Single-Cell Cloning and Transcriptomic Analysis Support a Myogenic Origin of Bovine Intramuscular Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Collection of Tissue Samples
2.3. Isolation of Stromal Vascular Fraction
2.4. Isolation of Satellite Cells
2.5. Single-Cell Cloning
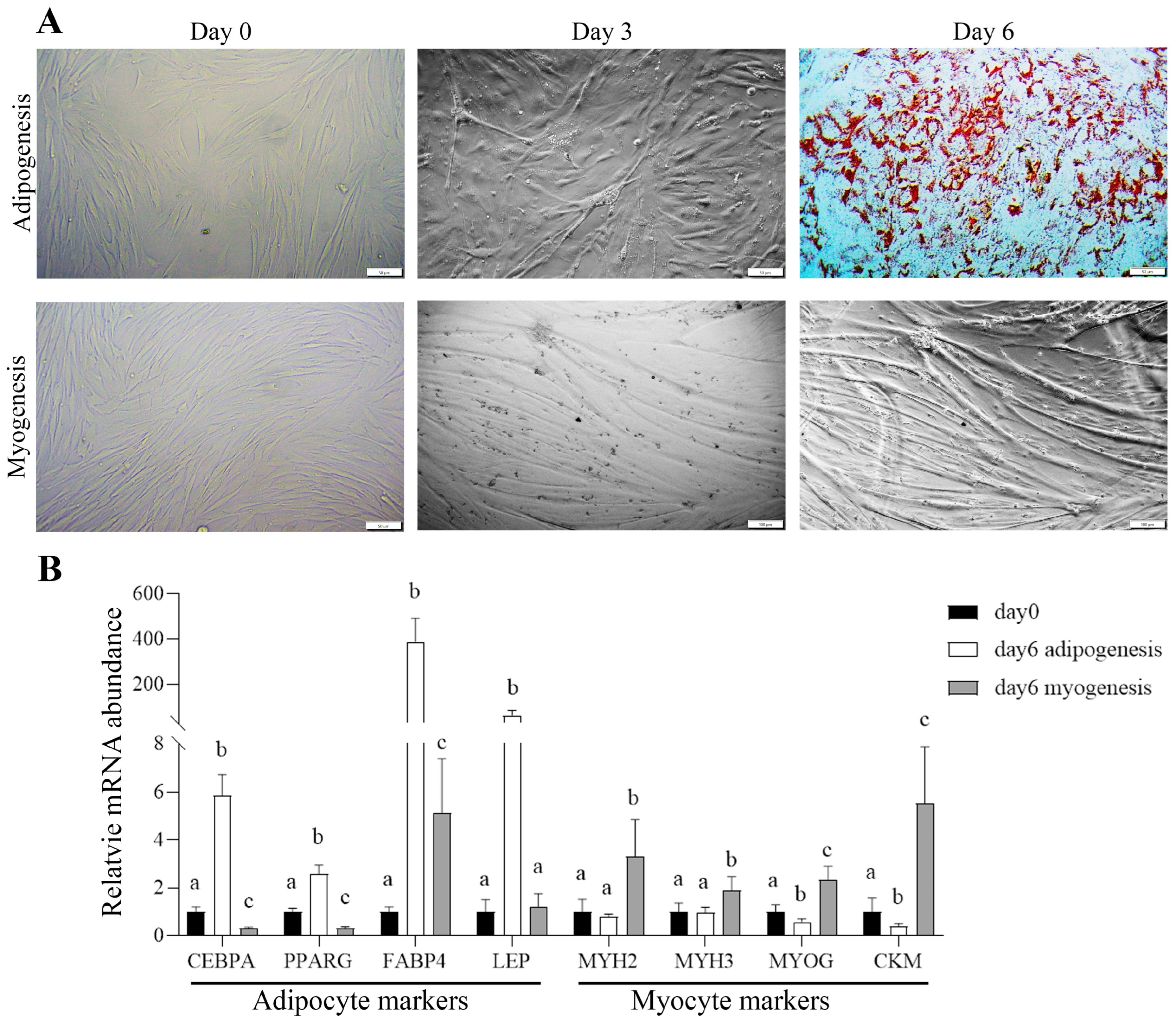
2.6. Adipogenic Differentiation Assay
2.7. Myogenic Differentiation Assay
2.8. Oil Red O Staining
2.9. RNA Sequencing and Bioinformatic Analyses
2.10. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.11. Statistical Analysis
3. Results
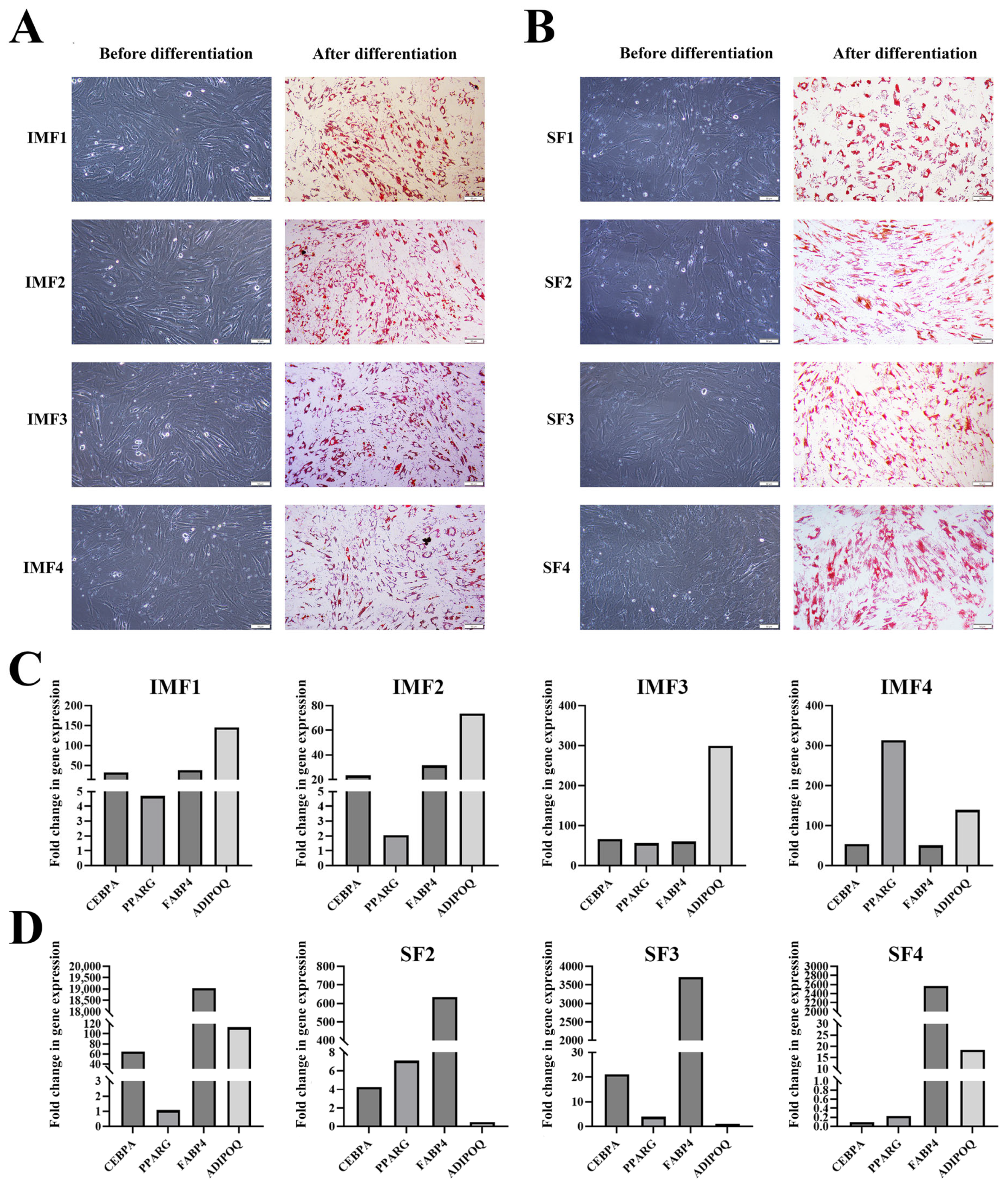
3.1. Cloning of Single Intramuscular and Subcutaneous Preadipocytes
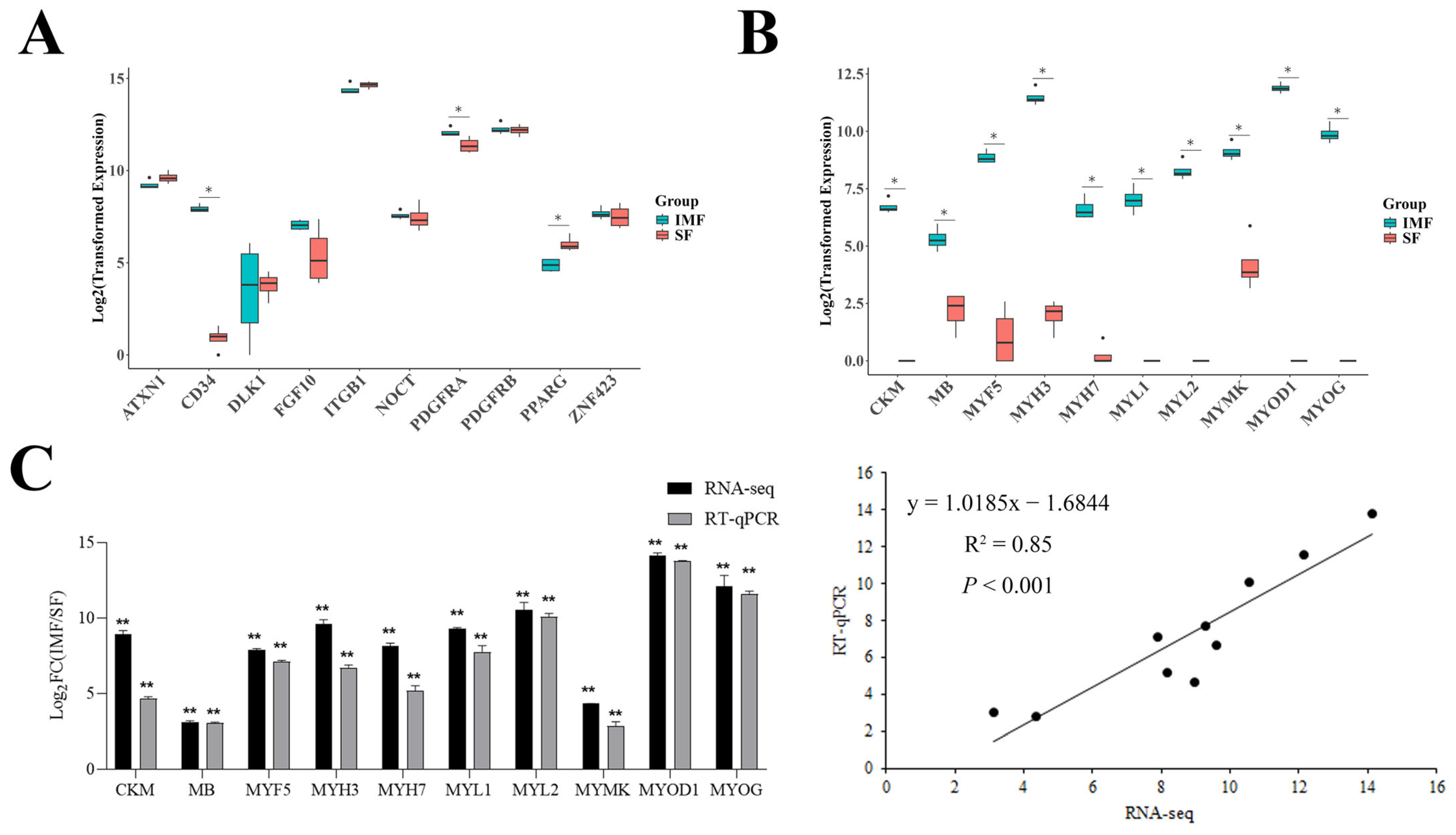
3.2. Different Gene Expression Profiles Between Intramuscular and Subcutaneous Preadipocyte Clones
3.3. Expression of Preadipocyte Marker Genes in Both Intramuscular and Subcutaneous Preadipocyte Clones
3.4. Expression of Many Skeletal Muscle-Specific Genes in Intramuscular but Not in Subcutaneous Preadipocyte Clones
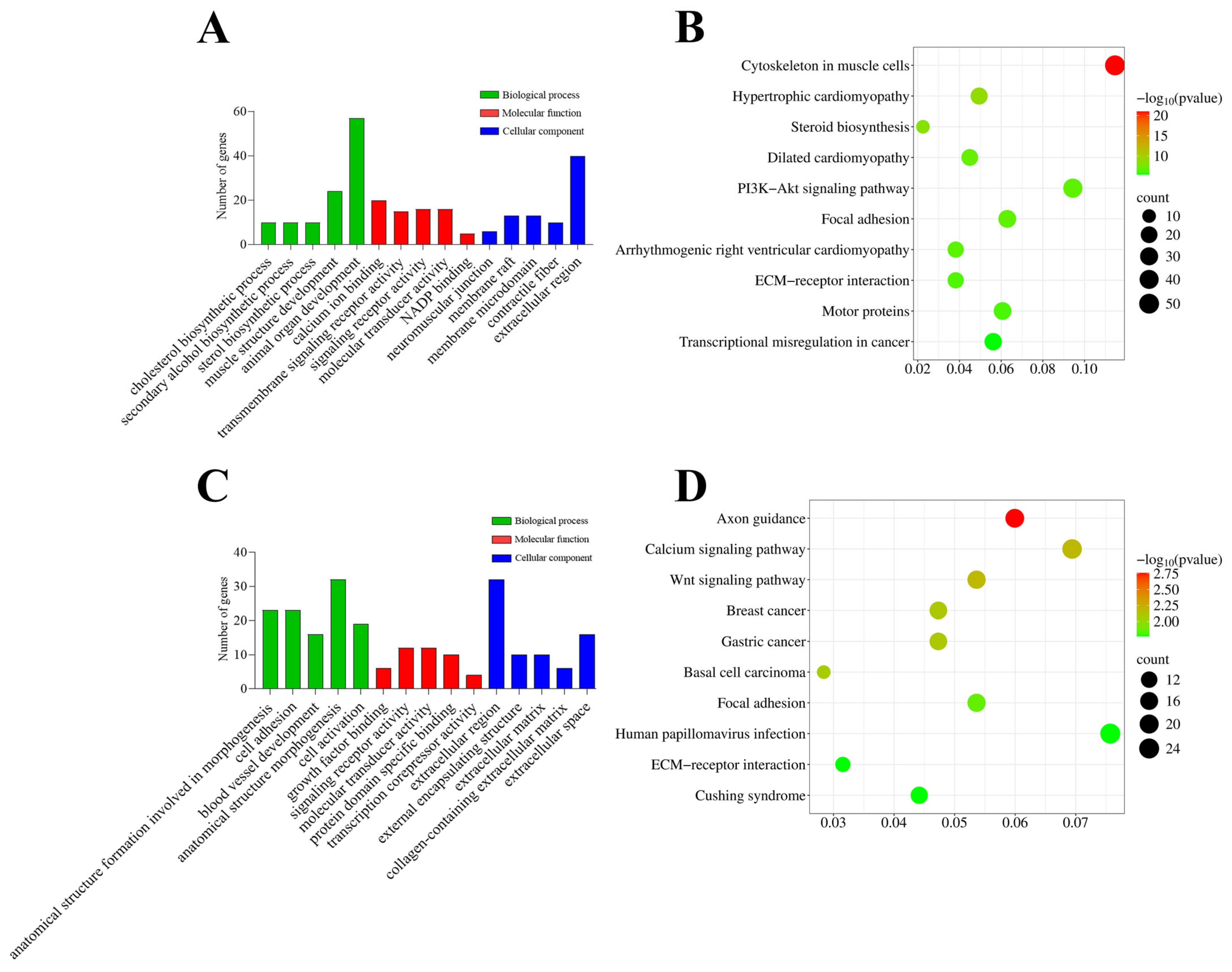
3.5. GO and KEGG Analyses Reveal Distinct Functional Signatures of Intramuscular and Subcutaneous Preadipocyte Clones
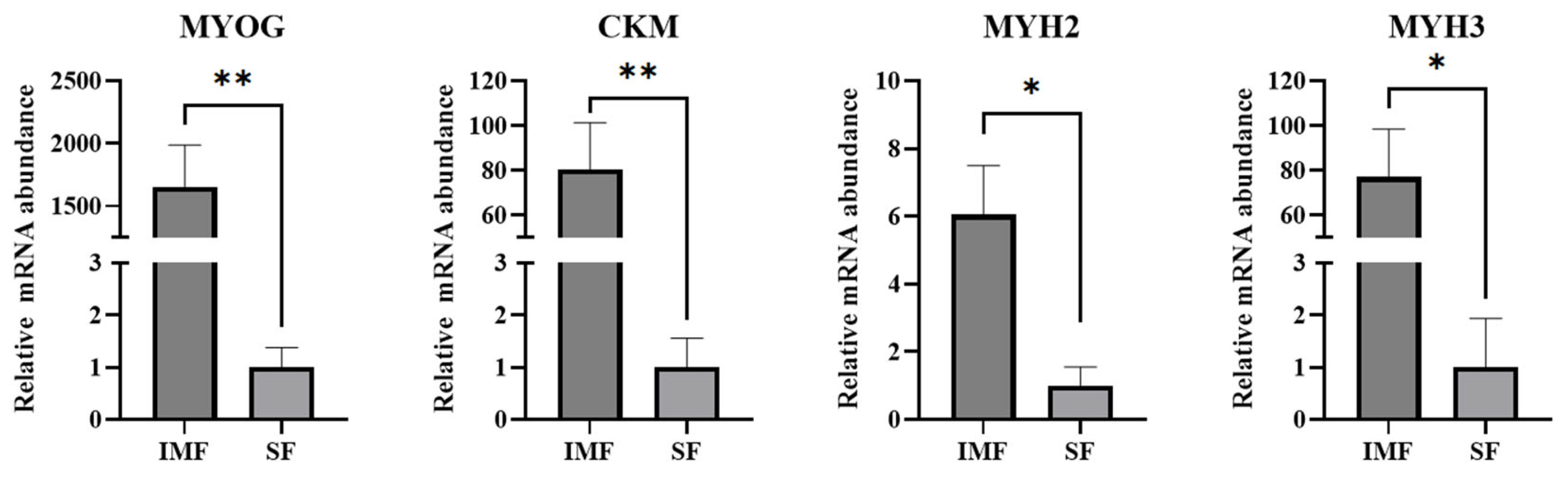
3.6. Persistent Expression of Muscle-Specific Genes in Adipocytes Differentiated from Intramuscular Preadipocytes
3.7. Bovine Satellite Cells Had Both Myogenic and Adipogenic Potentials
4. Discussion
5. Conclusions
6. Limitations of This Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMF | Intramuscular fat |
| SF | Subcutaneous fat |
| SVF | Stromal vascular fraction |
| bSCs | Bovine satellite cells |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| CEBPA | CCAAT/enhancer binding protein alpha |
| FABP4 | Fatty acid binding protein 4 |
| ADIPOQ | Adiponectin |
| MYOG | Myogenin |
| MYOD1 | Myogenic differentiation 1 |
| MYL2 | Myosin light chain 2 |
| DLK1 | Delta-like non-canonical Notch ligand 1 |
| PDGFRA | Platelet-derived growth factor receptor alpha |
| ZNF423 | Zinc finger protein 423 |
| FAP | Fibro/adipogenic progenitors |
| APC | Adipose progenitor cells |
References
- Thompson, J.M. The effects of marbling on flavour and juiciness scores of cooked beef, after adjusting to a constant tenderness. Aust. J. Exp. Agric. 2004, 44, 645–652. [Google Scholar] [CrossRef]
