect. Microbiol. 2023, 13, 1258275. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Corbett, D.; Goldrick, M.; Roberts, I.S.; Brough, D. Inhibition of Calpain Blocks the Phagosomal Escape of Listeria monocytogenes. PLoS ONE 2012, 7, e35936. [Google Scholar] [CrossRef]
- Dewamitta, S.R.; Nomura, T.; Kawamura, I.; Hara, H.; Tsuchiya, K.; Kurenuma, T.; Shen, Y.; Daim, S.; Yamamoto, T.; Qu, H.; et al. Listeriolysin O-Dependent Bacterial Entry into the Cytoplasm Is Required for Calpain Activation and Interleukin-1α Secretion in Macrophages Infected with Listeria monocytogenes. Infect. Immun. 2010, 78, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Wang, J.; Hou, D.; Liu, W.; Xie, C.; Shi, K.; Han, W.; Miao, X.; Chen, J.; Cai, Z.; et al. Calpain-2 facilitates infection of the intracellular bacteria Listeria monocytogenes and invasion intestinal immune barrier by impairing nitric oxide homeostasis. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Bonnardel, J.; Da Silva, C.; Henri, S.; Tamoutounour, S.; Chasson, L.; Montañana-Sanchis, F.; Gorvel, J.-P.; Lelouard, H. Innate and Adaptive Immune Functions of Peyer’s Patch Monocyte-Derived Cells. Cell Rep. 2015, 11, 770–784. [Google Scholar] [CrossRef]
- Jung, C.; Hugot, J.P.; Barreau, F. Peyer’s Patches: The Immune Sensors of the Intestine. Int. J. Inflam. 2010, 2010, 823710. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Choi, H.H.; Choi, J.A.; Jeong, J.A.; Cho, S.N.; Lee, J.H.; Park, J.B.; Kim, H.J.; Song, C.H. Mycobacterium kansasii-induced death of murine macrophages involves endoplasmic reticulum stress responses mediated by reactive oxygen species generation or calpain activation. Apoptosis 2013, 18, 150–159. [Google Scholar] [CrossRef]
- Castillo, E.F.; Dekonenko, A.; Arko-Mensah, J.; Mandell, M.A.; Dupont, N.; Jiang, S.; Delgado-Vargas, M.; Timmins, G.S.; Bhattacharya, D.; Yang, H.; et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, E3168–E3176. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Berliocchi, L.; Adornetto, A.; Varano, G.P.; Cavaliere, F.; Nucci, C.; Rotiroti, D.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011, 2, e144. [Google Scholar] [CrossRef]
- Sohn, H.; Kim, K.W.; Kang, H.B.; Won, C.J.; Kim, W.S.; Lee, B.; Kwon, O.J.; Koh, W.J.; Shin, S.J.; Kim, H.J. Induction of macrophage death by clinical strains of Mycobacterium kansasii. Microb. Pathog. 2010, 48, 160–167. [Google Scholar] [CrossRef]
- Ahn, D.; Peñaloza, H.; Wang, Z.; Wickersham, M.; Parker, D.; Patel, P.; Koller, A.; Chen, E.I.; Bueno, S.M.; Uhlemann, A.C.; et al. Acquired resistance to innate immune clearance promotes Klebsiella pneumoniae ST258 pulmonary infection. JCI Insight 2016, 1, e89704. [Google Scholar] [CrossRef]
- Lee, F.Y.; Kim, D.W.; Karmin, J.A.; Hong, D.; Chang, S.S.; Fujisawa, M.; Takayanagi, H.; Bigliani, L.U.; Blaine, T.A.; Lee, H.J. mu-Calpain regulates receptor activator of NF-kappaB ligand (RANKL)-supported osteoclastogenesis via NF-kappaB activation in RAW 264.7 cells. J. Biol. Chem. 2005, 280, 29929–29936. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, X.; Zhou, Y.; Xiao, Y. RANKL-induced M1 macrophages are involved in bone formation. Bone Res. 2017, 5, 17019. [Google Scholar] [CrossRef]
- Ahern, E.; Smyth, M.J.; Dougall, W.C.; Teng, M.W.L. Roles of the RANKL–RANK axis in antitumour immunity—Implications for therapy. Nat. Rev. Clin. Oncol. 2018, 15, 676–693. [Google Scholar] [CrossRef]
- Rigoni, T.S.; Vellozo, N.S.; Cabral-Piccin, M.; Fabiano-Coelho, L.; Lopes, U.G.; Filardy, A.A.; DosReis, G.A.; Lopes, M.F. RANK Ligand Helps Immunity to Leishmania major by Skewing M2-Like Into M1 Macrophages. Front. Immunol. 2020, 11, 00886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, D.; Yan, Y.; Yu, Y.; Chen, R.; Li, Z.; Greer, P.A.; Peng, T.; Wang, Q. Myeloid cell-specific deletion of Capns1 prevents macrophage polarization toward the M1 phenotype and reduces interstitial lung disease in the bleomycin model of systemic sclerosis. Arthritis Res. Ther. 2022, 24, 148. [Google Scholar] [CrossRef]
- Zhang, G.; Thomas, A.L.; Marshall, A.L.; Kernan, K.A.; Su, Y.; Zheng, Y.; Takano, J.; Saido, T.C.; Eddy, A.A. Nicotinic acetylcholine receptor α1 promotes calpain-1 activation and macrophage inflammation in hypercholesterolemic nephropathy. Lab. Investig. 2011, 91, 106–123. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A. Is neuroimmunomodulation a future therapeutic approach for sepsis? Int. Immunopharmacol. 2010, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors: Therapeutic targets for novel ligands to treat pain and inflammation. Pharmacol. Res. 2023, 190, 106715. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef]
