Improvement of Treg Selectivity and Stability for Diabetes Mellitus Type 1 Treatment: Complex Approach for Perspective Technologies
Highlights
- The efficacy of CAR-Treg therapies for type 1 diabetes is potentially limited by the instability of their phenotype in the inflammatory microenvironment caused by proinflammatory immune cells.
- Recent studies in immunology and translational medicine are aimed at Treg phe-notype stabilization.
- A complex approach based on different methods such as the genetic engineering of cytokine signaling pathways and the cAMP cascade, the management of FOXP3 splicing to ensure stable expression of a certain splice variant, and the use of some epigenetic modifications can be applied for effective Treg stabilization.
- A synergistic strategy based on CAR and stabilizing modifications of autologous Tregs with their subsequent transplantation is promising for type 1 diabetes therapy.
Abstract
1. Introduction
2. Genetic Modifications for Tregs Stabilization Through Cytokine and cAMP Signaling Control
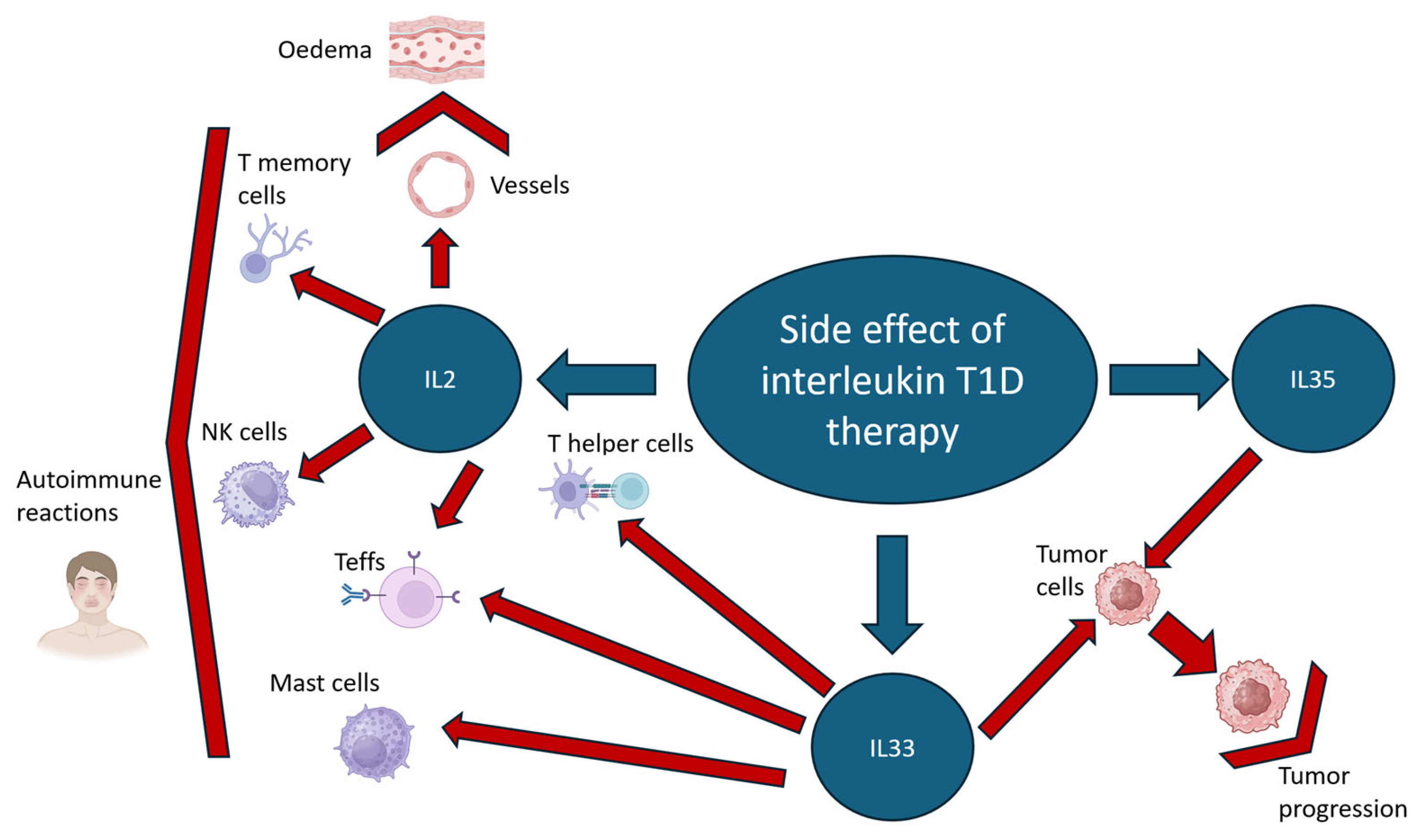
2.1. Interleukin 2 (IL2) Signaling
2.2. Interleukin 33 (IL33) Signaling
2.3. Interleukin 35 (IL35) Signaling
2.4. Cyclic AMP (cAMP) Signaling
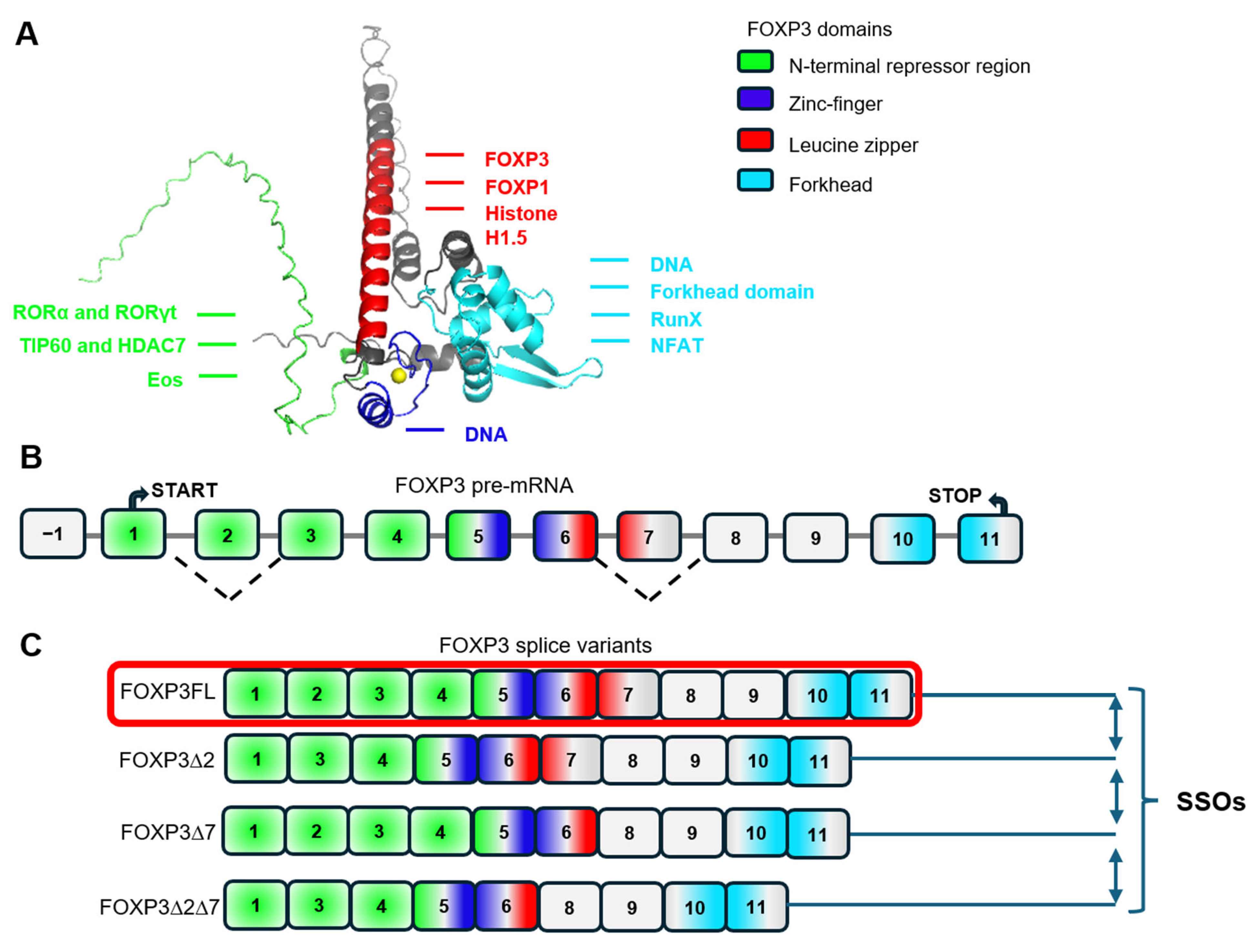
3. Modulation of Treg Stability, Suppressive and Proliferative Activity Through FOXP3 Alternative Splicing
4. Treg Stabilization by Epigenetic Modification
5. Complex Approach
6. The Application of Good Manufacturing Practice (GMP) in Genetically Modified Treg Therapy
6.1. Product Safety and Genomic Integrity
6.2. Compliance with GMP Standards
6.3. Building Trust Through Rigorous Practices
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leng, F.; Zhang, W.; Ramirez, R.N.; Leon, J.; Zhong, Y.; Hou, L.; Yuki, K.; van der Veeken, J.; Rudensky, A.Y.; Benoist, C.; et al. The transcription factor FoxP3 can fold into two dimerization states with divergent implications for regulatory T cell function and immune homeostasis. Immunity 2022, 55, 1354–1369.e8. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, L. Alternative splicing: Human disease and quantitative analysis from high-throughput sequencing. Comput. Struct. Biotechnol. J. 2021, 19, 183–195. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, L.; Wu, C. Alternative Splicing and Isoforms: From Mechanisms to Diseases. Genes 2022, 13, 401. [Google Scholar] [CrossRef]
- Hori, S. FOXP3 as a master regulator of Treg cells. Nat. Rev. Immunol. 2021, 21, 618–619. [Google Scholar] [CrossRef]
- Deng, G.; Song, X.; I Greene, M. FoxP3 in Treg cell biology: A molecular and structural perspective. Clin. Exp. Immunol. 2020, 199, 255–262. [Google Scholar] [CrossRef]
- Morgun, E.I.; Govorova, I.A.; Chernysheva, M.B.; Machinskaya, M.A.; Vorotelyak, E.A. Mini-Review: Tregs as a Tool for Therapy—Obvious and Non-Obvious Challenges and Solutions. Cells 2024, 13, 1680. [Google Scholar] [CrossRef]
- Marek-Trzonkowska, N.; Myśliwiec, M.; Iwaszkiewicz-Grześ, D.; Gliwiński, M.; Derkowska, I.; Żalińska, M.; Zieliński, M.; Grabowska, M.; Zielińska, H.; Piekarska, K.; et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J. Transl. Med. 2016, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Marek-Trzonkowska, N.; Myśliwiec, M.; Dobyszuk, A.; Grabowska, M.; Derkowska, I.; Juścińska, J.; Owczuk, R.; Szadkowska, A.; Witkowski, P.; Młynarski, W.; et al. Therapy of type 1 diabetes with CD4+CD25highCD127-regulatory T cells prolongs survival of pancreatic islets—Results of one year follow-up. Clin. Immunol. 2014, 153, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef] [PubMed]