- Drouillard, J.S. Current situation and future trends for beef production in the United States of America—A review. Asian Australas. J. Anim. 2018, 31, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Beak, S.H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.V.; Heo, G.N.; Park, M.R.; Nam, J.S.; Kim, N.K.; Yoon, D.; Kim, T.H.; Lee, H.J. Proteomic analysis of bovine omental, subcutaneous and intramuscular preadipocytes during adipogenic differentiation. Comp. Biochem. Physiol. Part D 2010, 5, 234–244. [Google Scholar] [CrossRef]
- Wan, R.; Du, J.; Ren, L.; Meng, Q. Selective adipogenic effects of propionate on bovine intramuscular and subcutaneous preadipocytes. Meat Sci. 2009, 82, 372–378. [Google Scholar] [CrossRef]
- Aldai, N.; Nájera, A.I.; Dugan, M.E.R.; Celaya, R.; Osoro, K. Characterisation of intramuscular, intermuscular and subcutaneous adipose tissues in yearling bulls of different genetic groups. Meat Sci. 2007, 76, 682–691. [Google Scholar] [CrossRef]
- Gardan, D.; Gondret, F.; Louveau, I. Lipid metabolism and secretory function of porcine intramuscular adipocytes compared with subcutaneous and perirenal adipocytes. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E372–E380. [Google Scholar] [CrossRef]
- Zhang, G.H.; Lu, J.X.; Chen, Y.; Zhao, Y.Q.; Guo, P.H.; Yang, J.T.; Zang, R.X. Comparison of the adipogenesis in intramuscular and subcutaneous adipocytes from Bamei and Landrace pigs. Biochem. Cell Biol. 2014, 92, 259–267. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef]
- Han, H.Y.; Wei, W.; Chu, W.W.; Liu, K.Q.; Tian, Y.; Jiang, Z.H.; Chen, J. Muscle Conditional Medium Reduces Intramuscular Adipocyte Differentiation and Lipid Accumulation through Regulating Insulin Signaling. Int. J. Mol. Sci. 2017, 18, 1799. [Google Scholar] [CrossRef]
- Singh, N.K.; Chae, H.S.; Hwang, I.H.; Yoo, Y.M.; Ahn, C.N.; Lee, S.H.; Lee, H.J.; Park, H.J.; Chung, H.Y. Transdifferentiation of porcine satellite cells to adipoblasts with ciglitizone. J. Anim. Sci. 2007, 85, 1126–1135. [Google Scholar] [CrossRef]
- Miao, Z.G.; Zhang, L.P.; Fu, X.; Yang, Q.Y.; Zhu, M.J.; Dodson, M.V.; Du, M. Invited review: Mesenchymal progenitor cells in intramuscular connective tissue development. Anim. An. Int. J. Anim. Biosci. 2016, 10, 75–81. [Google Scholar] [CrossRef]