- Mikulski, Z.; Hartmann, P.; Jositsch, G.; Zasłona, Z.; Lips, K.S.; Pfeil, U.; Kurzen, H.; Lohmeyer, J.; Clauss, W.G.; Grau, V.; et al. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir. Res. 2010, 11, 133. [Google Scholar] [CrossRef]
- Cauwels, A.; Rogge, E.; Vandendriessche, B.; Shiva, S.; Brouckaert, P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014, 5, e1102. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Vultaggio-Poma, V.; Falzoni, S.; Giuliani, A.L. Extracellular ATP: A powerful inflammatory mediator in the central nervous system. Neuropharmacology 2023, 224, 109333. [Google Scholar] [CrossRef]
- Savio, L.E.B. P2X receptors in the balance between inflammation and pathogen control in sepsis. Purinergic Signal. 2022, 18, 241–243. [Google Scholar] [CrossRef]
- Välimäki, E.; Cypryk, W.; Virkanen, J.; Nurmi, K.; Turunen, P.M.; Eklund, K.K.; Åkerman, K.E.; Nyman, T.A.; Matikainen, S. Calpain Activity Is Essential for ATP-Driven Unconventional Vesicle-Mediated Protein Secretion and Inflammasome Activation in Human Macrophages. J. Immunol. 2016, 197, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Letavernier, E.; Le Saux, C.J.; Houssaini, A.; Abid, S.; Czibik, G.; Sawaki, D.; Marcos, E.; Dubois-Rande, J.L.; Baud, L.; et al. Calpastatin overexpression impairs postinfarct scar healing in mice by compromising reparative immune cell recruitment and activation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1883–H1893. [Google Scholar] [CrossRef]
- Kumar, V.; Stewart IV, J.H. Obesity, bone marrow adiposity, and leukemia: Time to act. Obes. Rev. 2024, 25, e13674. [Google Scholar] [CrossRef]
- Kumar, V.; Kiran, S.; Kumar, S.; Singh, U.P. Extracellular vesicles in obesity and its associated inflammation. Int. Rev. Immunol. 2022, 41, 30–44. [Google Scholar] [CrossRef]
- Chakarov, S.; Blériot, C.; Ginhoux, F. Role of adipose tissue macrophages in obesity-related disorders. J. Exp. Med. 2022, 219, e20211948. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyazaki, A. Emerging roles of calpain proteolytic systems in macrophage cholesterol handling. Cell Mol. Life Sci. 2017, 74, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Thrum, S.; Sommer, M.; Raulien, N.; Gericke, M.; Massier, L.; Kovacs, P.; Krasselt, M.; Landgraf, K.; Körner, A.; Dietrich, A.; et al. Macrophages in obesity are characterised by increased IL-1β response to calcium-sensing receptor signals. Int. J. Obes. 2022, 46, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, L.; Xu, D.; Deng, W.; Yang, W.; Tang, F.; Da, M. Knockout of calpain-1 protects against high-fat diet-induced liver dysfunction in mouse through inhibiting oxidative stress and inflammation. Food Sci. Nutr. 2021, 9, 367–374. [Google Scholar] [CrossRef]
- Awad, M.A.; Gu, W.; Li, S.; Wei, Y.; Tang, Y.; Shi, Y.; Juvenal, H.; Jiang, Y.; Liu, T.; Shuai, L.; et al. Calpain-1 is required for abnormality of liver function and metabolism in apolipoprotein E knockout mouse evaluated noninvasively by small animal MRI and PET-CT. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159616. [Google Scholar] [CrossRef] [PubMed]
- Muniappan, L.; Javidan, A.; Jiang, W.; Mohammadmoradi, S.; Moorleghen, J.J.; Katz, W.S.; Balakrishnan, A.; Howatt, D.A.; Subramanian, V. Calpain Inhibition Attenuates Adipose Tissue Inflammation and Fibrosis in Diet-induced Obese Mice. Sci. Rep. 2017, 7, 14398. [Google Scholar] [CrossRef]
- Subramanian, V.; Balakrishnan, A.; Howatt, D.A.; Moorleghen, J.J.; Katz, W.S. Abstract 81: Pharmacological Inhibition of Calpain Attenuates Adipose Tissue Apoptosis, Macrophage Accumulation and Inflammation in Diet-induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33 (Suppl. 1), A81. [Google Scholar] [CrossRef]
- Hanouna, G.; Mesnard, L.; Vandermeersch, S.; Perez, J.; Placier, S.; Haymann, J.-P.; Campagne, F.; Moroch, J.; Bataille, A.; Baud, L.; et al. Specific calpain inhibition protects kidney against inflammaging. Sci. Rep. 2017, 7, 8016. [Google Scholar] [CrossRef]
- Lokuta, M.A.; Nuzzi, P.A.; Huttenlocher, A. Calpain regulates neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA 2003, 100, 4006–4011. [Google Scholar] [CrossRef]
- Katsube, M.; Kato, T.; Kitagawa, M.; Noma, H.; Fujita, H.; Kitagawa, S. Calpain-mediated regulation of the distinct signaling pathways and cell migration in human neutrophils. J. Leukoc. Biol. 2008, 84, 255–263. [Google Scholar] [CrossRef]