- Tenspolde, M.; Zimmermann, K.; Weber, L.C.; Hapke, M.; Lieber, M.; Dywicki, J.; Frenzel, A.; Hust, M.; Galla, M.; Buitrago-Molina, L.E.; et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J. Autoimmun. 2019, 103, 102289. [Google Scholar] [CrossRef]
- Spanier, J.A.; Fung, V.; Wardell, C.M.; Alkhatib, M.H.; Chen, Y.; Swanson, L.A.; Dwyer, A.J.; Weno, M.E.; Silva, N.; Mitchell, J.S.; et al. Insulin B peptide-MHC class II-specific chimeric antigen receptor-Tregs prevent autoimmune diabetes. J. Clin. Investig. 2023, 133, e168601. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.; Jaume, J. MON-LB033 Unleashing the Anti-Inflammatory Potential of Treg Cells Against Type I Diabetes Using Advanced Chimeric Antigen Receptor Technology. J. Endocr. Soc. 2019, 3, MON-LB033. [Google Scholar] [CrossRef]
- Radichev, I.A.; Yoon, J.; Scott, D.W.; Griffin, K.; Savinov, A.Y. Towards antigen-specific Tregs for type 1 diabetes: Construction and functional assessment of pancreatic endocrine marker, HPi2-based chimeric antigen receptor. Cell. Immunol. 2020, 358, 104224. [Google Scholar] [CrossRef] [PubMed]
- Barra, J.M.; Robino, R.A.; Castro-Gutierrez, R.; Proia, J.; Russ, H.A.; Ferreira, L.M. Combinatorial genetic engineering strategy for immune protection of stem cell-derived beta cells by chimeric antigen receptor regulatory T cells. Cell Rep. 2024, 43, 114994. [Google Scholar] [CrossRef]
- Tan, T.G.; Mathis, D.; Benoist, C. Singular role for T-BET+ CXCR3+ regulatory T cells in protection from autoimmune diabetes. Proc. Natl. Acad. Sci. USA 2016, 113, 14103–14108. [Google Scholar] [CrossRef]
- McClymont, S.A.; Putnam, A.L.; Lee, M.R.; Esensten, J.H.; Liu, W.; A Hulme, M.; Hoffmüller, U.; Baron, U.; Olek, S.; A Bluestone, J.; et al. Plasticity of Human Regulatory T Cells in Healthy Subjects and Patients with Type 1 Diabetes. J. Immunol. 2011, 186, 3918–3926. [Google Scholar] [CrossRef]
- Kressler, C.; Gasparoni, G.; Nordström, K.; Hamo, D.; Salhab, A.; Dimitropoulos, C.; Tierling, S.; Reinke, P.; Volk, H.-D.; Walter, J.; et al. Targeted De-Methylation of the FOXP3-TSDR is Sufficient to Induce Physiological FOXP3 Expression but Not a Functional Treg Phenotype. Front. Immunol. 2021, 11, 609891. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef]
- Eliseeva, D.D.; Lifshitz, G.V.; Lokhonina, A.V.; Zhdanov, D.D.; Zavalishin, I.A.; Bykovskaia, S.N. The treatment by expanded ex vivo autologous regulatory T-cells CD4+CD25+FoxP3+CD127low restores the balance of immune system in patients with remitting-relapsing multiple sclerosis. Zhurnal Nevrol. I Psikhiatrii Im. SS Korsakova 2016, 116, 54. [Google Scholar] [CrossRef]
- Boder, E.T. Tighter ties that bind. Nature 2012, 484, 463–464. [Google Scholar] [CrossRef]
- DiToro, D.; Winstead, C.J.; Pham, D.; Witte, S.; Andargachew, R.; Singer, J.R.; Wilson, C.G.; Zindl, C.L.; Luther, R.J.; Silberger, D.J.; et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 2018, 361, eaao2933. [Google Scholar] [CrossRef]
- Baeyens, A.; Saadoun, D.; Billiard, F.; Rouers, A.; Grégoire, S.; Zaragoza, B.; Grinberg-Bleyer, Y.; Marodon, G.; Piaggio, E.; Salomon, B.L. Effector T Cells Boost Regulatory T Cell Expansion by IL-2, TNF, OX40, and Plasmacytoid Dendritic Cells Depending on the Immune Context. J. Immunol. 2015, 194, 999–1010. [Google Scholar] [CrossRef]
