Brucella Immune Escape: TLR Subversion, Antigen Presentation Destruction and T Cell Disorder
Abstract
1. Introduction
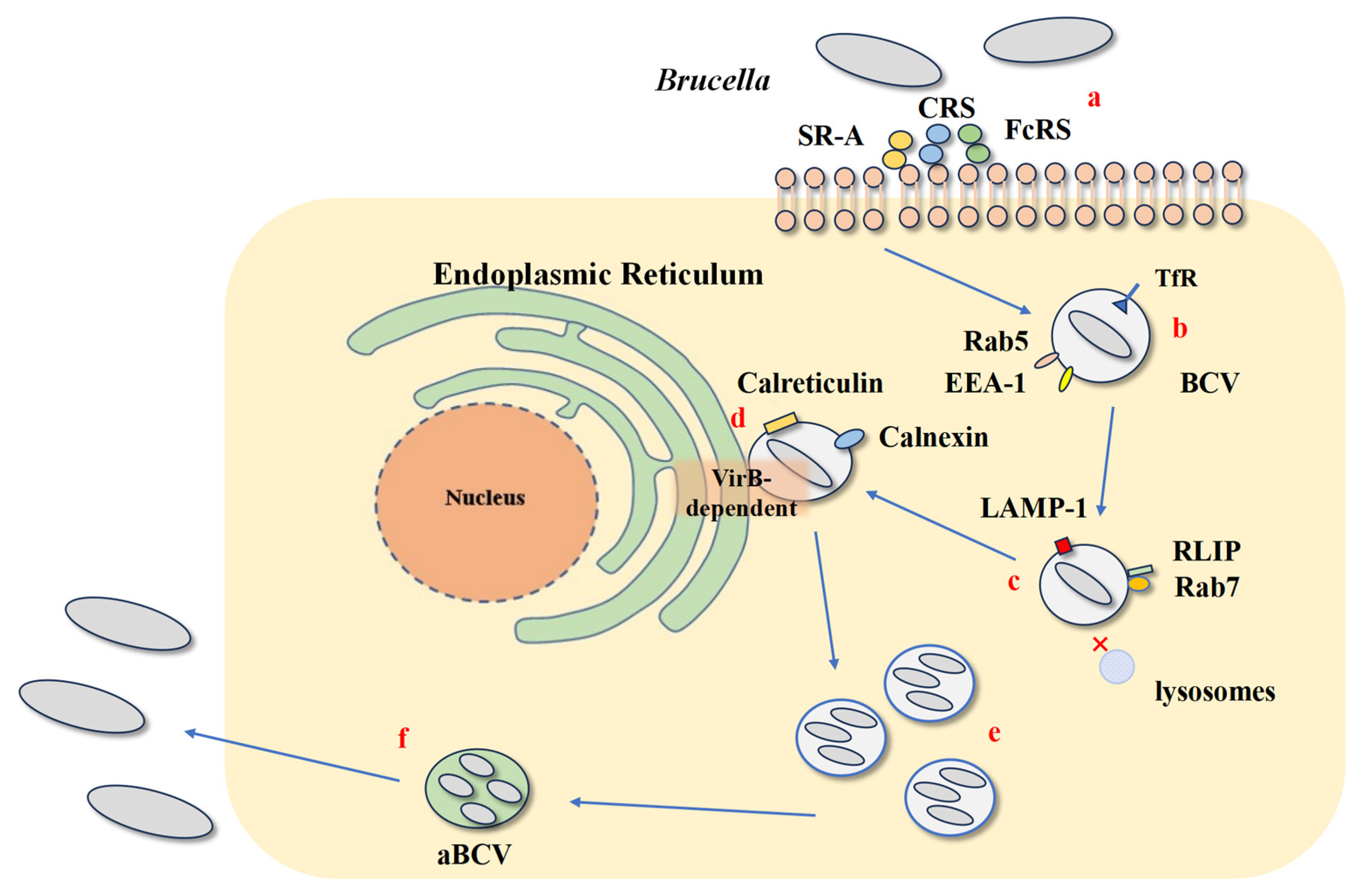
2. The Lifecycle of Brucella Within Host Cells
3. Brucella Interferes with the Recognition and Response of the Host’s Innate Immune System
3.1. Brucella LPS and Flagella Interfere with TLR Recognition
3.2. BtpA and BtpB of Brucella Interfere with TLR Pathways
3.3. Brucella Outer Membrane Proteins Regulate Immunity Through Various Pathways
4. Brucella Inhibits Host Antigen Presentation and Adaptive Immune Response
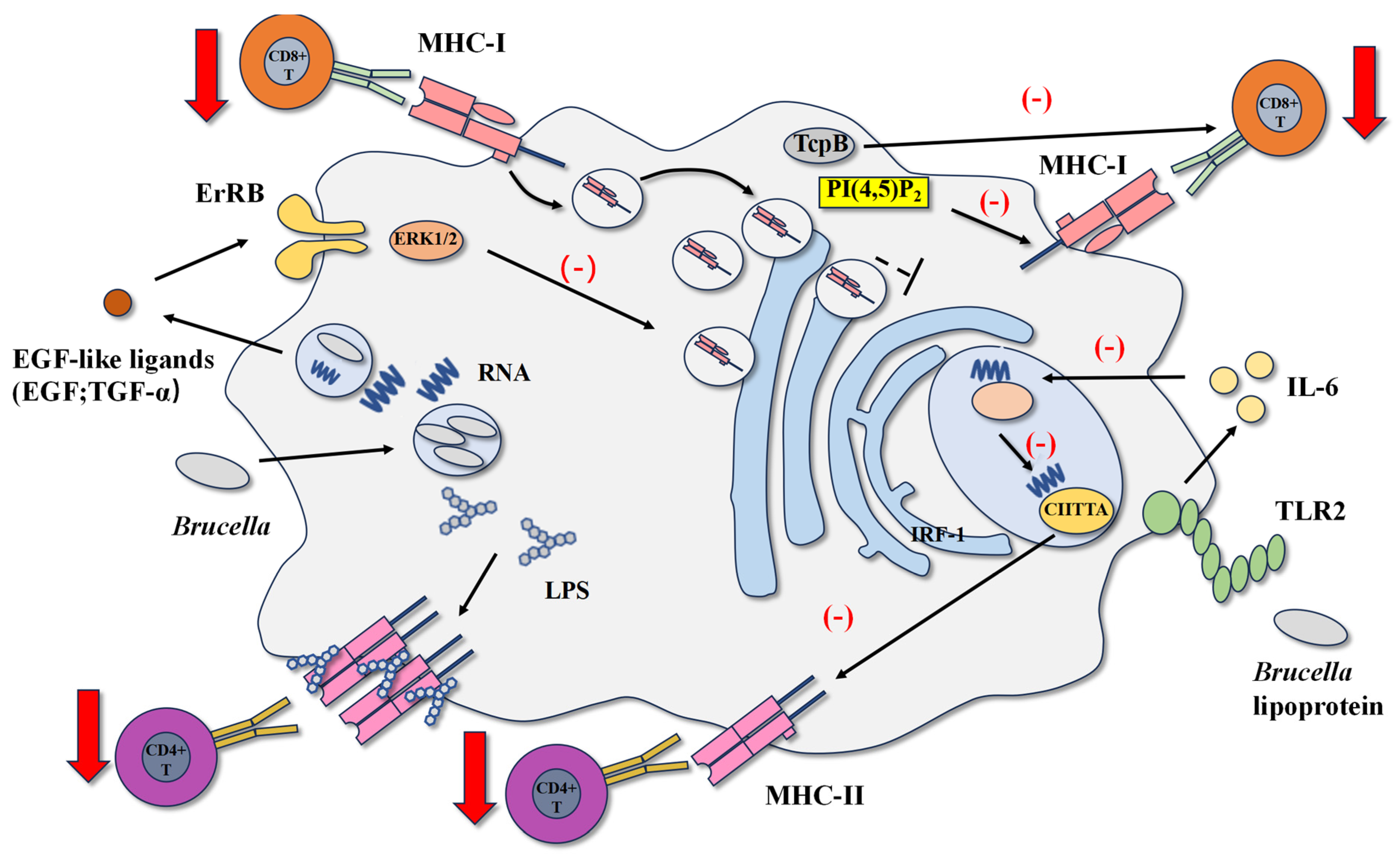
4.1. Brucella LPS and Lipoproteins Inhibit MHCII Antigen Presentation
4.2. Brucella RNA and Btp1/TcpB Inhibit CD8+ T Cell Response
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kurmanov, B.; Zincke, D.; Su, W.; Hadfield, T.L.; Aikimbayev, A.; Karibayev, T.; Berdikulov, M.; Orynbayev, M.; Nikolich, M.P.; Blackburn, J.K. Assays for Identification and Differentiation of Brucella Species: A Review. Microorganisms 2022, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; et al. Bovine brucellosis—A comprehensive review. Vet. Q. 2021, 41, 61–88. [Google Scholar] [CrossRef]
- González-Espinoza, G.; Arce-Gorvel, V.; Mémet, S.; Gorvel, J.-P. Brucella: Reservoirs and Niches in Animals and Humans. Pathogens 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Zhou, Z.; Li, B.; Xiao, Y.; Li, M.; Zeng, H.; Guo, X.; Gu, G. The Mechanism of Facultative Intracellular Parasitism of Brucella. Int. J. Mol. Sci. 2021, 22, 3673. [Google Scholar] [CrossRef]
- Martirosyan, A.; Moreno, E.; Gorvel, J. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 2011, 240, 211–234. [Google Scholar] [CrossRef]
- Godfroid, J.; Scholz, H.; Barbier, T.; Nicolas, C.; Wattiau, P.; Fretin, D.; Whatmore, A.; Cloeckaert, A.; Blasco, J.; Moriyon, I.; et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev. Vet. Med. 2011, 102, 118–131. [Google Scholar] [CrossRef]
- Durward, M.; Radhakrishnan, G.; Harms, J.; Bareiss, C.; Magnani, D.; Splitter, G.A. Active Evasion of CTL Mediated Killing and Low Quality Responding CD8+ T Cells Contribute to Persistence of Brucellosis. PLoS ONE 2012, 7, e34925. [Google Scholar] [CrossRef]
- Erkyihun, G.A.; Gari, F.R.; Kassa, G.M. Bovine Brucellosis and Its Public Health Significance in Ethiopia. Zoonoses 2022, 2, 985. [Google Scholar] [CrossRef]
- Roop, R.M.; Barton, I.S.; Hopersberger, D.; Martin, D.W. Uncovering the Hidden Credentials of Brucella Virulence. Microbiol. Mol. Biol. Rev. 2021, 85, e00021-19. [Google Scholar] [CrossRef] [PubMed]
- Roop, R.M.; Gaines, J.M.; Anderson, E.S.; Caswell, C.C.; Martin, D.W. Survival of the fittest: How Brucella strains adapt to their intracellular niche in the host. Med. Microbiol. Immunol. 2009, 198, 221–238. [Google Scholar] [CrossRef]
- Martirosyan, A.; Gorvel, J.-P. Brucella Evasion of Adaptive Immunity. Futur. Microbiol. 2013, 8, 147–154. [Google Scholar] [CrossRef]