- Loomis, T.; Smith, L.R. Thrown for a loop: Fibro-adipogenic progenitors in skeletal muscle fibrosis. Am. J. Physiol. Physiol. 2023, 325, C895–C906. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Lee, D.Y.; Lee, S.Y.; Lee, J.H.Y.; Mariano, E.J.; Joo, S.T.; Choi, I.; Choi, J.S.; Kim, G.D.; Hur, S.J. Improved culture procedure for bovine muscle satellite cells for cultured meat. Food Res. Int. 2023, 174, 113660. [Google Scholar] [CrossRef] [PubMed]
- Demerdash, Z.; El Baz, H.; Ali, N.; Mahmoud, F.; Mohamed, S.; Khalifa, R.; Hassan, M.; Shawky, S. Cloning of human cord blood-mesenchymal stem cells for isolation of enriched cell population of higher proliferation and differentiation potential. Mol. Biol. Rep. 2020, 47, 3963–3972. [Google Scholar] [CrossRef]
- Pokhrel, B.; Tan, Z.; Jiang, H. Identification of transcriptional regulators and signaling pathways mediating postnatal rumen growth and functional maturation in cattle. J. Anim. Sci. 2025, 103, skae367. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Research 2020, 9, 304. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cattaneo, P.; Mukherjee, D.; Spinozzi, S.; Zhang, L.; Larcher, V.; Stallcup, W.B.; Kataoka, H.; Chen, J.; Dimmeler, S.; Evans, S.M.; et al. Parallel Lineage-Tracing Studies Establish Fibroblasts as the Prevailing In Vivo Adipocyte Progenitor. Cell Rep. 2020, 30, 571–582.e572. [Google Scholar] [CrossRef]
- Whytock, K.L.; Sun, Y.; Divoux, A.; Yu, G.; Smith, S.R.; Walsh, M.J.; Sparks, L.M. Single cell full-length transcriptome of human subcutaneous adipose tissue reveals unique and heterogeneous cell populations. iScience 2022, 25, 104772. [Google Scholar] [CrossRef]
- Andersen, D.C.; Jensen, L.; Schrøder, H.D.; Jensen, C.H. “The preadipocyte factor” DLK1 marks adult mouse adipose tissue residing vascular cells that lack in vitro adipogenic differentiation potential. FEBS Lett. 2009, 583, 2947–2953. [Google Scholar] [CrossRef]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White fat progenitor cells reside in the adipose vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Mepani, R.J.; Kleiner, S.; Lo, J.C.; Khandekar, M.J.; Cohen, P.; Frontini, A.; Bhowmick, D.C.; Ye, L.; Cinti, S.; et al. Zfp423 Expression Identifies Committed Preadipocytes and Localizes to Adipose Endothelial and Perivascular Cells. Cell Metab. 2012, 15, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; MacDougald, O.A. Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. J. Lipid Res. 2012, 53, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef]
