Targeting PAK1 or PAK4 Uncovers Different Mechanisms of Vascular Reprogramming in Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Animal Studies
2.3. Immunohistochemistry
2.4. Immunofluorescence
2.5. Western Blot
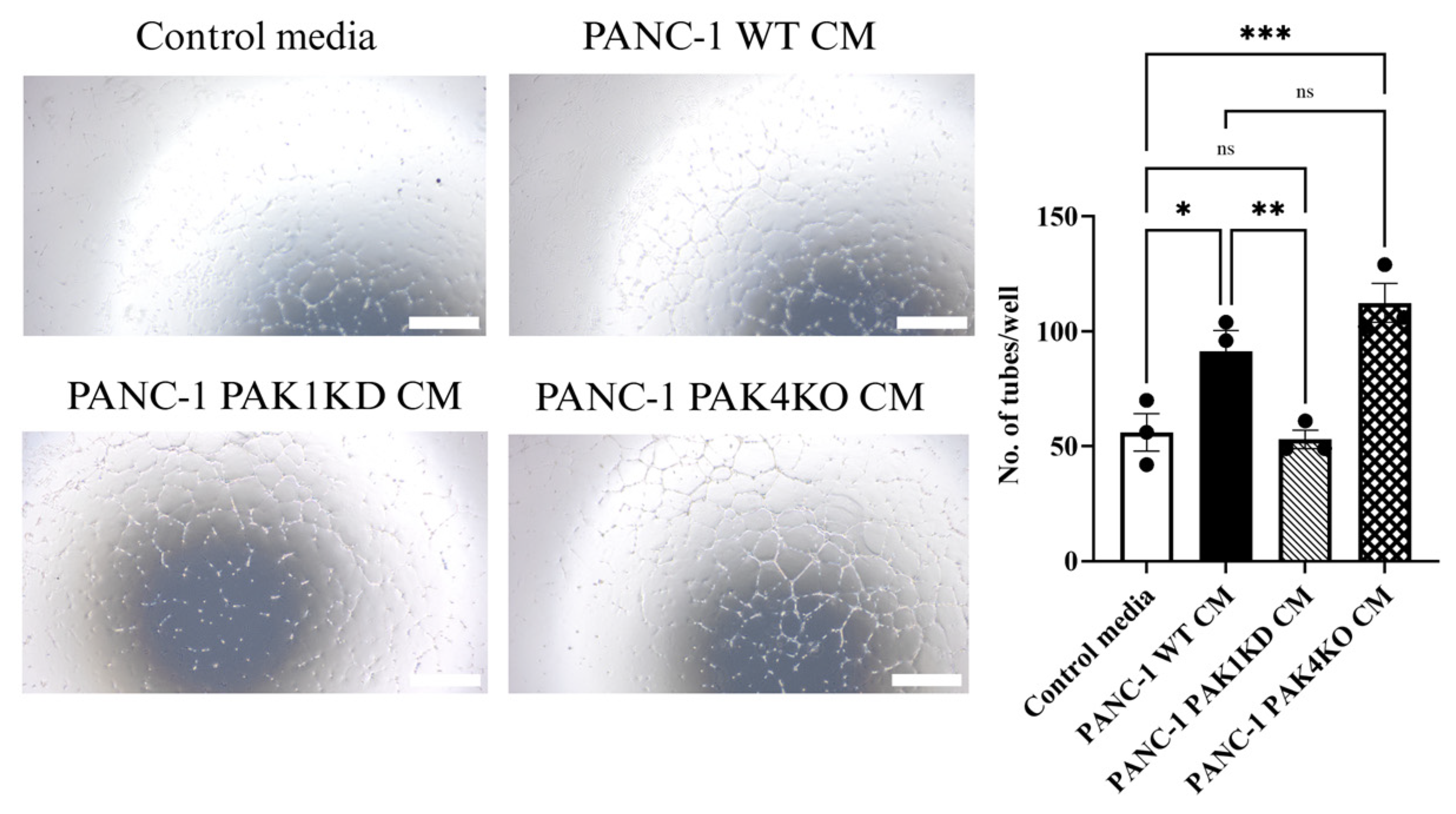
2.6. Tube Formation Assay
2.7. Proteomics
2.8. Statistical Analysis
3. Results
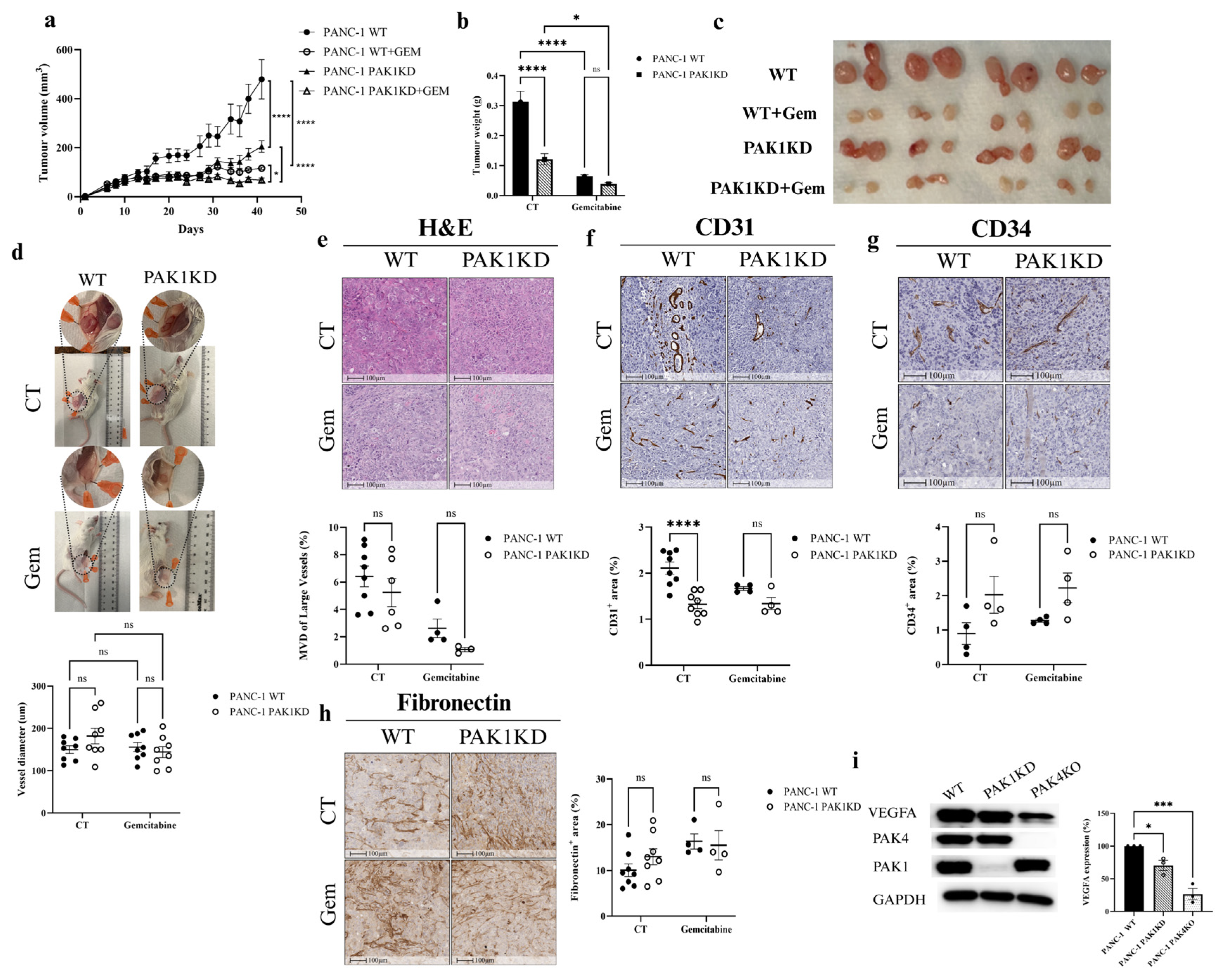
3.1. PAK1KD Reduced Tumour Growth and Angiogenesis, but Did Not Increase the Inhibitory Effect of Gemcitabine
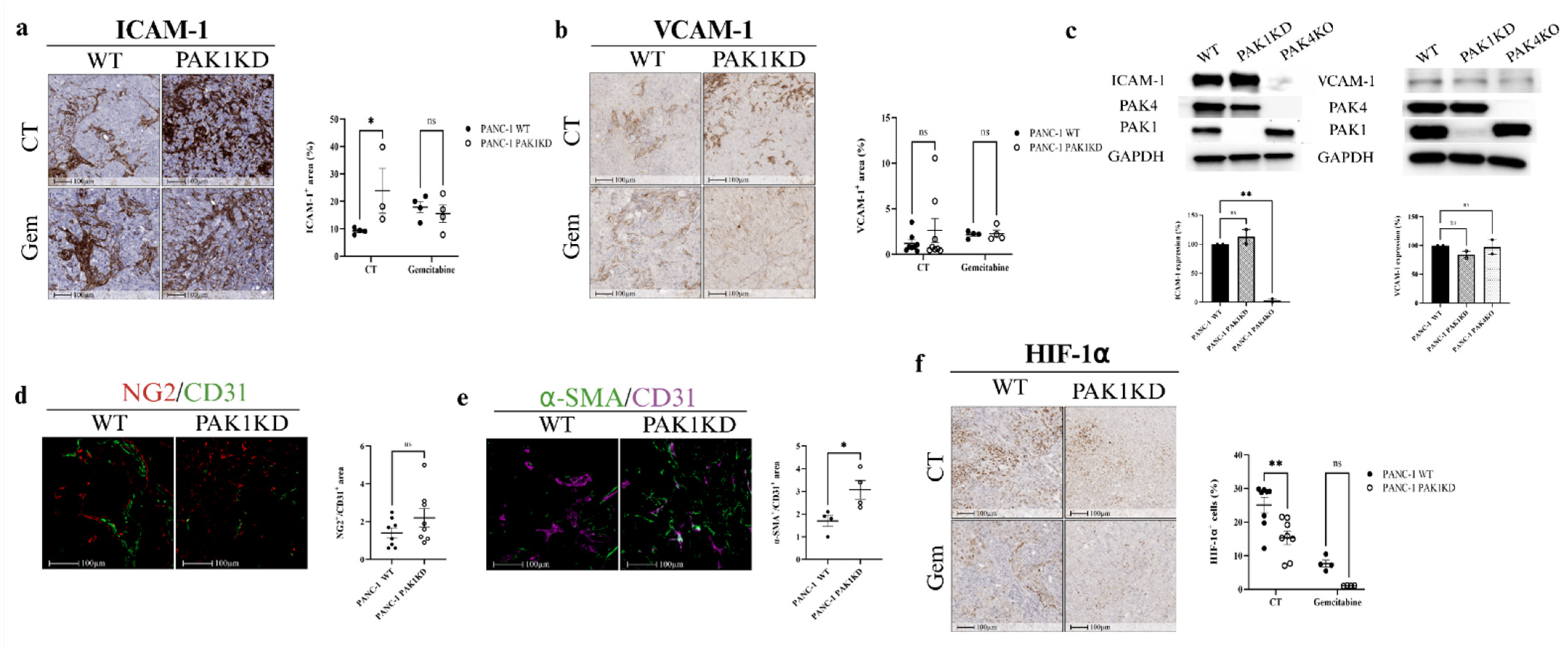
3.2. PAK1KD Increased ICAM-1 Expression and Promoted Vascular Normalisation, Followed by Reduced Hypoxia
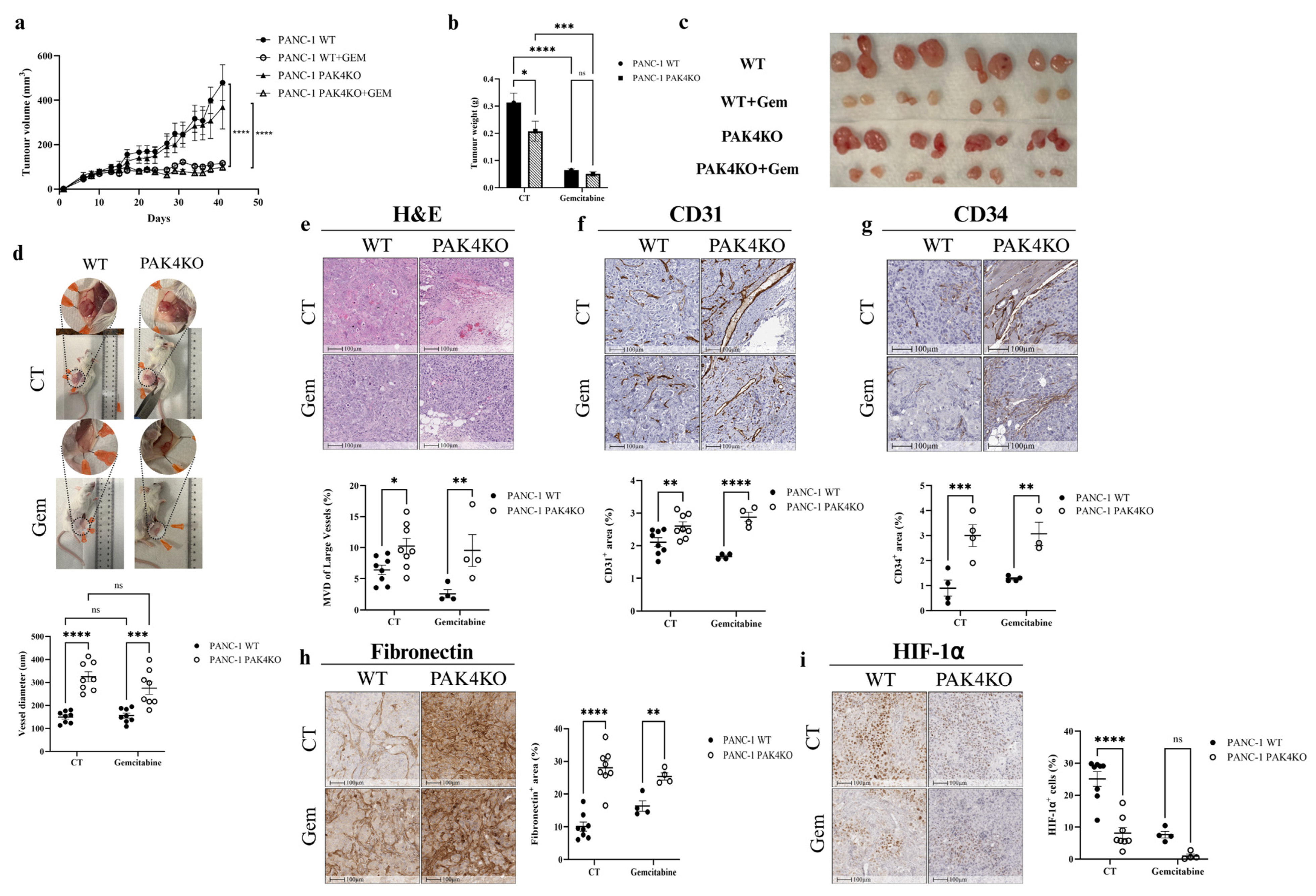
3.3. PAK4KO Reduced Tumour Growth and Increased Angiogenesis, but Did Not Increase the Inhibitory Effect of Gemcitabine
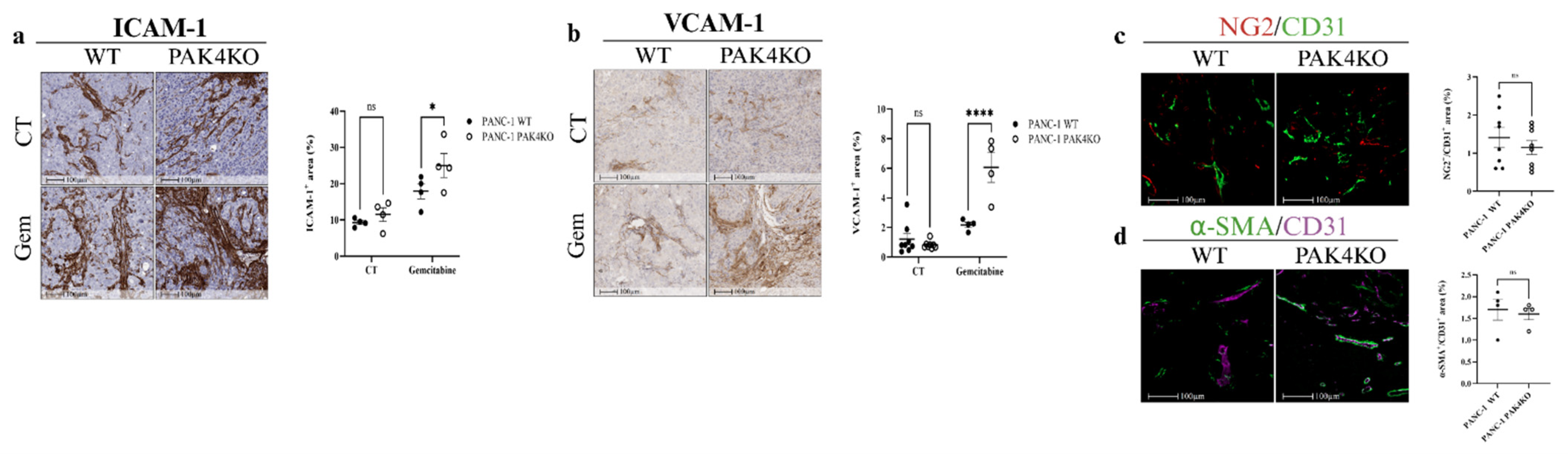
3.4. PAK4KO Increased ICAM-1 and VCAM-1 Expression in the Presence of Gemcitabine, but Did Not Promote Vascular Normalisation
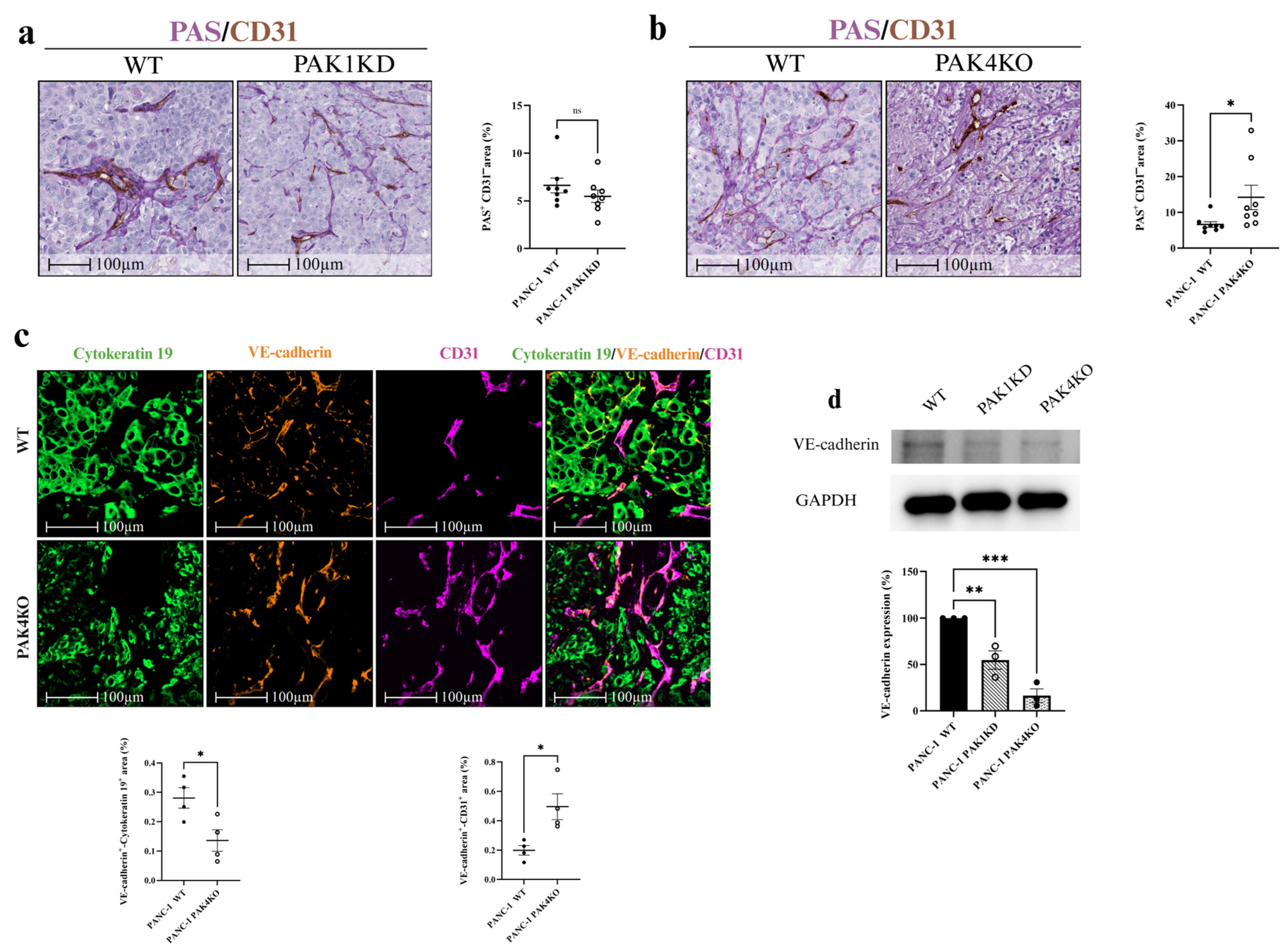
3.5. PAK4 Knockout Promoted Vascular Mimicry with Compromised Integrity in Tumour-Derived Vessels, but Enhanced Integrity in Endothelial-Derived Vessels
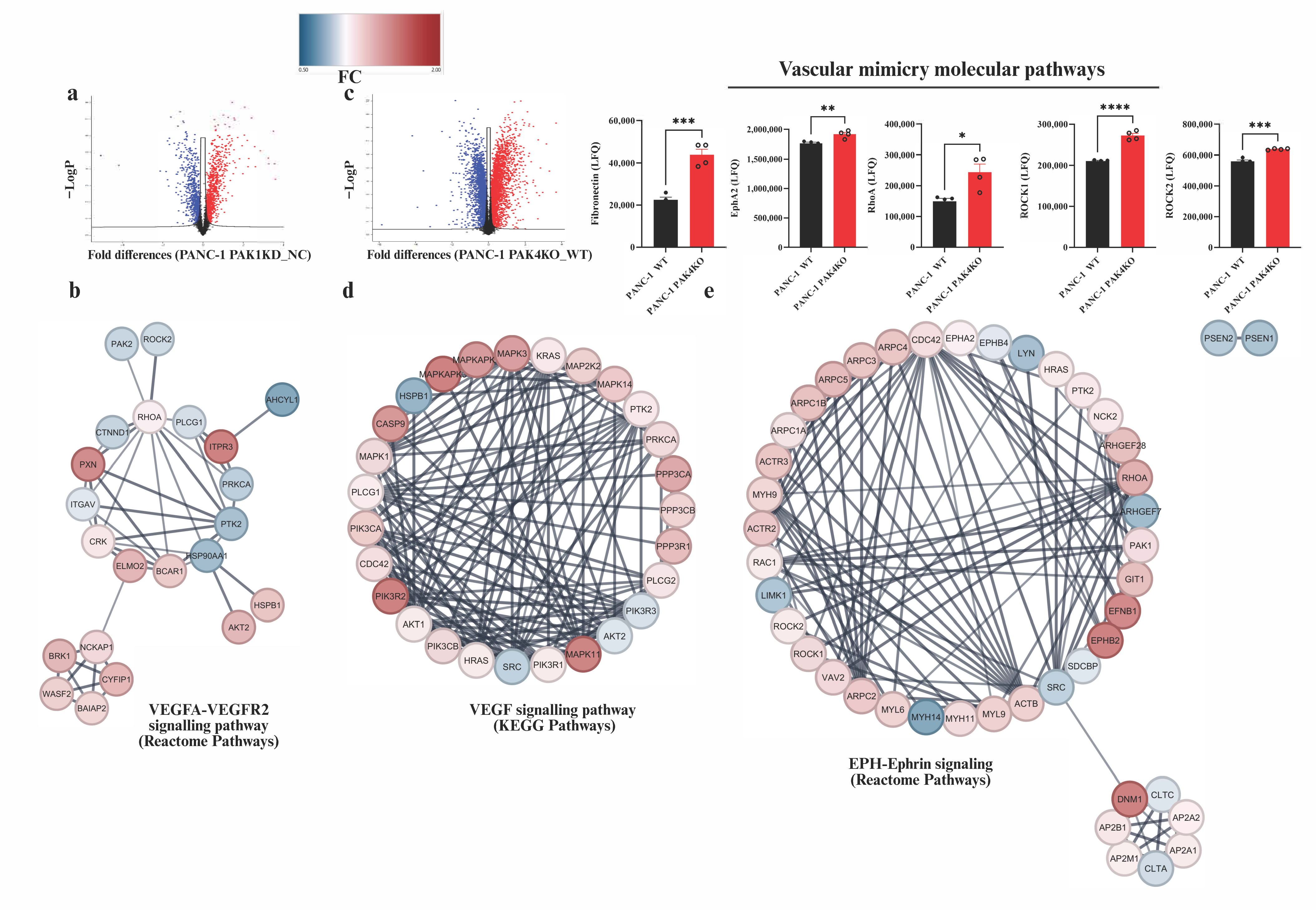
3.6. Global Proteomic Profiling Revealed Distinct Pathway Changes in PAK1KD and PAK4KO Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDA | Pancreatic ductal adenocarcinoma |
| TME | Tumour microenvironment |
| VEGFA | Vascular endothelial growth factor A |
| PAKs | P21-activated kinases |
| WT | Wild type |
| PAK1KD | PAK1 knockdown |
| PAK4KO | PAK4 knockout |
| NC | Negative control |
| sgRNAs | Single guide RNAs |
| FACS | Fluorescence-activated cell sorting |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Foetal bovine serum |
| NGS | Normal goat serum |
| BSA | Bovine serum albumin |
| MVD | Microvessel density |
| H&E | Haematoxylin and eosin |
| RBCs | Red blood cells |
| CM | Conditioned media |
| LC | Liquid chromatography |
| DIA | Data-independent acquisition |
| MS | Mass spectrometry |
| LFQ | Label-free quantification |
| FDR | False discovery rate |
| PPI | Protein–protein interaction |
| SEM | Standard error of the mean |
| LSD | Least significant difference |
| VM | Vascular mimicry |
| PAS | Periodic acid–Schiff |
| EphA2 | Ephrin type-A receptor 2 |
| RhoA | Ras homolog family member A |
| ROCK | Rho-associated coiled-coil containing kinase |
References
- Gharibi, A.; Adamian, Y.; Kelber, J. Cellular and molecular aspects of pancreatic cancer. Acta Histochem. 2016, 118, 305–316. [Google Scholar] [CrossRef]
