Retinal Neurovascular Coupling: From Mechanisms to a Diagnostic Window into Brain Disorders
Abstract
1. Introduction
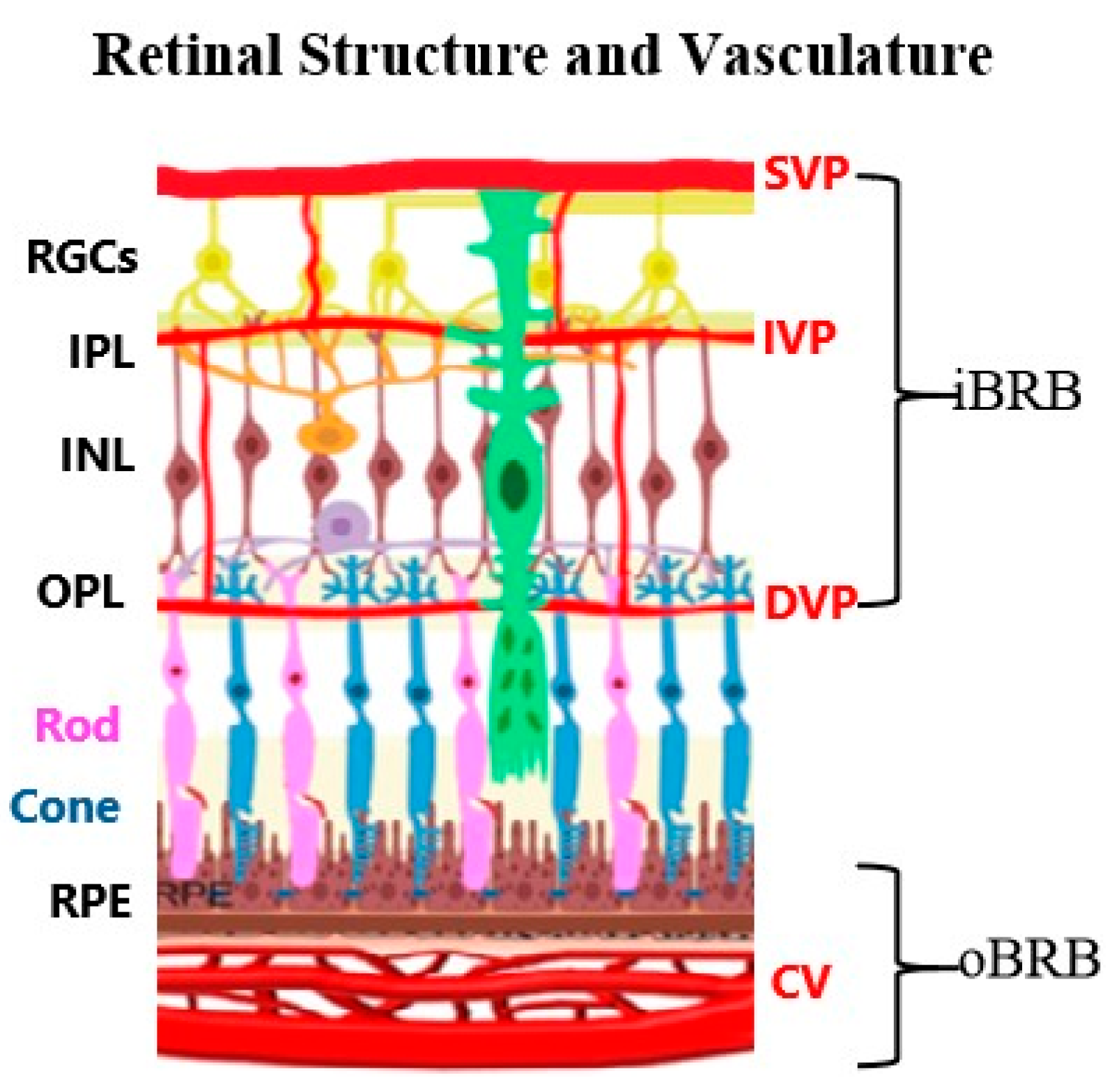
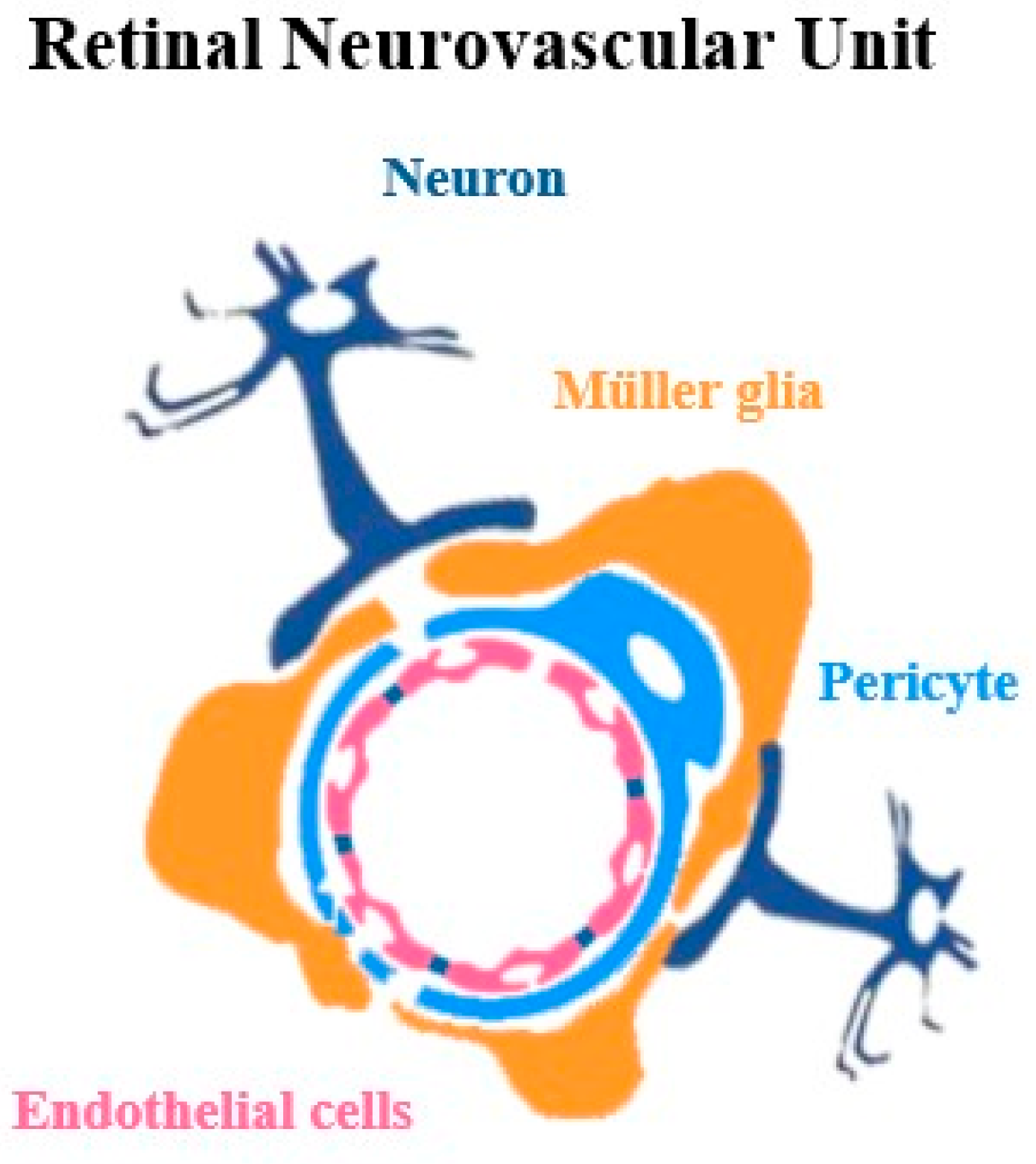
2. Retinal Neurovascular Unit: Mechanism and Diversity
2.1. Cellular Components of Neurovascular Unit
2.2. Heterogeneity of Neurovascular Unit
3. Signal Pathways of Neurovascular Coupling
3.1. NO Signaling Pathways
3.2. Signaling Pathways for ATP in Vasodilation and Constriction
3.3. Ions in Regulation of Blood Flow
3.4. VEGF Signaling Pathways
3.5. Signal Pathways of Pericytes
4. Neurovascular Coupling Dysfunction in Disease
4.1. Retinal Structure and Neurovascular Alterations in Alzheimer’s Disease
4.2. Using Biomarkers to Detect Early Signs of Parkinson’s Disease and Dementia
4.3. Detecting Huntington’s Disease Through the Retina
4.4. Retinal Structure Changes in Stroke
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NVU | Neurovascular unit |
| RGCs | Retinal ganglion cells |
| iBRB | inner blood–retina barrier |
| oBRB | outer blood–retina barrier |
| BBB | Blood–brain barrier |
| OCT | Optical coherence tomography |
| OCTA | Optical coherence tomography |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| ATP | Adenosine triphosphate |
| cAMP | Adenosine monophosphate |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| MAPK | Mitogen-activated protein kinase |
| PLC | Phospholipase C |
| PGE2 | prostaglandin E2 |
| EETs | epoxyeicosatrienoic acids |
References
- Alkayed, N.J.; Cipolla, M.J. The Ever-Evolving Concept of the Neurovascular Unit. Stroke 2023, 54, 2178–2180. [Google Scholar] [CrossRef]
- Grimes, W.N.; Berson, D.M.; Sabnis, A.; Hoon, M.; Sinha, R.; Tian, H.; Diamond, J.S. The retina’s neurovascular unit: Müller glial sheaths and neuronal contacts. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Eye: Anatomy, physiology and barriers to drug delivery. In Ocular Transporters and Receptors: Their Role in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–36. [Google Scholar] [CrossRef]
- Agarwal, N.; Carare, R.O. Cerebral Vessels: An Overview of Anatomy, Physiology, and Role in the Drainage of Fluids and Solutes. Front. Neurol. 2021, 11, 611485. [Google Scholar] [CrossRef] [PubMed]
- Grimes, W.N.; Berson, D.M.; Sabnis, A.; Hoon, M.; Sinha, R.; Tian, H.; Diamond, J.S. Layer-specific anatomical and physiological features of the retina’s neurovascular unit. Curr. Biol. 2024, 35, 109–120.e4. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Hussain, A.; Sheikh, Z.; Subramanian, M. The Eye as a Diagnostic Tool for Alzheimer’s Disease. Life 2023, 13, 726. [Google Scholar] [CrossRef]
- Subramaniam, M.D.; Aishwarya Janaki, P.; Abishek Kumar, B.; Gopalarethinam, J.; Nair, A.P.; Mahalaxmi, I.; Vellingiri, B. Retinal Changes in Parkinson’s Disease: A Non-invasive Biomarker for Early Diagnosis. Cell. Mol. Neurobiol. 2023, 43, 3983–3996. [Google Scholar] [CrossRef]
- Amini, E.; Moghaddasi, M.; Habibi, S.A.H.; Azad, Z.; Miri, S.; Nilforushan, N.; Mirshahi, R.; Cubo, E.; Mohammadzadeh, N.; Rohani, M. Huntington’s disease and neurovascular structure of retina. Neurol. Sci. 2022, 43, 5933–5941. [Google Scholar] [CrossRef]
- Cheung, C.Y.L.; Ikram, M.K.; Chen, C.; Wong, T.Y. Imaging retina to study dementia and stroke. Prog. Retin. Eye Res. 2017, 57, 89–107. [Google Scholar] [CrossRef]
- de Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Burns, S.A.; Miller, D.T.; Roorda, A. Evolution of adaptive optics retinal imaging [Invited]. Biomed. Opt. Express 2023, 14, 1307. [Google Scholar] [CrossRef] [PubMed]
- Peterfi, A.; Pinaffi-Langley, A.C.d.C.; Szarvas, Z.; Muranyi, M.; Kaposzta, Z.; Adams, C.; Pinto, C.B.; Mukli, P.; Kotliar, K.; Yabluchanshiy, A. Dynamic retinal vessel analysis: Flickering a light into the brain. Front. Aging Neurosci. 2024, 16, 1517368. [Google Scholar] [CrossRef] [PubMed]
- Mahroo, O.A. Visual electrophysiology and “the potential of the potentials”. Eye 2023, 37, 2399–2408. [Google Scholar] [CrossRef]
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140195. [Google Scholar] [CrossRef]
- Yoshioka, T.; Nagaoka, T.; Song, Y.; Yokota, H.; Tani, T.; Yoshida, A. Role of Neuronal Nitric Oxide Synthase in Regulating Retinal Blood Flow During Flicker-Induced Hyperemia in Cats. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3113–3120. [Google Scholar] [CrossRef]