- Knepper-Nicolai, B.; Savill, J.; Brown, S.B. Constitutive Apoptosis in Human Neutrophils Requires Synergy between Calpains and the Proteasome Downstream of Caspases. J. Biol. Chem. 1998, 273, 30530–30536. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Jundi, B.; Lee, D.-H.; Jeon, H.; Duvall, M.G.; Nijmeh, J.; Abdulnour, R.-E.E.; Pinilla-Vera, M.; Baron, R.M.; Han, J.; Voldman, J.; et al. Inflammation resolution circuits are uncoupled in acute sepsis and correlate with clinical severity. JCI Insight 2021, 6, 148866. [Google Scholar] [CrossRef]
- Delano, M.J.; Ward, P.A. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol. Rev. 2016, 274, 330–353. [Google Scholar] [CrossRef]
- Jennings, S.; Hallett, M.B. Single cell measurement of calpain activity in neutrophils reveals link to cytosolic Ca2+ elevation and individual phagocytotic events. Biochem. Biophys. Res. Commun. 2019, 515, 163–168. [Google Scholar] [CrossRef]
- Dewitt, S.; Francis, R.J.; Hallett, M.B. Ca2+ and calpain control membrane expansion during the rapid cell spreading of neutrophils. J. Cell Sci. 2013, 126, 4627–4635. [Google Scholar] [CrossRef]
- Singh, J.; Zlatar, L.; Muñoz-Becerra, M.; Lochnit, G.; Herrmann, I.; Pfister, F.; Janko, C.; Knopf, J.; Leppkes, M.; Schoen, J.; et al. Calpain-1 weakens the nuclear envelope and promotes the release of neutrophil extracellular traps. Cell Commun. Signal. 2024, 22, 435. [Google Scholar] [CrossRef]
- Gößwein, S.; Lindemann, A.; Mahajan, A.; Maueröder, C.; Martini, E.; Patankar, J.; Schett, G.; Becker, C.; Wirtz, S.; Naumann-Bartsch, N.; et al. Citrullination Licenses Calpain to Decondense Nuclei in Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 2481. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Borregaard, N. Neutrophil extracellular traps—The dark side of neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Danish, H.; Chheang, C.; Zhou, Y.; Huang, A.; Lai, A.; Khanmohammadi, M.; Quinn, K.M.; Khoshmanesh, K.; Peter, K. Piezo1 expression in neutrophils regulates shear-induced NETosis. Nat. Commun. 2024, 15, 7023. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Wiemer, A.J.; Lokuta, M.A.; Surfus, J.C.; Wernimont, S.A.; Huttenlocher, A. Calpain inhibition impairs TNF-alpha-mediated neutrophil adhesion, arrest and oxidative burst. Mol. Immunol. 2010, 47, 894–902. [Google Scholar] [CrossRef]
- Moser, M. Dendritic Cells in Immunity and Tolerance—Do They Display Opposite Functions? Immunity 2003, 19, 5–8. [Google Scholar] [CrossRef]
- Heras-Murillo, I.; Adán-Barrientos, I.; Galán, M.; Wculek, S.K.; Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.Y.; Belabed, M.; Park, M.D.; Mattiuz, R.; Puleston, D.; Merad, M. Dendritic cell maturation in cancer. Nat. Rev. Cancer 2025, 25, 225–248. [Google Scholar] [CrossRef]
- Calle, Y.; Carragher, N.O.; Thrasher, A.J.; Jones, G.E. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J. Cell Sci. 2006, 119, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, E.; Huber, S.M.; Lang, F. Ca2+ signaling in the regulation of dendritic cell functions. Am. J. Physiol.-Cell Physiol. 2011, 300, C1205–C1214. [Google Scholar] [CrossRef]
- Calle, Y.; Burns, S.; Thrasher, A.J.; Jones, G.E. The leukocyte podosome. Eur. J. Cell Biol. 2006, 85, 151–157. [Google Scholar] [CrossRef]
- Hidalgo, A.; Frenette, P.S. Leukocyte Podosomes Sense Their Way through the Endothelium. Immunity 2007, 26, 753–755. [Google Scholar] [CrossRef]
- Carman, C.V.; Sage, P.T.; Sciuto, T.E.; de la Fuente, M.A.; Geha, R.S.; Ochs, H.D.; Dvorak, H.F.; Dvorak, A.M.; Springer, T.A. Transcellular Diapedesis Is Initiated by Invasive Podosomes. Immunity 2007, 26, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zahir, N.; Jiang, Q.; Miliotis, H.; Heyraud, S.; Meng, X.; Dong, B.; Xie, G.; Qiu, F.; Hao, Z.; et al. The autoimmune disease–associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat. Genet. 2011, 43, 902–907. [Google Scholar] [CrossRef]
- Behrens, T.W. Lyp breakdown and autoimmunity. Nat. Genet. 2011, 43, 821–822. [Google Scholar] [CrossRef]
- Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Gauckler, P.; Li, H.; Shin, J.I.; Kronbichler, A. The role of PTPN22 in the pathogenesis of autoimmune diseases: A comprehensive review. Semin. Arthritis Rheum. 2021, 51, 513–522. [Google Scholar] [CrossRef]
- Hamel-Côté, G.; Gendron, D.; Rola-Pleszczynski, M.; Stankova, J. Regulation of platelet-activating factor-mediated protein tyrosine phosphatase 1B activation by a Janus kinase 2/calpain pathway. PLoS ONE 2017, 12, e0180336. [Google Scholar] [CrossRef] [PubMed]