- Nishimoto, A. Effective combinations of anti-cancer and targeted drugs for pancreatic cancer treatment. World J. Gastroenterol. 2022, 28, 3637–3643. [Google Scholar] [CrossRef]
- Binenbaum, Y.; Na’ara, S.; Gil, Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist. Updates 2015, 23, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget 2016, 7, 58649–58658. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Angiogenesis in pancreatic cancer: Pre-clinical and clinical studies. Cancers 2019, 11, 381. [Google Scholar] [CrossRef]
- Stickler, S.; Rath, B.; Hamilton, G. Targeting KRAS in pancreatic cancer. Oncol. Res. 2024, 32, 799–805. [Google Scholar] [CrossRef]
- Wang, K.; Baldwin, G.S.; Nikfarjam, M.; He, H. p21-activated kinase signalling in pancreatic cancer: New insights into tumour biology and immune modulation. World J. Gastroenterol. 2018, 24, 3709–3723. [Google Scholar] [CrossRef] [PubMed]
- Rane, C.K.; Minden, A. P21 activated kinase signaling in cancer. Semin. Cancer Biol. 2019, 54, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeshan, S.; Venkatraman, G.; Rayala, S.K. Targeting p21 activated kinase 1 (Pak1) to PAKup pancreatic Cancer. Expert Opin. Ther. Targets 2016, 20, 1283–1285. [Google Scholar] [CrossRef]
- Tyagi, N.; Bhardwaj, A.; Singh, A.P.; McClellan, S.; Carter, J.E.; Singh, S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT-and ERK-dependent activation of NF-κB pathway. Oncotarget 2014, 5, 8778–8789. [Google Scholar] [CrossRef]
- Ke, Y.; Lum, H.; Solaro, R.J. Inhibition of endothelial barrier dysfunction by P21-activated kinase-1. Can. J. Physiol. Pharmacol. 2007, 85, 281–288. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Li, D.; Li, Y.; Lin, F.; Wang, Y.; Song, H.; Liu, X.; Li, F.; Zhang, J. PAK4 in cancer development: Emerging player and therapeutic opportunities. Cancer Lett. 2022, 545, 215813. [Google Scholar] [CrossRef]
- Ansardamavandi, A.; Nikfarjam, M.; He, H. PAK in Pancreatic Cancer-Associated Vasculature: Implications for Therapeutic Response. Cells 2023, 12, 2692. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, Y.; Zhang, R.; Yang, F.; Zhang, D.; Huang, M.; Zhang, L.; Dorsey, J.F.; Binder, Z.A.; O’Rourke, D.M.; et al. Targeting PAK4 to reprogram the vascular microenvironment and improve CAR-T immunotherapy for glioblastoma. Nat. Cancer 2021, 2, 83–97. [Google Scholar] [CrossRef]
