Long Non-Coding RNA Analysis of Vitrified Porcine Immature Oocytes During Maturation and Early Parthenogenetic Embryo Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Oocyte Collection and Grouping
2.2. Oocyte Vitrification and Warming
2.3. Oocyte IVM
2.4. Vitro Parthenogenetic Activation and Embryo Culture
2.5. Sample Preparation, Library Construction, and RNA Sequencing
2.6. Quality Control and Transcriptome Assembly
2.7. Screening of Candidate LncRNA
2.8. Analysis of LncRNAs
2.9. Target Gene Prediction
2.10. Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. Identification and Distribution of lncRNAs
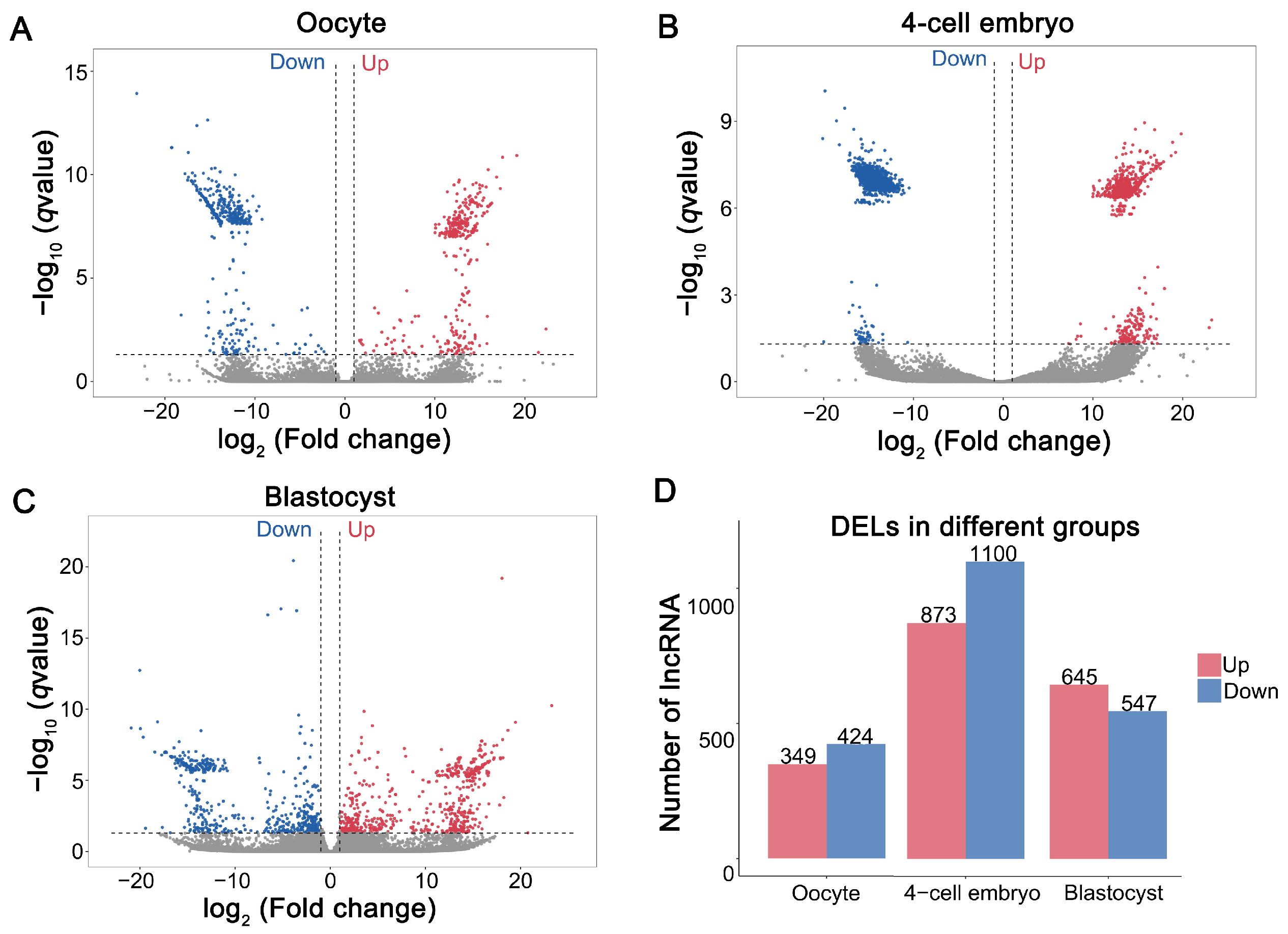
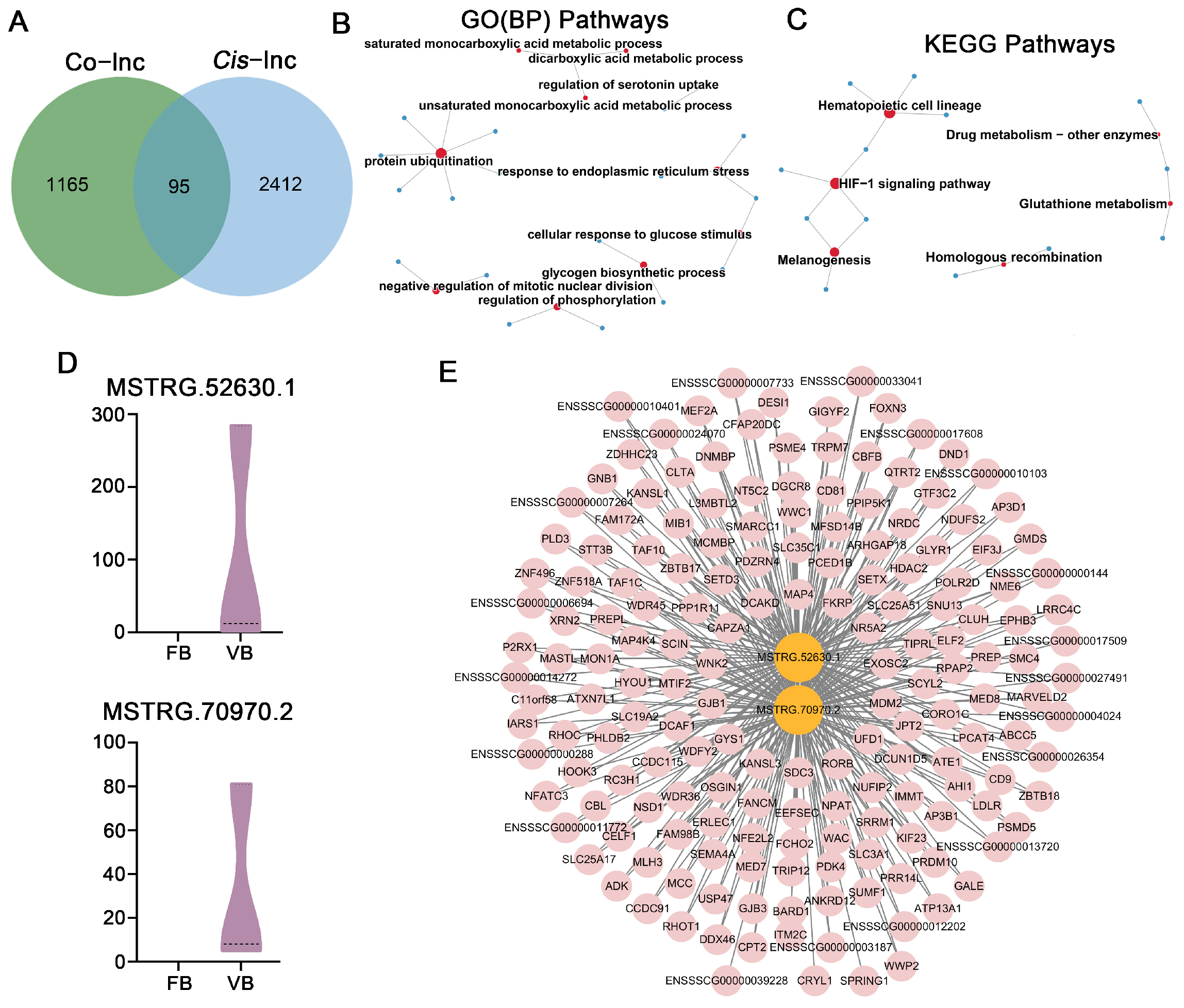
3.2. Differential Expression of LncRNAs in MII Oocytes and Parthenogenetic Embryos Derived from Oocytes Vitrified at the GV Stage
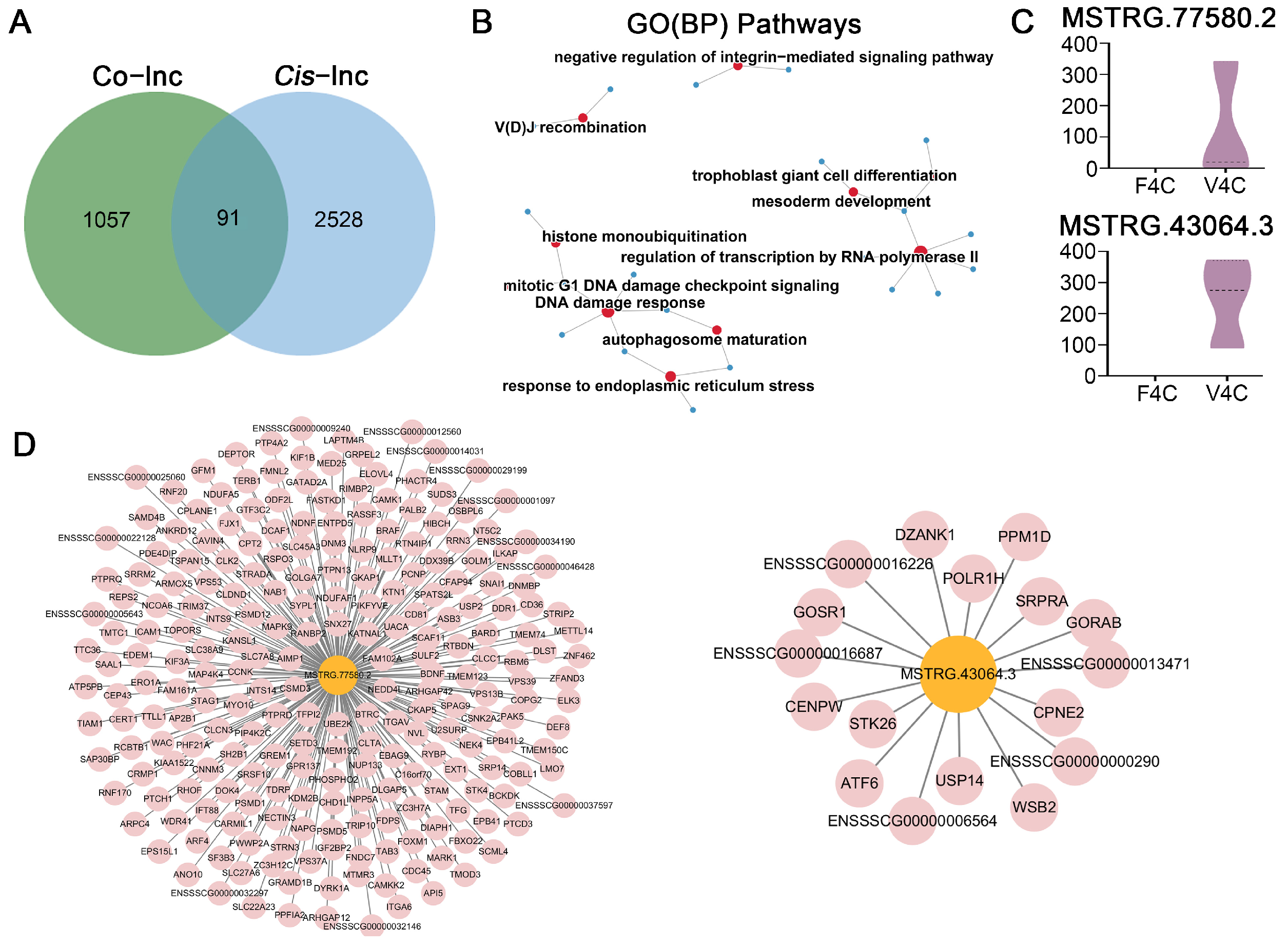
3.3. QPCR Validation of RNA-Seq Data
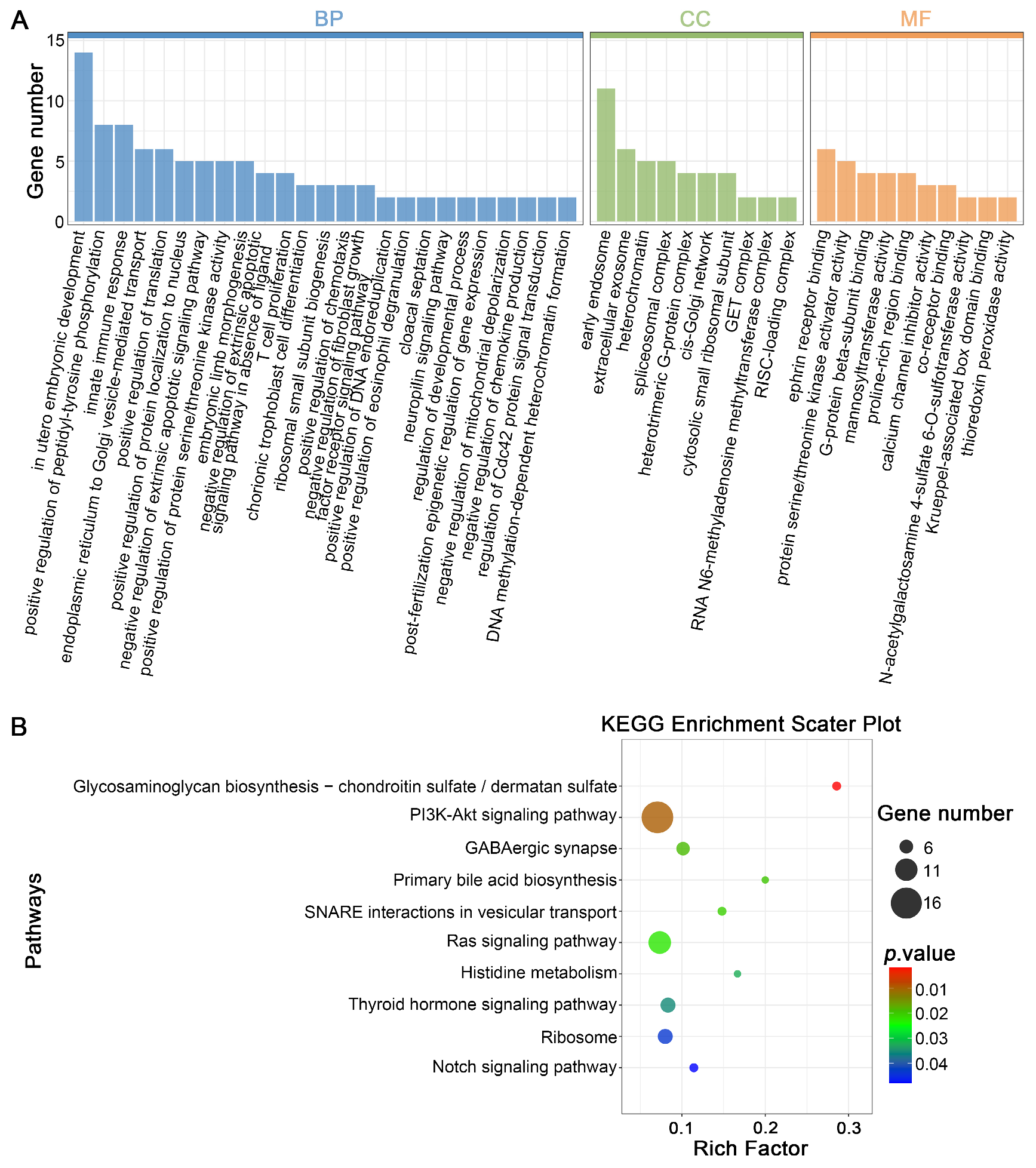
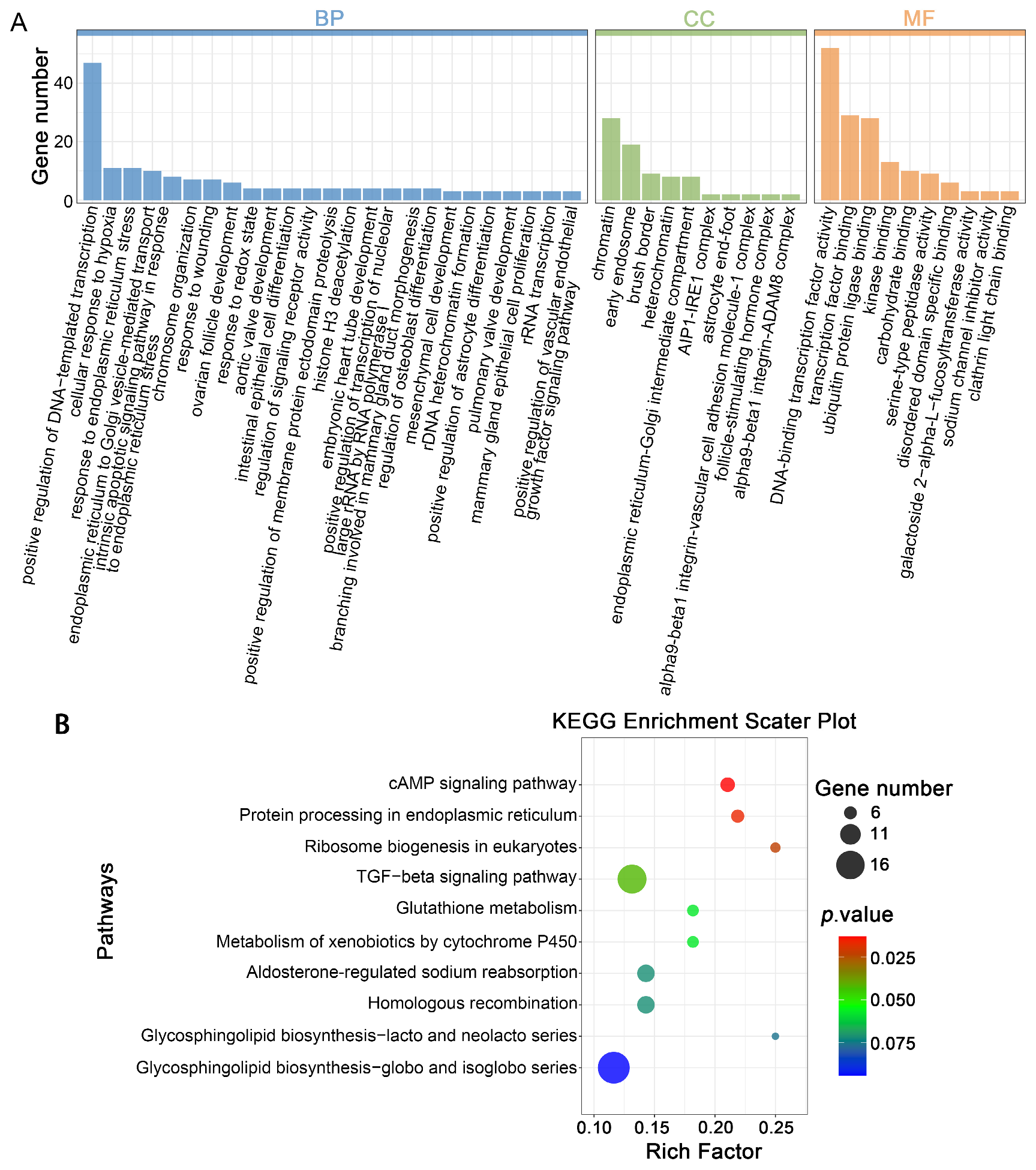
3.4. Prediction of Target Genes for DELs in Mature Oocytes Derived from Vitrified GV Oocytes and Enrichment Analysis
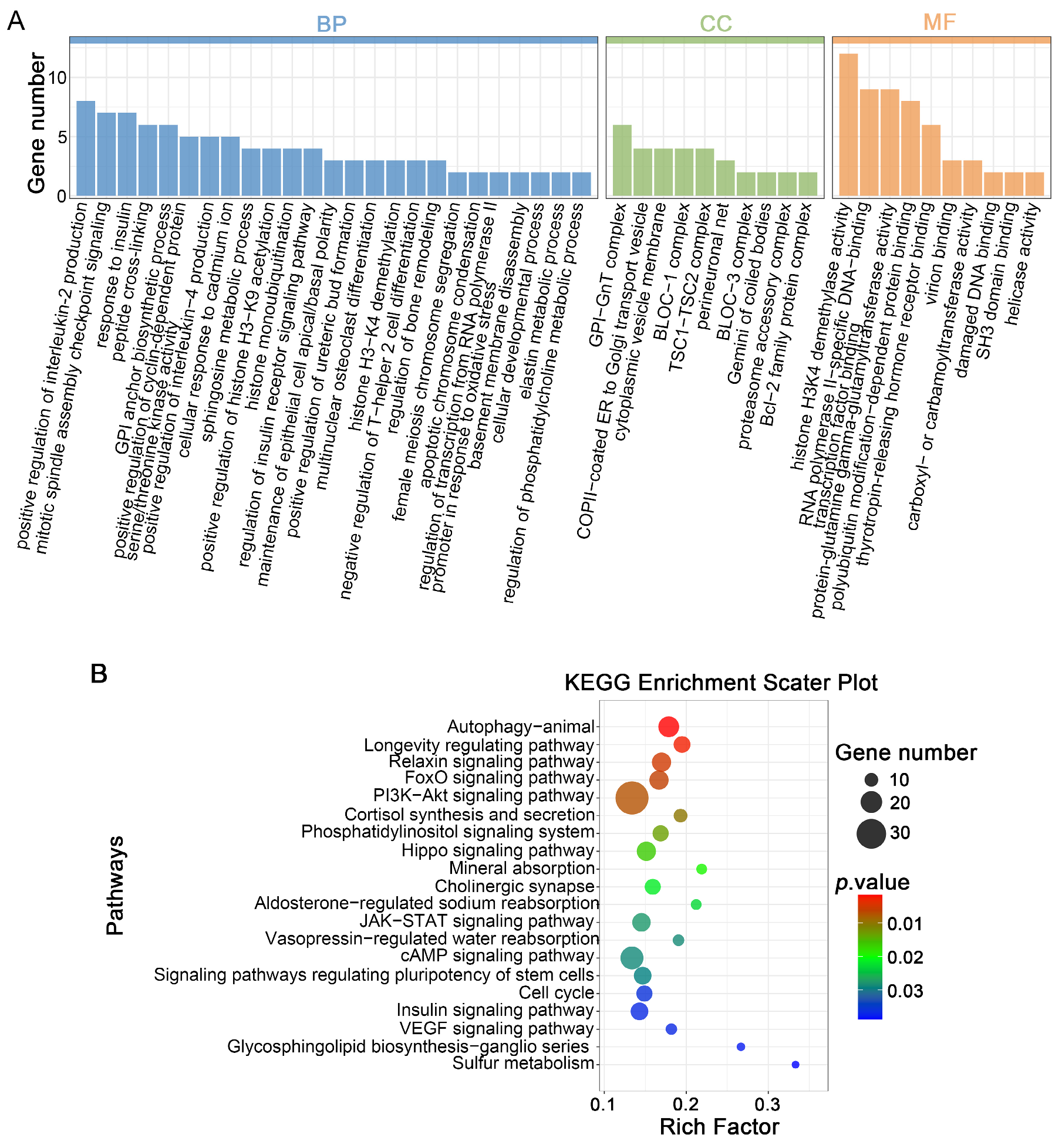
3.5. Prediction of Target Genes for DELs in Parthenogenetic 4-Cell Embryos Derived from Vitrified GV Stage Oo-Cytes and Enrichment Analysis
3.6. Prediction of Target Genes for DELs in Parthenogenetic Blastocysts Derived from Vitrified GV Stage Oocytes and Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVM | In vitro maturation |
| LncRNAs | Long non-coding RNAs |
| GV | Germinal vesicle |
| MII | Metaphase II |
| DELs | Differentially expressed lncRNAs |
| COCs | Cumulus–oocyte complexes |
| MZT | Maternal-to-zygotic transition |
| ZGA | Zygotic genome activation |
| PZM-3 | Porcine zygote medium-3 |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| DEGs | Differentially expressed genes |
References
- Lin, L.; Kragh, P.M.; Purup, S.; Kuwayama, M.; Du, Y.; Zhang, X.; Yang, H.; Bolund, L.; Callesen, H.; Vajta, G. Osmotic stress induced by sodium chloride, sucrose or trehalose improves cryotolerance and developmental competence of porcine oocytes. Reprod. Fertil. Dev. 2009, 21, 338–344. [Google Scholar] [CrossRef]
- Somfai, T.; Yoshioka, K.; Tanihara, F.; Kaneko, H.; Noguchi, J.; Kashiwazaki, N.; Nagai, T.; Kikuchi, K. Generation of live piglets from cryopreserved oocytes for the first time using a defined system for in vitro embryo production. PLoS ONE 2014, 9, e97731. [Google Scholar] [CrossRef] [PubMed]