- Raeber, M.E.; Rosalia, R.A.; Schmid, D.; Karakus, U.; Boyman, O. Interleukin-2 signals converge in a lymphoid–dendritic cell pathway that promotes anticancer immunity. Sci. Transl. Med. 2020, 12, eaba5464. [Google Scholar] [CrossRef]
- Churlaud, G.; Jimenez, V.; Ruberte, J.; Zin, M.A.; Fourcade, G.; Gottrand, G.; Casana, E.; Lambrecht, B.; Bellier, B.; Piaggio, E.; et al. Sustained stimulation and expansion of Tregs by IL2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin. Immunol. 2014, 151, 114–126. [Google Scholar] [CrossRef]
- Lykhopiy, V.; Malviya, V.; Humblet-Baron, S.; Schlenner, S.M. IL-2 immunotherapy for targeting regulatory T cells in autoimmunity. Genes Immun. 2023, 24, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Krieg, C.; Létourneau, S.; Pantaleo, G.; Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11906–11911. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.; Henschel, P.; Simon, D.; Riet, T.; Falk, C.; Hardtke-Wolenski, M.; Wedemeyer, H.; Noyan, F.; Jaeckel, E. Membrane-bound IL-2 improves the expansion, survival, and phenotype of CAR Tregs and confers resistance to calcineurin inhibitors. Front. Immunol. 2022, 13, 1005582. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.L.; Bolivar-Wagers, S.; Jin, S.; Thangavelu, G.; Simonetta, F.; Lin, P.-Y.; Hirai, T.; Saha, A.; Koehn, B.; Su, L.L.; et al. Prevention of acute GVHD using an orthogonal IL-2/IL-2Rβ system to selectively expand regulatory T cells in vivo. Blood 2023, 141, 1337–1352. [Google Scholar] [CrossRef]
- Peine, M.; Marek, R.M.; Löhning, M. IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends Immunol. 2016, 37, 321–333. [Google Scholar] [CrossRef]
- Pavlovic, S.; Petrovic, I.; Jovicic, N.; Ljujic, B.; Kovacevic, M.M.; Arsenijevic, N.; Lukic, M.L. IL-33 Prevents MLD-STZ Induction of Diabetes and Attenuate Insulitis in Prediabetic NOD Mice. Front. Immunol. 2018, 9, 2646. [Google Scholar] [CrossRef]
- Ryba-Stanisławowska, M.; Buksa, L.; Brandt, A.; Juhas, U.; Myśliwiec, M. IL-33 improves the suppressive potential of regulatory T cells in patients with type 1 diabetes. Diabetes Res. Clin. Pract. 2017, 128, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.; Liu, Z. Role of IL-33-ST2 pathway in regulating inflammation: Current evidence and future perspectives. J. Transl. Med. 2023, 21, 902. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef]
- Schiering, C.; Krausgruber, T.; Chomka, A.; Fröhlich, A.; Adelmann, K.; Wohlfert, E.A.; Pott, J.; Griseri, T.; Bollrath, J.; Hegazy, A.N.; et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014, 513, 564–568. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Moro, K.; Xin, A.; Liao, Y.; Gloury, R.; Kawamoto, S.; Fagarasan, S.; A Mielke, L.; Afshar-Sterle, S.; Masters, S.L.; et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue–resident regulatory T cells. Nat. Immunol. 2015, 16, 276–285. [Google Scholar] [CrossRef]
- Popovic, B.; Golemac, M.; Podlech, J.; Zeleznjak, J.; Bilic-Zulle, L.; Lukic, M.L.; Cicin-Sain, L.; Reddehase, M.J.; Sparwasser, T.; Krmpotic, A.; et al. IL-33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PLoS Pathog. 2017, 13, e1006345. [Google Scholar] [CrossRef] [PubMed]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef]