- Ansardamavandi, A.; Dumesny, C.; Ma, Y.; Dong, L.; Ellis, S.; Ang, C.-S.; Nikfarjam, M.; He, H. P-21 Kinase 1 or 4 Knockout Stimulated Anti-Tumour Immunity Against Pancreatic Cancer by Enhancing Vascular Normalisation. Int. J. Mol. Sci. 2025, 26, 8357. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Bankert, R.B.; Hess, S.D.; Egilmez, N.K. SCID mouse models to study human cancer pathogenesis and approaches to therapy: Potential, limitations, and future directions. Front. Biosci. 2002, 7, c44–c62. [Google Scholar] [CrossRef]
- Bosma, M.J.; Carroll, A.M. The SCID mouse mutant: Definition, characterization, and potential uses. Annu. Rev. Immunol. 1991, 9, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, C.-Y.; Liu, T.; Wu, B.; Zhou, F.; Xiong, J.-X.; Wu, H.-S.; Tao, J.; Zhao, G.; Yang, M.; et al. Persistence of side population cells with high drug efflux capacity in pancreatic cancer. World J. Gastroenterol. WJG 2008, 14, 925. [Google Scholar] [CrossRef]
- Amrutkar, M.; Vethe, N.T.; Verbeke, C.S.; Aasrum, M.; Finstadsveen, A.V.; Sántha, P.; Gladhaug, I.P. Differential gemcitabine sensitivity in primary human pancreatic cancer cells and paired stellate cells is driven by heterogenous drug uptake and processing. Cancers 2020, 12, 3628. [Google Scholar] [CrossRef]
- Yeo, D.; He, H.; Patel, O.; Lowy, A.M.; Baldwin, G.S.; Nikfarjam, M. FRAX597, a PAK1 inhibitor, synergistically reduces pancreatic cancer growth when combined with gemcitabine. BMC Cancer 2016, 16, 24. [Google Scholar] [CrossRef]
- Ma, Y.; Dumesny, C.; Dong, L.; Ang, C.-S.; Nikfarjam, M.; He, H. Knockout of p21-Activated Kinase 4 Stimulates MHC I Expression of Pancreatic Cancer Cells via an Autophagy-Independent Pathway. Cancers 2025, 17, 511. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’Er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Ma, Y.; Dumesny, C.; Dong, L.; Ang, C.-S.; Asadi, K.; Zhan, Y.; Nikfarjam, M.; He, H. Inhibition of P21-activated kinases 1 and 4 synergistically suppresses the growth of pancreatic cancer by stimulating anti-tumour immunity. Cell Commun. Signal. 2024, 22, 287. [Google Scholar] [CrossRef]
- Rudolph, J.D.; Cox, J.r. A network module for the Perseus software for computational proteomics facilitates proteome interaction graph analysis. J. Proteome Res. 2019, 18, 2052–2064. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Non-normal data: Is ANOVA still a valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Knief, U.; Forstmeier, W. Violating the normality assumption may be the lesser of two evils. Behav. Res. Methods 2021, 53, 2576–2590. [Google Scholar] [CrossRef]
