1. Introduction
Retinal degeneration (RD) is known to be caused by apoptosis or the impaired function of photoreceptors. The main types of RD are retinitis pigmentosa (RP) and age-related macular degeneration (AMD). The main characteristics of RD are progressive loss of photoreceptors and impairment of visual function, ultimately leading to blindness. There are many genes and mechanisms reported to cause RD. However, there are currently no particularly effective treatments for this disease in clinical practice. Many studies have found that unfolded protein response (UPR) activation has played a role in several retinal degenerative diseases, such as inherited retinal degeneration (IRD), Stargardt disease, Leber congenital amaurosis, AMD, and diabetic retinopathy (DR) [
1]. UPR activation has also been detected in many animal models. When UPR-related genes are knocked out in animal models, similar retinal degenerative diseases are produced [
2]. For example, ATF6 is crucial for human cone photoreceptors [
3]; its deficiency causes damage to both cone and rod cells, leading to retinal degenerative diseases. XBP1 plays a key role in photoreceptor synapses [
4]; once absent, retinal neurodegeneration accelerates in diabetic patients.
The ER plays an important role in organisms. The main functions of the ER include protein biosynthesis, and post-translational modification, folding, and transporting. Consequently, the ER has consistently been regarded as the protein factory of cells. Moreover, the ER possesses the ability to detect and rectify abnormal protein folding states to prevent pathological occurrences. The dysfunction of the ER can give rise to endoplasmic reticulum stress and the subsequent activation of the UPR intracellular signal transduction network [
5]. Chronic ER dysfunction can exert a significant influence on cells. The long-term activation of the UPR can result in cell death, inflammation, and oxidative stress, etc. [
6,
7] Numerous studies have indicated that many human diseases are associated with ER stress and the UPR, such as diabetes [
8], cancer [
9], vascular diseases [
10], neurodegenerative diseases [
11], etc. Among them, retinal diseases are mainly related to ER dysfunction [
1,
12,
13,
14].
B cell receptor-associated protein 31 (BAP31) is a widely expressed transmembrane protein that is localized in the ER. It is involved in regulating the synthesis, transportation, and degradation of multiple intracellular proteins and plays a crucial role in transporting protein from the ER to the Golgi apparatus. BAP31 is involved in the “ER quality control compartment”, which is a special perinuclear compartment [
15], and has also been reported to be involved in ER homeostasis. Moreover, the absence of BAP31 will cause ER stress and activate the UPR [
16], and this mechanism plays a core regulatory role in the pathological processes of various diseases. Previous studies by our team have revealed that BAP31 has many important functions, such as participating in the proteasome degradation pathway [
17], T cell activation [
18], insulin resistance [
19], promoting apoptosis [
20], activating autophagy [
21], and the progression of Alzheimer’s disease, etc. [
22]. In particular, previous research conducted by our team has shown that BAP31 has an inhibitory effect on neurodegenerative diseases. It can protect against neuroinflammation and the associated memory deficits [
23]. Recently, our team has further expanded this understanding; we found that BAP31 can regulate superoxide levels in microglia by modulating the p22phox and Keap1/Nrf2/HO-1 signaling pathways, thereby participating in the pathological regulation of neurodegenerative diseases [
24]. Qin et al. [
25] provided a comprehensive elucidation of the core mechanism by which BAP31 mitigates neurodegenerative lesions, such as those observed in Parkinson’s disease, through the inhibition of ER stress-mediated apoptosis. This research offered substantial theoretical support for its neuroprotective role. Notably, this mechanism aligns closely with the established pathway of “excessive ER stress and neuronal apoptosis” seen in retinal degenerative diseases [
26,
27,
28], thereby offering significant insights for investigating the function of BAP31 within the retina.
BAP31 plays an important role not only in the nervous system but also in metabolism and liver diseases. Studies have confirmed that BAP31 deficiency inhibits adipogenesis, blocks lipolysis, and promotes abnormal enlargement of lipid droplets by reducing the proteasomal degradation of Perilipin1, directly associated with the occurrence and development of lipid metabolism disorders [
29]. In the context of liver health, relevant investigations have further elucidated the regulatory role of BAP31. For instance, one study constructed a hepatocyte-specific BAP31-deficient mouse model and found that BAP31 deficiency significantly exacerbates acetaminophen-induced hepatotoxicity by impairing Nrf2 signaling activation [
30]. Another independent study complemented this finding by revealing that in chronic alcoholic liver injury, the deacetylase Sirtuin 2 can alleviate endoplasmic reticulum (ER) stress-induced hepatocyte apoptosis by deacetylating BAP31 [
31]. The above studies suggest that BAP31, as a key molecule regulating the physiological functions of multiple systems and disease progression in the body, plays an important role in processes such as maintaining body homeostasis, regulating cellular metabolism, and mediating the occurrence and development of diseases.
In addition, BAP31 dysfunction mutations are the cause of “deafness, dystonia, and cerebral/central hypomelination” (DDCH) syndrome, which is characterized by severe neurological symptoms and early death. This disease is a rare autosomal dominant disorder, caused by missense mutations in the BAP31 gene, which is located in the q28 region of the X chromosome [
32,
33]. The pathological changes in DDCH patients mainly involve the auditory system and the central nervous system. However, no tests on systematic retinal function and structure have been conducted on these patients in existing reports. Although all these systemic diseases rely on capacity of BAP31 to sustain cellular homeostasis, such as regulating ER stress and facilitating protein folding, there is a lack of clear evidence on whether BAP31 mutations have effects on the retina. Notably, the retina is an organ with extremely high metabolic demands, and its phototransduction process also relies heavily on robust ER function. Age-related retinal diseases are the leading cause of irreversible blindness worldwide, and their pathogenesis is closely associated with the decline of cellular homeostasis, including impaired ER stress resolution, accumulation of misfolded proteins, and accelerated loss of photoreceptor cells [
34]. These diseases share core pathogenic features with BAP31-related systemic diseases (such as ER stress, apoptosis, and protein misfolding), but whether BAP31 is involved in maintaining retinal health or retinal disease progression, and whether it can serve as a protective factor against age-related retinal degeneration, remains completely unknown.