- Gajda, B.; Skrzypczak-Zielińska, M.; Gawrońska, B.; Słomski, R.; Smorąg, Z. Successful production of piglets derived from mature oocytes vitrified using OPS method. Cryo Lett. 2015, 36, 8–18. [Google Scholar] [PubMed]
- Wu, C.; Rui, R.; Dai, J.; Zhang, C.; Ju, S.; Xie, B.; Lu, X.; Zheng, X. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol. Reprod. Dev. 2006, 73, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Tharasanit, T.; Thuwanut, P. Oocyte Cryopreservation in Domestic Animals and Humans: Principles, Techniques and Updated Outcomes. Animals 2021, 11, 2949. [Google Scholar] [CrossRef] [PubMed]
- Somfai, T.; Men, N.T.; Noguchi, J.; Kaneko, H.; Kashiwazaki, N.; Kikuchi, K. Optimization of cryoprotectant treatment for the vitrification of immature cumulus-enclosed porcine oocytes: Comparison of sugars, combinations of permeating cryoprotectants and equilibration regimens. J. Reprod. Dev. 2015, 61, 571–579. [Google Scholar] [CrossRef]
- Wu, G.; Jia, B.; Quan, G.; Xiang, D.; Zhang, B.; Shao, Q.; Hong, Q. Vitrification of porcine immature oocytes: Association of equilibration manners with warming procedures, and permeating cryoprotectants effects under two temperatures. Cryobiology 2017, 75, 21–27. [Google Scholar] [CrossRef]
- Casillas, F.; Ducolomb, Y.; Lemus, A.E.; Cuello, C.; Betancourt, M. Porcine embryo production following in vitro fertilization and intracytoplasmic sperm injection from vitrified immature oocytes matured with a granulosa cell co-culture system. Cryobiology 2015, 71, 299–305. [Google Scholar] [CrossRef]
- Casillas, F.; Betancourt, M.; Cuello, C.; Ducolomb, Y.; López, A.; Juárez-Rojas, L.; Retana-Márquez, S. An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porc. Health Manag. 2018, 4, 16. [Google Scholar] [CrossRef]
- Jia, B.Y.; Xiang, D.C.; Zhang, B.; Quan, G.B.; Shao, Q.Y.; Hong, Q.H.; Wu, G.Q. Quality of vitrified porcine immature oocytes is improved by coculture with fresh oocytes during in vitro maturation. Mol. Reprod. Dev. 2019, 86, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. A lipid-phase separation model of low-temperature damage to biological membranes. Cryobiology 1985, 22, 128–146. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, G.L.; Ma, J.Y.; Qi, S.T.; Wang, Z.B.; Wang, Z.W.; Luo, Y.B.; Jiang, Z.Z.; Schatten, H.; Sun, Q.Y. Effects of DNA damage and short-term spindle disruption on oocyte meiotic maturation. Histochem. Cell Biol. 2014, 142, 185–194. [Google Scholar] [CrossRef]
