Obesity-Related Inflammatory Biomarkers in the Elderly Population
Abstract
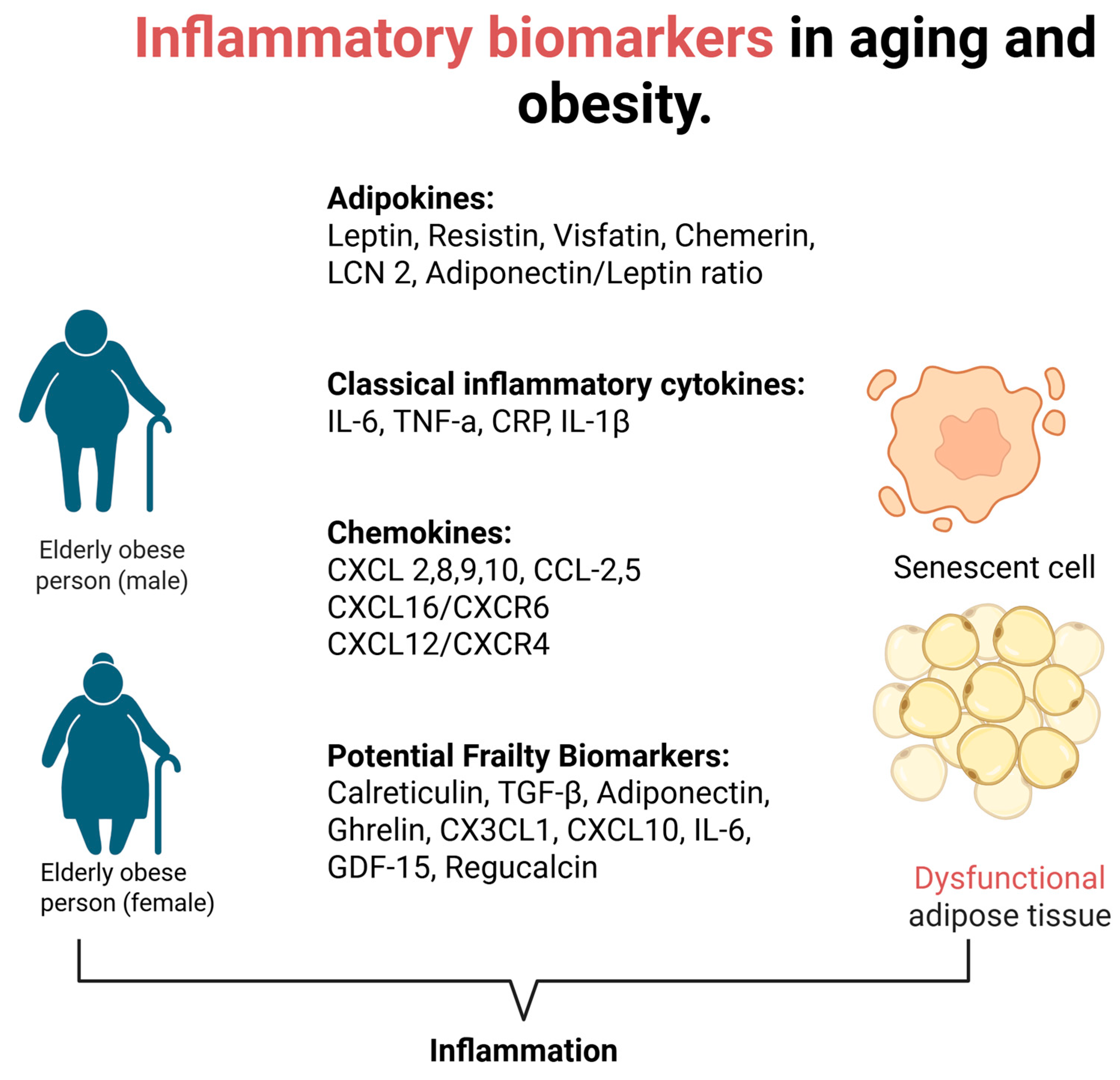
1. Introduction
2. Methodology
3. Changes in Adipose Tissue During Aging
4. Plasma Visfatin Levels in Obesity and Aging
5. Leptin
6. Retinol Binding Protein 4 (RBP4) in Obesity and Aging
7. Chemerin in Obesity and Aging
8. Resistin in Obesity and Aging
9. Lipocalin 2 in Obesity and Aging
10. TNF-a, IL-1β, and IL-6 in Obesity and Aging
11. CRP Levels Under the Scope of Obesity and Aging
12. The Role of Chemokines in Obesity and Aging
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AL | Adiponectin/Leptin |
| BMI | Body mass index |
| CRP | C-reactive protein |
| GI tract | Gastrointestinal tract |
| HOMA | Homeostasis model assessment |
| IL | Interleukin |
| LCN2 | Lipocalin 2 |
| MetS | Metabolic syndrome |
| RBP4 | Retinol binding protein 4 |
| SAT | Subcutaneous adipose tissue |
| VAT | Visceral adipose tissue |
| VSF | Visfatin |
| WC | Waist circumference |
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 May 2025).