Given the above, we hypothesize that BAP31 plays a crucial role in maintaining retinal homeostasis. To verify our hypothesis, we investigated the roles of BAP31 in rod cells by generating BAP31 rod-specific conditional knockdown mice (Rho-iCre-BAP31fl/fl(−/−)). We found that conditional knockdown of BAP31 in the retina induced ER stress, which led to a reduction in the ONL of the retina, apoptosis of photoreceptor cells, and ultimately impaired the visual function of the mice. At the same time, the expression of ER stress markers increased, and the misfolding of proteins in the retina increased. This result is highly consistent with the mechanism by which BAP31 regulates ER homeostasis to function in other systems, initially suggesting that BAP31 may be involved in retinal degenerative diseases through similar pathways. Additionally, in 12-month-old mice, the expression of glial fibrillary acidic protein (GFAP) was increased and gliosis was severe, indicating that BAP31 is crucial for the long-term survival of photoreceptor cells. This study not only preliminarily explored the potential mechanism by which BAP31 affects retinal function but also verified its role in retinal degenerative diseases. In turn, this provides a new direction for subsequent research and offers hope for identifying new therapeutic targets for age-related retinal diseases.
2. Materials and Methods
2.1. Animals and Genotyping
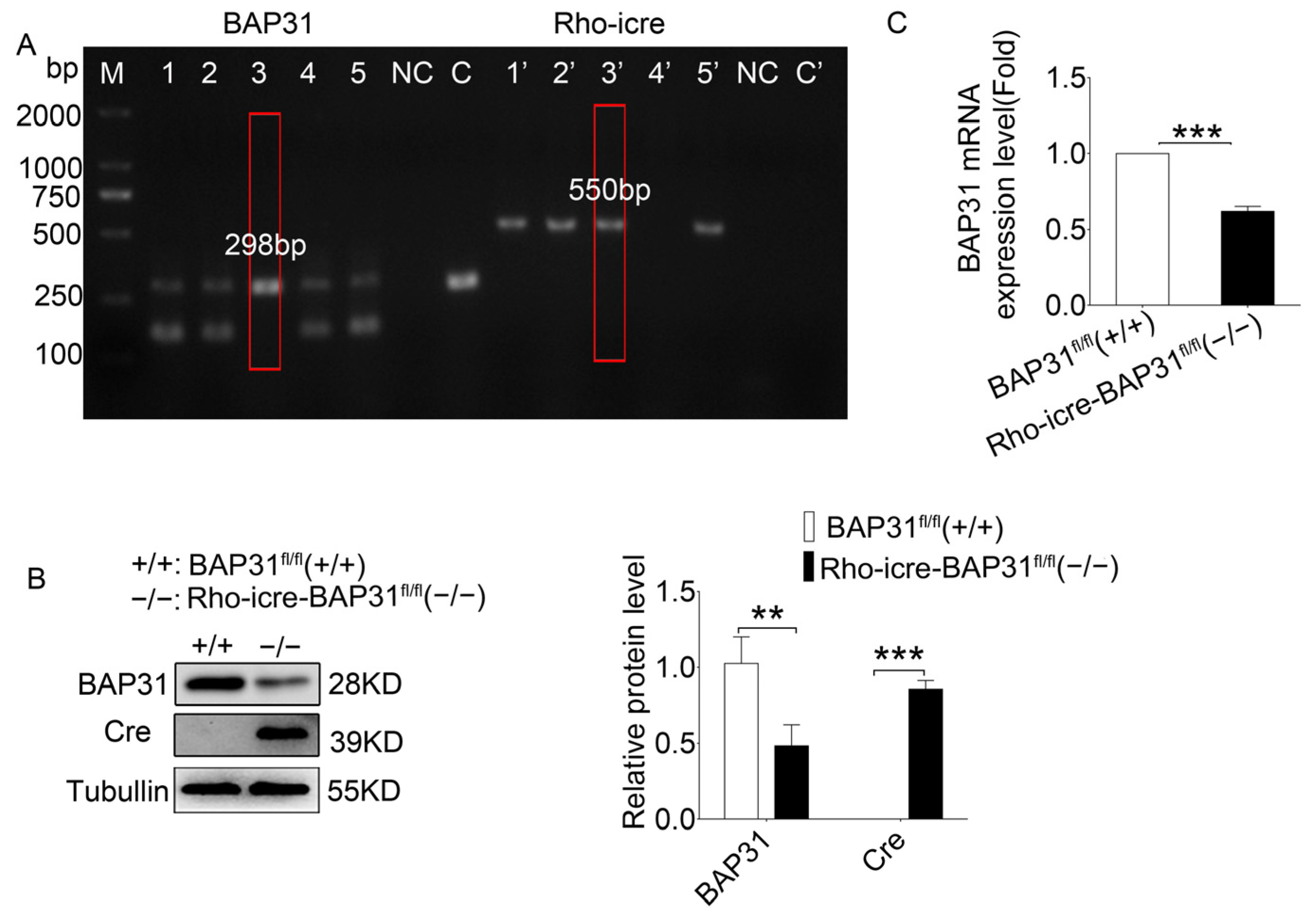
Conditional knockdown of BAP31 in the retina was achieved by crossing mice with LoxP sites flanking BAP31 [
35] with a retina-specific Rho-iCre line [
36]. The Rho-iCre line carries an improved Cre recombinase (iCre), which exhibits higher recombination efficiency than the traditional Cre recombinase and mitigates the risks of background recombination and non-specific expression. This line is widely used in the study of gene functions related to photoreceptor cells and eye diseases. The two independent lines have been interbred and maintained as BAP31
fl/fl(+/+) mice and Rho-iCre-BAP31
fl/fl(−/−). The F1 offspring were backcrossed to BAP31
fl/fl(+/+) parental mice for more than 3 generations to obtain conditional knockdown mice (Rho-iCre-BAP31
fl/fl(−/−)). During subsequent experimental procedures, sib-mating within the same genotype was performed every 2 generations. Meanwhile, genotyping was regularly conducted by PCR to ensure consistent genetic background and effectively prevent genetic drift. We also verified the expression level of BAP31 at different time points using Western blot, confirming that BAP31 was maintained at a low expression level (
Figure S1).
Genotyping was performed by PCR using the primers lox-S-5’-GAGAAGCTAATGGTCTGTGACCCTGA-3′ and loxA-5′-CTACAGAGCAAGTGCCATGACATCC-3′, resulting in a 170 bp band for WT and a 298 bp band for BAP31 fl/fl(+/+). The presence of the Rho-iCre allele was determined by PCR with Cre-F 5′-TCAGTGCCTGGAGTTGCGCTGTGG-3′ and Cre-R 5′-CTTAAAGGCCAGGGCCTGCTTGGC-3′, resulting in a 550 bp band. At each time point (3, 6, 9, and 12 months of age), there were 6–8 animals, with an equal number of males and females.
All animals were illuminated by fluorescent lamps with 12 h light/12 h dark cycle, and the ambient temperature was controlled at 25 ± 2 °C. All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals and the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. They were monitored with approval from the Institutional Animal Care and Use Committee (IACUC) of He University.