- Zarei Moradi, S.; Mohseni Meybodi, A.; Gourabi, H.; Mozdarani, H.; Mansouri, Z. Chromosome abnormalities and viability of vitrified eight-cell mouse embryos at presence of two different cryoprotectants at different storage durations. Cell J. 2013, 14, 254–263. [Google Scholar] [CrossRef]
- Lei, T.; Guo, N.; Liu, J.Q.; Tan, M.H.; Li, Y.F. Vitrification of in vitro matured oocytes: Effects on meiotic spindle configuration and mitochondrial function. Int. J. Clin. Exp. Pathol. 2014, 7, 1159–1165. [Google Scholar] [CrossRef]
- Xiang, D.C.; Jia, B.Y.; Fu, X.W.; Guo, J.X.; Hong, Q.H.; Quan, G.B.; Wu, G.Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef]
- López, A.; Ducolomb, Y.; Casas, E.; Retana-Márquez, S.; Betancourt, M.; Casillas, F. Effects of Porcine Immature Oocyte Vitrification on Actin Microfilament Distribution and Chromatin Integrity During Early Embryo Development in vitro. Front. Cell Dev. Biol. 2021, 9, 636765. [Google Scholar] [CrossRef]
- Somfai, T.; Nguyen, H.T.; Nguyen, M.T.; Dang-Nguyen, T.Q.; Kaneko, H.; Noguchi, J.; Kikuchi, K. Vitrification of porcine cumulus-oocyte complexes at the germinal vesicle stage does not trigger apoptosis in oocytes and early embryos, but activates anti-apoptotic Bcl-XL gene expression beyond the 4-cell stage. J. Reprod. Dev. 2020, 66, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Jia, B.; Guo, J.; Shao, Q.; Hong, Q.; Wei, H.; Quan, G.; Wu, G. Transcriptome Analysis of mRNAs and Long Non-Coding RNAs During Subsequent Embryo Development of Porcine Cloned Zygotes After Vitrification. Front. Genet. 2021, 12, 753327. [Google Scholar] [CrossRef]
- Cai, M.D.; Xu, Z.Q.; Liu, Y.H.; Liu, J.Q.; Zhao, S.Y.; Wang, X.J.; Li, Y.H.; Yu, X.L.; Li, X.X. LncRNA-mediated effects of vitrification temperatures and cryoprotectant concentrations on bovine oocyte development following vitrification at the GV stage. Theriogenology 2022, 186, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Iyyappan, R.; Aleshkina, D.; Zhu, L.; Jiang, Z.; Kinterova, V.; Susor, A. Oocyte specific lncRNA variant Rose influences oocyte and embryo development. Noncoding RNA Res. 2021, 6, 107–113. [Google Scholar] [CrossRef]
- Pang, K.C.; Frith, M.C.; Mattick, J.S. Rapid evolution of noncoding RNAs: Lack of conservation does not mean lack of function. Trends Genet. 2006, 22, 1–5. [Google Scholar] [CrossRef]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef]