- De Jong, M.F.; Rolán, H.G.; Tsolis, R.M. Microreview: Innate immune encounters of the (Type) 4th kind: Brucella. Cell. Microbiol. 2010, 12, 1195–1202. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Cassataro, J.; Delpino, M.V.; Zwerdling, A.; Pasquevich, K.A.; Samartino, C.G.; Wallach, J.C.; Fossati, C.A.; Giambartolomei, G.H. Brucella abortus Inhibits Major Histocompatibility Complex Class II Expression and Antigen Processing through Interleukin-6 Secretion via Toll-Like Receptor 2. Infect. Immun. 2008, 76, 250–262. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Delpino, M.V.; Pozner, R.G.; Velásquez, L.N.; Cassataro, J.; Giambartolomei, G.H. Brucella abortus induces intracellular retention of MHC-I molecules in human macrophages down-modulating cytotoxic CD8+T cell responses. Cell. Microbiol. 2013, 15, 487–502. [Google Scholar] [CrossRef]
- Copin, R.; Vitry, M.-A.; Mambres, D.H.; Machelart, A.; De Trez, C.; Vanderwinden, J.-M.; Magez, S.; Akira, S.; Ryffel, B.; Carlier, Y.; et al. In Situ Microscopy Analysis Reveals Local Innate Immune Response Developed around Brucella Infected Cells in Resistant and Susceptible Mice. PLoS Pathog. 2012, 8, e1002575. [Google Scholar] [CrossRef] [PubMed]
- Huy, T.X.N.; Nguyen, T.T.; Kim, H.; Reyes, A.W.B.; Kim, S. Brucella Phagocytosis Mediated by Pathogen-Host Interactions and Their Intracellular Survival. Microorganisms 2022, 10, 2003. [Google Scholar] [CrossRef] [PubMed]
- Bialer, M.G.; Sycz, G.; González, F.M.; Ferrero, M.C.; Baldi, P.C.; Zorreguieta, A. Adhesins of Brucella: Their Roles in the Interaction with the Host. Pathogens 2020, 9, 942. [Google Scholar] [CrossRef]
- Köhler, S.; Michaux-Charachon, S.; Porte, F.; Ramuz, M.; Liautard, J.-P. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 2003, 11, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, M.; Guo, X.; Zeng, H.; Shuai, X.; Guo, J.; Huang, Q.; Chu, Y.; Zhou, B.; Wen, J.; et al. Inflammatory Mechanism of Brucella Infection in Placental Trophoblast Cells. Int. J. Mol. Sci. 2022, 23, 13417. [Google Scholar] [CrossRef]
- Del Giudice, M.G.; Ugalde, J.E.; Czibener, C. A Lysozyme-Like Protein in Brucella abortus Is Involved in the Early Stages of Intracellular Replication. Infect. Immun. 2013, 81, 956–964. [Google Scholar] [CrossRef]
- de Bolle, X.; Letesson, J.-J.; Gorvel, J.-P. Small GTPases and Brucella entry into the endoplasmic reticulum. Biochem. Soc. Trans. 2012, 40, 1348–1352. [Google Scholar] [CrossRef]
- Pandey, A.; Lin, F.; Cabello, A.L.; da Costa, L.F.; Feng, X.; Feng, H.-Q.; Zhang, M.-Z.; Iwawaki, T.; Rice-Ficht, A.; Ficht, T.A.; et al. Activation of Host IRE1α-Dependent Signaling Axis Contributes the Intracellular Parasitism of Brucella melitensis. Front. Cell. Infect. Microbiol. 2018, 8, 103. [Google Scholar] [CrossRef]
- Starr, T.; Child, R.; Wehrly, T.D.; Hansen, B.; Hwang, S.; López-Otin, C.; Virgin, H.W.; Celli, J. Selective Subversion of Autophagy Complexes Facilitates Completion of the Brucella Intracellular Cycle. Cell Host Microbe 2012, 11, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Tsai, A.Y.; Walker, G.T.; Miller, C.N.; Young, B.M.; English, B.C.; Seyffert, N.; Kerrinnes, T.; de Jong, M.F.; Atluri, V.L.; et al. Brucella abortus Infection of Placental Trophoblasts Triggers Endoplasmic Reticulum Stress-Mediated Cell Death and Fetal Loss via Type IV Secretion System-Dependent Activation of CHOP. mBio 2019, 10, e01538-19. [Google Scholar] [CrossRef]