- Siede, J.; Fröhlich, A.; Datsi, A.; Hegazy, A.N.; Varga, D.V.; Holecska, V.; Saito, H.; Nakae, S.; Löhning, M. IL-33 Receptor-Expressing Regulatory T Cells Are Highly Activated, Th2 Biased and Suppress CD4 T Cell Proliferation through IL-10 and TGFβ Release. PLoS ONE 2016, 11, e0161507. [Google Scholar] [CrossRef]
- Lam, A.J.; MacDonald, K.N.; Pesenacker, A.M.; Juvet, S.C.; Morishita, K.A.; Bressler, B.; Pan, J.G.; Sidhu, S.S.; Rioux, J.D.; Levings, M. K et al. Innate Control of Tissue-Reparative Human Regulatory T Cells. J. Immunol. 2019, 202, 2195–2209. [Google Scholar] [CrossRef]
- Hattori, K.; Tanaka, S.; Hashiba, D.; Tamura, J.; Etori, K.; Kageyama, T.; Ito, T.; Meguro, K.; Iwata, A.; Suto, A.; et al. Synovial regulatory T cells expressing ST2 deteriorate joint inflammation through the suppression of immunoregulatory eosinophils. J. Autoimmun. 2024, 149, 103333. [Google Scholar] [CrossRef]
- Battut, L.; Leveque, E.; Valitutti, S.; Cenac, N.; Dietrich, G.; Espinosa, E. IL-33-primed human mast cells drive IL-9 production by CD4+ effector T cells in an OX40L-dependent manner. Front. Immunol. 2024, 15, 1470546. [Google Scholar] [CrossRef] [PubMed]
- Selvan, R.S.; Nagarkatti, P.S.; Nagarkatti, M. Role of IL-2, IL-4 and IL-6 in the growth and differentiation of tumor-specific CD4+ T helper and CD8+ T cytotoxic cells. Int. J. Cancer 1990, 45, 1096–1104. [Google Scholar] [CrossRef]
- Cristinziano, L.; Poto, R.; Criscuolo, G.; Ferrara, A.L.; Galdiero, M.R.; Modestino, L.; Loffredo, S.; de Paulis, A.; Marone, G.; Spadaro, G.; et al. IL-33 and Superantigenic Activation of Human Lung Mast Cells Induce the Release of Angiogenic and Lymphangiogenic Factors. Cells 2021, 10, 145. [Google Scholar] [CrossRef]
- Zhang, Y.; Davis, C.; Shah, S.; Hughes, D.; Ryan, J.C.; Altomare, D.; Peña, M.M.O. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis: IL-33 Promotes Colon Cancer Growth and Liver Metastasis. Mol. Carcinog. 2017, 56, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. IL-12 Family Cytokines: General Characteristics, Pathogenic Microorganisms, Receptors, and Signalling Pathways. Acta Microbiol. Immunol. Hung. 2016, 63, 1–25. [Google Scholar] [CrossRef]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef]
- Huang, A.; Cheng, L.; He, M.; Nie, J.; Wang, J.; Jiang, K. Interleukin-35 on B cell and T cell induction and regulation. J. Inflamm. 2017, 14, 16. [Google Scholar] [CrossRef]
- Huang, A.; Liu, K.; Yin, Z.; Liu, J.; Wei, H.; Xing, S.; Qu, Y.; Huang, L.; Li, L.; Li, C.; et al. IL-35 Stabilizes Treg Phenotype to Protect Cardiac Allografts in Mice. Transplantation 2024, 108, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Deng, K.; Chong, W.P. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front. Immunol. 2024, 15, 1387975. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kadesjö, E.; Lindroos, J.; Hjort, M.; Lundberg, M.; Espes, D.; Carlsson, P.-O.; Sandler, S.; Thorvaldson, L. Interleukin-35 administration counteracts established murine type 1 diabetes-possible involvement of regulatory T cells. Sci. Rep. 2015, 5, 12633. [Google Scholar] [CrossRef]
- Hu, S.; Lian, P.-P.; Hu, Y.; Zhu, X.-Y.; Jiang, S.-W.; Ma, Q.; Li, L.-Y.; Yang, J.-F.; Yang, L.; Guo, H.-Y.; et al. The Role of IL-35 in the Pathophysiological Processes of Liver Disease. Front. Pharmacol. 2021, 11, 569575. [Google Scholar] [CrossRef]