- Sengenès, C.; Lolmède, K.; Zakaroff-Girard, A.; Busse, R.; Bouloumié, A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J. Cell. Physiol. 2005, 205, 114–122. [Google Scholar] [CrossRef]
- Rodeheffer, M.S.; Birsoy, K.; Friedman, J.M. Identification of white adipocyte progenitor cells in vivo. Cell 2008, 135, 240–249. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2004, 14, 311–324. [Google Scholar] [CrossRef]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kim, S.; Li, L.; Chattopadhyay, S.; Rando, T.A.; Feldman, B.J. Identification of an adipose tissue-resident pro-preadipocyte population. Cell Rep. 2023, 42, 112440. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, H.; Hu, P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. 2015, 72, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef]
- Hudak, C.S.; Sul, H.S. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. 2013, 4, 79. [Google Scholar] [CrossRef]
- Ritter, A.; Friemel, A.; Roth, S.; Kreis, N.N.; Hoock, S.C.; Safdar, B.K.; Fischer, K.; Möllmann, C.; Solbach, C.; Louwen, F.; et al. Subcutaneous and Visceral Adipose-Derived Mesenchymal Stem Cells: Commonality and Diversity. Cells 2019, 8, 1288. [Google Scholar] [CrossRef]
- Zuk, P. Adipose-Derived Stem Cells in Tissue Regeneration: A Review. ISRN Stem Cells 2013, 2013, 1–35. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dessels, C.; Durandt, C.; Pepper, M.S. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 2016, 16, 725–734. [Google Scholar] [CrossRef]
- Wafer, R.; Tandon, P.; Minchin, J.E.N. The Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARG) in Adipogenesis: Applying Knowledge from the Fish Aquaculture Industry to Biomedical Research. Front. Endocrinol. 2017, 8, 102. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the Roles of PPARgamma in Adipocytes via Dynamic Change of Transcription Complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Dang, T.N.; Tiongco, R.P.; Brown, L.M.; Taylor, J.L.; Lyons, J.M.; Lau, F.H.; Floyd, Z.E. Expression of the preadipocyte marker ZFP423 is dysregulated between well-differentiated and dedifferentiated liposarcoma. BMC Cancer 2022, 22, 300. [Google Scholar] [CrossRef]