- Read, N.E.; Wilson, H.M. Recent Developments in the Role of Protein Tyrosine Phosphatase 1B (PTP1B) as a Regulator of Immune Cell Signalling in Health and Disease. Int. J. Mol. Sci. 2024, 25, 7207. [Google Scholar] [CrossRef] [PubMed]
- Braquet, P.; Rola-Pleszcynski, M. Platelet-activating factor and cellular immune responses. Immunol. Today 1987, 8, 345–351. [Google Scholar] [CrossRef]
- Stafforini, D.M.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit. Rev. Clin. Lab. Sci. 2003, 40, 643–672. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Terlizzi, M.; Di Crescenzo, V.G.; Popolo, A.; Pecoraro, M.; Perillo, G.; Galderisi, A.; Pinto, A. Human lung cancer-derived immunosuppressive plasmacytoid dendritic cells release IL-1α in an AIM2 inflammasome-dependent manner. Am. J. Pathol. 2015, 185, 3115–3124. [Google Scholar] [CrossRef]
- Abraham, S.N.; St. John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef]
- Plum, T.; Feyerabend, T.B.; Rodewald, H.-R. Beyond classical immunity: Mast cells as signal converters between tissues and neurons. Immunity 2024, 57, 2723–2736. [Google Scholar] [CrossRef]
- Noto, C.N.; Hoft, S.G.; DiPaolo, R.J. Mast Cells as Important Regulators in Autoimmunity and Cancer Development. Front. Cell Dev. Biol. 2021, 9, 752350. [Google Scholar] [CrossRef]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast cell-mediated immune regulation in health and disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef]
- Jones, M.K.; Nair, A.; Gupta, M. Mast Cells in Neurodegenerative Disease. Front. Cell. Neurosci. 2019, 13, 00171. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Yu, J.; Liu, Y.; Wang, F.; Gou, G.; Wen, M.; Luo, C.; Lu, X.; Hu, Y.; Du, Q.; et al. Mast cells: Key players in digestive system tumors and their interactions with immune cells. Cell Death Discov. 2025, 11, 8. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Liu, F.; Chen, W.; Wu, P.; Wieschhaus, A.J.; Chishti, A.H.; Roche, P.A.; Chen, W.M.; Lin, T.J. Calpain-1 contributes to IgE-mediated mast cell activation. J. Immunol. 2014, 192, 5130–5139. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.P.; Ahmed, M.E.; Thangavel, R.; Kempuraj, D.; Dubova, I.; Raikwar, S.P.; Zaheer, S.; Iyer, S.S.; Zaheer, A. A role for glia maturation factor dependent activation of mast cells and microglia in MPTP induced dopamine loss and behavioural deficits in mice. Brain Behav. Immun. 2020, 87, 429–443. [Google Scholar] [CrossRef]
- Crocker, S.J.; Smith, P.D.; Jackson-Lewis, V.; Lamba, W.R.; Hayley, S.P.; Grimm, E.; Callaghan, S.M.; Slack, R.S.; Melloni, E.; Przedborski, S.; et al. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J. Neurosci. 2003, 23, 4081–4091. [Google Scholar] [CrossRef]
- Gao, A.; McCoy, H.M.; Zaman, V.; Shields, D.C.; Banik, N.L.; Haque, A. Calpain activation and progression of inflammatory cycles in Parkinson’s disease. Front. Biosci. 2022, 27, 20. [Google Scholar] [CrossRef]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; Zaheer, A. Mast Cell Activation, Neuroinflammation, and Tight Junction Protein Derangement in Acute Traumatic Brain Injury. Mediat. Inflamm. 2020, 2020, 4243953. [Google Scholar] [CrossRef]
- Huang, X.; Lan, Z.; Hu, Z. Role and mechanisms of mast cells in brain disorders. Front. Immunol. 2024, 15, 1445867. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef]
- Forsythe, P.; Befus, A.D. Inhibition of calpain is a component of nitric oxide-induced down-regulation of human mast cell adhesion. J. Immunol. 2003, 170, 287–293. [Google Scholar] [CrossRef]
- Kumar, V. Innate lymphoid cells: New paradigm in immunology of inflammation. Immunol. Lett. 2014, 157, 23–37. [Google Scholar] [CrossRef]
- Kumar, V. Innate lymphoid cell and adaptive immune cell cross-talk: A talk meant not to forget. J. Leukoc. Biol. 2020, 108, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Innate Lymphoid Cells: Immunoregulatory Cells of Mucosal Inflammation. Eur. J. Inflamm. 2014, 12, 11–20. [Google Scholar] [CrossRef]
- Kumar, V. Innate Lymphoid Cells and Adaptive Immune Cells Cross-Talk: A Secret Talk Revealed in Immune Homeostasis and Different Inflammatory Conditions. Int. Rev. Immunol. 2021, 40, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, A.; Colonna, M.; Koyasu, S. Development, Differentiation, and Diversity of Innate Lymphoid Cells. Immunity 2014, 41, 354–365. [Google Scholar] [CrossRef]
- Deshpande, R.V.; Goust, J.M.; Banik, N.L. Differential distribution of calpain in human lymphoid cells. Neurochem. Res. 1993, 18, 767–773. [Google Scholar] [CrossRef]