- Zou, J.-M.; Qin, J.; Li, Y.-C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.-S.; Chi, G.; Guo, F.; et al. IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 2017, 8, 33501–33514. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Huang, A.; Nie, J.; Tan, J.; Xing, S.; Qu, Y.; Jiang, K. IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front. Immunol. 2021, 12, 683332. [Google Scholar] [CrossRef]
- Anandagoda, N.; Willis, J.C.; Hertweck, A.; Roberts, L.B.; Jackson, I.; Gökmen, M.R.; Jenner, R.G.; Howard, J.K.; Lord, G.M. microRNA-142–mediated repression of phosphodiesterase 3B critically regulates peripheral immune tolerance. J. Clin. Investig. 2019, 129, 1257–1271. [Google Scholar] [CrossRef]
- Gavin, M.A.; Torgerson, T.R.; Houston, E.; Deroos, P.; Ho, W.Y.; Stray-Pedersen, A.; Ocheltree, E.L.; Greenberg, P.D.; Ochs, H.D.; Rudensky, A.Y. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl. Acad. Sci. USA 2006, 103, 6659–6664. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, J.; Lei, Z.; Shen, S.; Li, D.; Shen, G.; Zhang, G.; Feng, Z. miR-142-3p restricts cAMP production in CD4+ CD25− T cells and CD4+ CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009, 10, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bopp, T.; Dehzad, N.; Reuter, S.; Klein, M.; Ullrich, N.; Stassen, M.; Schild, H.; Buhl, R.; Schmitt, E.; Taube, C. Inhibition of cAMP Degradation Improves Regulatory T Cell-Mediated Suppression. J. Immunol. 2009, 182, 4017–4024. [Google Scholar] [CrossRef]
- Kim, H.-P.; Leonard, W.J. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: A role for DNA methylation. J. Exp. Med. 2007, 204, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, S.H.; Hriczko, J.T.; Schulz, S.; Look, T.; Goodarzi, T.; Clarner, T.; Scheld, M.; Kipp, M.; Verjans, E.; Böll, S.; et al. CREB regulates Foxp3+ST-2+ TREGS with enhanced IL-10 production. Front. Immunol. 2025, 16, 1601008. [Google Scholar] [CrossRef]
- Pagni, P.P.; Chaplin, J.; Wijaranakula, M.; Wesley, J.D.; Granger, J.; Cracraft, J.; O’Brien, C.; Perdue, N.; Kumar, V.; Li, S.; et al. Multicomponent Plasmid Protects Mice from Spontaneous Autoimmune Diabetes. Diabetes 2022, 71, 157–169. [Google Scholar] [CrossRef]
- Lopes, J.E.; Torgerson, T.R.; Schubert, L.A.; Anover, S.D.; Ocheltree, E.L.; Ochs, H.D.; Ziegler, S.F. Analysis of FOXP3 Reveals Multiple Domains Required for Its Function as a Transcriptional Repressor. J. Immunol. 2006, 177, 3133–3142. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.-L.; Liu, S.; Dahlberg, C.I.; Mailer, R.K.; Westerberg, L.S.; Andersson, J. Foxp3 lacking exons 2 and 7 is unable to confer suppressive ability to regulatory T cells in vivo. J. Autoimmun. 2015, 63, 23–30. [Google Scholar] [CrossRef]
- Mailer, R.K.W.; Falk, K.; Rötzschke, O. Absence of Leucine Zipper in the Natural FOXP3Δ2Δ7 Isoform Does Not Affect Dimerization but Abrogates Suppressive Capacity. PLoS ONE 2009, 4, e6104. [Google Scholar] [CrossRef]
- Weinstein, K.N.; Domeier, P.P.; Ziegler, S.F. A splice of life: The discovery, function, and clinical implications of FOXP3 isoforms in autoimmune disease. Int. Immunol. 2024, 37, 83–90. [Google Scholar] [CrossRef]
- Blinova, V.G.; Novachly, N.S.; Gippius, S.N.; Hilal, A.; Gladilina, Y.A.; Eliseeva, D.D.; Zhdanov, D.D. Phenotypical and Functional Characteristics of Human Regulatory T Cells during Ex Vivo Maturation from CD4+ T Lymphocytes. Appl. Sci. 2021, 11, 5776. [Google Scholar] [CrossRef]