- Yamasaki, M.; Emoto, H.; Konishi, M.; Mikami, T.; Ohuchi, H.; Nakao, K.; Itoh, N. FGF-10 Is a Growth Factor for Preadipocytes in White Adipose Tissue. Biochem. Biophys. Res. Commun. 1999, 258, 109–112. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, S.; Wang, Y.; Zhu, J.; Lin, Y. Fibroblast growth factor 10 (FGF10) promotes the adipogenesis of intramuscular preadipocytes in goat. Mol. Biol. Rep. 2018, 45, 1881–1888. [Google Scholar] [CrossRef]
- Nielsen, R.; Pedersen, T.A.; Hagenbeek, D.; Moulos, P.; Siersbaek, R.; Megens, E.; Denissov, S.; Børgesen, M.; Francoijs, K.J.; Mandrup, S.; et al. Genome-wide profiling of PPARγ: RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008, 22, 2953–2967. [Google Scholar] [CrossRef]
- Luo, S.; Shi, Q.; Li, W.; Wu, W.; Zha, Z. ITGB1 promotes the chondrogenic differentiation of human adipose-derived mesenchymal stem cells by activating the ERK signaling. J. Mol. Histol. 2020, 51, 729–739. [Google Scholar] [CrossRef]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. Isolation of adipose and bone marrow mesenchymal stem cells using CD29 and CD90 modifies their capacity for osteogenic and adipogenic differentiation. J. Tissue Eng. 2015, 6, 2041731415592356. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ebihara, Y.; Xu, M.-J.; Ishii, T.; Sugiyama, D.; Yoshino, H.; Ueda, T.; Manabe, A.; Tanaka, R.; Ikeda, Y.; et al. CD34 expression on long-term repopulating hematopoietic stem cells changes during developmental stages. Blood 2001, 97, 419–425. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Li, Z.; Tian, Y.; Li, Z.; Lu, A.; Hsu, C.-Y.; Negri, S.; Tang, C.; Tower, R.J.; et al. PDGFRα reporter activity identifies periosteal progenitor cells critical for bone formation and fracture repair. Bone Res. 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr. Opin. Genet. Dev. 1995, 5, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2012, 13, 127–133. [Google Scholar] [CrossRef]
- Timmons, J.A.; Wennmalm, K.; Larsson, O.; Walden, T.B.; Lassmann, T.; Petrovic, N.; Hamilton, D.L.; Gimeno, R.E.; Wahlestedt, C.; Baar, K.; et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA 2007, 104, 4401–4406. [Google Scholar] [CrossRef]
- Wang, W.; Kissig, M.; Rajakumari, S.; Huang, L.; Lim, H.W.; Won, K.J.; Seale, P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl. Acad. Sci. USA 2014, 111, 14466–14471. [Google Scholar] [CrossRef]