- Macvicar, B.A.; Newman, E.A. Astrocyte Regulation of Blood Flow in the Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front. Cell Dev. Biol. 2021, 9, 732820. [Google Scholar] [CrossRef]
- Newman, E.A. Glial Cell Inhibition of Neurons by Release of ATP. J. Neurosci. 2003, 23, 1659. [Google Scholar] [CrossRef]
- Kur, J.; Newman, E.A.; Kur, J.; Physiol, J. Purinergic control of vascular tone in the retina. J. Physiol. C 2014, 592, 491–504. [Google Scholar] [CrossRef]
- Karwoski, C.J.; Lu, H.-K.; Newman, E.A.; Karwoski, J.; Lu, H.-K.; Newman, E.A. Spatial buffering of light-evoked potassium increases by retinal Müller (glial) cells. Science 1989, 244, 578–580. [Google Scholar] [CrossRef]
- Stratman, A.N.; Davis, G.E. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: Influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 2012, 18, 68–80. [Google Scholar] [CrossRef]
- Hill, M.A.; Zou, H.; Potocnik, S.J.; Meininger, G.A.; Davis, M.J. Invited Review: Arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J. Appl. Physiol. 2001, 91, 973–985. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, F.C.T.; van Sloten, T.T.; Willekens, N.; Stehouwer, C.D.A. Neurovascular coupling unit dysfunction and dementia: Retinal measurements as tools to move towards population-based evidence. Front. Endocrinol. 2022, 13, 1014287. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lim, J.S.; Park, J.H.; Lee, J. Molecular, Cellular, and Functional Heterogeneity of Retinal and Choroidal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2023, 64, 35. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; Brunner, C.; Cottarelli, A.; Balbi, M. Location Matters: Navigating Regional Heterogeneity of the Neurovascular Unit. Front. Cell. Neurosci. 2021, 15, 696540. [Google Scholar] [CrossRef]
- Yang, S.; Webb, A.J.S. Associations between neurovascular coupling and cerebral small vessel disease: A systematic review and meta-analysis. Eur. Stroke J. 2023, 8, 895. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Mäe, M.A.; He, L.; Nordling, S.; Vazquez-Liebanas, E.; Nahar, K.; Jung, B.; Li, X.; Tan, B.C.; Chin Foo, J.; Cazenave-Gassiot, A.; et al. Single-Cell Analysis of Blood-Brain Barrier Response to Pericyte Loss. Circ. Res. 2021, 128, E46–E62. [Google Scholar] [CrossRef]
- Niu, R.Z.; Xu, H.Y.; Tian, H.; Zhang, D.; He, C.Y.; Li, X.L.; Li, Y.Y.; He, J. Single-cell transcriptome unveils unique transcriptomic signatures of human organ-specific endothelial cells. Basic Res. Cardiol. 2024, 119, 973–999. [Google Scholar] [CrossRef]
- Blom, J.; Giove, T.; Deshpande, M.; Eldred, W.D. Characterization of nitric oxide signaling pathways in the mouse retina. J. Comp. Neurol. 2012, 520, 4204–4217. [Google Scholar] [CrossRef]
- Agurto, A.; Vielma, A.H.; Cadiz, B.; Couve, E.; Schmachtenberg, O. NO signaling in retinal bipolar cells. Exp. Eye Res. 2017, 161, 30–35. [Google Scholar] [CrossRef]
- Goldstein, I.M.; Ostwald, P.; Roth, S. Nitric Oxide: A Review of Its Role in Retinal Function and Disease. Vis. Res. 1996, 36, 2979–2994. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Laranjinha, J. Nitric Oxide Pathways in Neurovascular Coupling Under Normal and Stress Conditions in the Brain: Strategies to Rescue Aberrant Coupling and Improve Cerebral Blood Flow. Front. Physiol. 2021, 12, 729201. [Google Scholar] [CrossRef]
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef]
- Noonan, J.E.; Lamoureux, E.L.; Sarossy, M. Neuronal activity-dependent regulation of retinal blood flow. Clin. Exp. Ophthalmol. 2015, 43, 673–682. [Google Scholar] [CrossRef]
- Kuhlencordt, P.J.; Rosel, E.; Gerszten, R.E.; Morales-Ruiz, M.; Dombkowski, D.; Aikinson, W.J.; Han, F.; Preffer, F.; Rosenzweig, A.; Sessa, W.C.; et al. Role of endothelial nitric oxide synthase in endothelial activation: Insights from eNOS knockout endothelial cells. Am. J. Physiol. Cell Physiol. 2004, 286, 1195–1202. [Google Scholar] [CrossRef]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble Guanylate Cyclase as an Emerging Therapeutic Target in Cardiopulmonary Disease. Circulation 2011, 123, 2263. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, J.; Nath, A.; Jessen, Z.F.; Schwartz, G.W. A Self-Regulating Gap Junction Network of Amacrine Cells Controls Nitric Oxide Release in the Retina. Neuron 2018, 100, 1149–1162.e5. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic regulation of vascular tone and remodelling. Auton. Autacoid Pharmacol. 2009, 29, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Pannicke, T.; Iandiev, I.; Francke, M.; Hollborn, M.; Wiedemann, P.; Reichenbach, A.; Osborne, N.N.; Bringmann, A. Purinergic signaling involved in Müller cell function in the mammalian retina. Prog. Retin. Eye Res. 2011, 30, 324–342. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kida, T.; Hori, M.; Ozaki, H.; Murata, T. Multiple roles of the PGE2-EP receptor signal in vascular permeability. Br. J. Pharmacol. 2014, 171, 4879. [Google Scholar] [CrossRef] [PubMed]