- Blinova, V.G.; Gladilina, Y.A.; Abramova, A.A.; Eliseeva, D.D.; Vtorushina, V.V.; Shishparenok, A.N.; Zhdanov, D.D. Modulation of Suppressive Activity and Proliferation of Human Regulatory T Cells by Splice-Switching Oligonucleotides Targeting FoxP3 Pre-mRNA. Cells 2023, 13, 77. [Google Scholar] [CrossRef]
- Zhdanov, D.D.; Gladilina, Y.A.; Blinova, V.G.; Abramova, A.A.; Shishparenok, A.N.; Eliseeva, D.D. Induction of FoxP3 Pre-mRNA Alternative Splicing to Enhance the Suppressive Activity of Regulatory T Cells from Amyotrophic Lateral Sclerosis Patients. Biomedicines 2024, 12, 1022. [Google Scholar] [CrossRef]
- Du, J.; Wang, Q.; Yang, S.; Chen, S.; Fu, Y.; Spath, S.; Domeier, P.; Hagin, D.; Anover-Sombke, S.; Haouili, M.; et al. FOXP3 exon 2 controls Treg stability and autoimmunity. Sci. Immunol. 2022, 7, eabo5407. [Google Scholar] [CrossRef]
- Serena, G.; Yan, S.; Camhi, S.; Patel, S.; Lima, R.S.; Sapone, A.; Leonard, M.M.; Mukherjee, R.; Nath, B.J.; Lammers, K.M.; et al. Proinflammatory cytokine interferon-γ and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin. Exp. Immunol. 2017, 187, 490–506. [Google Scholar] [CrossRef]
- Crooke, S.T.; Liang, X.-H.; Baker, B.F.; Crooke, R.M. Antisense technology: A review. J. Biol. Chem. 2021, 296, 100416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Josefowicz, S.; Chaudhry, A.; Peng, X.P.; Forbush, K.; Rudensky, A.Y. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 2010, 463, 808–812. [Google Scholar] [CrossRef]
- Polansky, J.K.; Schreiber, L.; Thelemann, C.; Ludwig, L.; Krüger, M.; Baumgrass, R.; Cording, S.; Floess, S.; Hamann, A.; Huehn, J. Methylation matters: Binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J. Mol. Med. 2010, 88, 1029–1040. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; Zong, X.; He, M.; Fan, Y.; Cross, R.; Hanna, J.H.; Feng, Y. DNA demethylation switches the drivers of Foxp3 expression to maintain regulatory T cell identity. Cell Rep. 2021, 37, 110124. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Arvey, A.; Chinen, T.; van der Veeken, J.; Gasteiger, G.; Rudensky, A.Y. Control of the Inheritance of Regulatory T Cell Identity by a cis Element in the Foxp3 Locus. Cell 2014, 158, 749–763. [Google Scholar] [CrossRef]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.-D.; Bopp, T.; Schmitt, E.; et al. Epigenetic Control of the foxp3 Locus in Regulatory T Cells. PLoS Biol. 2007, 5, e38. [Google Scholar] [CrossRef]
- Ngalamika, O.; Liang, G.; Zhao, M.; Yu, X.; Yang, Y.; Yin, H.; Liu, Y.; Yung, S.; Chan, T.M.; Lu, Q. Peripheral whole blood FOXP3 TSDR methylation: A potential marker in severity assessment of autoimmune diseases and chronic infections. Immunol. Investig. 2015, 44, 126–136. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, Y.; Liu, Y.; Zhang, B.; Li, X.; Guo, F.; Zhao, Y. Induction of Foxp3 demethylation increases regulatory CD4+CD25+ T cells and prevents the occurrence of diabetes in mice. J. Mol. Med. 2009, 87, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.D.; Chandel, N.S. Immunometabolism of pro-repair cells. J. Clin. Investig. 2019, 129, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Arvey, A.; van der Veeken, J.; Plitas, G.; Rich, S.S.; Concannon, P.; Rudensky, A.Y. Genetic and epigenetic variation in the lineage specification of regulatory T cells. eLife 2015, 4, e07571. [Google Scholar] [CrossRef]