- Ahmed, W.; Zheng, K.; Liu, Z.-F. Establishment of Chronic Infection: Brucella’s Stealth Strategy. Front. Cell. Infect. Microbiol. 2016, 6, 30. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.G.; Macedo, G.C.; Azevedo, V.; Oliveira, S.C. Brucella spp. noncanonical LPS: Structure, biosynthesis, and interaction with host immune system. Microb. Cell Factories 2006, 5, 13. [Google Scholar] [CrossRef]
- Bryant, C.E.; Spring, D.R.; Gangloff, M.; Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010, 8, 8–14. [Google Scholar] [CrossRef]
- Lapaque, N.; Takeuchi, O.; Corrales, F.; Akira, S.; Moriyon, I.; Howard, J.C.; Gorvel, J.-P. Differential inductions of TNF-α and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell. Microbiol. 2006, 8, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.-S.; Lee, H.; Lee, J.-O. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Parent, M.A.; Goenka, R.; Murphy, E.; LeVier, K.; Carreiro, N.; Golding, B.; Ferguson, G.; Roop, R.M.; Walker, G.C.; Baldwin, C.L. Brucella abortus bacA mutant induces greater pro-inflammatory cytokines than the wild-type parent strain. Microbes Infect. 2007, 9, 55–62. [Google Scholar] [CrossRef]
- Fontana, C.; Conde-Álvarez, R.; Ståhle, J.; Holst, O.; Iriarte, M.; Zhao, Y.; Arce-Gorvel, V.; Hanniffy, S.; Gorvel, J.-P.; Moriyón, I.; et al. Structural Studies of Lipopolysaccharide-defective Mutants from Brucella melitensis Identify a Core Oligosaccharide Critical in Virulence. J. Biol. Chem. 2016, 291, 7727–7741. [Google Scholar] [CrossRef] [PubMed]
- Conde-Álvarez, R.; Arce-Gorvel, V.; Iriarte, M.; Manček-Keber, M.; Barquero-Calvo, E.; Palacios-Chaves, L.; Chacón-Díaz, C.; Chaves-Olarte, E.; Martirosyan, A.; von Bargen, K.; et al. The Lipopolysaccharide Core of Brucella abortus Acts as a Shield Against Innate Immunity Recognition. PLoS Pathog. 2012, 8, e1002675. [Google Scholar] [CrossRef]
- Gil-Ramírez, Y.; Conde-Álvarez, R.; Palacios-Chaves, L.; Zúñiga-Ripa, A.; Grilló, M.-J.; Arce-Gorvel, V.; Hanniffy, S.; Moriyón, I.; Iriarte, M. The identification of wadB, a new glycosyltransferase gene, confirms the branched structure and the role in virulence of the lipopolysaccharide core of Brucella abortus. Microb. Pathog. 2014, 73, 53–59. [Google Scholar] [CrossRef]
- Terwagne, M.; Ferooz, J.; Rolán, H.G.; Sun, Y.-H.; Atluri, V.; Xavier, M.N.; Franchi, L.; Núñez, G.; Legrand, T.; Flavell, R.A.; et al. Innate immune recognition of flagellin limits systemic persistence of Brucella. Cell. Microbiol. 2013, 15, 942–960. [Google Scholar] [CrossRef]
- Zhai, Y.; Fang, J.; Zheng, W.; Hao, M.; Chen, J.; Liu, X.; Zhang, M.; Qi, L.; Zhou, D.; Liu, W.; et al. A potential virulence factor: Brucella flagellin FliK does not affect the main biological properties but inhibits the inflammatory response in RAW264.7 cells. Int. Immunopharmacol. 2024, 133, 112119. [Google Scholar] [CrossRef]