- Ndandala, C.B.; Guo, Y.; Ju, Z.; Fachri, M.; Mwemi, H.M.; Chen, H. Integrated lncRNA and mRNA Transcriptome Analyses of IGF1 and IGF2 Stimulated Ovaries Reveal Genes and Pathways Potentially Associated with Ovarian Development and Oocyte Maturation in Golden Pompano (Trachinotus ovatus). Animals 2025, 15, 1134. [Google Scholar] [CrossRef]
- Wang, J.J.; Niu, M.H.; Zhang, T.; Shen, W.; Cao, H.G. Genome-Wide Network of lncRNA-mRNA During Ovine Oocyte Development from Germinal Vesicle to Metaphase II in vitro. Front. Physiol. 2020, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pan, Y.; Wang, M.; Wang, J.; Wang, Y.; Han, X.; Wang, J.; Zhang, T.; Zhao, T.; He, H.; et al. Integrated analysis of the expression profiles of the lncRNA-miRNA-mRNA ceRNA network in granulosa and cumulus cells from yak ovaries. BMC Genom. 2022, 23, 633. [Google Scholar] [CrossRef]
- Dholpuria, S.; Kumar, S.; Kumar, M.; Sarwalia, P.; Kumar, R.; Datta, T.K. A novel lincRNA identified in buffalo oocytes with protein binding characteristics could hold the key for oocyte competence. Mol. Biol. Rep. 2021, 48, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cai, M.; Du, K.; Bai, X.; Tang, L.; Jia, X.; Chen, S.; Wang, J.; Lai, S. Dynamics of Known Long Non-Coding RNAs during the Maternal-to-Zygotic Transition in Rabbit. Animals 2021, 11, 3592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, Y.; Huang, R.; Zhai, Y.; Wu, D.; An, X.; Zhang, S.; Shi, L.; Li, Q.; Kong, X.; et al. LncRNA affects epigenetic reprogramming of porcine embryo development by regulating global epigenetic modification and the downstream gene SIN3A. Front. Physiol. 2022, 13, 971965. [Google Scholar] [CrossRef]
- Wei, R.; Yue, Y.; Wu, Y.; Zhang, C.; Jin, J.X.; Liu, Z.; Wang, J. A long noncoding RNA with enhancer-like function in pig zygotic genome activation. J. Mol. Cell Biol. 2025, 17, mjae061. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, L.; Yang, H.; Zhao, J.; Ma, H.; Chen, Y.; Zhang, J.; Luo, Y.; Zhang, Y.; Liu, J. A long non-coding RNA essential for early embryonic development improves somatic cell nuclear transfer somatic cell nuclear transfer efficiency in goats. Reproduction 2023, 166, 285–297. [Google Scholar] [CrossRef]
- Cao, S.; Han, J.; Wu, J.; Li, Q.; Liu, S.; Zhang, W.; Pei, Y.; Ruan, X.; Liu, Z.; Wang, X.; et al. Specific gene-regulation networks during the pre-implantation development of the pig embryo as revealed by deep sequencing. BMC Genom. 2014, 15, 4. [Google Scholar] [CrossRef]