2.2. Electroretinography (ERG)
All the mice were dark-adapted for 12 h prior to the start of ERG recordings, and all procedures were performed under dim red light. Mice were anesthetized with Tiletamine hydrochloride and Zolazepam hydrochloride (16 mg/mL) at a dose of 50–75 mg/kg, and 1% topical tropicamide was used to dilate the pupils. After the anesthesia, each mouse was placed on a test bench. The contact lens electrodes were placed on each cornea, reference electrodes were placed in the mouth, and the ground electrode was placed intradermally next to the tail. The a- and b-wave amplitudes were recorded at intensities of 0.015, 1.5, 3.0, and 10.0 cd·s/m2. The recordings were analyzed using Espion e2 software.
2.3. OCT
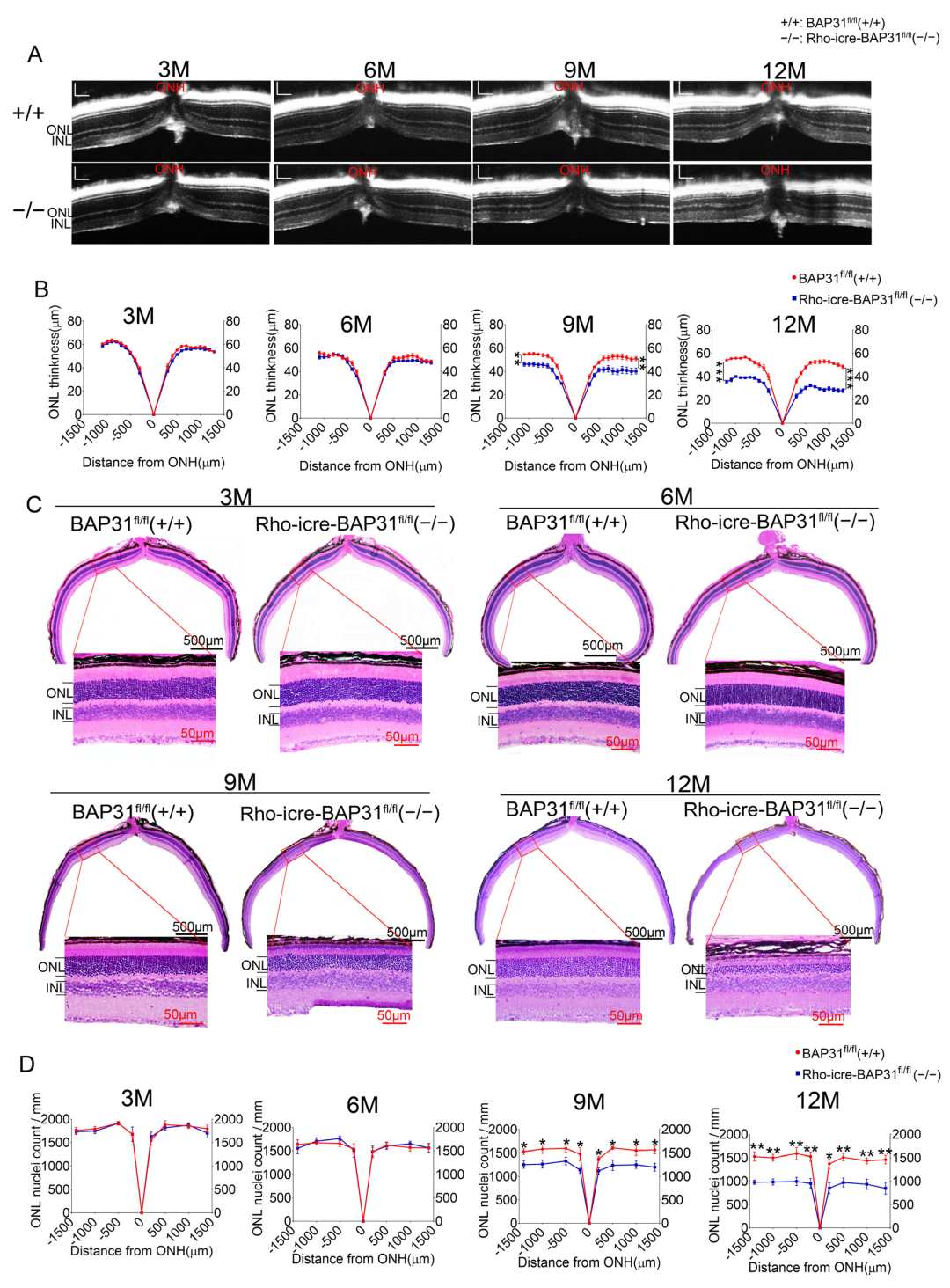
Mice were anesthetized via intraperitoneal injection of a ketamine (Jiangsu, China) and xylazine (Sichuan, China) mixture (100 mg/kg and 10 mg/kg, respectively). Retinal thickness was captured using rectangular volume scans, with the optic nerve serving as the positional reference. The outer nuclear layer (ONL) and retinal thickness were measured using the built-in calipers of the imaging instrument (Optoprobe Science LTD, London, UK) before the images were saved for further analysis. The thickness of the ONL measured by OCT at different age points was analyzed using two-way ANOVA with replicates for multiple comparisons.
2.4. Immunohistochemistry and Retinal Staining
The mice were euthanized, and eyeballs were enucleated. The eyes were fixed with 4% paraformaldehyde for 24 h at 4 °C. After removing the cornea and lens, the eyes were embedded in OCT and directly frozen. They were then sectioned at 8 µm thickness through the optic nerve head using a cryostat. The slices were taken from the −80 °C freezer and dried at room temperature for 10 min before soaking them in PBS to remove the OCT. They were fixed with 4% paraformaldehyde for 30 min, then blocked with 1% bovine serum albumin for 1 h at room temperature. The primary antibodies were diluted in accordance with the optimal dilution ratio and incubated the slices overnight at 4 °C. The following primary antibodies were used: RHO (abcam, ab98887, Mouse, 1:200, Cambridge, UK), GNAT1 (proteintech, Rabbit, 1:200, Rosemont, IL, USA), PDE6A (HUABIO, HA500264, Rabbit, 1:500, Hangzhou, China), PDE6B (santa, sc-377486, Mouse, 1:500, Dallas, TX, USA), GFAP (abcam, ab7260, Rabbit, 1:600, Cambridge, UK).
2.5. HE
The mice were euthanized, and eyeballs were enucleated. Immediately, the eyeballs were immersion fixed using Davidson’s fixative (PH0975, Phygene, Fuzhou, China) and placed in a refrigerator at 4 °C overnight. Before dehydration, the cornea and iris were removed. After gradient dehydration in a tissue dehydrator, the lens was removed and the tissue block was immersed in paraffin. Then, the embedded eyes were sliced into 6 um sections using a paraffin slicing machine (BIOCUT, Leica Microsystems, Wetzlar, Germany). The retina sections encompassing the optic nerve head (ONH) were carefully selected and subjected to hematoxylin–eosin (HE) staining for subsequent histological examination and analysis.