- Someya, E.; Akagawa, M.; Mori, A.; Morita, A.; Yui, N.; Asano, D.; Sakamoto, K.; Nakahara, T. Role of Neuron–Glia Signaling in Regulation of Retinal Vascular Tone in Rats. Int. J. Mol. Sci. 2019, 20, 1952. [Google Scholar] [CrossRef] [PubMed]
- Metea, M.R.; Newman, E.A. Glial Cells Dilate and Constrict Blood Vessels: A Mechanism of Neurovascular Coupling. J. Neurosci. 2006, 26, 2862–2870. [Google Scholar] [CrossRef]
- Rocic, P.; Schwartzman, M.L. 20-HETE in the regulation of vascular and cardiac function. Pharmacol. Ther. 2018, 192, 74. [Google Scholar] [CrossRef]
- Faria, R.X.; Freitas, H.R.; Reis, R.A.M. P2X7 receptor large pore signaling in avian Müller glial cells. J. Bioenerg. Biomembr. 2017, 49, 215–229. [Google Scholar] [CrossRef]
- Filosa, J.A.; Morrison, H.W.; Iddings, J.A.; Du, W.; Kim, K.J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 2016, 323, 96–109. [Google Scholar] [CrossRef]
- Mishra, A.; Reynolds, J.P.; Chen, Y.; Gourine, A.V.; Rusakov, D.A.; Attwell, D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016, 19, 1619–1627. [Google Scholar] [CrossRef]
- Grant, M.B.; Tarnuzzer, R.W.; Caballero, S.; Ozeck, M.J.; Davis, M.I.; Spoerri, P.E.; Feoktistov, I.; Biaggioni, I.; Shryock, J.C.; Belardinelli, L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ. Res. 1999, 85, 699–706. [Google Scholar] [CrossRef]
- Riis-Vestergaard, M.J.; Misfeldt, M.W.; Bek, T. Dual effects of adenosine on the tone of porcine retinal arterioles in vitro. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1630–1636. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Puro, D.G. Adenosine activates ATP-sensitive K+ currents in pericytes of rat retinal microvessels: Role of A1 and A2a receptors. Brain Res. 2001, 907, 93–99. [Google Scholar] [CrossRef]
- Filosa, J.A.; Bonev, A.D.; Straub, S.V.; Meredith, A.L.; Wilkerson, M.K.; Aldrich, R.W.; Nelson, M.T. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006, 9, 1397–1403. [Google Scholar] [CrossRef]
- Metea, M.R.; Newman, E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, X.; Wei, J.; Zhang, G.; Zhang, J.; Xie, P.; Xu, L.; Wang, L.; Zhao, L.; Li, L.; et al. NaHCO3 dilates mouse afferent arteriole via Na+/HCO3 cotransporters NBCs. Hypertension 2019, 74, 1104. [Google Scholar] [CrossRef]
- García-Llorca, A.; Carta, F.; Supuran, C.T.; Eysteinsson, T. Carbonic anhydrase, its inhibitors and vascular function. Front. Mol. Biosci. 2024, 11, 1338528. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor–Associated Hypertension and Vascular Disease. Hypertension 2018, 71, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dentler, W.L.; Borchardt, R.T. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H434–H440. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Suzuma, K.; Matsui, S.; Kurimoto, M.; Kiryu, J.; Kita, M.; Suzuma, I.; Ohashi, H.; Ojima, T.; Murakami, T.; et al. Erythropoietin as a Retinal Angiogenic Factor in Proliferative Diabetic Retinopathy. Nippon. Ganka Gakkai Zasshi 2005, 111, 892–898. [Google Scholar] [CrossRef]
- Callan, A.; Jha, S.; Valdez, L.; Tsin, A. Cellular and Molecular Mechanisms of Neuronal Degeneration in Early-Stage Diabetic Retinopathy. Curr. Vasc. Pharmacol. 2024, 22, 301–315. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Van Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Coelho-Santos, V.; Shih, A.Y. Pericyte Control of Blood Flow across Microvascular Zones in the Central Nervous System. Annu. Rev. Physiol. 2021, 84, 331. [Google Scholar] [CrossRef]