- Gluhovschi, C.; Gluhovschi, G.; Potencz, E.; Herman, D.; Petrica, L.; Velciov, S.; Bozdog, G.; Bob, F.; Vernic, C.; Cioca, D. What is the significance of CD34 immunostaining in the extraglomerular and intraglomerular mesangium? Virchows Arch. 2008, 453, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.S.; Papadopoulos, J.; Wook Kim, S.; Maya, M.; Zhang, F.; He, J.; Fan, D.; Langley, R.; Fidler, I.J. Circulating monocytes expressing CD31: Implications for acute and chronic angiogenesis. Am. J. Pathol. 2009, 174, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. 2006, 54, 385–395. [Google Scholar] [CrossRef]
- McCloskey, K.E.; Smith, D.A.; Jo, H.; Nerem, R.M. Embryonic stem cell-derived endothelial cells may lack complete functional maturation in vitro. J. Vasc. Res. 2006, 43, 411–421. [Google Scholar] [CrossRef]
- Kang, Y.; Li, H.; Liu, Y.; Li, Z. Regulation of VEGF-A expression and VEGF-A-targeted therapy in malignant tumors. J. Cancer Res. Clin. Oncol. 2024, 150, 221. [Google Scholar] [CrossRef]
- Sarelius, I.H.; Glading, A.J. Control of vascular permeability by adhesion molecules. Tissue Barriers 2015, 3, e985954. [Google Scholar] [CrossRef]
- Aurrand-Lions, M.; Johnson-Léger, C.; Imhof, B.A. Role of interendothelial adhesion molecules in the control of vascular functions. Vasc. Pharmacol. 2002, 39, 239–246. [Google Scholar] [CrossRef]
- Hamzah, J.; Jugold, M.; Kiessling, F.; Rigby, P.; Manzur, M.; Marti, H.H.; Rabie, T.; Kaden, S.; Gröne, H.-J.; Hämmerling, G.J.; et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 2008, 453, 410–414. [Google Scholar] [CrossRef]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”. Front. Oncol. 2019, 9, 803. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhou, H.; Fan, G.; Li, Q. Molecular Mechanisms and Anticancer Therapeutic Strategies in Vasculogenic Mimicry. J. Cancer 2019, 10, 6327–6340. [Google Scholar] [CrossRef]
- Seftor, R.E.; Hess, A.R.; Seftor, E.A.; Kirschmann, D.A.; Hardy, K.M.; Margaryan, N.V.; Hendrix, M.J. Tumor cell vasculogenic mimicry: From controversy to therapeutic promise. Am. J. Pathol. 2012, 181, 1115–1125. [Google Scholar] [CrossRef]
- Nan, W.; He, Y.; Wang, S.; Zhang, Y. Molecular mechanism of VE-cadherin in regulating endothelial cell behaviour during angiogenesis. Front. Physiol. 2023, 14, 1234104. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef]
- Cheng, N.; Brantley, D.M.; Chen, J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002, 13, 75–85. [Google Scholar] [CrossRef]
- Héroult, M.; Schaffner, F.; Augustin, H.G. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp. Cell Res. 2006, 312, 642–650. [Google Scholar] [CrossRef]