- Lee, M.T.; Bonneau, A.R.; Giraldez, A.J. Zygotic genome activation during the maternal-to-zygotic transition. Annu. Rev. Cell Dev. Biol. 2014, 30, 581–613. [Google Scholar] [CrossRef]
- Watson, A.J.; Barcroft, L.C. Regulation of blastocyst formation. Front. Biosci. 2001, 6, 708–730. [Google Scholar] [CrossRef]
- Pennarossa, G.; Gandolfi, F.; Brevini, T.A.L. Biomechanical Signaling in Oocytes and Parthenogenetic Cells. Front. Cell Dev. Biol. 2021, 9, 646945. [Google Scholar] [CrossRef]
- Funahashi, H.; Cantley, T.C.; Day, B.N. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol. Reprod. 1997, 57, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.Y.; Xiang, D.C.; Quan, G.B.; Zhang, B.; Shao, Q.Y.; Hong, Q.H.; Wu, G.Q. Transcriptome analysis of porcine immature oocytes and surrounding cumulus cells after vitrification and in vitro maturation. Theriogenology 2019, 134, 90–97. [Google Scholar] [CrossRef]
- Wu, G.Q.; Quan, G.B.; Shao, Q.Y.; Lv, C.R.; Jiang, Y.T.; Zhao, Z.Y.; Hong, Q.H. Cryotop vitrification of porcine parthenogenetic embryos at the early developmental stages. Theriogenology 2016, 85, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Xiang, D.; Yang, H.; Liang, J.; Lv, C.; Yang, Q.; Huang, X.; Quan, G.; Wu, G. Transcriptome analysis of porcine embryos derived from oocytes vitrified at the germinal vesicle stage. Theriogenology 2024, 218, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Liu, L.; Yang, Y.; Wang, Y.; Xu, B. Glycine and Melatonin Improve Preimplantation Development of Porcine Oocytes Vitrified at the Germinal Vesicle Stage. Front. Cell Dev. Biol. 2022, 10, 856486. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Somfai, T.; Hirao, Y.; Dang-Nguyen, T.Q.; Linh, N.V.; Nguyen, B.X.; Nguyen, N.T.; Nguyen, H.T.; Nguyen, V.H.; Kaneko, H.; et al. Dibutyryl-cAMP and roscovitine differently affect premature meiotic resumption and embryo development of vitrified immature porcine oocytes. Anim. Sci. J. 2022, 93, e13795. [Google Scholar] [CrossRef] [PubMed]
- Crichton, J.H.; Playfoot, C.J.; Adams, I.R. The role of chromatin modifications in progression through mouse meiotic prophase. J. Genet. Genom. 2014, 41, 97–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guttman, M.; Garber, M.; Levin, J.Z.; Donaghey, J.; Robinson, J.; Adiconis, X.; Fan, L.; Koziol, M.J.; Gnirke, A.; Nusbaum, C.; et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 2010, 28, 503–510. [Google Scholar] [CrossRef]