- Xiong, X.; Li, B.; Zhou, Z.; Gu, G.; Li, M.; Liu, J.; Jiao, H. The VirB System Plays a Crucial Role in Brucella Intracellular Infection. Int. J. Mol. Sci. 2021, 22, 13637. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.A.; Deredge, D.; Waldhuber, A.; Fresquez, T.; Wilkins, D.Z.; Smith, P.T.; Durr, S.; Cirl, C.; Jiang, J.; Jennings, W.; et al. Crystal Structures of the Toll/Interleukin-1 Receptor (TIR) Domains from the Brucella Protein TcpB and Host Adaptor TIRAP Reveal Mechanisms of Molecular Mimicry. J. Biol. Chem. 2014, 289, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Cirl, C.; Wieser, A.; Yadav, M.; Duerr, S.; Schubert, S.; Fischer, H.; Stappert, D.; Wantia, N.; Rodriguez, N.; Wagner, H.; et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain–containing proteins. Nat. Med. 2008, 14, 399–406. [Google Scholar] [CrossRef]
- Avila-Calderón, E.D.; Flores-Romo, L.; Sharon, W.; Donis-Maturano, L.; Becerril-García, M.A.; Arreola, M.G.A.; Reynoso, B.A.; Güemes, F.S.; Contreras-Rodríguez, A. Dendritic cells and Brucella spp. interaction: The sentinel host and the stealthy pathogen. Folia Microbiol. 2020, 65, 1–16. [Google Scholar] [CrossRef]
- Salcedo, S.P.; Marchesini, M.I.; Lelouard, H.; Fugier, E.; Jolly, G.; Balor, S.; Muller, A.; Lapaque, N.; Demaria, O.; Alexopoulou, L.; et al. Brucella Control of Dendritic Cell Maturation Is Dependent on the TIR-Containing Protein Btp1. PLoS Pathog. 2008, 4, e21. [Google Scholar] [CrossRef]
- Billard, E.; Dornand, J.; Gross, A. Brucella suis Prevents Human Dendritic Cell Maturation and Antigen Presentation through Regulation of Tumor Necrosis Factor Alpha Secretion. Infect. Immun. 2007, 75, 4980–4989. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.M.; Salunkhe, P.; Godzik, A.; Reed, J.C. Identification and Characterization of a Novel Bacterial Virulence Factor That Shares Homology with Mammalian Toll/Interleukin-1 Receptor Family Proteins. Infect. Immun. 2006, 74, 594–601. [Google Scholar] [CrossRef]
- Chaudhary, A.; Ganguly, K.; Cabantous, S.; Waldo, G.S.; Micheva-Viteva, S.N.; Nag, K.; Hlavacek, W.S.; Tung, C.-S. The Brucella TIR-like protein TcpB interacts with the death domain of MyD88. Biochem. Biophys. Res. Commun. 2012, 417, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Zhi, F.; Zhai, Y.; Zhou, D.; Chen, H.; Lin, P.; Tang, K.; Liu, W.; Jin, Y.; et al. BtpB inhibits innate inflammatory responses in goat alveolar macrophages through the TLR/NF-κB pathway and NLRP3 inflammasome during Brucella infection. Microb. Pathog. 2022, 166, 105536. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, S.P.; Marchesini, M.I.; Degos, C.; Terwagne, M.; Von Bargen, K.; Lepidi, H.; Herrmann, C.K.; Santos Lacerda, T.L.; Imbert, P.R.C.; Pierre, P.; et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front. Cell. Infect. Microbiol. 2013, 3, 28. [Google Scholar] [CrossRef]
- Mol, J.P.S.; Costa, E.A.; Carvalho, A.F.; Sun, Y.-H.; Tsolis, R.M.; Paixão, T.A.; Santos, R.L. Early Transcriptional Responses of Bovine Chorioallantoic Membrane Explants to Wild Type, ΔvirB2 or ΔbtpB Brucella abortus Infection. PLoS ONE 2014, 9, e108606. [Google Scholar] [CrossRef]
- Felix, C.; Türköz, B.K.; Ranaldi, S.; Koelblen, T.; Terradot, L.; O’cAllaghan, D.; Vergunst, A.C. The Brucella TIR domain containing proteins BtpA and BtpB have a structural WxxxE motif important for protection against microtubule depolymerisation. Cell Commun. Signal. 2014, 12, 53. [Google Scholar] [CrossRef]