- Seftor, E.A.; Meltzer, P.S.; Schatteman, G.C.; Gruman, L.M.; Hess, A.R.; Kirschmann, D.A.; Seftor, R.E.B.; Hendrix, M.J.C. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: Role in vasculogenic mimicry. Crit. Rev. Oncol./Hematol. 2002, 44, 17–27. [Google Scholar] [CrossRef]
- Kirschmann, D.A.; Seftor, E.A.; Hardy, K.M.; Seftor, R.E.; Hendrix, M.J. Molecular pathways: Vasculogenic mimicry in tumor cells: Diagnostic and therapeutic implications. Clin. Cancer Res. 2012, 18, 2726–2732. [Google Scholar] [CrossRef]
- Zhang, J.G.; Li, X.Y.; Wang, Y.Z.; Zhang, Q.D.; Gu, S.Y.; Wu, X.; Zhu, G.H.; Li, Q.; Liu, G.L. ROCK is involved in vasculogenic mimicry formation in hepatocellular carcinoma cell line. PLoS ONE 2014, 9, e107661. [Google Scholar] [CrossRef]
- Xia, Y.; Cai, X.; Fan, J.; Zhang, L.; Li, Z.; Ren, J.; Wu, G.; Zhu, F. RhoA/ROCK pathway inhibition by fasudil suppresses the vasculogenic mimicry of U2OS osteosarcoma cells in vitro. Anti-Cancer Drugs 2017, 28, 514–521. [Google Scholar] [CrossRef]
- Xia, Y.; Cai, X.Y.; Fan, J.Q.; Zhang, L.L.; Ren, J.H.; Li, Z.Y.; Zhang, R.G.; Zhu, F.; Wu, G. The role of sema4D in vasculogenic mimicry formation in non-small cell lung cancer and the underlying mechanisms. Int. J. Cancer 2019, 144, 2227–2238. [Google Scholar] [CrossRef]
- Rane, C.K.; Minden, A. P21 activated kinases. Small GTPases 2014, 5, e28003. [Google Scholar] [CrossRef]
- Qian, C.; Yang, C.; Tang, Y.; Zheng, W.; Zhou, Y.; Zhang, S.; Song, M.; Cheng, P.; Wei, Z.; Zhong, C.; et al. Pharmacological manipulation of Ezh2 with salvianolic acid B results in tumor vascular normalization and synergizes with cisplatin and T cell-mediated immunotherapy. Pharmacol. Res. 2022, 182, 106333. [Google Scholar] [CrossRef]
- Takano, E.A.; Jana, M.K.; Lara Gonzalez, L.E.; Pang, J.-M.B.; Salgado, R.; Loi, S.; Fox, S.B. Preliminary characterisation of the spatial immune and vascular environment in triple negative basal breast carcinomas using multiplex fluorescent immunohistochemistry. PLoS ONE 2025, 20, e0317331. [Google Scholar] [CrossRef]
- Greenberg, J.I.; Cheresh, D.A. VEGF as an inhibitor of tumor vessel maturation: Implications for cancer therapy. Expert Opin. Biol. Ther. 2009, 9, 1347–1356. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing tumor vasculature to reduce hypoxia, enhance perfusion, and optimize therapy uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Roy, S.; Kumaravel, S.; Sharma, A.; Duran, C.L.; Bayless, K.J.; Chakraborty, S. Hypoxic tumor microenvironment: Implications for cancer therapy. Exp. Biol. Med. 2020, 245, 1073–1086. [Google Scholar] [CrossRef]