- Salazar-Tortosa, D.F.; Enard, D.; Itan, Y.; Ruiz, J.R. Novel brown adipose tissue candidate genes predicted by the human gene connectome. Sci. Rep. 2022, 12, 7614. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.J.; Goldhamer, D.J. Skeletal muscle stem cells. Reprod. Biol. Endocrinol. 2003, 1, 101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-Derived Mesenchymal Stem Cells: Biology and Potential Applications. In Mesenchymal Stem Cells—Basics and Clinical Application I; Weyand, B., Dominici, M., Hass, R., Jacobs, R., Kasper, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 59–71. [Google Scholar]
- Lyu, P.; Qi, Y.; Tu, Z.J.; Jiang, H. Single-cell RNA Sequencing Reveals Heterogeneity of Cultured Bovine Satellite Cells. Front. Genet. 2021, 12, 742077. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Feng, M.; Zhu, J.; Yu, H.; He, Z.; Zhang, Z.; Zhao, T.; Zhang, Q.; Pang, W. Adipocyte Progenitor Pools Composition and Cellular Niches Affect Adipogenesis Divergence in Porcine Subcutaneous and Intramuscular Fat. J. Agric. Food Chem. 2024, 72, 14044–14056. [Google Scholar] [CrossRef]
- Uyen, N.T.; Cuong, D.V.; Thuy, P.D.; Son, L.H.; Ngan, N.T.; Quang, N.H.; Tuan, N.D.; Hwang, I.H. A Comparative Study on the Adipogenic and Myogenic Capacity of Muscle Satellite Cells, and Meat Quality Characteristics between Hanwoo and Vietnamese Yellow Steers. Food Sci. Anim. Resour. 2023, 43, 563–579. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.-I.; Yamamoto, N.; Takeda, S.I.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Lou, P.-H.; Gustavsson, N.; Wang, Y.; Radda, G.K.; Han, W. Increased Lipolysis and Energy Expenditure in a Mouse Model with Severely Impaired Glucagon Secretion. PLoS ONE 2011, 6, e26671. [Google Scholar] [CrossRef]
- Norris, A.M.; Palzkill, V.R.; Appu, A.B.; Fierman, K.E.; Noble, C.D.; Ryan, T.E.; Kopinke, D. Intramuscular adipose tissue restricts functional muscle recovery. Cell Rep. 2025, 44, 116021. [Google Scholar] [CrossRef]
- Ford, H.; Liu, Q.; Fu, X.; Strieder-Barboza, C. White Adipose Tissue Heterogeneity in the Single-Cell Era: From Mice and Humans to Cattle. Biology 2023, 12, 1289. [Google Scholar] [CrossRef]
- Wang, L.; Gao, P.; Li, C.; Liu, Q.; Yao, Z.; Li, Y.; Zhang, X.; Sun, J.; Simintiras, C.; Welborn, M.; et al. A single-cell atlas of bovine skeletal muscle reveals mechanisms regulating intramuscular adipogenesis and fibrogenesis. J. Cachexia Sarcopenia Muscle 2023, 14, 2152–2167. [Google Scholar] [CrossRef] [PubMed]