- Tanuma, S.-I.; Kawashima, K.; Endo, H. Comparison of ADP-ribosylation of chromosomal proteins between intact and broken cells. Biochem. Biophys. Res. Commun. 1985, 127, 896–902. [Google Scholar] [CrossRef]
- Dahiya, S.; Beier, U.H.; Wang, L.; Han, R.; Jiao, J.; Akimova, T.; Angelin, A.; Wallace, D.C.; Hancock, W.W. HDAC10 deletion promotes Foxp3+ T-regulatory cell function. Sci. Rep. 2020, 10, 424. [Google Scholar] [CrossRef]
- Nikolouli, E.; Hardtke-Wolenski, M.; Hapke, M.; Beckstette, M.; Geffers, R.; Floess, S.; Jaeckel, E.; Huehn, J. Alloantigen-Induced Regulatory T Cells Generated in Presence of Vitamin C Display Enhanced Stability of Foxp3 Expression and Promote Skin Allograft Acceptance. Front. Immunol. 2017, 8, 748. [Google Scholar] [CrossRef]
- Marek-Trzonkowska, N.; Piekarska, K.; Filipowicz, N.; Piotrowski, A.; Gucwa, M.; Vogt, K.; Sawitzki, B.; Siebert, J.; Trzonkowski, P. Mild hypothermia provides Treg stability. Sci. Rep. 2017, 7, 11915. [Google Scholar] [CrossRef]
- Joudi, A.M.; Flores, C.P.R.; Singer, B.D. Epigenetic Control of Regulatory T Cell Stability and Function: Implications for Translation. Front. Immunol. 2022, 13, 861607. [Google Scholar] [CrossRef]
- Salazar-Fontana, L.I. A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges with Current Regulatory Expectations. Front. Med. 2022, 9, 855100. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.A.M.; Dijkgraaf, E.M.; Battaglia, A.; Beckhove, P.; Britten, C.M.; Gallimore, A.; Godkin, A.; Gouttefangeas, C.; de Gruijl, T.D.; Koenen, H.J.P.M.; et al. Monitoring regulatory T cells in clinical samples: Consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol. Immunother. 2015, 64, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Vignali, D.A.A. In vitro Treg suppression assays. Methods Mol. Biol. 2011, 707, 21–37. [Google Scholar] [CrossRef]
- Koopman, G.; Reutelingsperger, C.P.; Kuijten, G.A.; Keehnen, R.M.; Pals, S.T.; van Oers, M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, F.; Cossarizza, A.; Hayday, A.C. To Ki or Not to Ki: Re-Evaluating the Use and Potentials of Ki-67 for T Cell Analysis. Front. Immunol. 2021, 12, 653974. [Google Scholar] [CrossRef]
- Pandiyan, P.; Zhu, J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine 2015, 76, 13–24. [Google Scholar] [CrossRef]
- Ortega-Mejia, I.; Romero-López, N.; Casasola-Vargas, J.; Burgos-Vargas, R.; Domínguez-López, M.; Romero-López, J. Treg cell plasticity as a driver of inflammation in spondyloarthritis and psoriasis. Front. Immunol. 2025, 16, 1621396. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Medeiros, L.J.; Young, K.H. Diagnostic and predictive biomarkers for lymphoma diagnosis and treatment in the era of precision medicine. Mod. Pathol. 2016, 29, 1118–1142. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays: Methods and Protocols; Gilbert, D.F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar] [CrossRef]
- Salmikangas, P.; Chamberlain, P.; Lima, B.S.; Toivonen, M. Immunogenicity of advanced therapy medicinal products: Risk factors and mitigation measures. Cell Gene Ther. Insights 2019, 5, 829–857. [Google Scholar] [CrossRef]
- Cell Culture: Parameters for Healthy Cells. Available online: https://ibidi.com/content/435-parameters-for-healthy-cells (accessed on 5 November 2025).