- Blom, W.M.; de Bont, H.J.; Mulder, G.J.; Nagelkerke, J.F. The role of calpains in apoptotic changes in isolated hepatocytes after attack by Natural Killer cells. Environ. Toxicol. Pharmacol. 2002, 11, 159–165. [Google Scholar] [CrossRef]
- Shenoy, A.M.; Brahmi, Z. Inhibition of the calpain-mediated proteolysis of protein kinase C enhances lytic activity in human NK cells. Cell Immunol. 1991, 138, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kwon, S. Enhanced cytotoxic activity of natural killer cells from increased calcium influx induced by electrical stimulation. PLoS ONE 2024, 19, e0302406. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Ramírez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Pérez, A.; Oñate, C.; Andrés-Tovar, A.; Garzón-Tituaña, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228. [Google Scholar] [CrossRef]
- Kumar, V. Cytotoxic T Cells: Kill, Memorize, and Mask to Maintain Immune Homeostasis. Int. J. Mol. Sci. 2025, 26, 8788. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Park, K.; Gola, A.; Baptista, A.P.; Miller, C.H.; Deep, D.; Lou, M.; Boyd, L.F.; Rudensky, A.Y.; Savage, P.A.; et al. A local regulatory T cell feedback circuit maintains immune homeostasis by pruning self-activated T cells. Cell 2021, 184, 3981–3997.e22. [Google Scholar] [CrossRef]
- Barnes, M.J.; Powrie, F. Regulatory T Cells Reinforce Intestinal Homeostasis. Immunity 2009, 31, 401–411. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Kuchay, S.; Nunez, R.; Bartholomew, A.M.; Chishti, A.H. Calpain I Null Mice Display Lymphoid Hyperplasia. Blood 2004, 104, 1268. [Google Scholar] [CrossRef]
- Deshpande, R.V.; Goust, J.-M.; Chakrabarti, A.K.; Barbosa, E.; Hogan, E.L.; Banik, N.L. Calpain Expression in Lymphoid Cells: Increased mRNA and Protein Levels After Cell Activation. J. Biol. Chem. 1995, 270, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Selliah, N.; Brooks, W.H.; Roszman, T.L. Proteolytic cleavage of α-actinin by calpain in T cells stimulated with anti-CD3 monoclonal antibody. J. Immunol. 1996, 156, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Mikosik, A.; Jasiulewicz, A.; Daca, A.; Henc, I.; Frąckowiak, J.E.; Ruckemann-Dziurdzińska, K.; Foerster, J.; Le Page, A.; Bryl, E.; Fulop, T.; et al. Roles of calpain-calpastatin system (CCS) in human T cell activation. Oncotarget 2016, 7, 76479–76495. [Google Scholar] [CrossRef] [PubMed]
- Rock, M.T.; Brooks, W.H.; Roszman, T.L. Calcium-dependent Signaling Pathways in T Cells: Potential Role of Calpain, Protein Tyrosine Phosphatase 1B, And p130Cas in Integrin-Mediated Signaling Events. J. Biol. Chem. 1997, 272, 33377–33383. [Google Scholar] [CrossRef]
- Wiede, F.; Lu, K.H.; Du, X.; Zeissig, M.N.; Xu, R.; Goh, P.K.; Xirouchaki, C.E.; Hogarth, S.J.; Greatorex, S.; Sek, K.; et al. PTP1B Is an Intracellular Checkpoint that Limits T-cell and CAR T-cell Antitumor Immunity. Cancer Discov. 2022, 12, 752–773. [Google Scholar] [CrossRef]
- Rock, M.T.; Dix, A.R.; Brooks, W.H.; Roszman, T.L. β1 Integrin-Mediated T Cell Adhesion and Cell Spreading Are Regulated by Calpain. Exp. Cell Res. 2000, 261, 260–270. [Google Scholar] [CrossRef]
- Penna, D.; Müller, S.; Martinon, F.; Demotz, S.; Iwashima, M.; Valitutti, S. Degradation of ZAP-70 following antigenic stimulation in human T lymphocytes: Role of calpain proteolytic pathway. J. Immunol. 1999, 163, 50–56. [Google Scholar] [CrossRef]
- Damen, H.; Tebid, C.; Viens, M.; Roy, D.C.; Dave, V.P. Negative Regulation of Zap70 by Lck Forms the Mechanistic Basis of Differential Expression in CD4 and CD8 T Cells. Front. Immunol. 2022, 13, 935367. [Google Scholar] [CrossRef]
- Liu, C.S.C.; Raychaudhuri, D.; Paul, B.; Chakrabarty, Y.; Ghosh, A.R.; Rahaman, O.; Talukdar, A.; Ganguly, D. Cutting Edge: Piezo1 Mechanosensors Optimize Human T Cell Activation. J. Immunol. 2018, 200, 1255–1260. [Google Scholar] [CrossRef]
- Liu, C.S.C.; Mandal, T.; Biswas, P.; Hoque, M.A.; Bandopadhyay, P.; Sinha, B.P.; Sarif, J.; D’Rozario, R.; Sinha, D.K.; Sinha, B.; et al. Piezo1 mechanosensing regulates integrin-dependent chemotactic migration in human T cells. eLife 2024, 12, RP91903. [Google Scholar] [CrossRef]
- Wernimont, S.A.; Simonson, W.T.N.; Greer, P.A.; Seroogy, C.M.; Huttenlocher, A. Calpain 4 Is Not Necessary for LFA-1-Mediated Function in CD4+ T Cells. PLoS ONE 2010, 5, e10513. [Google Scholar] [CrossRef]
- Chen, J.; Ganguly, A.; Mucsi, A.D.; Meng, J.; Yan, J.; Detampel, P.; Munro, F.; Zhang, Z.; Wu, M.; Hari, A.; et al. Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy. J. Exp. Med. 2017, 214, 327–338. [Google Scholar] [CrossRef]