- Coronas-Serna, J.M.; Louche, A.; Rodríguez-Escudero, M.; Roussin, M.; Imbert, P.R.C.; Rodríguez-Escudero, I.; Terradot, L.; Molina, M.; Gorvel, J.-P.; Cid, V.J.; et al. The TIR-domain containing effectors BtpA and BtpB from Brucella abortus impact NAD metabolism. PLoS Pathog. 2020, 16, e1007979. [Google Scholar] [CrossRef]
- Li, J.; Yuan, N.; Zhai, Y.; Wang, M.; Hao, M.; Liu, X.; Zhou, D.; Liu, W.; Jin, Y.; Wang, A. Protein disulfide isomerase A4 binds to Brucella BtpB and mediates intracellular NAD+/NADH metabolism in RAW264.7 cells. Int. Immunopharmacol. 2024, 142, 113046. [Google Scholar] [CrossRef] [PubMed]
- Rollauer, S.E.; Sooreshjani, M.A.; Noinaj, N.; Buchanan, S.K. Outer membrane protein biogenesis in Gram-negative bacteria. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150023. [Google Scholar] [CrossRef]
- Jubier-Maurin, V.; Boigegrain, R.-A.; Cloeckaert, A.; Gross, A.; Alvarez-Martinez, M.-T.; Terraza, A.; Liautard, J.; KöhLer, S.; Rouot, B.; Dornand, J.; et al. Major Outer Membrane Protein Omp25 of Brucella suis Is Involved in Inhibition of Tumor Necrosis Factor Alpha Production during Infection of Human Macrophages. Infect. Immun. 2001, 69, 4823–4830. [Google Scholar] [CrossRef]
- Degos, C.; Hysenaj, L.; Gonzalez-Espinoza, G.; Arce-Gorvel, V.; Gagnaire, A.; Papadopoulos, A.; Pasquevich, K.A.; Méresse, S.; Cassataro, J.; Mémet, S.; et al. Omp25-dependent engagement of SLAMF1 by Brucella abortus in dendritic cells limits acute inflammation and favours bacterial persistence in vivo. Cell. Microbiol. 2020, 22, e13164. [Google Scholar] [CrossRef]
- Cui, B.; Liu, W.; Wang, X.; Chen, Y.; Du, Q.; Zhao, X.; Zhang, H.; Liu, S.-L.; Tong, D.; Huang, Y. Brucella Omp25 Upregulates miR-155, miR-21-5p, and miR-23b to inhibit interleukin-12 Production via Modulation of Programmed Death-1 signaling in human Mono-cyte/Macrophages. Front. Immunol. 2017, 8, 708. [Google Scholar] [CrossRef]
- Ortega, M.A.; Boaru, D.L.; De Leon-Oliva, D.; Fraile-Martinez, O.; García-Montero, C.; Rios, L.; Garrido-Gil, M.J.; Barrena-Blázquez, S.; Minaya-Bravo, A.M.; Rios-Parra, A.; et al. PD-1/PD-L1 axis: Implications in immune regulation, cancer progression, and translational applications. J. Mol. Med. 2024, 102, 987–1000. [Google Scholar] [CrossRef]
- Godlewska, R.; Wiåniewska, K.; Pietras, Z.; Jagusztyn-Krynicka, E.K. Peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria: Function, structure, role in pathogenesis and potential application in immunoprophylaxis. FEMS Microbiol. Lett. 2009, 298, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhi, F.; Fang, J.; Zheng, W.; Li, J.; Zhang, G.; Chen, L.; Jin, Y.; Wang, A. RNA-Seq Analysis Reveals the Role of Omp16 in Brucella-Infected RAW264.7 Cells. Front. Vet. Sci. 2021, 8, 646839. [Google Scholar] [CrossRef]
- Giambartolomei, G.H.; Zwerdling, A.; Cassataro, J.; Bruno, L.; Fossati, C.A.; Philipp, M.T. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 2004, 173, 4635–4642. [Google Scholar] [CrossRef] [PubMed]
- Zwerdling, A.; Delpino, M.V.; Barrionuevo, P.; Cassataro, J.; Pasquevich, K.A.; Samartino, C.G.; Fossati, C.A.; Giambartolomei, G.H. Brucella lipoproteins mimic dendritic cell maturation induced by Brucella abortus. Microbes Infect. 2008, 10, 1346–1354. [Google Scholar] [CrossRef]