- Eguchi, R.; Kawabe, J.-i.; Wakabayashi, I. VEGF-independent angiogenic factors: Beyond VEGF/VEGFR2 signaling. J. Vasc. Res. 2022, 59, 78–89. [Google Scholar] [CrossRef]
- Nicosia, R.F.; Bonanno, E.; Smith, M. Fibronectin promotes the elongation of microvessels during angiogenesis in vitro. J. Cell. Physiol. 1993, 154, 654–661. [Google Scholar] [CrossRef]
- Rijken, P.F.; Bernsen, H.J.; Peters, J.P.; Hodgkiss, R.J.; Raleigh, J.A.; van der Kogel, A.J. Spatial relationship between hypoxia and the (perfused) vascular network in a human glioma xenograft: A quantitative multi-parameter analysis. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 571–582. [Google Scholar] [CrossRef]
- Siegel, G.; Malmsten, M. The role of the endothelium in inflammation and tumor metastasis. Int. J. Microcirc. 1997, 17, 257–272. [Google Scholar] [CrossRef]
- Mollà, M.; Gironella, M.; Miquel, R.; Tovar, V.; Engel, P.; Biete, A.; Piqué, J.M.; Panés, J. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 264–273. [Google Scholar] [CrossRef]
- Ferreras, C.; Cole, C.L.; Urban, K.; Jayson, G.C.; Avizienyte, E. Segregation of late outgrowth endothelial cells into functional endothelial CD34- and progenitor-like CD34+ cell populations. Angiogenesis 2015, 18, 47–68. [Google Scholar] [CrossRef]
- Benjakul, N.; Prakobphol, N.; Tangshewinsirikul, C.; Dulyaphat, W.; Svasti, J.; Charngkaew, K.; Kangsamaksin, T. Notch signaling regulates vasculogenic mimicry and promotes cell morphogenesis and the epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma. PLoS ONE 2022, 17, e0279001. [Google Scholar] [CrossRef]
- Zhuo, M.; Yuan, C.; Han, T.; Hu, H.; Cui, J.; Jiao, F.; Wang, L. JQ1 effectively inhibits vasculogenic mimicry of pancreatic ductal adenocarcinoma cells via the ERK1/2-MMP-2/9 signaling pathway both in vitro and in vivo. Am. J. Transl. Res. 2019, 11, 1030–1039. [Google Scholar]
- Yang, J.; Zhu, D.-M.; Zhou, X.-G.; Yin, N.; Zhang, Y.; Zhang, Z.-X.; Li, D.-C.; Zhou, J. HIF-2α promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget 2017, 8, 47801. [Google Scholar] [CrossRef]
- Festuccia, C.; Gravina, G.L.; Giorgio, C.; Mancini, A.; Pellegrini, C.; Colapietro, A.; Delle Monache, S.; Maturo, M.G.; Sferra, R.; Chiodelli, P.; et al. UniPR1331, a small molecule targeting Eph/ephrin interaction, prolongs survival in glioblastoma and potentiates the effect of antiangiogenic therapy in mice. Oncotarget 2018, 9, 24347–24363. [Google Scholar] [CrossRef] [PubMed]