2.6. Western Blot Analysis
After the animals were euthanized, the eyeballs were enucleated and retinas were quickly isolated on ice. The retinas were washed with cold PBS and lysed using RIPA lysis buffer (Solarbio, Shanghai, China) supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF) and protease inhibitors for 30 min. Following protein quantification using the BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China), 20 µg of protein was added to each lane and separated by 10% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Then, the samples were transferred to polyvinylidene fluoride (PVDF) transfer membranes (Merck KGaA, Darmstadt, Germany) and blocked by blocking buffer for 1 h. PVDF membranes containing specific proteins were then carefully incubated in primary antibodies overnight at 4 °C. The following day, PVDF membranes were washed and incubated with the corresponding HRP-conjugated secondary antibody for 1 h at room temperature. The immunoreactivity was detected using an ECL Kit (Beyotime Institute of Biotechnology, Shanghai, China). ImageJ software (ImageJ1.47, Media Cybernetics, Baltimore, MD, USA) was used to assess the protein levels, which were normalized to the relative density of tubulin. The following primary antibodies were used: BAP31 (our lab, Rabbit, 1:3000, Shenyang, China), Cre (abcam, ab216262, Rabbit, 1:2000, Cambridge, UK), RHO (abcam, ab98887, Mouse, 1:1000, Cambridge, UK), RCVRN (Abways, AY3894, Rabbit, 1:1000, Shanghai, China), GNAT1 (proteintech, Rabbit, 1:2000, Rosemont, USA), PDE6A (HUABIO, HA500264, Rabbit, 1:500, Hangzhou, China), PDE6B (santa, sc-377486, Mouse, 1:500, Dallas, USA), CRX (abcam, ab140603, Rabbit, 1:1000, Cambridge, UK), GFAP (abcam, ab7260, Rabbit, 1:10000, Cambridge, UK), BIP (proteintech, 66574-1-Ig, Mouse, 1:2000, Rosemont, USA), ATF4 (beyotime, AF2560, Rabbit, 1:1000, Shanghai, China), ATF6 (Affinity, DF6009, Rabbit, 1:1000, Cinti, OH, USA), XBPI (HUABIO, ET1703-23, Rabbit, 1:500, Hangzhou, China), CHOP (HUABIO, HA722854, Rabbit, 1:500, Hangzhou, China), and TUBULIN (abcam, ab18207, Rabbit, 1:5000, Cambridge, UK).
2.7. Optomotor Response Test
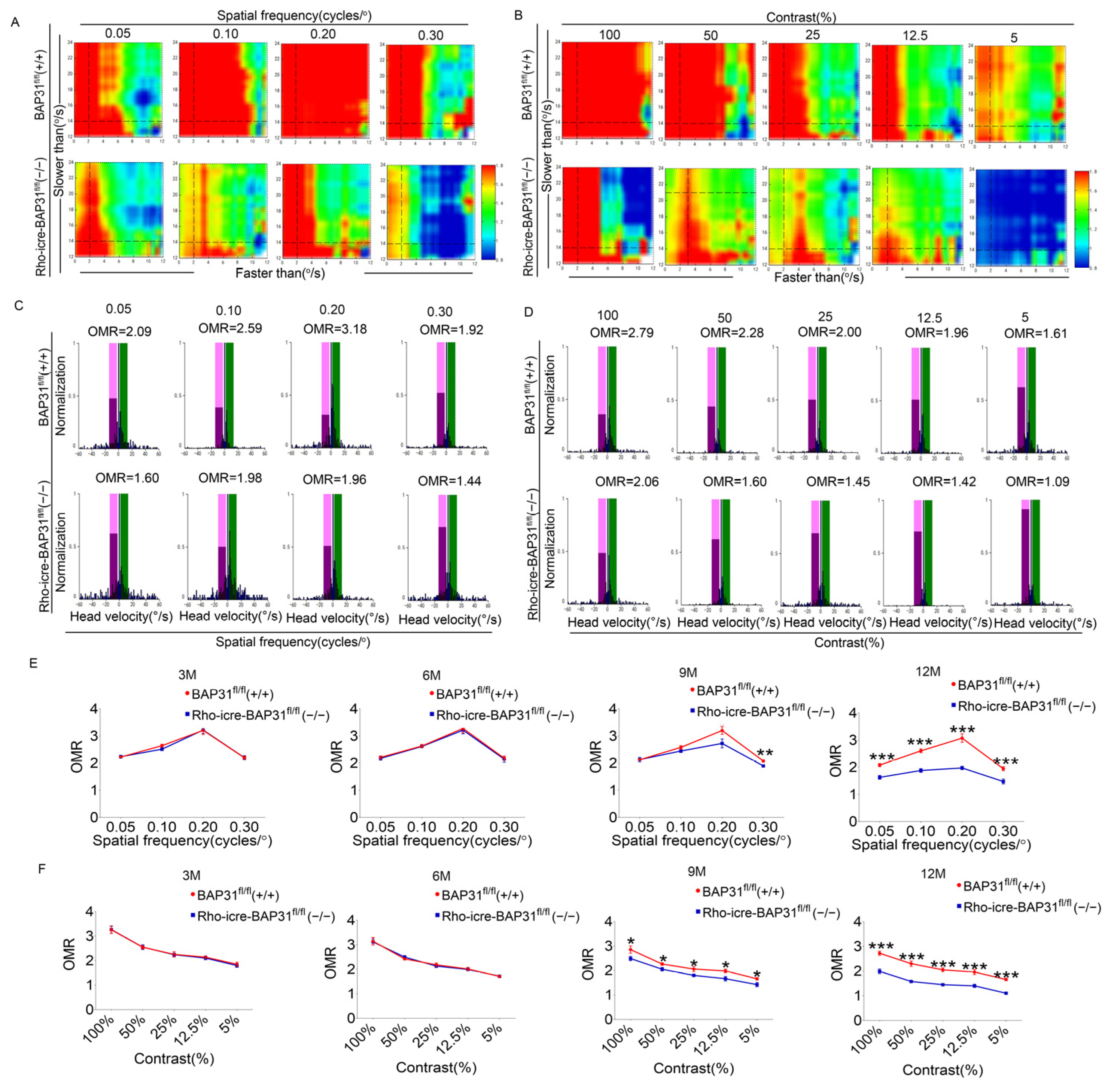
The visual function was measured by an automated head tracking system, according to previously published methods [
37]. The mice were adapted to darkness for 12 h in advance. In a dark and quiet environment, the mice were placed on the central platform of the machine where they were allowed to move freely. After starting the detection, the mice were presented with vertical black and white stripes generated by the surrounding LCD monitors located around the machine in a random clockwise or counter-clockwise cylindrical image. We selected four different spatial frequencies (0.1, 0.15, 0.2, and 0.3 cycles/degree) or presented the best spatial frequency (0.2 cycles/degree) with five different contrast levels (100%, 50%, 25%, 12.5%, and 5%). The head movements were monitored using a camera.