- Yan, J.; Liu, B.; Shi, Y.; Qi, H. Class II MHC-independent suppressive adhesion of dendritic cells by regulatory T cells in vivo. J. Exp. Med. 2017, 214, 319–326. [Google Scholar] [CrossRef]
- Simonson, W.T.N.; Franco, S.J.; Huttenlocher, A. Talin1 Regulates TCR-Mediated LFA-1 Function1. J. Immunol. 2006, 177, 7707–7714. [Google Scholar] [CrossRef]
- Franco, S.J.; Rodgers, M.A.; Perrin, B.J.; Han, J.; Bennin, D.A.; Critchley, D.R.; Huttenlocher, A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 2004, 6, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Franz, F.; Tapia-Rojo, R.; Winograd-Katz, S.; Boujemaa-Paterski, R.; Li, W.; Unger, T.; Albeck, S.; Aponte-Santamaria, C.; Garcia-Manyes, S.; Medalia, O.; et al. Allosteric activation of vinculin by talin. Nat. Commun. 2023, 14, 4311. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Said, R.; Nguyen-Vigouroux, C.; Henriot, V.; Gebhardt, P.; Pernier, J.; Grosse, R.; Le Clainche, C. Talin and vinculin combine their activities to trigger actin assembly. Nat. Commun. 2024, 15, 9497. [Google Scholar] [CrossRef] [PubMed]
- Mikosik, A.; Foerster, J.; Jasiulewicz, A.; Frąckowiak, J.; Colonna-Romano, G.; Bulati, M.; Buffa, S.; Martorana, A.; Caruso, C.; Bryl, E.; et al. Expression of calpain-calpastatin system (CCS) member proteins in human lymphocytes of young and elderly individuals; pilot baseline data for the CALPACENT project. Immun. Ageing 2013, 10, 27. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Bryl, E. Paradoxical age-related cell cycle quickening of human CD4(+) lymphocytes: A role for cyclin D1 and calpain. Exp. Gerontol. 2004, 39, 577–585. [Google Scholar] [CrossRef]
- Perez, J.; Dansou, B.; Hervé, R.; Levi, C.; Tamouza, H.; Vandermeersch, S.; Demey-Thomas, E.; Haymann, J.-P.; Zafrani, L.; Klatzmann, D.; et al. Calpains Released by T Lymphocytes Cleave TLR2 To Control IL-17 Expression. J. Immunol. 2016, 196, 168–181. [Google Scholar] [CrossRef]
- Frangié, C.; Zhang, W.; Perez, J.; Dubois, Y.C.; Haymann, J.P.; Baud, L. Extracellular calpains increase tubular epithelial cell mobility. Implications for kidney repair after ischemia. J. Biol. Chem. 2006, 281, 26624–26632. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Oppenheim, J.J. Chemerin reveals its chimeric nature. J. Exp. Med. 2008, 205, 2187–2190. [Google Scholar] [CrossRef]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.; Greaves, D.R. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-Y.; Leung, L.L.K. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim. Biophys. Sin. 2009, 41, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Oda, N.; Sato, Y. Cell-associated activation of latent transforming growth factor-beta by calpain. J. Cell Physiol. 1998, 174, 186–193. [Google Scholar] [CrossRef]
- Letavernier, B.; Zafrani, L.; Nassar, D.; Perez, J.; Levi, C.; Bellocq, A.; Mesnard, L.; Sachon, E.; Haymann, J.P.; Aractingi, S.; et al. Calpains contribute to vascular repair in rapidly progressive form of glomerulonephritis: Potential role of their externalization. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 335–342. [Google Scholar] [CrossRef]
- Mikosik, A.; Zaremba, A.; Puchalska, Z.; Daca, A.; Smolenska, Z.; Lopatniuk, P.; Mital, A.; Hellman, A.; Bryl, E.; Witkowski, J.M. Ex vivo measurement of calpain activation in human peripheral blood lymphocytes by detection of immunoreactive products of calpastatin degradation. Folia Histochem. Cytobiol. 2007, 45, 343–347. [Google Scholar]
- Mimori, T.; Suganuma, K.; Tanami, Y.; Nojima, T.; Matsumura, M.; Fujii, T.; Yoshizawa, T.; Suzuki, K.; Akizuki, M. Autoantibodies to calpastatin (an endogenous inhibitor for calcium-dependent neutral protease, calpain) in systemic rheumatic diseases. Proc. Natl. Acad. Sci. USA 1995, 92, 7267–7271. [Google Scholar] [CrossRef]
- Després, N.; Talbot, G.; Plouffe, B.; Boire, G.; Ménard, H.A. Detection and expression of a cDNA clone that encodes a polypeptide containing two inhibitory domains of human calpastatin and its recognition by rheumatoid arthritis sera. J. Clin. Investig. 1995, 95, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Iguchi-Hashimoto, M.; Usui, T.; Yoshifuji, H.; Shimizu, M.; Kobayashi, S.; Ito, Y.; Murakami, K.; Shiomi, A.; Yukawa, N.; Kawabata, D.; et al. Overexpression of a minimal domain of calpastatin suppresses IL-6 production and Th17 development via reduced NF-κB and increased STAT5 signals. PLoS ONE 2011, 6, e27020. [Google Scholar] [CrossRef] [PubMed]