- Rodríguez, A.M.; Delpino, M.V.; Miraglia, M.C.; Franco, M.M.C.; Barrionuevo, P.; Dennis, V.A.; Oliveira, S.C.; Giambartolomei, G.H. Brucella abortus-activated microglia induce neuronal death through primary phagocytosis. Glia 2017, 65, 1137–1151. [Google Scholar] [CrossRef]
- Pasquevich, K.A.; Carabajal, M.V.; Guaimas, F.F.; Bruno, L.; Roset, M.S.; Coria, L.M.; Serrantes, D.A.R.; Comerci, D.J.; Cassataro, J. Omp19 Enables Brucella abortus to Evade the Antimicrobial Activity from Host’s Proteolytic Defense System. Front. Immunol. 2019, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.C.; Hielpos, M.S.; Carvalho, N.B.; Barrionuevo, P.; Corsetti, P.P.; Giambartolomei, G.H.; Oliveira, S.C.; Baldi, P.C. Key Role of Toll-Like Receptor 2 in the Inflammatory Response and Major Histocompatibility Complex Class II Downregulation in Brucella abortus-Infected Alveolar Macrophages. Infect. Immun. 2014, 82, 626–639. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Forestier, C.; Deleuil, F.; Lapaque, N.; Moreno, E.; Gorvel, J.-P. Brucella abortus Lipopolysaccharide in Murine Peritoneal Macrophages Acts as a Down-Regulator of T Cell Activation. J. Immunol. 2000, 165, 5202–5210. [Google Scholar] [CrossRef] [PubMed]
- Lapaque, N.; Forquet, F.; de Chastellier, C.; Mishal, Z.; Jolly, G.; Moreno, E.; Moriyon, I.; Heuser, J.E.; He, H.-T.; Gorvel, J.-P. Characterization of Brucella abortus lipopolysaccharide macrodomains as mega rafts. Cell. Microbiol. 2006, 8, 197–206. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Giambartolomei, G.H. Inhibition of antigen presentation by Brucella: Many more than many ways. Microbes Infect. 2019, 21, 136–142. [Google Scholar] [CrossRef]
- Velásquez, L.N.; Milillo, M.A.; Delpino, M.V.; Trotta, A.; Fernández, P.; Pozner, R.G.; Lang, R.; Balboa, L.; Giambartolomei, G.H.; Barrionuevo, P. Brucella abortus down-regulates MHC class II by the IL-6-dependent inhibition of CIITA through the downmodulation of IFN regulatory factor-1 (IRF-1). J. Leukoc. Biol. 2017, 101, 759–773. [Google Scholar] [CrossRef]
- Barquero-Calvo, E.; Chaves-Olarte, E.; Weiss, D.S.; Guzmán-Verri, C.; Chacón-Díaz, C.; Rucavado, A.; Moriyón, I.; Moreno, E. Brucella abortus Uses a Stealthy Strategy to Avoid Activation of the Innate Immune System during the Onset of Infection. PLoS ONE 2007, 2, e631. [Google Scholar] [CrossRef]
- Zhan, Y.; Cheers, C. Endogenous Gamma Interferon Mediates Resistance to Brucella abortus Infection. Infect. Immun. 1993, 61, 4899–4901. [Google Scholar] [CrossRef]
- Murphy, E.A.; Sathiyaseelan, J.; Parent, M.A.; Zou, B.; Baldwin, C.L. Interferon-γ is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 2001, 103, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, L.N.; Milillo, M.A.; Delpino, M.V.; Trotta, A.; Mercogliano, M.F.; Pozner, R.G.; Schillaci, R.; Elizalde, P.V.; Giambartolomei, G.H.; Barrionuevo, P. Inhibition of MHC-I by Brucella abortus is an early event during infection and involves EGFR pathway. Immunol. Cell Biol. 2017, 95, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Milillo, M.A.; Velásquez, L.N.; Trotta, A.; Delpino, M.V.; Marinho, F.V.; Balboa, L.; Vermeulen, M.; Espindola, S.L.; Rodriguez-Rodrigues, N.; Fernández, G.C.; et al. B. abortus RNA is the component involved in the down-modulation of MHC-I expression on human monocytes via TLR8 and the EGFR pathway. PLoS Pathog. 