2.8. Quantitative Real-Time PCR
Total RNA was isolated from the retinas using TRIzol
® Reagent (Ambion, Austin, TX, USA). Subsequently, the extracted RNA was reverse-transcribed into complementary DNA (cDNA) using a cDNA synthesis kit (BioFlux, Hangzhou Bioer Technology Co., Ltd., Hangzhou, China). For quantitative real-time PCR, SYBR Green qPCR Master Mix (BioFlux, Hangzhou Bioer Technology Co., Ltd., Hangzhou, China) was used, with the cycle time, temperature, and number of cycles set in accordance with the manufacturer’s protocol. The reaction was performed in qTOWER
3G (Analytik Jena, Jena, Germany). The sequences of the primers used for qPCR are provided in
Table S1.
2.9. RNA Sequencing
Following inhalation anesthesia, the eyeballs of the mice were promptly enucleated. The corneas were incised, and the retinas were quickly dissected after removing the lenses. All procedures were carried out on ice to ensure sample integrity. Total RNA was extracted using Trizol Reagent (Invitrogen Life Technologies, Waltham, MA, US), and its concentration, quality, and integrity were assessed using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). Three micrograms of RNA were used as the starting material for RNA sample preparation. Sequencing libraries were constructed following standard operating procedures (SOP) and subsequently sequenced on the NovaSeq 6000 platform (Illumina, San Diego, CA, USA) at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
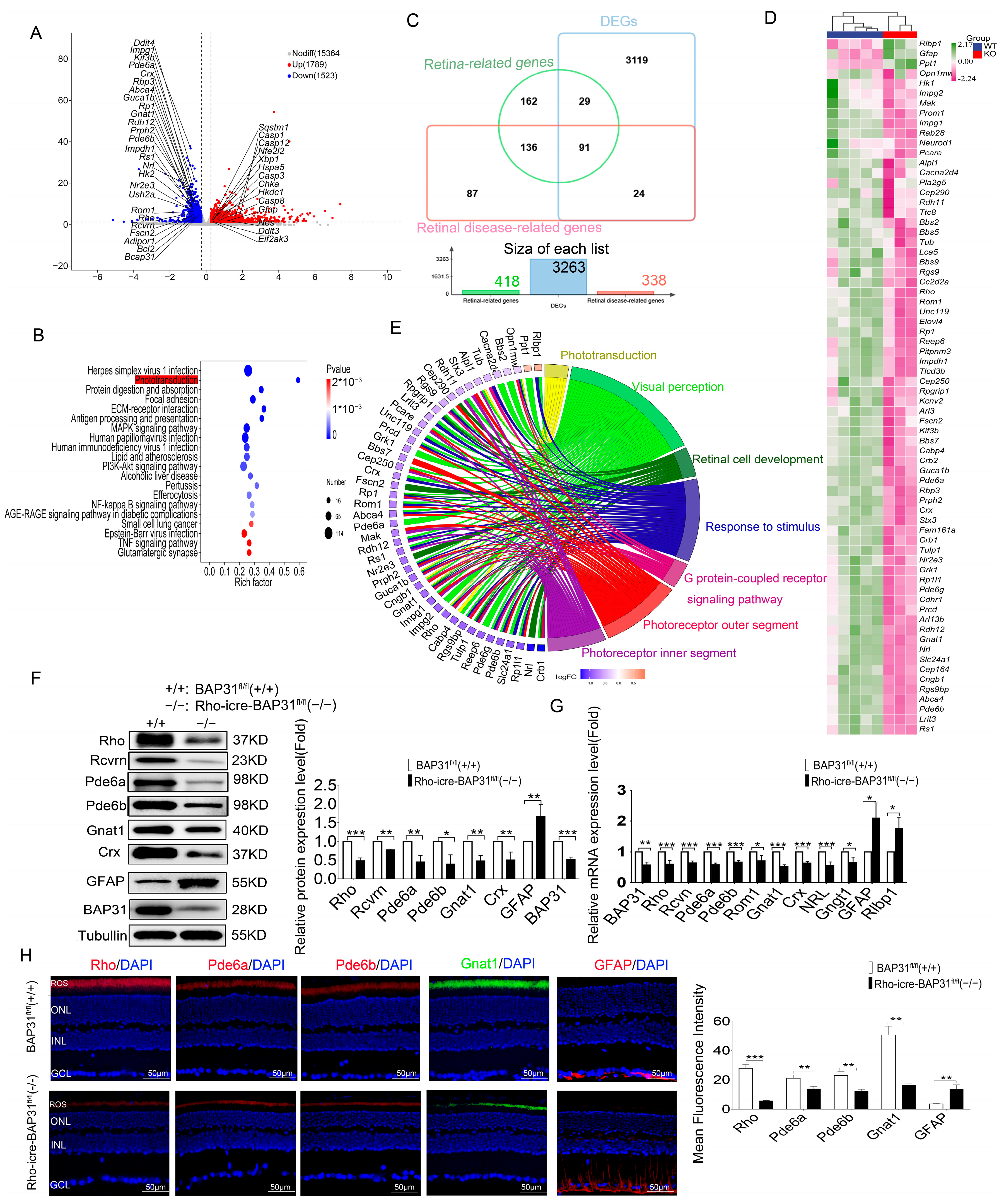
Samples were sequenced to obtain image files, which were transformed by the software of the sequencing platform. The original data was generated in FASTQ format (Raw Data). Sequencing data contains a number of connectors and low-quality reads, so we filtered it using the fastp (0.22.0) software to obtain high-quality sequence data (Clean Data) for further analysis. The reference genome and gene annotation files were downloaded from the genome website. The filtered reads were mapping to the reference genome GRCm39 (Ensembl 108.39) using HISAT2 (v2.1.0). We used HTSeq (v0.9.1) statistics to compare the Read Count values on each gene as the original expression of the gene, and then used FPKM (Fragments Per Kilo bases per Million fragments)/TPM (Transcripts per Million) to standardize the expression. The difference in expression of genes was analyzed by DESeq2 (v1.38.3), with screened conditions as follows: expression difference multiple |log2FoldChange| > 1, significant p-value < 0.05. At the same time, we used the ComplexHeatmap (v2.16.0) software package to perform bi-directional clustering analysis of all different genes in the samples. We generated a heatmap according to the expression level of the same gene in different samples and the expression patterns of different genes in the same sample, using the Euclidean method to calculate the distance and the Complete Linkage method to cluster.