- Salmons, B.; Hauser, O.; Günzburg, W.; Tabotta, W. GMP Production of an Encapsulated Cell Therapy Product: Issues and Considerations. BioProcess. J. 2007, 6, 37–44. [Google Scholar] [CrossRef]
- Pörtner, R.; Parida, S.K.; Schaffer, C.; Hoffmeister, H. Landscape of Manufacturing Process of ATMP Cell Therapy Products for Unmet Clinical Needs. In Stem Cells in Clinical Practice and Tissue Engineering; Sharma, R., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Weber, T.; Malakpour-Permlid, A.; Chary, A.; D’alessandro, V.; Haut, L.; Seufert, S.; Wenzel, E.V.; Hickman, J.; Bieback, K.; Wiest, J.; et al. Fetal bovine serum: How to leave it behind in the pursuit of more reliable science. Front. Toxicol. 2025, 7, 1612903. [Google Scholar] [CrossRef]
- Narula, P.; Lokshman, M.K.; Pathak, S.B.; Mukherjee, S.; Banerjee, M. Chemical inactivation of two non-enveloped viruses results in distinct thermal unfolding patterns and morphological alterations. BMC Microbiol. 2024, 24, 413. [Google Scholar] [CrossRef] [PubMed]
- Poletti, V.; Mavilio, F. Interactions between Retroviruses and the Host Cell Genome. Mol. Ther. Methods Clin. Dev. 2018, 8, 31–41. [Google Scholar] [CrossRef] [PubMed]

| Epitope/Antigen | Study Date | Mouse Strain | Model In Vivo | Results/Efficiency | Reference |
|---|---|---|---|---|---|
| Insulin | 2019 | NOD/LtJ | Spontaneous autoimmune diabetes | Insulin-specific CAR-Tregs were functional in vitro but failed to prevent diabetes in NOD/LtJ mice. | Tenspolde et al., 2019 [10] |
| HPi2 (pancreatic marker) | 2020 | - | - | HPi2-specific CAR-Tregs failed due to off-target CD98 binding and consequent exhaustion | Radichev et al., 2020 [13] |
| EGFRt | 2024 | NSG | Graft rejection was modeled in NSG mice by challenging established EGFRt-sBC transplants with an adoptive immune transfer of CAR-Teffs ± CAR-Tregs. | EGFRt-specific CAR-Tregs, generated against an engineered inert target on hPSCs, demonstrated potent suppression of innate and adaptive immune responses in vitro and completely prevented the immune rejection of stem cell-derived pancreatic beta-like cell grafts in vivo. | Barra et al., 2024 [14] |
| Insulin beta chain (AA 10-23) | 2023 | NOD | Spontaneous autoimmune diabetes Diabetes induced in immunodeficient NOD mice by BDC2.5 T cell transfer | CAR-Treg therapy completely prevented diabetes in both models, showing stability and a potent suppressive effect. | Spanier et al., 2023 [11] |
| Signaling Pathway | Methodology | The Result of Therapy | References |
|---|---|---|---|
| IL2 | Overexpression of IL2 by adenovirus | Prevention T1D development in NOD mice | Churlaud et al., 2014 [24] |
| Creation of a vaccine based on autologous T-regs after culturing in the presence of IL2, antibodies to CD3/CD28, TGF-β | The rate of T1D exacerbations more than halved, and the EDSS score increased by about 10%. | Eliseeva et al., 2016 [19] | |
| Introduction of a plasmid expressing proinsulin 2 and a combination of immunomodulatory cytokines (transforming growth factor-β1, interleukin IL10 and IL2). | Reduction in the incidence of T1D development in the NOD line of mice prone to this disease to 0 | Pagni et al., 2022 [60] | |
| IL33 | Direct injection of IL33 | Prevention of T1D development in lymph nodes and pancreatic islets in a streptoztocin-induced T1D model through increased ST2+Foxp3+ Treg proliferation | Pavlovic et al., 2018 [30] |
| Il35 | Direct injection of exogenous IL35 into c T1D mice | T-reg stabilization | Singh et al., 2015 [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riabinin, A.A.; Zhdanov, D.D.; Blinova, V.G.; Permyakova, A.A.; Stulova, A.A.; Rzhanova, L.A.; Nikitochkina, S.Y.; Morgun, E.I.; Vorotelyak, E.A. Improvement of Treg Selectivity and Stability for Diabetes Mellitus Type 1 Treatment: Complex Approach for Perspective Technologies. Cells 2025, 14, 1803. https://doi.org/10.3390/cells14221803
Riabinin AA, Zhdanov DD, Blinova VG, Permyakova AA, Stulova AA, Rzhanova LA, Nikitochkina SY, Morgun EI, Vorotelyak EA. Improvement of Treg Selectivity and Stability for Diabetes Mellitus Type 1 Treatment: Complex Approach for Perspective Technologies. Cells. 2025; 14(22):1803. https://doi.org/10.3390/cells14221803
Chicago/Turabian StyleRiabinin, Andrei A., Dmitry D. Zhdanov, Varvara G. Blinova, Alena A. Permyakova, Alina A. Stulova, Lyubov A. Rzhanova, Sofya Y. Nikitochkina, Elena I. Morgun, and Ekaterina A. Vorotelyak. 2025. "Improvement of Treg Selectivity and Stability for Diabetes Mellitus Type 1 Treatment: Complex Approach for Perspective Technologies" Cells 14, no. 22: 1803. https://doi.org/10.3390/cells14221803
APA StyleRiabinin, A. A., Zhdanov, D. D., Blinova, V. G., Permyakova, A. A., Stulova, A. A., Rzhanova, L. A., Nikitochkina, S. Y., Morgun, E. I., & Vorotelyak, E. A. (2025). Improvement of Treg Selectivity and Stability for Diabetes Mellitus Type 1 Treatment: Complex Approach for Perspective Technologies. Cells, 14(22), 1803. https://doi.org/10.3390/cells14221803