- Pauli, A.; Valen, E.; Lin, M.F.; Garber, M.; Vastenhouw, N.L.; Levin, J.Z.; Fan, L.; Sandelin, A.; Rinn, J.L.; Regev, A.; et al. Systematic identification of long noncoding RNAs expressed during zebrafish embryogenesis. Genome Res. 2012, 22, 577–591. [Google Scholar] [CrossRef]
- Anchamparuthy, V.M.; Pearson, R.E.; Gwazdauskas, F.C. Expression pattern of apoptotic genes in vitrified-thawed bovine oocytes. Reprod. Domest. Anim. 2010, 45, e83–e90. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Ding, J.; Wu, J.; Smith, G.D. Regulation of spindle and chromatin dynamics during early and late stages of oocyte maturation by aurora kinases. Mol. Humam Reprod. 2008, 14, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.O.; Lee, H.J.; Selesniemi, K.; Styer, A.K.; Rueda, B.R. The impact of vitrification on murine germinal vesicle oocyte In vitro maturation and aurora kinase A protein expression. J. Assist. Reprod. Genet. 2014, 31, 1695–1702. [Google Scholar] [CrossRef][Green Version]
- Jiao, Y.; Zhu, S.; Li, J.; Jam Zaheer, A.; Li, M.; Huang, B. PS48 promotes in vitro maturation and developmental competence of porcine oocytes through activating PI3K/Akt signalling pathway. Reprod. Domest. Anim. 2020, 55, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Kalous, J.; Aleshkina, D.; Anger, M. A Role of PI3K/Akt Signaling in Oocyte Maturation and Early Embryo Development. Cells 2023, 12, 1830. [Google Scholar] [CrossRef]
- Guo, S.; Quan, S.; Zou, S. Roles of the Notch Signaling Pathway in Ovarian Functioning. Reprod. Sci. 2021, 28, 2770–2778. [Google Scholar] [CrossRef]
- Zhong, D.; Pan, Y.; Wang, M.; Zhang, H.; Xu, R.; Zuo, Q.; Li, T.; Yu, S.; Cui, Y. Activation of YAP1 and TAZ enhances the in vitro maturation of yak oocytes and influences subsequent embryonic development. Theriogenology 2025, 246, 117548. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, S.; Yang, Y.; Guo, T.; Ke, H.; Mi, X.; Qin, Y.; Chen, Z.J.; Zhao, S. TP63 gain-of-function mutations cause premature ovarian insufficiency by inducing oocyte apoptosis. J. Clin. Invest. 2023, 133, e162315. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, S.; Wan, Y.; Song, W.; Jin, H.; Sun, Y. Single-cell RNA sequencing of human oocytes reveals a differential transcriptomic profile associated with agar-like zona pellucida. J. Ovarian Res. 2024, 17, 132. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Wang, Z.; Chang, H.; Xie, X.; Fu, L.; Zhang, Y.; Quan, F. Resveratrol improved the developmental potential of oocytes after vitrification by modifying the epigenetics. Mol. Reprod. Dev. 2019, 86, 862–870. [Google Scholar] [CrossRef]