2.10. Statistical Analysis
The GraphPad Prism 5 software was used to analyze the dates obtained from at least three independent experiments. A two-tailed Student’s t-test was used for comparison. Before conducting the two-tailed Student’s t-test, the Shapiro–Wilk test was performed on each group of data using GraphPad Prism 5.0 software. The test results for all groups met the criterion of p > 0.05, indicating that the data conformed to a normal distribution. Each group of data was presented as mean ± SEM. * p < 0.05, ** p < 0.01, and *** p < 0.001 were considered statistically significant.
4. Discussion
BAP31 is a transmembrane protein located in the ER that plays a significant role in various cellular processes, including protein trafficking, apoptosis, and ER stress responses. Given the current lack of direct evidence for the involvement of BAP31 in retinal degeneration, our study is the first to identify that retina-specific knockdown of BAP31 causes age-dependent retinal degenerative disease. However, the specific regulatory pathways still require further exploration, which also clarifies directions for future research. The significance of our study was that we used a BAP31 rod cell-specific knockdown mouse model, and systematically investigated the long-term effects of BAP31 deficiency on retinal neuronal development and visual function for the first time.
The OCT analysis revealed that the thickness of the ONL was significantly reduced in Rho-iCre-BAP31fl/fl(−/−) mice at 9 months of age, and this reduction became more severe at 12 months, which indicated progressive photoreceptor cell loss. This degenerative phenotype became more significant with advancing age, suggesting that the deterioration of retinal structure was time-dependent. ERG and OCT are standard ophthalmic tests used to assess retinal function and structure. Consistent with the results of the OCT test, the ERG results demonstrated a gradual decline in retinal function of BAP31 knockdown mice beginning at 9 months of age, with the functional impairment becoming increasingly severe over time. We used optomotor response testing to further evaluate the visual function. The results were in agreement with those of the OCT and ERG, showing that there were no significant differences in OMR between Rho-iCre-BAP31fl/fl(−/−) mice and BAP31fl/fl(+/+) mice prior to 6 months of age. However, a significant decrease in visual dynamic response indices was observed beginning at 9 months of age, with the disparity becoming increasingly evident by 12 months of age. Collectively, these findings demonstrate that retina-specific knockdown of BAP31 induces age-dependent retinal degeneration, characterized by progressive ONL thinning, photoreceptor loss, and visual function impairment. This proved that the lack of BAP31 in rod cell does not affect the early development of the retina but plays a critical role in maintaining retinal integrity during aging. The loss of BAP31 may cause the damaging retina response to chronic stress over a long time period, which may be due to the cumulative impact on cells, finally leading to the accelerated decline of visual function and the gradual deterioration of retina structures.
Transcriptomic profiling analysis of 12-month-old mice demonstrated that the specific ablation of BAP31 exerts a profound impact on the phototransduction pathway. The reduction in the expression of key phototransduction genes (
Rho,
Rcvrn,
Grk1,
Gnat1,
Gnb1,
Gngt1,
Pde6a,
Pde6b,
Pde6g,
Gucy2e,
Gucy2f,
Guca1b,
Slc24A1,
Cngb1 and
Rgs9) suggests a potential disruption in the phototransduction pathway, which could have significant implications for retinal function and vision [
39,
43]. The phototransduction pathway is a critical biochemical process by which photoreceptor cells in the retina (rods and cones) convert light into electrical signals. This pathway is essential for vision and involves a series of molecular events [
44,
45,
46]. Reduced expression of any of these genes can disrupt this process, leading to decrease in sensitivity to light, delayed response to light stimuli, and impaired vision in low-light conditions (scotopic vision). This effect may be associated with photoreceptor loss, which leads to downregulated expression of phototransduction-related genes and consequently results in visual functional impairment in the animal model.
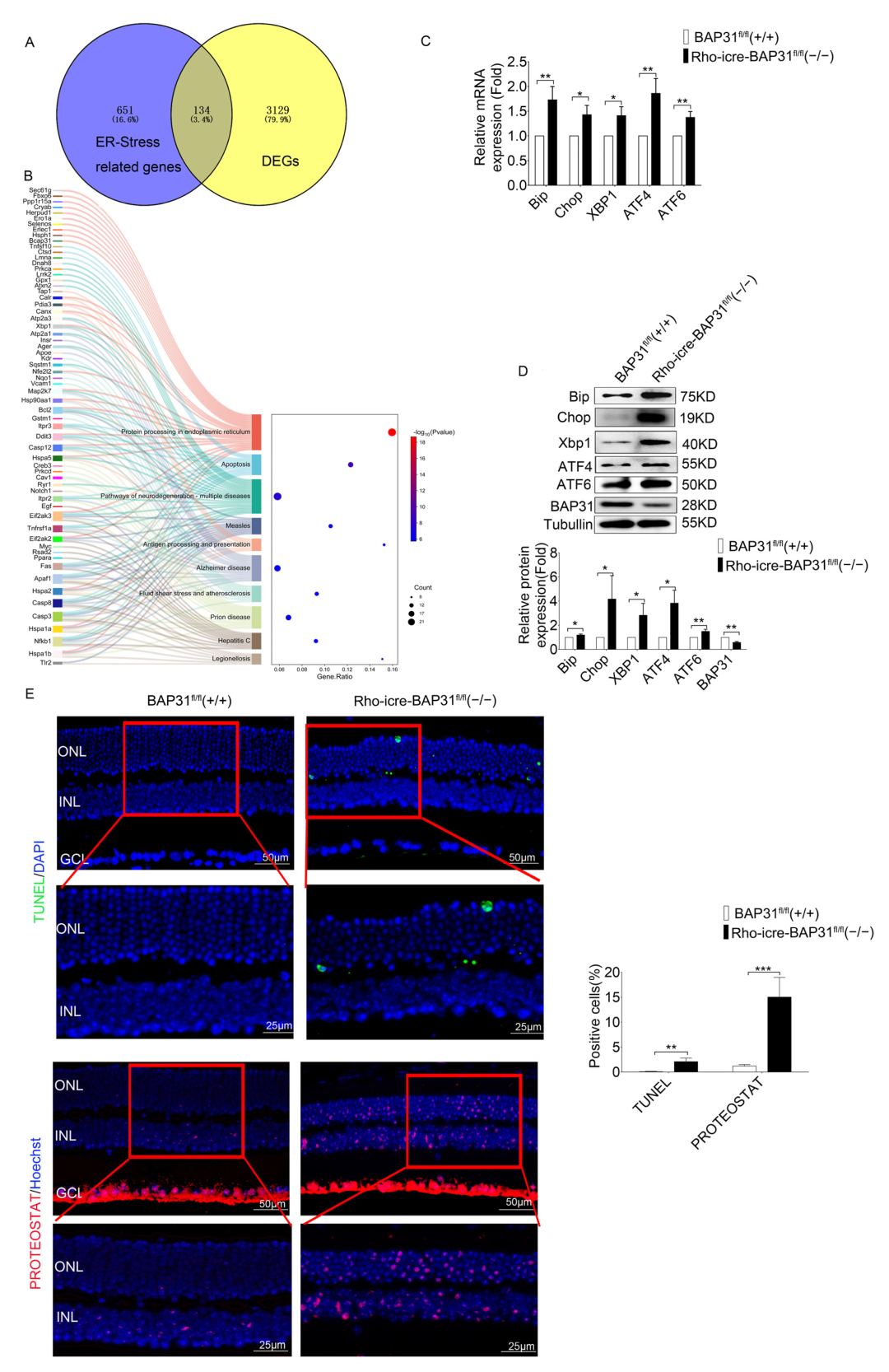
Another important finding in our study was the observation of upregulation in the expression of ER stress-associated marker genes, including Bip, Chop, Xbp1, Atf6 and Atf4, which were the core components of the ER stress pathway. The high expression of Bip, a molecular chaperone, reflected the cell’s attempt to enhance protein folding capacity and prevent aggregation of misfolded proteins. Concurrently, Chop is a pro-apoptotic transcription factor, and Atf4 is a regulator of stress-responsive genes; the increase in the two genes indicates a shift toward apoptotic signaling under prolonged ER stress conditions. Additionally, ATF6 and XBP1 are major transcription factors in the core pathway of the UPR; they both regulate protein homeostasis in cells in response to ER stress.