- Scarda, A.; Franzin, C.; Milan, G.; Sanna, M.; Dal Prà, C.; Pagano, C.; Boldrin, L.; Piccoli, M.; Trevellin, E.; Granzotto, M.; et al. Increased adipogenic conversion of muscle satellite cells in obese Zucker rats. Int. J. Obes. 2010, 34, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, Z.; Loughna, P.T. Adipogenic Differentiation of Muscle Derived Cells is Repressed by Inhibition of GSK-3 Activity. Front. Vet. Sci. 2018, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.G.; Coy, C.S. Research Note: Effect of selection for body weight on the adipogenic conversion of turkey myogenic satellite cells by Syndecan-4 and its covalently attached N-glycosylation chains. Poult. Sci. 2020, 99, 1209–1215. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Yan, Y.; Sun, B.; Wang, Y.; Tang, L.; Wang, E.; Yu, J.; Nogoy, K.M.C.; Li, X.; et al. Effect of ciglitazone on adipogenic transdifferentiation of bovine skeletal muscle satellite cells. J. Anim. Sci. Technol. 2021, 63, 934–953. [Google Scholar] [CrossRef]

| Gene ID | Primer Sequence * | GenBank Accession Number | Product Size |
|---|---|---|---|
| HMBS | F: CTTTGGAGAGGAATGAAGTGG R: AATGGTGAAGCCAGGAGGAA | NM_001046207.1 | 80 |
| PPARG | F: CAGTGTCTGCAAGGACCTCA R: ATAGTGCGGAGTGGAAATGC | NM_181024.2 | 176 |
| CEBPA | F: ATCGACATCAGCGCCTACAT R: CGGGTAGTCAAAGTCGTTGC | NM_176784.2 | 138 |
| FABP4 | F: AATTGGGCCAGGAATTTGAT R: TGGTGGTTGATTTTCCATCC | NM_174314.2 | 117 |
| ADIPOQ | F: AGGTTGGATGGCAGGCATT R: GGACCTTCGATCCCAGTGATT | XM_024995232.2 | 162 |
| LEP | F: GACATCTCACACACGCAGTC R: ATCGCCAATGTCTGGTCCAT | NM_173928.2 | 113 |
| MYH2 | F: CTGGCTGGAGAAGAACAAGG R: CACCGTCTGGAAAGAAGAGC | NM_001166227.1 | 172 |
| MYH3 | F: CTGGAGGAAATGAGGGATGA R: CACTCTTGAGAAGGGGCTTG | NM_001101835.2 | 211 |
| MYOG | F: TGGGCGTGTAAGGTGTGTAA R: TATGGGAGCTGCATTCACTG | NM_001111325.1 | 295 |
| CKM | F: TGGAGATGATCTGGACCCCA | NM_174773.4 | 166 |
| R: TTTCCCCTTGAACTCACCCG | |||
| MYOD1 | F: GACGGCTCTCTCTGCAACTT | NM_001040478.2 | 271 |
| R: TAGTCGTCTTGCGTTTGCAC | |||
| MYL1 | F: ACCCCAGCAATGAAGAGATGA | NM_001079578.1 | 128 |
| R: GAAGACACGCAGACCCTCAA | |||
| MYL2 | F:TCGGGGAGAAACTTAAAGGAGC | NM_001035025.2 | 192 |
| R: AGTTGCCAGTCACATCAGGG | |||
| MYH7 | F: CTTCAACCACCACATGTTCG | NM_174727.1 | 233 |
| R: GCTTCTGGAAGTTGCTGGAC | |||