2017, 13, e1006527. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Rosinha, G.M.S.; Almeida, I.C.; Salgueiro, X.S.; Jarvis, B.W.; Splitter, G.A.; Qureshi, N.; Bruna-Romero, O.; Gazzinelli, R.T.; Oliveira, S.C. Role of Toll-Like Receptor 4 in Induction of Cell-Mediated Immunity and Resistance to Brucella abortus Infection in Mice. Infect. Immun. 2004, 72, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Pääbo, S.; Nilsson, T.; Peterson, P.A. Impaired Intracellular Transport of Class I MHC Antigens as a Possible Means for Adenoviruses to Evade Immune Surveillance. Cell 1985, 43, 215–222. [Google Scholar] [CrossRef]
- Radhakrishnan, G.K.; Yu, Q.; Harms, J.S.; Splitter, G.A. Brucella TIR Domain-containing Protein Mimics Properties of the Toll-like Receptor Adaptor Protein TIRAP. J. Biol. Chem. 2009, 284, 9892–9898. [Google Scholar] [CrossRef]
- Ashrafi, G.H.; Tsirimonaki, E.; Marchetti, B.; O’Brien, P.M.; Sibbet, G.J.; Andrew, L.; Campo, M.S. Down-regulation of MHC class I by bovine papillomavirus E5 oncoproteins. Oncogene 2002, 21, 248–259. [Google Scholar] [CrossRef]
- Marchetti, B.; Ashrafi, G.H.; Tsirimonaki, E.; O’Brien, P.M.; Campo, M.S. The bovine papillomavirus oncoprotein E5 retains MHC class I molecules in the Golgi apparatus and prevents their transport to the cell surface. Oncogene 2002, 21, 7808–7816. [Google Scholar] [CrossRef][Green Version]
- Fooksman, D.R.; Shaikh, S.R.; Boyle, S.; Edidin, M. Cutting Edge: Phosphatidylinositol 4,5-Bisphosphate Concentration at the APC Side of the Immunological Synapse Is Required for Effector T Cell Function. J. Immunol. 2009, 182, 5179–5182. [Google Scholar] [CrossRef]
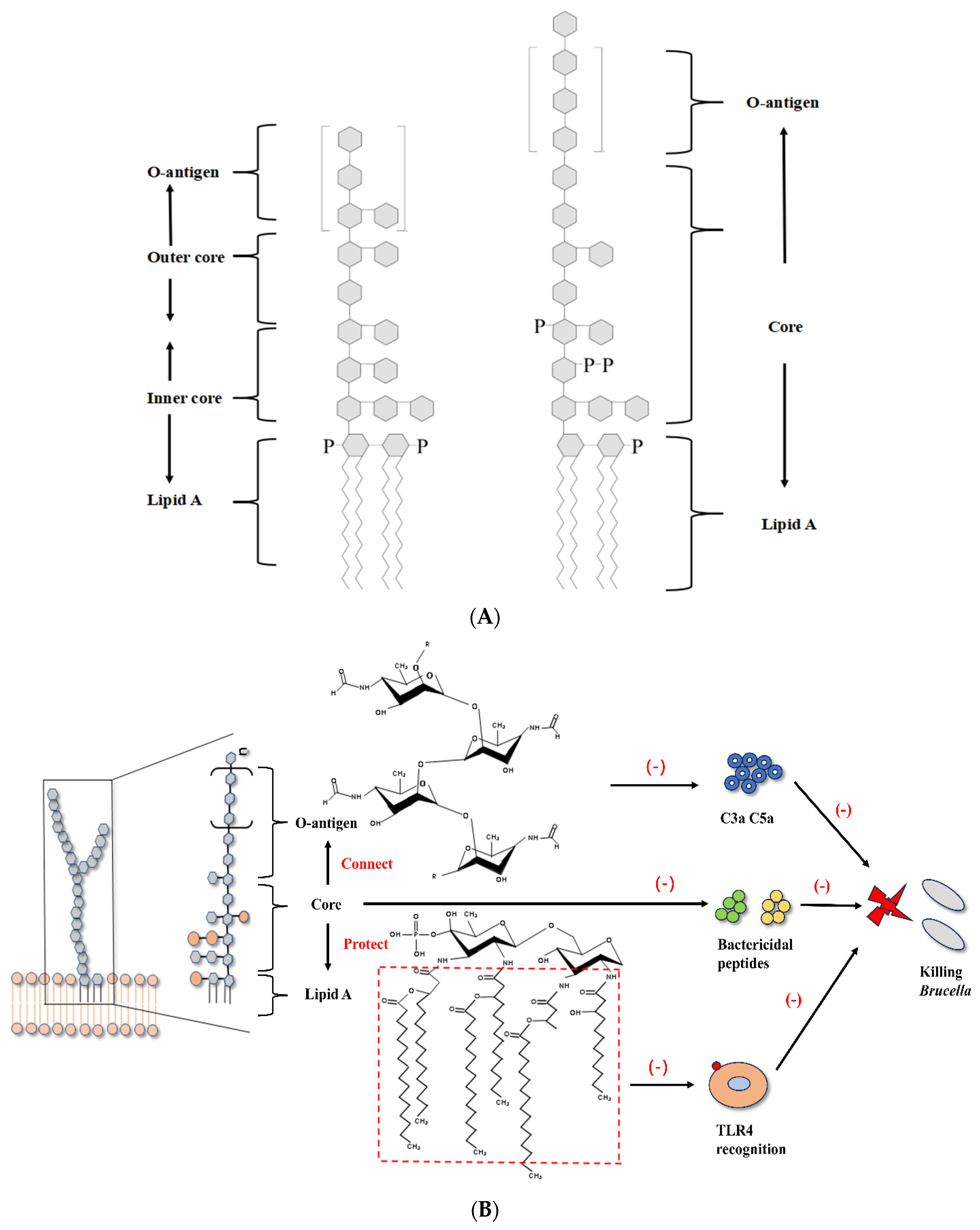

| LPS Component | Structural Feature | Receptor Affected | In Vivo Outcome |
|---|---|---|---|
| Lipid A | Presence of very long-chain fatty acids (VLCFAs; e.g., C28) | TLR4/MD-2 (weak agonist) | Attenuated pro-inflammatory response; facilitates chronic infection |
| Core | Positively charged; branched oligosaccharides | TLR4/MD-2 (steric hindrance); Antimicrobial peptides; Complement | Resistance to cationic antimicrobial peptides and complement-mediated killing |
| O-Polysaccharide (O-PS) | Composed of N-formylperosamine, lacking free hydroxyl groups | Complement component C3 | Impairs complement activation and opsonization; reduces phagocyte recruitment |
| Effector Protein | Target/Receptor | Main Action/Effect | Effector Protein |
|---|---|---|---|
| BtpA (TcpB) | TIRAP, MyD88 | Degrades TIRAP; inhibits MyD88-dependent TLR2/4 signaling; reduces IL-12 and TNF-α production; inhibits DC maturation and CD8+ T cell function | BtpA (TcpB) |
| BtpB | MyD88, TLR2/4/5/9 | Strong inhibitor of multiple TLR pathways; interacts strongly with MyD88; suppresses NF-κB and NLRP3 inflammasome; possesses NAD+ hydrolase activity | BtpB |