- United Nations. World Population Ageing, 2019 Highlights; United Nations: New York, NY, USA, 2020. [Google Scholar]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and All Cause Mortality: Systematic Review and Non-Linear Dose-Response Meta-Analysis of 230 Cohort Studies with 3.74 Million Deaths among 30.3 Million Participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global Prevalence of Sarcopenic Obesity in Older Adults: A Systematic Review and Meta-Analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, C.; Zhao, R.; Dong, C.; Gu, Z.; Gao, J. The Association between Sarcopenic Obesity, Sarcopenia and Functional Dependence, Malnutrition, and Mortality: The Phenomenon of Obesity Paradox in Sarcopenic Obesity. Eur. Geriatr. Med. 2025, 16, 89–97. [Google Scholar] [CrossRef]
- Gkrinia, E.M.M.; Belančić, A. The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 357. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Trim, W.V.; Walhin, J.-P.; Koumanov, F.; Bouloumié, A.; Lindsay, M.A.; Chen, Y.-C.; Travers, R.L.; Turner, J.E.; Thompson, D. Divergent Immunometabolic Changes in Adipose Tissue and Skeletal Muscle with Ageing in Healthy Humans. J. Physiol. 2022, 600, 921–947. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and Ageing: Two Sides of the Same Coin. Obes. Rev. 2020, 21, e12991. [Google Scholar] [CrossRef]
- Pérez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Gálvez, B.G. “Adipaging”: Ageing and Obesity Share Biological Hallmarks Related to a Dysfunctional Adipose Tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Schosserer, M.; Grillari, J.; Wolfrum, C.; Scheideler, M. Age-Induced Changes in White, Brite, and Brown Adipose Depots: A Mini-Review. Gerontology 2017, 64, 229–236. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White Adipose Tissue Dysfunction in Obesity and Aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef]
- Cinti, S. The Adipose Organ at a Glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular Adipose Tissue as a Source of Therapeutic Targets and Clinical Biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-Related Changes in Total and Regional Fat Distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat Tissue, Aging, and Cellular Senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef]
- Srikakulapu, P.; McNamara, C.A. B Lymphocytes and Adipose Tissue Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1110–1122. [Google Scholar] [CrossRef]
- Pfannenberg, C.; Werner, M.K.; Ripkens, S.; Stef, I.; Deckert, A.; Schmadl, M.; Reimold, M.; Häring, H.-U.; Claussen, C.D.; Stefan, N. Impact of Age on the Relationships of Brown Adipose Tissue With Sex and Adiposity in Humans. Diabetes 2010, 59, 1789–1793. [Google Scholar] [CrossRef]
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of Brown/Beige Adipose Tissues in Obesity and Metabolic Disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary Atherosclerosis Is Associated with Macrophage Polarization in Epicardial Adipose Tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Spray, L.; Richardson, G.; Haendeler, J.; Altschmied, J.; Rumampouw, V.; Wallis, S.B.; Georgiopoulos, G.; White, S.; Unsworth, A.; Stellos, K.; et al. Cardiovascular Inflammaging: Mechanisms, Consequences, and Therapeutic Perspectives. Cell Rep. Med. 2025, 6, 102264. [Google Scholar] [CrossRef]
- Curat, C.A.; Wegner, V.; Sengenès, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumié, A. Macrophages in Human Visceral Adipose Tissue: Increased Accumulation in Obesity and a Source of Resistin and Visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef]
- Kamińska, A.; Kopczyńska, E.; Bronisz, A.; Żmudzińska, M.; Bieliński, M.; Borkowska, A.; Tyrakowski, T.; Junik, R. An Evaluation of Visfatin Levels in Obese Subjects. Endokrynol. Pol. 2010, 61, 169–173. [Google Scholar]
- Pagano, C.; Pilon, C.; Olivieri, M.; Mason, P.; Fabris, R.; Serra, R.; Milan, G.; Rossato, M.; Federspil, G.; Vettor, R. Reduced Plasma Visfatin/Pre-B Cell Colony-Enhancing Factor in Obesity Is Not Related to Insulin Resistance in Humans. J. Clin. Endocrinol. Metab. 2006, 91, 3165–3170. [Google Scholar] [CrossRef]
- Jurdana, M.; Petelin, A.; Černelič Bizjak, M.; Bizjak, M.; Biolo, G.; Jenko-Pražnikar, Z. Increased Serum Visfatin Levels in Obesity and Its Association with Anthropometric/Biochemical Parameters, Physical Inactivity and Nutrition. e-SPEN J. 2013, 8, e59–e67. [Google Scholar] [CrossRef]