- Letavernier, E.; Dansou, B.; Lochner, M.; Perez, J.; Bellocq, A.; Lindenmeyer, M.T.; Cohen, C.D.; Haymann, J.P.; Eberl, G.; Baud, L. Critical role of the calpain/calpastatin balance in acute allograft rejection. Eur. J. Immunol. 2011, 41, 473–484. [Google Scholar] [CrossRef]
- Smith, A.W.; Doonan, B.P.; Tyor, W.R.; Abou-Fayssal, N.; Haque, A.; Banik, N.L. Regulation of Th1/Th17 cytokines and IDO gene expression by inhibition of calpain in PBMCs from MS patients. J. Neuroimmunol. 2011, 232, 179–185. [Google Scholar] [CrossRef]
- Imam, S.A.; Guyton, M.K.; Haque, A.; Vandenbark, A.; Tyor, W.R.; Ray, S.K.; Banik, N.L. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J. Neuroimmunol. 2007, 190, 139–145. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Rossini, S.; Suvieri, C.; Coletti, A.; Orabona, C.; Macchiarulo, A.; Volpi, C.; Grohmann, U. Indoleamine 2,3-dioxygenase 1 (IDO1): An up-to-date overview of an eclectic immunoregulatory enzyme. FEBS J. 2022, 289, 6099–6118. [Google Scholar] [CrossRef]
- Forteza, M.J.; Polyzos, K.A.; Baumgartner, R.; Suur, B.E.; Mussbacher, M.; Johansson, D.K.; Hermansson, A.; Hansson, G.K.; Ketelhuth, D.F.J. Activation of the Regulatory T-Cell/Indoleamine 2,3-Dioxygenase Axis Reduces Vascular Inflammation and Atherosclerosis in Hyperlipidemic Mice. Front. Immunol. 2018, 9, 00950. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F.; Fennell, E.; Murray, P.G. Calcium-dependent signalling in B-cell lymphomas. Oncogene 2021, 40, 6321–6328. [Google Scholar] [CrossRef]
- Ulbricht, C.; Leben, R.; Rakhymzhan, A.; Kirchhoff, F.; Nitschke, L.; Radbruch, H.; Niesner, R.A.; Hauser, A.E. Intravital quantification reveals dynamic calcium concentration changes across B cell differentiation stages. eLife 2021, 10, e56020. [Google Scholar] [CrossRef]
- Kitahara, A.; Ohtsuki, H.; Kirihata, Y.; Yamagata, Y.; Takano, E.; Kannagi, R.; Murachi, T. Selective localization of calpain I (the low-Ca2+-requiring form of Ca2+-dependent cysteine proteinase) in B-cells of human pancreatic islets. FEBS Lett. 1985, 184, 120–124. [Google Scholar] [CrossRef]
- Ruiz-Vela, A.; Serrano, F.; González, M.A.; Abad, J.L.; Bernad, A.; Maki, M.; Martínez-A, C. Transplanted Long-Term Cultured Pre-Bi Cells Expressing Calpastatin Are Resistant to B Cell Receptor–Induced Apoptosis. J. Exp. Med. 2001, 194, 247–254. [Google Scholar] [CrossRef]
- Ruiz-Vela, A.; González de Buitrago, G.; Martínez, A.C. Implication of calpain in caspase activation during B cell clonal deletion. Embo J. 1999, 18, 4988–4998. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; Eisenman, R.N. Post-translational control of Myc function during differentiation. Cell Cycle 2011, 10, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Habib, T.; Park, H.; Tsang, M.; de Alborán, I.M.; Nicks, A.; Wilson, L.; Knoepfler, P.S.; Andrews, S.; Rawlings, D.J.; Eisenman, R.N.; et al. Myc stimulates B lymphocyte differentiation and amplifies calcium signaling. J. Cell Biol. 2007, 179, 717–731. [Google Scholar] [CrossRef] [PubMed]
- de Barrios, O.; Meler, A.; Parra, M. MYC’s Fine Line Between B Cell Development and Malignancy. Cells 2020, 9, 523. [Google Scholar] [CrossRef]
- Calado, D.P.; Sasaki, Y.; Godinho, S.A.; Pellerin, A.; Köchert, K.; Sleckman, B.P.; de Alborán, I.M.; Janz, M.; Rodig, S.; Rajewsky, K. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat. Immunol. 2012, 13, 1092–1100. [Google Scholar] [CrossRef]
- Mikosik, A.; Henc, I.; Ruckemann-Dziurdzińska, K.; Frąckowiak, J.E.; Płoszyńska, A.; Balcerska, A.; Bryl, E.; Witkowski, J.M. Increased μ-Calpain Activity in Blasts of Common B-Precursor Childhood Acute Lymphoblastic Leukemia Correlates with Their Lower Susceptibility to Apoptosis. PLoS ONE 2015, 10, e0136615. [Google Scholar] [CrossRef] [PubMed]
- Łopatniuk, P.; Puchalska, Z.; Mital, A.; Mikosik, A.; Frąckowiak, J.; Hellmann, A.; Daca, A.; Bryl, E.; Fulop, T.; Witkowski, J.M. Excessive amount and activity of μ-calpain affects apoptotic machinery in chronic B-cell leukemia cells and influences the course of the disease. Acta Biochim. Pol. 2020, 67, 247–257. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Zmuda-Trzebiatowska, E.; Swiercz, J.M.; Cichorek, M.; Ciepluch, H.; Lewandowski, K.; Bryl, E.; Hellmann, A. Modulation of the activity of calcium-activated neutral proteases (calpains) in chronic lymphocytic leukemia (B-CLL) cells. Blood 2002, 100, 1802–1809. [Google Scholar] [CrossRef]
- Zhu, D.M.; Uckun, F.M. Calpain inhibitor II induces caspase-dependent apoptosis in human acute lymphoblastic leukemia and non-Hodgkin’s lymphoma cells as well as some solid tumor cells. Clin. Cancer Res. 2000, 6, 2456–2463. [Google Scholar] [PubMed]