- Haefiiger, I.O.; Zschauer, A.; Anderson, D.R. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Investig. Ophthalmol. Vis. Sci. 1994, 35, 991–997. [Google Scholar]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.S.P.; Prazeres, P.H.D.M.; Mintz, A.; Birbrair, A. Role of pericytes in the retina. Eye 2018, 32, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Rangasamy, S.; Monickaraj, F.; Legendre, C.; Cabrera, A.P.; Llaci, L.; Bilagody, C.; McGuire, P.; Das, A. Transcriptomics analysis of pericytes from retinas of diabetic animals reveals novel genes and molecular pathways relevant to blood-retinal barrier alterations in diabetic retinopathy. Exp. Eye Res. 2020, 195, 108043. [Google Scholar] [CrossRef]
- Joyal, J.S.; Gantner, M.L.; Smith, L.E.H. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res. 2017, 64, 131. [Google Scholar] [CrossRef]
- Weiner, G.A.; Shah, S.H.; Angelopoulos, C.M.; Bartakova, A.B.; Pulido, R.S.; Murphy, A.; Nudleman, E.; Daneman, R.; Goldberg, J.L. Cholinergic neural activity directs retinal layer-specific angiogenesis and blood retinal barrier formation. Nat. Commun. 2019, 10, 2477. [Google Scholar] [CrossRef]
- Hammes, H.P.; Lin, J.; Wagner, P.; Feng, Y.; Vom Hagen, F.; Krzizok, T.; Renner, O.; Breier, G.; Brownlee, M.; Deutsch, U. Angiopoietin-2 causes pericyte dropout in the normal retina: Evidence for involvement in diabetic retinopathy. Diabetes 2004, 53, 1104–1110. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Tang, F.; Tham, C.C.Y.; Young, A.L.; Cheung, C.Y. Retinal vasculature in glaucoma: A review. BMJ Open Ophthalmol. 2017, 1, e000032. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.H.; Schwartz, S. Diabetic retinopathy: New concepts of screening, monitoring, and interventions. Surv. Ophthalmol. 2024, 69, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Venkata, K.; Teja, R.; Berendschot, T.; Steinbusch, H.; Webers, C.; Murthy, P.; Ps, M. Cerebral and Retinal Neurovascular Changes: A Biomarker for Alzheimer’s Disease. J. Gerontol. Geriatr. Res. 2017, 6, 447. [Google Scholar]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Fuchs, D.-T.; Sheyn, J.; Black, K.L.; Mirzaei, N.; Koronyo-Hamaoui, M. Retinal Vasculopathy in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 731614. [Google Scholar] [CrossRef]
- Cao, Q.; Yang, S.; Wang, X.; Sun, H.; Chen, W.; Wang, Y.; Gao, J.; Wu, Y.; Yang, Q.; Chen, X.; et al. Transport of β-amyloid from brain to eye causes retinal degeneration in Alzheimer’s disease. J. Exp. Med. 2024, 221, e20240386. [Google Scholar] [CrossRef]
- Hamilton, N.B. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenergetics 2010, 2, 1453. [Google Scholar] [CrossRef]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; DeBuc, D.C.; Campbell, M.C.W.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimer’s Dement. 2020, 17, 103–111. [Google Scholar] [CrossRef]
- Chiasseu, M.; Alarcon-Martinez, L.; Belforte, N.; Quintero, H.; Dotigny, F.; Destroismaisons, L.; Vande Velde, C.; Panayi, F.; Louis, C.; Di Polo, A. Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 58. [Google Scholar] [CrossRef]
- Xia, F.; Ha, Y.; Shi, S.; Li, Y.; Li, S.; Luisi, J.; Kayed, R.; Motamedi, M.; Liu, H.; Zhang, W. Early alterations of neurovascular unit in the retina in mouse models of tauopathy. Acta Neuropathol. Commun. 2021, 9, 51. [Google Scholar] [CrossRef]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.H.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011, 54, S204–S217. [Google Scholar] [CrossRef]
- Yuan, A.; Lee, C.S. Retinal Biomarkers for Alzheimer’s Disease: The Facts and the Future. Asia Pac. J. Ophthalmol. 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Ning, A.; Cui, J.; To, E.; Ashe, K.H.; Matsubara, J. Amyloid-β Deposits Lead to Retinal Degeneration in a Mouse Model of Alzheimer Disease. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5136–5143. [Google Scholar] [CrossRef] [PubMed]
- Solis, E.; Hascup, K.N.; Hascup, E.R. Alzheimer’s Disease: The Link Between Amyloid-β and Neurovascular Dysfunction. J. Alzheimers Dis. 2020, 76, 1179. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Regis, G.C.; Sheyn, J.; Fuchs, D.T.; Kramerov, A.A.; Ljubimov, A.V.; Dumitrascu, O.M.; Rodriguez, A.R.; et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020, 139, 813–836. [Google Scholar] [CrossRef]
- Xu, Q.A.; Boerkoel, P.; Hirsch-Reinshagen, V.; Mackenzie, I.R.; Hsiung, G.Y.R.; Charm, G.; To, E.F.; Liu, A.Q.; Schwab, K.; Jiang, K.; et al. Müller cell degeneration and microglial dysfunction in the Alzheimer’s retina. Acta Neuropathol. Commun. 2022, 10, 145. [Google Scholar] [CrossRef]