| Omp25 | SLAMF1, PD-1 pathway | Binds SLAMF1 to inhibit NF-κB; upregulates miR-155, miR-23b, miR-21-5p and PD-1; inhibits IL-12 production and T cell priming | Omp25 |
| Omp16 | Macrophage signaling | Deficiency upregulates pro-inflammatory cytokines (e.g., IL-6, CCL2); highly conserved peptidoglycan-associated lipoprotein | Omp16 |
| Omp19 | TLR2 | Recombinant form inhibits MHC-II via IL-6/IRF-1/CIITA axis; protects Omp25 from proteolysis; may modulate autophagy | Omp19 |
| Mechanism Category | Key Molecule/Component | Main Action/Effect | Selected References |
|---|---|---|---|
| TLR Recognition Subversion | Lipopolysaccharide (LPS) | Weak TLR4/MD-2 agonist due to VLCFAs in Lipid A; O-PS impairs complement opsonization. | [4,9,12,31,33,34] |
| Flagellin | Lacks TLR5-interacting residues, evading detection and pro-inflammatory responses. | [36] | |
| BtpA/TcpB | Mimics TIR domain, degrades TIRAP, interacts with MyD88, inhibiting TLR2/4 signaling. | [39,41,45] | |
| BtpB | Broad TLR inhibitor (TLR2/4/5/9); strong MyD88 interaction; possesses NADase activity. | [46,47,50,51] | |
| Innate Immune Modulation | Outer Membrane Protein Omp25 | Binds SLAMF1, inhibits NF-κB; upregulates miRNAs and PD-1, inhibiting IL-12 production. | [53,54,55] |
| Outer Membrane Protein Omp16 | Deficiency upregulates pro-inflammatory cytokines in macrophages. | [58] | |
| Outer Membrane Protein Omp19 | TLR2 agonist; recombinant form inhibits MHC-II via IL-6; protects Omp25 from proteolysis. | [8,14,63,64] | |
| Antigen Presentation Disruption | LPS (MHC-II interference) | Forms macrodomains with MHC-II on macrophage surface, hindering CD4+ T cell recognition. | [5,65,66,67] |
| Lipoproteins (e.g., Omp19) | TLR2/IL-6 dependent downregulation of CIITA and MHC-II expression. | [14,64,67] | |
| Bacterial RNA (MHC-I interference) | Retains MHC-I molecules in the Golgi via TLR8-EGFR-ERK1/2 pathway. | [15,73,76,77] | |
| T Cell Response Interference | Btp1/TcpB (CD8+ T cells) | Sequesters PI(4,5)P2 at immunological synapse; directly impairs CD8+ T cell function. | [7,79] |
| Omp25 (Indirect T cell inhibition) | Upregulates PD-1 on macrophages, contributing to T cell exhaustion. | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, H.; Zhou, G.; Wu, S.; Meng, C.; Wang, L.; Fan, C.; Li, J.; Chu, Y. Brucella Immune Escape: TLR Subversion, Antigen Presentation Destruction and T Cell Disorder. Cells 2025, 14, 1809. https://doi.org/10.3390/cells14221809
Jiao H, Zhou G, Wu S, Meng C, Wang L, Fan C, Li J, Chu Y. Brucella Immune Escape: TLR Subversion, Antigen Presentation Destruction and T Cell Disorder. Cells. 2025; 14(22):1809. https://doi.org/10.3390/cells14221809
Chicago/Turabian StyleJiao, Hanwei, Gengxu Zhou, Shengping Wu, Chi Meng, Lingjie Wang, Cailiang Fan, Jixiang Li, and Yuefeng Chu. 2025. "Brucella Immune Escape: TLR Subversion, Antigen Presentation Destruction and T Cell Disorder" Cells 14, no. 22: 1809. https://doi.org/10.3390/cells14221809
APA StyleJiao, H., Zhou, G., Wu, S., Meng, C., Wang, L., Fan, C., Li, J., & Chu, Y. (2025). Brucella Immune Escape: TLR Subversion, Antigen Presentation Destruction and T Cell Disorder. Cells, 14(22), 1809. https://doi.org/10.3390/cells14221809