- Movahed, E.; Soleimani, M.; Hosseini, S.; Akbari Sene, A.; Salehi, M. Aberrant expression of miR-29a/29b and methylation level of mouse embryos after in vitro fertilization and vitrification at two-cell stage. J. Cell. Physiol. 2019, 234, 18942–18950. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Huang, J.; Liu, W.; Han, W.; Huang, G. Long-Term Storage Does Not Affect the Expression Profiles of mRNA and Long Non-Coding RNA in Vitrified-Warmed Human Embryos. Front. Genet. 2021, 12, 751467. [Google Scholar] [CrossRef]
- Lin, T.; Lee, J.E.; Kang, J.W.; Shin, H.Y.; Lee, J.B.; Jin, D.I. Endoplasmic Reticulum (ER) Stress and Unfolded Protein Response (UPR) in Mammalian Oocyte Maturation and Preimplantation Embryo Development. Int. J. Mol. Sci. 2019, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Barrera, N.; Dos Santos Neto, P.C.; Cuadro, F.; Bosolasco, D.; Mulet, A.P.; Crispo, M.; Menchaca, A. Impact of delipidated estrous sheep serum supplementation on in vitro maturation, cryotolerance and endoplasmic reticulum stress gene expression of sheep oocytes. PLoS ONE 2018, 13, e0198742. [Google Scholar] [CrossRef]
- Khatun, H.; Ihara, Y.; Takakura, K.; Egashira, J.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.I. Role of endoplasmic reticulum stress on developmental competency and cryo-tolerance in bovine embryos. Theriogenology 2020, 142, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Crasta, D.N.; Nair, R.; Kumari, S.; Dutta, R.; Adiga, S.K.; Zhao, Y.; Kannan, N.; Kalthur, G. Haploid Parthenogenetic Embryos Exhibit Unique Stress Response to pH, Osmotic and Oxidative Stress. Reprod. Sci. 2023, 30, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Mutalik, S.; Dasappa, J.P.; Kalthur, G.; Adiga, S.K. Haploid parthenotes express differential response to in vitro exposure of ammonia compared to normally fertilized embryos. Biochem. Biophys. Res. Commun. 2017, 486, 88. [Google Scholar] [CrossRef]
- Minet, E.; Michel, G.; Remacle, J.; Michiels, C. Role of HIF-1 as a transcription factor involved in embryonic development, cancer progression and apoptosis (review). Int. J. Mol. Med. 2000, 5, 253–259. [Google Scholar] [CrossRef]