- López-de-Eguileta, A.; López-García, S.; Lage, C.; Pozueta, A.; García-Martínez, M.; Kazimierczak, M.; Bravo, M.; Irure, J.; López-Hoyos, M.; Muñoz-Cacho, P.; et al. The retinal ganglion cell layer reflects neurodegenerative changes in cognitively unimpaired individuals. Alzheimer’s Res. Ther. 2022, 14, 57. [Google Scholar] [CrossRef]
- Czakó, C.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; Csipo, T.; Lipecz, A.; Horváth, H.; Sándor, G.L.; Kovács, T.; et al. Retinal biomarkers for Alzheimer’s disease and vascular cognitive impairment and dementia (VCID): Implication for early diagnosis and prognosis. Geroscience 2020, 42, 1499–1525. [Google Scholar] [CrossRef]
- Mirzaei, N.; Shi, H.; Oviatt, M.; Doustar, J.; Rentsendorj, A.; Fuchs, D.T.; Sheyn, J.; Black, K.L.; Koronyo, Y.; Koronyo-Hamaoui, M. Alzheimer’s Retinopathy: Seeing Disease in the Eyes. Front. Neurosci. 2020, 14, 921. [Google Scholar] [CrossRef]
- Alber, J.; Bouwman, F.; den Haan, J.; Rissman, R.A.; De Groef, L.; Koronyo-Hamaoui, M.; Lengyel, I.; Thal, D.R. Retina pathology as a target for biomarkers for Alzheimer’s disease: Current status, ophthalmopathological background, challenges, and future directions. Alzheimer’s Dement. 2024, 20, 728–740. [Google Scholar] [CrossRef]
- Gaire, B.P.; Koronyo, Y.; Fuchs, D.T.; Shi, H.; Rentsendorj, A.; Danziger, R.; Vit, J.P.; Mirzaei, N.; Doustar, J.; Sheyn, J.; et al. Alzheimer’s disease pathophysiology in the Retina. Prog. Retin. Eye Res. 2024, 101, 101273. [Google Scholar] [CrossRef]
- Kaštelan, S.; Gverović Antunica, A.; Puzović, V.; Didović Pavičić, A.; Čanović, S.; Kovačević, P.; Vučemilović, P.A.F.; Konjevoda, S. Non-Invasive Retinal Biomarkers for Early Diagnosis of Alzheimer’s Disease. Biomedicines 2025, 13, 283. [Google Scholar] [CrossRef]
- Ashraf, G.; McGuinness, M.; Khan, M.A.; Obtinalla, C.; Hadoux, X.; van Wijngaarden, P. Retinal imaging biomarkers of Alzheimer’s disease: A systematic review and meta-analysis of studies using brain amyloid beta status for case definition. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2023, 15, e12421. [Google Scholar] [CrossRef] [PubMed]
- Hart de Ruyter, F.J.; Morrema, T.H.J.; den Haan, J.; Gase, G.; Twisk, J.W.R.; de Boer, J.F.; Scheltens, P.; Bouwman, F.H.; Verbraak, F.D.; Rozemuller, A.J.M.; et al. α-Synuclein pathology in post-mortem retina and optic nerve is specific for α-synucleinopathies. NPJ Parkinsons Dis. 2023, 9, 124. [Google Scholar] [CrossRef]
- Deng, Y.; Jie, C.; Wang, J.; Liu, Z.; Li, Y.; Hou, X. Evaluation of retina and microvascular changes in the patient with Parkinson’s disease: A systematic review and meta-analysis. Front. Med. 2022, 9, 957700. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, R.; Huang, L.; Zhang, H.; Gao, M.; Sun, B.; Tan, Y.; Ye, J.; Ding, Z.; Gu, Y.; et al. Transocular detection of premotor Parkinson’s disease via retinal capillary neurovascular coupling through functional OCT angiography. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kivrak, U.; Özen Barut, B.; Sungu, İ.; Güçlü Altun, İ.; Telek, B.; Şimşek, Ş. Neurovascular Changes in the Retina of Parkinson’s Disease Patients: A Comprehensive Study on Disease Severity, Levodopa Dosage, and Stroke Risk. Noro Psikiyatr. Ars 2025, 62, 241–248. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, Y.; Zhang, P.; He, C.; Li, R.; Wang, L.; Zhang, H.; Zhang, Y. Retinal microvascular impairment in Parkinson’s disease with cognitive dysfunction. Park. Relat. Disord. 2022, 98, 27–31. [Google Scholar] [CrossRef]
- Tran, K.K.N.; Wong, V.H.Y.; Hoang, A.; Finkelstein, D.I.; Bui, B.V.; Nguyen, C.T.O. Retinal alpha-synuclein accumulation correlates with retinal dysfunction and structural thinning in the A53T mouse model of Parkinson’s disease. Front. Neurosci. 2023, 17, 1146979. [Google Scholar] [CrossRef]
- Weil, R.S.; Schrag, A.E.; Warren, J.D.; Crutch, S.J.; Lees, A.J.; Morris, H.R. Visual dysfunction in Parkinson’s disease. Brain 2016, 139, 2827–2843. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, J.Y.; Kim, T.W.; Yoon, E.J.; Oh, S.; Kim, Y.K.; Kim, J.M.; Woo, S.J.; Kim, K.W.; Jeon, B. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 2018, 91, e1003–e1012. [Google Scholar] [CrossRef]