Collectively, these findings suggest that BAP31 may play a critical role in maintaining ER homeostasis. Our result confirms that BAP31 deficiency would impair the function of ER, leading to abnormalities in key processes such as protein folding and processing. Furthermore, ER dysfunction triggers ER stress. When this ER stress state accumulates over the long term, it ultimately induces the apoptosis of photoreceptor cells which involves the disorders of the transduction. The literature provides partial support for this mechanism; for example, 4-PBA treatment can effectively rescue the expression of phototransduction-related genes in EYS-RP cells [
47]. However, simply blocking ER stress cannot fully prevent neuroinflammation-mediated loss of retinal RGC cells [
48]. This indicates that retinal damage may be the result of the synergistic effect of multiple factors, rather than being caused by a single mechanism. Combined with existing research conclusions—that both 4-PBA and TUDCA exert their therapeutic effects in retinal degenerative diseases mainly by inhibiting ER stress, and further inhibiting apoptosis and neuroinflammation through this pathway—we infer that ER stress and apoptosis may have a synergistic effect, jointly promoting the progression of retinal damage.
Interestingly, no retinal degeneration or visual functional impairments were observed in younger animal models. Structural and functional deficits started at 9 months of age. These results were consistent with observations from previous studies, in which structural and functional defects were detected only at around 12–14 months of age after the deletion of XBP1 in the retina [
49]. A similar phenotype was also observed in Atf6(−/−) mice, where the retinal morphology and function were normal in childhood, but developed rod and cone dysfunction with increasing age until 18 months [
50]. These results suggest that BAP31, XBP1, and ATF6 deficiencies exhibit delayed-onset phenotypes, with retinal degeneration and functional decline becoming apparent only at advanced ages. This late-onset phenotype is also observed in IRE1 knockout model mice [
12]. This phenomenon is highly consistent with the previous research results of our team in the Alzheimer’s disease model [
22]. In this model, animals only showed obvious symptoms after reaching 10 months of age, and this result indicates that the simple knockout of BAP31 is not sufficient to induce pathological changes in the early stage of the disease. Although mice with ATF6, XBP1, or IRE1 gene knockout all display delayed-onset retinal phenotypes, their disease onset timelines differ significantly from our model. Specifically, the ATF6 knockout model manifests pathological changes at 18 months of age, while the XBP1 knockout model exhibits disease onset between 12 and 14 months of age. Both models exhibit later onset compared to our BAP31 knockout model (onset at 9 months of age), thus classifying them as late-stage animal models. In contrast, although the IRE1 knockout model is also categorized as delayed-onset, its disease onset occurs earlier at 6 months of age, preceding our model. Notably, the BAP31 knockdown model in our study initiates phenotypic changes at 9–12 months of age, which precisely fills the gap in the 9–12 months age-dependent onset window among ER stress-related models. This characteristic aligns more closely with the clinical onset pattern of human age-related retinal degenerative diseases, which typically commence in the late middle-aged period. Furthermore, ATF6, XBP1, and IRE1 are direct effectors of the ER stress pathway; their mutation or knockout directly disrupts key components of this pathway. In contrast, BAP31 functions as an indirect regulator localized on the ER membrane, modulating ER stress pathway homeostasis by controlling ER-associated protein transport. This mechanistic distinction highlights that our model is uniquely suited for investigating retinal degeneration induced by “non-direct pathway disruption”, thereby offering a novel perspective to decipher the diverse mechanisms underlying ER stress-mediated diseases. Collectively, the successful establishment of this BAP31 knockdown model not only advances the research framework of ER stress-related animal models but also enriches the theoretical basis for understanding how ER stress regulates retinal degenerative diseases. Previous studies have found that the fidelity of protein quality control and proteostasis regulatory mechanisms was deteriorated with advancing age in
Caenorhabditis elegans, resulting in elevated ER stress levels and the accumulation of misfolded proteins [
51,
52]. The results of our study further confirm the critical role of BAP31 in the regulation of protein processing and folding in retinal cells. The retina cells were highly susceptible to disruptions in proteostasis due to their high metabolic demands and specialized functions; however, dysfunction of BAP31 may impair ER function, causing abnormalities in key processes such as protein folding and processing, ultimately triggering ER stress. It is important to note that BAP31 is not a direct trigger of ER stress; instead, BAP31 acts as a regulator of ER function. This differs from models such as those induced by tunicamycin, rhodopsin misfolding, or Tulp1 mutants, pathogenic factors that are direct triggers of ER stress. Thus, the deficiency of BAP31 may not trigger acute ER stress immediately; rather, functional defects gradually manifest over time. Additionally, during retinal development, a compensatory mechanism may exist in the context of BAP31 deficiency, which can temporarily offset its functional defects. For example, the compensation may occur through the activation of the IRE1 and ATF6 pathways. The IRE1 could enhance the clearance efficiency of abnormal proteins by upregulating the expression of ER-associated degradation (ERAD)-related proteins [
2]; after translocating to the nucleus, ATF6 promotes the transcription of ER chaperone proteins, thereby enhancing the ability of ER to fold unfolded proteins [
53]. This compensatory mechanism is sufficient to maintain normal retinal structure and function in young animals. Only when animals age and their compensatory capacity gradually declines does retinal degeneration begin to manifest. Specifically, dysfunction of the ER may first trigger ER stress; when this stress state accumulates over the long term and exceeds the regulatory capacity of other compensatory mechanisms during development, it will eventually induce apoptosis in the photoreceptor. This process is accompanied by impairments in the phototransduction pathway, which in turn leads to visual dysfunction in animal models. Since this pathological process is associated with animal development and aging, it ultimately presents as a delayed-onset phenotype. Our results offer mechanistic insight into retinal degenerative diseases such as retinitis pigmentosa and age-related macular degeneration with the potential contribution of BAP31 dysfunction.