- Margaryan, N.V.; Strizzi, L.; Abbott, D.E.; Seftor, E.A.; Rao, M.S.; Hendrix, M.J.; Hess, A.R. EphA2 as a promoter of melanoma tumorigenicity. Cancer Biol. Ther. 2009, 8, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Brantley-Sieders, D.M.; Caughron, J.; Hicks, D.; Pozzi, A.; Ruiz, J.C.; Chen, J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J. Cell Sci. 2004, 117, 2037–2049. [Google Scholar] [CrossRef]
- Beauchamp, A.; Debinski, W. Ephs and ephrins in cancer: Ephrin-A1 signalling. Semin. Cell Dev. Biol. 2012, 23, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Brantley, D.M.; Cheng, N.; Thompson, E.J.; Lin, Q.; Brekken, R.A.; Thorpe, P.E.; Muraoka, R.S.; Cerretti, D.P.; Pozzi, A.; Jackson, D.; et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene 2002, 21, 7011–7026. [Google Scholar] [CrossRef]
- Lim, D.; Do, Y.; Kwon, B.S.; Chang, W.; Lee, M.S.; Kim, J.; Cho, J.G. Angiogenesis and vasculogenic mimicry as therapeutic targets in ovarian cancer. BMB Rep. 2020, 53, 291–298. [Google Scholar] [CrossRef]
- Kim, H.S.; Won, Y.J.; Shim, J.H.; Kim, H.J.; Kim, J.; Hong, H.N.; Kim, B.S. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci. Rep. 2019, 9, 3414. [Google Scholar] [CrossRef]
- Hess, A.R.; Seftor, E.A.; Gardner, L.M.; Carles-Kinch, K.; Schneider, G.B.; Seftor, R.E.; Kinch, M.S.; Hendrix, M.J. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: Role of epithelial cell kinase (Eck/EphA2). Cancer Res. 2001, 61, 3250–3255. [Google Scholar]
- Pasquale, E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008, 133, 38–52. [Google Scholar] [CrossRef]
- Itoh, K.; Yoshioka, K.; Akedo, H.; Uehata, M.; Ishizaki, T.; Narumiya, S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat. Med. 1999, 5, 221–225. [Google Scholar] [CrossRef]
- Xia, Y.; Cai, X.Y.; Fan, J.Q.; Zhang, L.L.; Ren, J.H.; Chen, J.; Li, Z.Y.; Zhang, R.G.; Zhu, F.; Wu, G. Rho Kinase Inhibitor Fasudil Suppresses the Vasculogenic Mimicry of B16 Mouse Melanoma Cells Both In Vitro and In Vivo. Mol. Cancer Ther. 2015, 14, 1582–1590. [Google Scholar] [CrossRef]
- Szczepanowska, J. Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim. Pol. 2009, 56, 225–234. [Google Scholar] [CrossRef]
- Masuo, H.; Kubota, K.; Shimizu, A.; Notake, T.; Miyazaki, S.; Yoshizawa, T.; Sakai, H.; Hayashi, H.; Soejima, Y. Increased mitochondria are responsible for the acquisition of gemcitabine resistance in pancreatic cancer cell lines. Cancer Sci. 2023, 114, 4388–4400. [Google Scholar] [CrossRef]