- Wagner, S.K.; Romero-Bascones, D.; Cortina-Borja, M.; Williamson, D.J.; Struyven, R.R.; Zhou, Y.; Patel, S.; Weil, R.S.; Antoniades, C.A.; Topol, E.J.; et al. Retinal Optical Coherence Tomography Features Associated with Incident and Prevalent Parkinson Disease. Neurology 2023, 101, E1581–E1593. [Google Scholar] [CrossRef]
- Cubo, E.; López Peña, M.J.; Diez-Feijo Varela, E.; Pérez Gil, O.; Garcia Gutierrez, P.; Araus González, E.; Prieto Tedejo, R.; Mariscal Pérez, N.; Armesto, D. Lack of association of morphologic and functional retinal changes with motor and non-motor symptoms severity in Parkinson’s disease. J. Neural. Transm. 2014, 121, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.K.N.; Wong, V.H.Y.; Vessey, K.A.; Finkelstein, D.I.; Bui, B.V.; Nguyen, C.T.O. Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson’s Disease. Biomedicines 2024, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Huemer, K.H.; Zawinka, C.; Garhöfer, G.; Golestani, E.; Litschauer, B.; Dorner, G.T.; Schmetterer, L. Effects of dopamine on retinal and choroidal blood flow parameters in humans. Br. J. Ophthalmol. 2007, 91, 1194. [Google Scholar] [CrossRef] [PubMed]
- Murueta-Goyena, A.; del Pino, R.; Reyero, P.; Galdós, M.; Arana, B.; Lucas-Jiménez, O.; Acera, M.; Tijero, B.; Ibarretxe-Bilbao, N.; Ojeda, N.; et al. Parafoveal thinning of inner retina is associated with visual dysfunction in Lewy body diseases. Mov. Disord. 2019, 34, 1315–1324. [Google Scholar] [CrossRef]
- Marrocco, E.; Indrieri, A.; Esposito, F.; Tarallo, V.; Carboncino, A.; Alvino, F.G.; De Falco, S.; Franco, B.; De Risi, M.; De Leonibus, E. α-synuclein overexpression in the retina leads to vision impairment and degeneration of dopaminergic amacrine cells. Sci. Rep. 2020, 10, 9619. [Google Scholar] [CrossRef]
- Dhaliwal, K.K.; Imani, E.; Hudson, C.; Wright, T.; Ballios, B.; Bizheva, K.K. Visually-evoked changes in human retinal blood flow measured with OCT. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2537. [Google Scholar]
- Pescosolido, N.; Barbato, A.; Stefanucci, A.; Buomprisco, G. Role of Electrophysiology in the Early Diagnosis and Follow-Up of Diabetic Retinopathy. J. Diabetes Res. 2015, 2015, 319692. [Google Scholar] [CrossRef]
- Tagawa, K.; Hoshino, M.; Okuda, T.; Ueda, H.; Hayashi, H.; Engemann, S.; Okado, H.; Ichikawa, M.; Wanker, E.E.; Okazawa, H. Distinct aggregation and cell death patterns among different types of primary neurons induced by mutant huntingtin protein. J. Neurochem. 2004, 89, 974–987. [Google Scholar] [CrossRef]
- Dhalla, A.; Pallikadavath, S.; Hutchinson, C.V. Visual Dysfunction in Huntington’s Disease: A Systematic Review. J. Huntingtons Dis. 2019, 8, 233–242. [Google Scholar] [CrossRef]
- Murueta-Goyena, A.; Del Pino, R.; Acera, M.; Teijeira-Portas, S.; Romero, D.; Ayala, U.; Fernández-Valle, T.; Tijero, B.; Gabilondo, I.; Gómez Esteban, J.C. Retinal thickness as a biomarker of cognitive impairment in manifest Huntington’s disease. J. Neurol. 2023, 270, 3821–3829. [Google Scholar] [CrossRef]
- Xu, H.; Ajayan, A.; Langen, R.; Chen, J. Pleiotropic effects of mutant huntingtin on retinopathy in two mouse models of Huntington’s disease. Neurobiol. Dis. 2025, 205, 106780. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cano, F.; Martín-Loro, F.; Gallardo-Orihuela, A.; González-Montelongo, M.d.C.; Ortuño-Miquel, S.; Hervás-Corpión, I.; de la Villa, P.; Ramón-Marco, L.; Navarro-Calvo, J.; Gómez-Jaramillo, L.; et al. Retinal dysfunction in Huntington’s disease mouse models concurs with local gliosis and microglia activation. Sci. Rep. 2024, 14, 4176. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Huang, C.; Guo, X.; Li, Y.; Wu, J.; Zhang, Z.; Yan, S.; Xu, Y. Abnormal outer and inner retina in a mouse model of Huntington’s disease with age. Front. Aging Neurosci. 2024, 16, 1434551. [Google Scholar] [CrossRef]
- Ragauskas, S.; Leinonen, H.; Puranen, J.; Rö Nkkö, S.; Nymark, S.; Gurevicius, K.; Lipponen, A.; Kontkanen, O.; Puoliväli, J.; Tanila, H.; et al. Early Retinal Function Deficit without Prominent Morphological Changes in the R6/2 Mouse Model of Huntington’s Disease. PLoS ONE 2014, 9, e113317. [Google Scholar] [CrossRef]
- Helmlinger, D.; Yvert, G.; Picaud, S.; Merienne, K.; Sahel, J.; Mandel, J.L.; Devys, D. Progressive retinal degeneration and dysfunction in R6 Huntington’s disease mice. Human Mol. Genet. 2002, 11, 3351–3359. [Google Scholar] [CrossRef]