Consequently, our findings suggest that BAP31 is either non-essential or its deficiency can be compensated for under conditions of photoreceptor homeostasis or normal ER stress. However, BAP31 assumes increasing significance during aging when cells are subjected to heightened ER stress, emphasizing its critical role in the regulation of ER mechanisms in aged animals. It may potentially implicate shared mechanisms related to age-dependent stress responses or protein homeostasis dysregulation. While our study has shed light on the transcriptional changes in the phototransduction pathway and the ER stress pathway associated with BAP31 deficiency, the intrinsic mechanisms linking these pathways remains to be fully elucidated. Combining published research and our experimental findings, we hypothesize that the ER dysfunction caused by BAP31 deficiency may exert a synergistic effect with aging pathways. Specifically, aging accelerates ER stress-induced cellular damage, while ER stress further exacerbates the aging-related disruption of protein homeostasis; these two factors collectively drive the manifestation of phenotypes in mice aged 9–12 months. Thus, the BAP31 knockdown model serves as an ideal experimental model for studying age-related retinal neurodegenerative diseases.
Another significant feature of this study is that it provides a new animal model for investigating the pathogenesis of age-related retinal diseases. Experimental models have played a crucial role in elucidating genetic and pharmacological mechanisms, particularly in the study of familial neurodegenerative diseases. However, whether these models were suitable for explaining the etiology of age-related neurodegenerative diseases remained questionable. As is widely known, rd1 and rd10 are mouse models established for the study of retinitis pigmentosa. Both models arise from the mutations in the Pde6b gene, which trigger apoptosis of rod photoreceptors and ultimately lead to RP [
54]. However, these two models have significant limitations regarding age: rd1 exhibits an exceptionally rapid disease progression, with severe outer retinal degeneration evident as early as postnatal day 14. In contrast, at this same developmental stage, most rd10 mice display normal spectral-domain optical coherence tomography (SD-OCT) images, with only a subset showing subtle structural alterations at the inner segment/outer segment (IS/OS) junction, reflecting a comparatively slower disease course. Notably, the peak of rod photoreceptor loss in rd10 mice occurs around three weeks of age; the onset rate of the disease is also relatively fast [
55]. Given the established age correspondence between mice and humans [
56], the early onset of retinal degeneration in both models limits their suitability as representative systems for studying age-related forms of RP. In contrast, the BAP31 knockdown model developed in our study offers significant advantages. Retinal degeneration in this model begins at approximately 9 months of age (equivalent to 38–47 years in human age), which aligns closely with the characteristics of age-dependent disease onset. More importantly, therapeutic interventions in rd1 and rd10 models have primarily targeted correction of phototransduction defects, while our model highlights pathways involving regulation of endoplasmic reticulum homeostasis and enhancement of cellular compensatory mechanisms. These findings suggest a promising therapeutic avenue for late-onset RP and provide critical experimental support for the development of age-adapted treatment strategies tailored to elderly patients. In previous studies, through the overexpression of mutant proteins or the use of neurotoxins, certain pathological features of diseases could be induced in animals. However, the pathogenesis of these diseases may be different from those of age-related diseases. These experimental models have certain limitations. For instance, overexpression of mutant proteins could lead to abnormal protein aggregation and cellular stress, but this may not be fully consistent with the gradual dysregulation of protein homeostasis observed in sporadic diseases. Neurotoxins (such as MPTP and 6-OHDA) could rapidly induce pathological changes, but these phenotypes were different from the chronic progression of age-related diseases. Additionally, most experimental models used were young animals, which cannot fully replicate the cellular and molecular changes that occur during the aging process. Therefore, whether the mechanisms revealed in these models are relevant to the etiology of the majority of age-related neurodegenerative diseases remains an unresolved question. The retinal degenerative disease model induced by the specific deletion of BAP31 in our study exhibits characteristics of age-related diseases and mimics the pathogenesis of this disease. Thus, it serves as an ideal experimental model for studying age-related retinal neurodegenerative diseases.
Furthermore, recent studies have also confirmed that a programmed cell death mechanism independent of caspases—PARP1-dependent programmed cell death (parthanatos)—that plays a key role in the rd10 mouse model [
57]. Moreover, this mechanism may be independent of disease-causing mutant genes and directly involved in the death process of photoreceptor cells in RP. The core mechanism of parthanatos is that DNA damage signals trigger the recruitment and activation of poly (ADP-ribose) polymerase 1 (PARP1) in the nucleus. The abnormal activation of PARP1 has been clearly confirmed to be a key driver of retinal degeneration [
58].
Based on the findings of this study, BAP31 deficiency disrupts the membrane protein transport function of the ER, triggering persistent ER stress. We hypothesize that this chronic ER stress may also induce DNA damage, which in turn activates parthanatos. To verify this hypothesis and clarify the regulatory role of parthanatos, we will use the PARP1-specific inhibitor Olaparib to intervene in BAP31-deficient model mice in future studies. By detecting the thickness of the ONL, visual function (ERG/OMR), and cell death ratio, we will evaluate the alleviating effect of parthanatos inhibition on the retinal degeneration phenotype induced by BAP31 deficiency. Meanwhile, considering that excessive activation of PARP1 consumes a large amount of intracellular NAD+, leading to energy metabolism disorders and further aggravating cell death, we also plan to use NAD+ precursor substances (e.g., β-nicotinamide mononucleotide, NMN) for intervention in subsequent studies. By detecting the NAD+ content in retinal tissue, ER stress markers (e.g., GRP78, PERK phosphorylation), and parthanatos-related indicators (e.g., PAR polymer levels), we will observe whether NAD+ supplementation can synergistically improve ER stress and parthanatos-mediated cell death, providing a potential target reference for the clinical development of targeted intervention strategies.