- Huanwen, W.; Zhiyong, L.; Xiaohua, S.; Xinyu, R.; Kai, W.; Tonghua, L. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Mol. Cancer 2009, 8, 125. [Google Scholar] [CrossRef]
- Amrutkar, M.; Gladhaug, I.P. Pancreatic cancer chemoresistance to gemcitabine. Cancers 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Fryer, R.A.; Barlett, B.; Galustian, C.; Dalgleish, A.G. Mechanisms underlying gemcitabine resistance in pancreatic cancer and sensitisation by the iMiD™ lenalidomide. Anticancer Res. 2011, 31, 3747–3756. [Google Scholar] [PubMed]
- Chang, Z.; Zhang, Y.; Liu, J.; Guan, C.; Gu, X.; Yang, Z.; Ye, Q.; Ding, L.; Liu, R. GATA1 promotes gemcitabine resistance in pancreatic cancer through antiapoptotic pathway. J. Oncol. 2019, 2019, 9474273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Luo, B.; Wang, X.; Sun, H.; Liu, S.; Cui, Y.; Xu, X. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma 2009, 56, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huynh, N.; Wang, X.; Pajic, M.; Parkin, A.; Man, J.; Baldwin, G.S.; Nikfarjam, M.; He, H. PAK inhibition by PF-3758309 enhanced the sensitivity of multiple chemotherapeutic reagents in patient-derived pancreatic cancer cell lines. Am. J. Transl. Res. 2019, 11, 3353–3364. [Google Scholar] [PubMed]
- Ansardamavandi, A.; Dumesny, C.; Ma, Y.; Dong, L.; Ellis, S.; Ang, C.-S.; Nikfarjam, M.; He, H. The Effects of PAK-Regulated Tumour Vasculature on Gemcitabine Response of Pancreatic Cancer. Cancers 2025, 17, 3434. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansardamavandi, A.; Dumesny, C.; Ellis, S.; Ang, C.-S.; Nikfarjam, M.; He, H. Targeting PAK1 or PAK4 Uncovers Different Mechanisms of Vascular Reprogramming in Pancreatic Cancer. Cells 2025, 14, 1806. https://doi.org/10.3390/cells14221806
Ansardamavandi A, Dumesny C, Ellis S, Ang C-S, Nikfarjam M, He H. Targeting PAK1 or PAK4 Uncovers Different Mechanisms of Vascular Reprogramming in Pancreatic Cancer. Cells. 2025; 14(22):1806. https://doi.org/10.3390/cells14221806
Chicago/Turabian StyleAnsardamavandi, Arian, Chelsea Dumesny, Sarah Ellis, Ching-Seng Ang, Mehrdad Nikfarjam, and Hong He. 2025. "Targeting PAK1 or PAK4 Uncovers Different Mechanisms of Vascular Reprogramming in Pancreatic Cancer" Cells 14, no. 22: 1806. https://doi.org/10.3390/cells14221806
APA StyleAnsardamavandi, A., Dumesny, C., Ellis, S., Ang, C.-S., Nikfarjam, M., & He, H. (2025). Targeting PAK1 or PAK4 Uncovers Different Mechanisms of Vascular Reprogramming in Pancreatic Cancer. Cells, 14(22), 1806. https://doi.org/10.3390/cells14221806