- Di Maio, L.G.; Montorio, D.; Peluso, S.; Dolce, P.; Salvatore, E.; De Michele, G.; Cennamo, G. Optical coherence tomography angiography findings in Huntington’s disease. Neurol. Sci. 2021, 42, 995–1001. [Google Scholar] [CrossRef]
- Shah, A.; Fuller, S.; Criswell, S.; Apte, R.S. Dark Adaptometry and Optical Coherence Tomography Angiography in Huntington Disease. J. Ophthalmic Vis. Res. 2024, 19, 18. [Google Scholar] [CrossRef]
- Dusek, P.; Kopal, A.; Brichova, M.; Roth, J.; Ulmanova, O.; Klempir, J.; Preiningerova, J.L. Is retina affected in Huntington’s disease? Is optical coherence tomography a good biomarker? PLoS ONE 2023, 18, e0282175. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V. Understanding Retinal Changes after Stroke. Open J. Ophthalmol. 2017, 7, 281–292. [Google Scholar] [CrossRef]
- Lee, D.; Kopal, A.; Brichova, M.; Roth, J.; Ulmanova, O.; Klempir, J.; Preiningerova, J.L. Retinal Degeneration in a Murine Model of Retinal Ischemia by Unilateral Common Carotid Artery Occlusion. BioMed Res. Int. 2021, 2021, 7727648. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Pan, S.J.; Verma, R.; Wizeman, J.; Crapser, J.; Patel, A.R.; Lieberman, R.; Mohan, R.; McCullough, L.D. Early retinal inflammatory biomarkers in the middle cerebral artery occlusion model of ischemic stroke. Mol. Vis. 2016, 22, 575. [Google Scholar] [PubMed]
- Conti, E.; Carlini, N.; Piccardi, B.; Mascaro, A.L.A.; Pavone, F.S. Photothrombotic Middle Cerebral Artery Occlusion in Mice: A Novel Model of Ischemic Stroke. eNeuro 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.S.; Sayeed, I.; Cale, H.A.; Morrison, K.C.; Boatright, J.H.; Pardue, M.T.; Stein, D.G. Severity of middle cerebral artery occlusion determines retinal deficits in rats. Exp. Neurol. 2014, 254, 206. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schmidt-Kastner, R.; Hamasaki, D.I.; Yamamoto, H.; Parel, J.M. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res. 2006, 82, 767–779. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, M.; Deng, T.; Luo, D.; Zi, Y.; Pan, L.; Wang, Z.; Jin, M. Functional and morphologic study of retinal hypoperfusion injury induced by bilateral common carotid artery occlusion in rats OPEN. Sci. Rep. 2019, 9, 80. [Google Scholar] [CrossRef]
- Lee, D.; Kang, H.; Yoon, K.Y.; Chang, Y.Y.; Song, H.B. A mouse model of retinal hypoperfusion injury induced by unilateral common carotid artery occlusion. Exp. Eye Res. 2020, 201, 108275. [Google Scholar] [CrossRef]
- Mosinger, J.L.; Olney, J.W. Photothrombosis-Induced Lschemic Neuronal Degeneration in the Rat Retina. Exp. Neurol. 1989, 105, 110–113. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, B.; Xiao, Y.; Zeng, X.; Wu, G.; Du, Z.; Fang, Y.; Hu, Y.; Yang, X.; Yu, H. Retinal Neurovascular Changes in Patients With Ischemic Stroke Investigated by Optical Coherence Tomography Angiography. Front. Aging Neurosci. 2022, 14, 834560. [Google Scholar] [CrossRef]
- Kapupara, K.; Huang, T.L.; Wen, Y.T.; Huang, S.P.; Tsai, R.K. Optic nerve head width and retinal nerve fiber layer changes are proper indexes for validating the successful induction of experimental anterior ischemic optic neuropathy. Exp. Eye Res. 2019, 181, 105–111. [Google Scholar] [CrossRef]
- Kim, B.J.; Braun, T.A.; Wordinger, R.J.; Clark, A.F. Progressive morphological changes and impaired retinal function associated with temporal regulation of gene expression after retinal ischemia/reperfusion injury in mice. Mol. Neurodegener. 2013, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.M.; Kim, B.J.; Howell, G.R.; Miller, J.; John, S.W.M.; Wordinger, R.J.; Clark, A.F. C1q propagates microglial activation and neurodegeneration in the visual axis following retinal ischemia/reperfusion injury. Mol. Neurodegener. 2016, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, L.; Cai, R.; Zheng, L.; Guo, L. Identification of Protein Network Alterations upon Retinal Ischemia-Reperfusion Injury by Quantitative Proteomics Using a Rattus norvegicus Model. PLoS ONE 2014, 9, e116453. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J. Cereb. Blood Flow Metab. 2013, 33, 1685–1695. [Google Scholar] [CrossRef]
- Kern, T.S. Interrelationships between the Retinal Neuroglia and Vasculature in Diabetes. Diabetes Metab. J. 2014, 38, 163. [Google Scholar] [CrossRef]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Garhöfer, G.; Chua, J.; Tan, B.; Wong, D.; Schmidl, D.; Schmetterer, L. Retinal Neurovascular Coupling in Diabetes. J. Clin. Med. 2020, 9, 2829. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—From eye research to CNS disorders. Nat. Rev. Neurol. 2012, 9, 44–53. [Google Scholar] [CrossRef]