- Nothelfer, K.; Arena, E.T.; Pinaud, L.; Neunlist, M.; Mozeleski, B.; Belotserkovsky, I.; Parsot, C.; Dinadayala, P.; Burger-Kentischer, A.; Raqib, R.; et al. B lymphocytes undergo TLR2-dependent apoptosis upon Shigella infection. J. Exp. Med. 2014, 211, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Arizmendi, O.; Picking, W.D.; Picking, W.L. Macrophage Apoptosis Triggered by IpaD from Shigella flexneri. Infect. Immun. 2016, 84, 1857–1865. [Google Scholar] [CrossRef]
- Poon, M.M.L.; Farber, D.L. The Whole Body as the System in Systems Immunology. iScience 2020, 23, 101509. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Pederzoli-Ribeil, M.; Gabillet, J.; Witko-Sarsat, V. Proteases from Inflammatory Cells: Regulation of Inflammatory Response. In Proteases and Their Receptors in Inflammation; Vergnolle, N., Chignard, M., Eds.; Springer: Basel, Switzerland, 2011; pp. 73–100. [Google Scholar]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Dókus, L.E.; Yousef, M.; Bánóczi, Z. Modulators of calpain activity: Inhibitors and activators as potential drugs. Expert Opin. Drug Discov. 2020, 15, 471–486. [Google Scholar] [CrossRef]
- Leloup, L.; Wells, A. Calpains as potential anti-cancer targets. Expert Opin. Ther. Targets 2011, 15, 309–323. [Google Scholar] [CrossRef]
- Shapovalov, I.; Harper, D.; Greer, P.A. Calpain as a therapeutic target in cancer. Expert Opin. Ther. Targets 2022, 26, 217–231. [Google Scholar] [CrossRef]
- Cagmat, E.B.; Guingab-Cagmat, J.D.; Vakulenko, A.V.; Hayes, R.L.; Anagli, J. Frontiers in Neuroengineering Potential Use of Calpain Inhibitors as Brain Injury Therapy. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2015. [Google Scholar]
- Hu, J.; Chen, L.; Huang, X.; Wu, K.; Ding, S.; Wang, W.; Wang, B.; Smith, C.; Ren, C.; Ni, H.; et al. Calpain inhibitor MDL28170 improves the transplantation-mediated therapeutic effect of bone marrow-derived mesenchymal stem cells following traumatic brain injury. Stem Cell Res. Ther. 2019, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Wang, Z.; Zhang, G.; Yang, L.; Gan, J.; Jiang, X. Calpain: The regulatory point of cardiovascular and cerebrovascular diseases. Biomed. Pharmacother. 2024, 179, 117272. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.S.; Mookherjee, N.; Hancock, R.E.; Bleackley, R.C. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol. Cancer Res. 2009, 7, 689–702. [Google Scholar] [CrossRef]
- Barlow, P.G.; Beaumont, P.E.; Cosseau, C.; Mackellar, A.; Wilkinson, T.S.; Hancock, R.E.; Haslett, C.; Govan, J.R.; Simpson, A.J.; Davidson, D.J. The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium. Am. J. Respir. Cell Mol. Biol. 2010, 43, 692–702. [Google Scholar] [CrossRef]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Saberian, E.; Jenča, A.; Ghanipour-Meybodi, R.; Jenča, A.; Petrášová, A.; Jenčová, J. From defense to offense: Antimicrobial peptides as promising therapeutics for cancer. Front. Oncol. 2024, 14, 1463088. [Google Scholar] [CrossRef]
- Teng, X.; Ji, C.; Zhong, H.; Zheng, D.; Ni, R.; Hill, D.J.; Xiong, S.; Fan, G.-C.; Greer, P.A.; Shen, Z.; et al. Selective deletion of endothelial cell calpain in mice reduces diabetic cardiomyopathy by improving angiogenesis. Diabetologia 2019, 62, 860–872. [Google Scholar] [CrossRef]
- Sreenan, S.K.; Zhou, Y.-P.; Otani, K.; Hansen, P.A.; Currie, K.P.M.; Pan, C.-Y.; Lee, J.-P.; Ostrega, D.M.; Pugh, W.; Horikawa, Y.; et al. Calpains Play a Role in Insulin Secretion and Action. Diabetes 2001, 50, 2013–2020. [Google Scholar] [CrossRef]
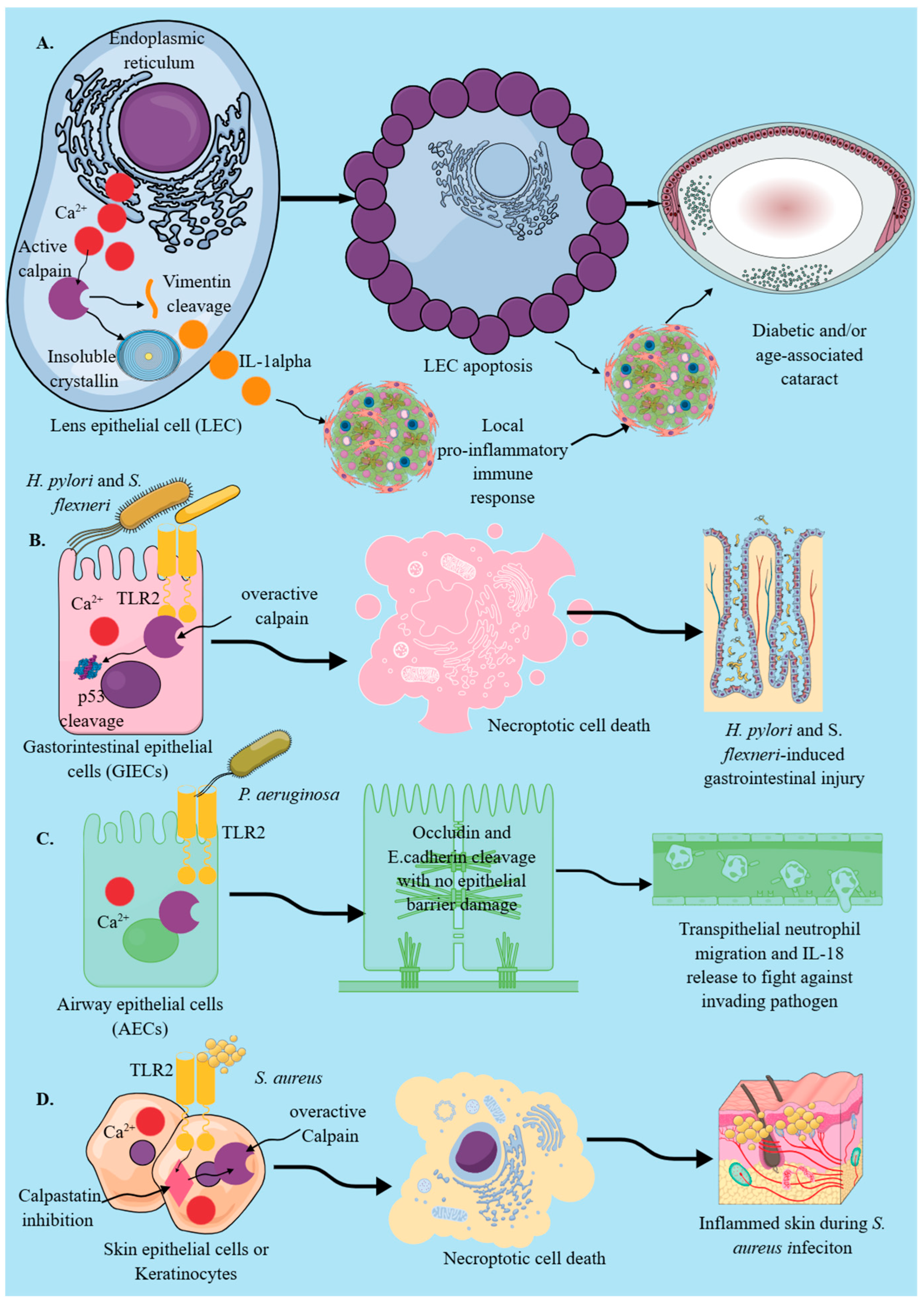
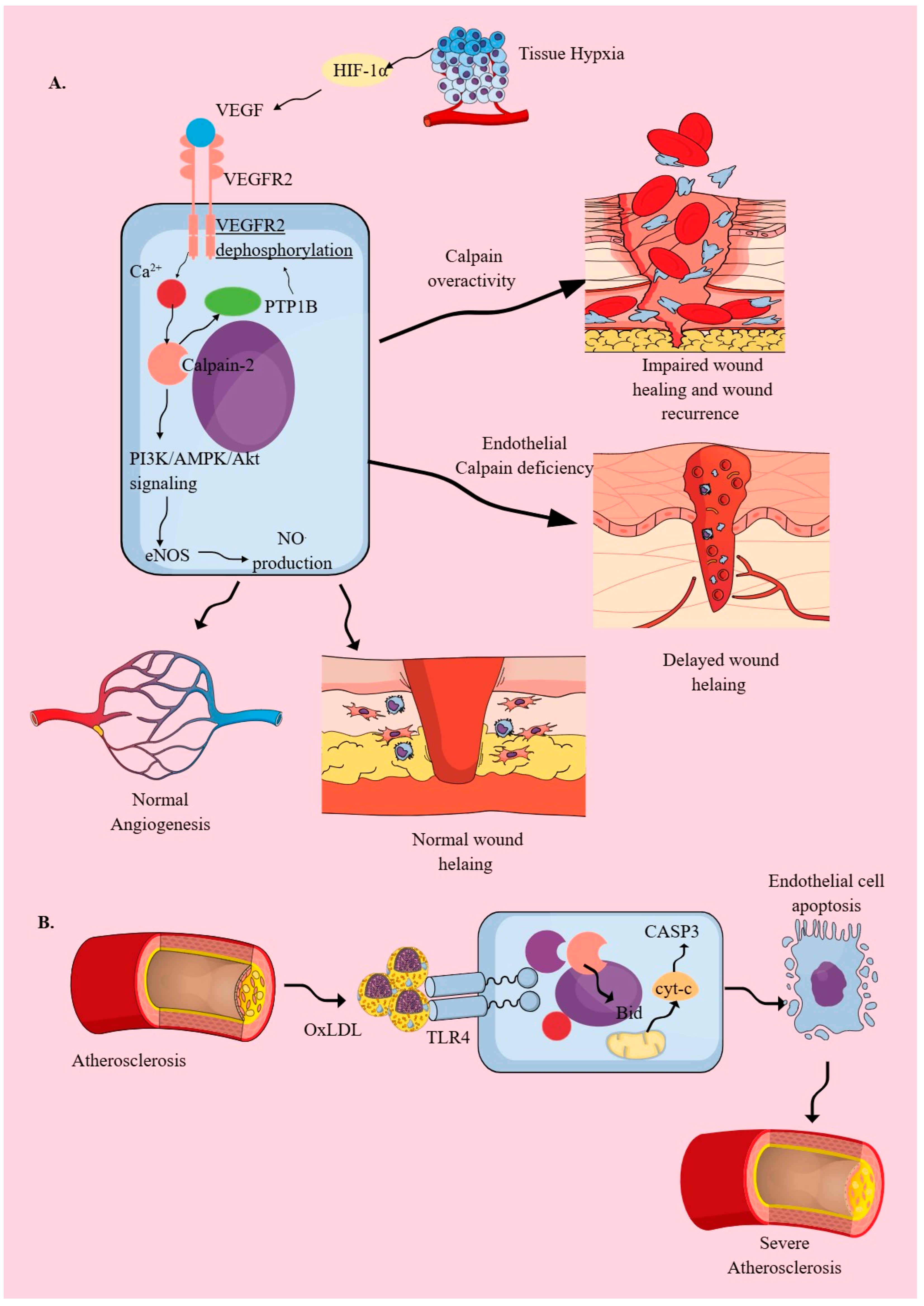
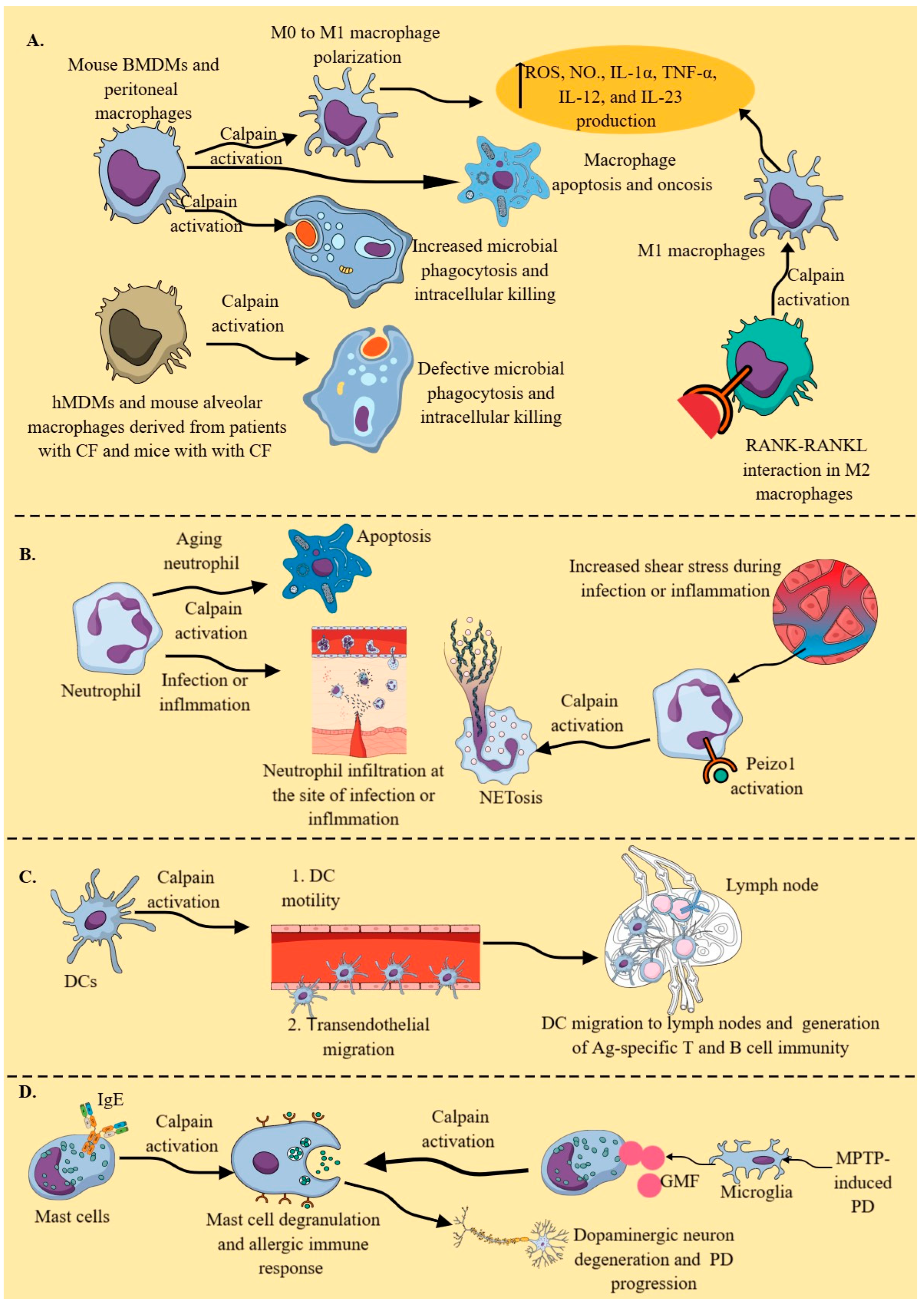
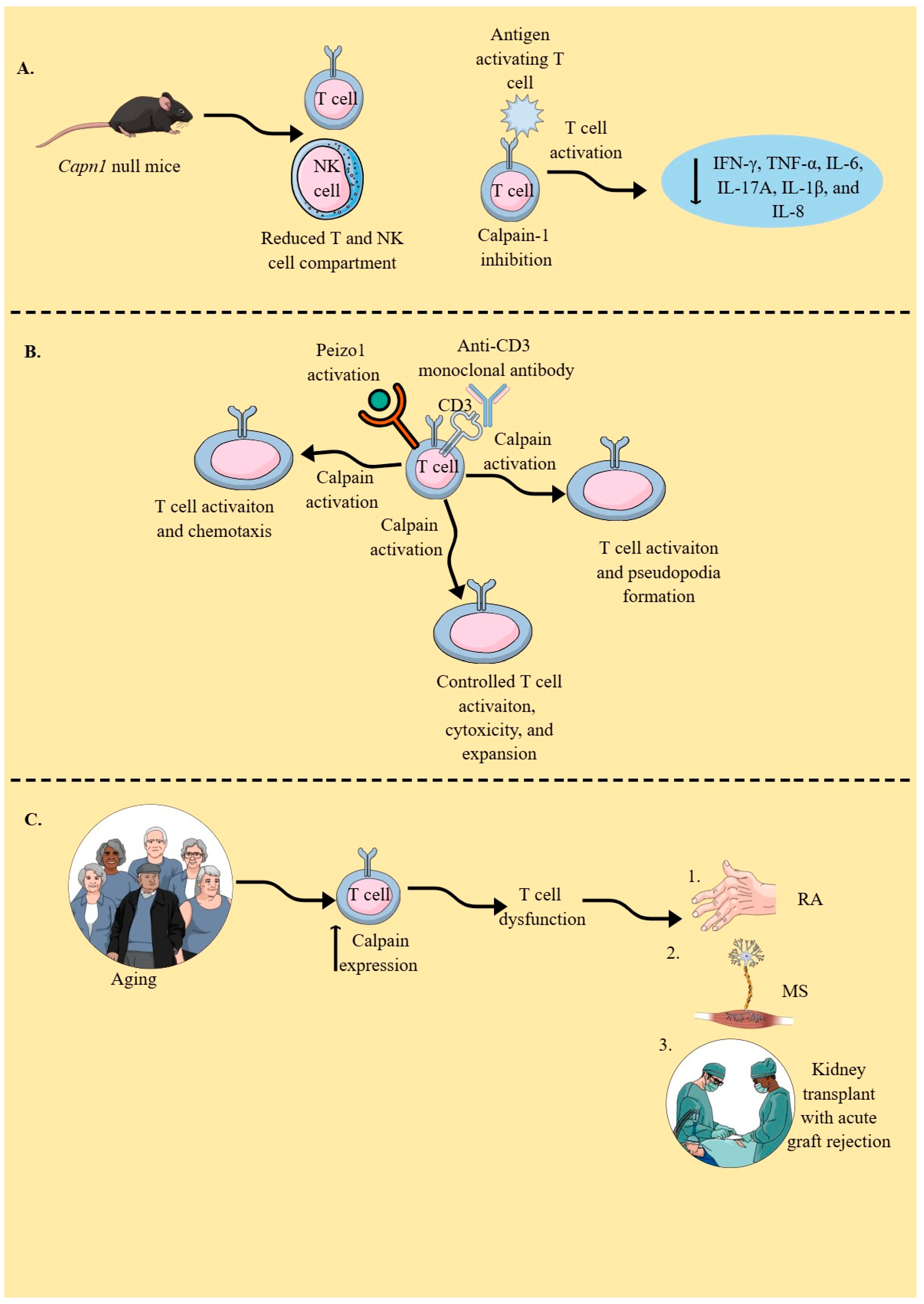
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Stewart, J.H., IV. Viewing Inflammation and Immunoregulation Under the Calpain System Lens. Cells 2025, 14, 1814. https://doi.org/10.3390/cells14221814
Kumar V, Stewart JH IV. Viewing Inflammation and Immunoregulation Under the Calpain System Lens. Cells. 2025; 14(22):1814. https://doi.org/10.3390/cells14221814
Chicago/Turabian StyleKumar, Vijay, and John H. Stewart, IV. 2025. "Viewing Inflammation and Immunoregulation Under the Calpain System Lens" Cells 14, no. 22: 1814. https://doi.org/10.3390/cells14221814
APA StyleKumar, V., & Stewart, J. H., IV. (2025). Viewing Inflammation and Immunoregulation Under the Calpain System Lens. Cells, 14(22), 1814. https://doi.org/10.3390/cells14221814