| MYF5 | F: AGACGCCTGAAGAAGGTCAA | NM_174116.1 | 134 |
| R: AGCAGCTCCTGCAGACTCTC | |||
| MYMK | F: GCTCGGCCATCCTCATCATT | NM_001193046.1 | 158 |
| R: GTCCCAGTCCTCGAAGAAGAA | |||
| MB | F: AGTCACATGCCAACAAGCAC | NM_173881.2 | 108 |
| R: CATCAGCACCGAAGTCTGAA |
| Gene ID | Log2FC (IMF/SF) | Padj-Value | Gene Description |
|---|---|---|---|
| MYOD1 | 14.1 | 0.001 | Myogenic differentiation 1 |
| SELP | 13.3 | 0.001 | Selectin P |
| MYOG | 12.1 | 0.001 | Myogenin |
| CHRNG | 12.0 | 0.001 | Cholinergic receptor nicotinic gamma subunit |
| MYL11 | 11.9 | 0.001 | Myosin light chain 11 |
| PDGFB | 11.8 | 0.001 | Platelet-derived growth factor subunit B |
| SELE | 11.3 | 0.001 | Selectin E |
| CBLN4 | 11.2 | 0.001 | Cerebellin 4 |
| CD93 | 11.1 | 0.001 | CD93 molecule |
| MYO18B | 11.0 | 0.001 | Myosin XVIIIB |
| Gene ID | Log2FC (IMF/SF) | Padj-Value | Gene Description |
|---|---|---|---|
| KCNJ5 | −10.6 | 0.001 | Potassium inwardly rectifying channel subfamily J member 5 |
| SP9 | −9.3 | 0.001 | Sp9 transcription factor |
| TBX5 | −8.3 | 0.001 | T-box transcription factor 5 |
| ASIP | −8.0 | 0.001 | Agouti signaling protein |
| LOC105605854 | −7.4 | 0.001 | Uncharacterized gene |
| DCDC1 | −6.7 | 0.001 | Doublecortin domain containing 1 |
| LOC101103380 | −6.7 | 0.001 | Uncharacterized gene |
| APCDD1L | −6.5 | 0.001 | APC downregulated 1 like |
| LOC790312 | −6.4 | 0.001 | Uncharacterized gene |
| CDH20 | −6.2 | 0.001 | Cadherin 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Pokhrel, B.; Jiang, H. Single-Cell Cloning and Transcriptomic Analysis Support a Myogenic Origin of Bovine Intramuscular Adipocytes. Cells 2025, 14, 1807. https://doi.org/10.3390/cells14221807
Tan Z, Pokhrel B, Jiang H. Single-Cell Cloning and Transcriptomic Analysis Support a Myogenic Origin of Bovine Intramuscular Adipocytes. Cells. 2025; 14(22):1807. https://doi.org/10.3390/cells14221807
Chicago/Turabian StyleTan, Zhendong, Binod Pokhrel, and Honglin Jiang. 2025. "Single-Cell Cloning and Transcriptomic Analysis Support a Myogenic Origin of Bovine Intramuscular Adipocytes" Cells 14, no. 22: 1807. https://doi.org/10.3390/cells14221807
APA StyleTan, Z., Pokhrel, B., & Jiang, H. (2025). Single-Cell Cloning and Transcriptomic Analysis Support a Myogenic Origin of Bovine Intramuscular Adipocytes. Cells, 14(22), 1807. https://doi.org/10.3390/cells14221807