- Olexiková, L.; Dujíčková, L.; Makarevich, A.V.; Bezdíček, J.; Sekaninová, J.; Nesvadbová, A.; Chrenek, P. Glutathione during Post-Thaw Recovery Culture Can Mitigate Deleterious Impact of Vitrification on Bovine Oocytes. Antioxidants 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.; Dresner, E.; Mandel, S.; Gozes, I. Silencing of the ADNP-family member, ADNP2, results in changes in cellular viability under oxidative stress. J. Neurochem. 2008, 105, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Y.; Deng, G.; Zhu, W.Z.; Sun, M.; Jiang, L.Y.; Li, W.H.; Liu, Y.B.; Guo, L.; Song, B.L.; Zhao, X. Defects in CYB5A and CYB5B impact sterol-C4 oxidation in cholesterol biosynthesis and demonstrate regulatory roles of dimethyl sterols. Cell Rep. 2024, 43, 114912. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.R.; Xie, B.T.; Zhang, H.; Li, J.Y.; Huang, T.Q.; Wei, R.Y.; Liu, Z.H. RE1-silencing Transcription Factor (REST) Is Required for Nuclear Reprogramming by Inhibiting Transforming Growth Factor β Signaling Pathway. J. Biol. Chem. 2016, 291, 27334–27342. [Google Scholar] [CrossRef]
- Voellenkle, C.; Fuschi, P.; Mutoli, M.; Carrara, M.; Righini, P.; Nano, G.; Gaetano, C.; Martelli, F. CircANKRD12 Is Induced in Endothelial Cell Response to Oxidative Stress. Cells 2022, 11, 3546. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, D.-C.; He, Z.; Pu, S.-Q.; Mu, D.-M.; Fu, J.; Chen, W.-J.; Jiang, J.-Y.; Li, X.-M.; Jia, B.-Y.; Wu, G.-Q. Long Non-Coding RNA Analysis of Vitrified Porcine Immature Oocytes During Maturation and Early Parthenogenetic Embryo Development. Cells 2025, 14, 1808. https://doi.org/10.3390/cells14221808
Xiang D-C, He Z, Pu S-Q, Mu D-M, Fu J, Chen W-J, Jiang J-Y, Li X-M, Jia B-Y, Wu G-Q. Long Non-Coding RNA Analysis of Vitrified Porcine Immature Oocytes During Maturation and Early Parthenogenetic Embryo Development. Cells. 2025; 14(22):1808. https://doi.org/10.3390/cells14221808
Chicago/Turabian StyleXiang, De-Cai, Zhen He, Shi-Qi Pu, De-Meng Mu, Jing Fu, Wen-Juan Chen, Jun-Yu Jiang, Xue-Mei Li, Bao-Yu Jia, and Guo-Quan Wu. 2025. "Long Non-Coding RNA Analysis of Vitrified Porcine Immature Oocytes During Maturation and Early Parthenogenetic Embryo Development" Cells 14, no. 22: 1808. https://doi.org/10.3390/cells14221808
APA StyleXiang, D.-C., He, Z., Pu, S.-Q., Mu, D.-M., Fu, J., Chen, W.-J., Jiang, J.-Y., Li, X.-M., Jia, B.-Y., & Wu, G.-Q. (2025). Long Non-Coding RNA Analysis of Vitrified Porcine Immature Oocytes During Maturation and Early Parthenogenetic Embryo Development. Cells, 14(22), 1808. https://doi.org/10.3390/cells14221808