- Frost, S.; Kanagasingam, Y.; Sohrabi, H.; Vignarajan, J.; Bourgeat, P.; Salvado, O.; Villemagne, V.; Rowe, C.C.; Lance MacAulay, S.; Szoeke, C.; et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl. Psychiatry 2013, 3, e233. [Google Scholar] [CrossRef]
| Signaling Pathway | Shared Features (Retina and Brain) | Retina-Specific Features/Distinctions |
|---|---|---|
| Nitric Oxide (NO) | Produced by nNOS/iNOS/eNOS in neurons, glial and endothelial cells, respectively; induces vasodilation and increases local blood flow | Layer-specific modulation across superficial, intermediate, and deep capillary plexuses; rapid responses to visual stimulation; higher integration with retinal glia (Müller cells) |
| ATP/ Purinergic Signaling | Released from neurons and glia; acts on A1/A2 and P2X/P2Y receptors on pericytes and endothelial cells to modulate vascular tone | Stratified effect: deep plexus more sensitive to ATP/adenosine modulation; tightly coordinated with glial Ca2+ signaling; spatially precise regulation of blood flow to match synaptic activity |
| Ionic Regulation (K+, Ca2+) | Extracellular K+ and intracellular Ca2+ regulate vascular tone in both brain and retina; involved in neurovascular signaling | Layer-specific ionic dynamics mediated by Müller glia; K+ buffering and Ca2+ waves directly influence pericytes across capillary plexuses; rapid adjustments to localized visual activity |
| VEGF Signaling | Supports endothelial survival and angiogenesis; involved in adaptive vascular remodeling | Retina-specific VEGF gradients across layers; finely tuned to inner retinal metabolic demands; dysregulation leads to pathological angiogenesis (e.g., diabetic retinopathy, AMD) [69,70] |
| Pericytes | Modulate capillary diameter and blood flow; interact with endothelial cells via NO, ATP, and growth factors | Highly stratified and closely associated with synaptic layers; exhibit layer-specific contractility; integrate signals from neurons and Müller cells; coordinate local vascular remodeling; the retina is particularly susceptible to pericyte loss–related vascular leakage [5,71] |
| Disease | Key Retinal Neuronal Changes | Glial/Microglial Responses | Vascular Alterations | Distinct Retinal Features/Biomarkers |
|---|---|---|---|---|
| Alzheimer’s disease (AD) | Loss of RGCs; synaptic dysfunction; Aβ and tau deposition | Reactive gliosis (Müller and astrocytes); microglial activation | Loss of pericytes; vasoconstriction; reduced flow; iBRB disruption | Retinal thinning (OCT); Aβ plaques; impaired flicker-induced vasodilation |
| Parkinson’s disease (PD) | Dopaminergic amacrine cell loss; altered contrast sensitivity; α-syn aggregates | Elevated oxidative stress and glial reactivity | Microvascular rarefaction; reduced capillary density | ERG deficits; reduced retinal dopamine markers |
| Huntington’s disease (HD) | Progressive loss of photoreceptors and RGCs; early synaptic loss in the inner retina | Microglial activation; increased inflammatory glial signaling [116] | ↓ Capillary density; vascular remodeling and leakage | Reduced b-wave amplitude; impaired NVU response; thinning of retina |
| Stroke/ Ischemic injury | Loss of nerve fibers; ischemic RGC death; inner retinal layer thinning | Astrocyte and Müller glia swelling; microglial recruitment | iBRB disruption; vessel occlusion; reduced autoregulation | ERG deficits; Inner retinal infarcts (OCT); NVU uncoupling in post-stroke recovery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W. Retinal Neurovascular Coupling: From Mechanisms to a Diagnostic Window into Brain Disorders. Cells 2025, 14, 1798. https://doi.org/10.3390/cells14221798
Shen W. Retinal Neurovascular Coupling: From Mechanisms to a Diagnostic Window into Brain Disorders. Cells. 2025; 14(22):1798. https://doi.org/10.3390/cells14221798
Chicago/Turabian StyleShen, Wen. 2025. "Retinal Neurovascular Coupling: From Mechanisms to a Diagnostic Window into Brain Disorders" Cells 14, no. 22: 1798. https://doi.org/10.3390/cells14221798
APA StyleShen, W. (2025). Retinal Neurovascular Coupling: From Mechanisms to a Diagnostic Window into Brain Disorders. Cells, 14(22), 1798. https://doi.org/10.3390/cells14221798