- Haider, D.G.; Schindler, K.; Schaller, G.; Prager, G.; Wolzt, M.; Ludvik, B. Increased Plasma Visfatin Concentrations in Morbidly Obese Subjects Are Reduced after Gastric Banding. J. Clin. Endocrinol. Metab. 2006, 91, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- García-Fuentes, E.; García-Almeida, J.M.; García-Arnés, J.; García-Serrano, S.; Rivas-Marín, J.; Gallego-Perales, J.L.; Rojo-Martínez, G.; Garrido-Sánchez, L.; Bermudez-Silva, F.J.; de Fonseca, F.R.; et al. Plasma Visfatin Concentrations in Severely Obese Subjects Are Increased After Intestinal Bypass. Obesity 2007, 15, 2391–2395. [Google Scholar] [CrossRef]
- Berndt, J.; Klöting, N.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Plasma Visfatin Concentrations and Fat Depot–Specific mRNA Expression in Humans. Diabetes 2005, 54, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Gonzalez Sagrado, M.; Conde, R.; Aller, R.; Izaola, O.; Romero, E. Effect of a Hypocaloric Diet on Serum Visfatin in Obese Non-Diabetic Patients. Nutrition 2008, 24, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Gavrieli, A.; Mantzoros, C.S. Leptin Applications in 2015: What Have We Learned about Leptin and Obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 353. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, R.; Feingold, K.R.; Grunfeld, C. Leptin Regulation of the Immune Response and the Immunodeficiency of Malnutrition. FASEB J. 2001, 15, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Heymsfield, S.B.; Haft, C.; Kahn, B.B.; Laughlin, M.; Leibel, R.L.; Tschöp, M.H.; Yanovski, J.A. Challenges and Opportunities of Defining Clinical Leptin Resistance. Cell Metab. 2012, 15, 150–156. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef]
- La Cava, A.; Matarese, G. The Weight of Leptin in Immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Laule, C.; Rahmouni, K. Leptin and Associated Neural Pathways Underlying Obesity-Induced Hypertension. Compr. Physiol. 2025, 15, e8. [Google Scholar] [CrossRef]
- Kohara, K.; Ochi, M.; Tabara, Y.; Nagai, T.; Igase, M.; Miki, T. Leptin in Sarcopenic Visceral Obesity: Possible Link between Adipocytes and Myocytes. PLoS ONE 2011, 6, e24633. [Google Scholar] [CrossRef]
- Zhuo, Q.; Wang, Z.; Fu, P.; Piao, J.; Tian, Y.; Xu, J.; Yang, X. Comparison of Adiponectin, Leptin and Leptin to Adiponectin Ratio as Diagnostic Marker for Metabolic Syndrome in Older Adults of Chinese Major Cities. Diabetes Res. Clin. Pract. 2009, 84, 27–33. [Google Scholar] [CrossRef]
- Rizza, R.A. Pathogenesis of Fasting and Postprandial Hyperglycemia in Type 2 Diabetes: Implications for Therapy. Diabetes 2010, 59, 2697–2707. [Google Scholar] [CrossRef]
- Bik, W.; Baranowska-Bik, A.; Wolinska-Witort, E.; Kalisz, M.; Broczek, K.; Mossakowska, M.; Baranowska, B. Assessment of Adiponectin and Its Isoforms in Polish Centenarians. Exp. Gerontol. 2013, 48, 401–407. [Google Scholar] [CrossRef]
- Senkus, K.E.; Crowe-White, K.M.; Bolland, A.C.; Locher, J.L.; Ard, J.D. Changes in Adiponectin:Leptin Ratio among Older Adults with Obesity Following a 12-Month Exercise and Diet Intervention. Nutr. Diabetes 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Vieira, P.M.; Yore, M.M.; Dwyer, P.M.; Syed, I.; Aryal, P.; Kahn, B.B. RBP4 Activates Antigen-Presenting Cells, Leading to Adipose Tissue Inflammation and Systemic Insulin Resistance. Cell Metab. 2014, 19, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Kadoglou, N.P.; Stasinos, V.; Maratou, E.; Antoniadis, A.; Kolokathis, F.; Parissis, J.; Hatziagelaki, E.; Iliodromitis, E.K.; Dimitriadis, G. Serum Levels of Retinol-Binding Protein-4 Are Associated with the Presence and Severity of Coronary Artery Disease. Cardiovasc. Diabetol. 2014, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, E.; Lind, L. Circulating Retinol-Binding Protein 4 and Subclinical Cardiovascular Disease in the Elderly. Diabetes Care 2009, 32, 733–735. [Google Scholar] [CrossRef]
- Zabetian-Targhi, F.; Mahmoudi, M.J.; Rezaei, N.; Mahmoudi, M. Retinol Binding Protein 4 in Relation to Diet, Inflammation, Immunity, and Cardiovascular Diseases. Adv. Nutr. 2015, 6, 748–762. [Google Scholar] [CrossRef]
- Gavi, S.; Qurashi, S.; Stuart, L.M.; Lau, R.; Melendez, M.M.; Mynarcik, D.C.; McNurlan, M.A.; Gelato, M.C. Influence of Age on the Association of Retinol-Binding Protein 4 with Metabolic Syndrome. Obesity 2008, 16, 893–895. [Google Scholar] [CrossRef]
- Lee, J.-W.; Im, J.-A.; Park, K.D.; Lee, H.-R.; Shim, J.-Y.; Lee, D.-C. Retinol Binding Protein 4 and Insulin Resistance in Apparently Healthy Elderly Subjects. Clin. Chim. Acta 2009, 400, 30–32. [Google Scholar] [CrossRef]
- Sell, H.; Laurencikiene, J.; Taube, A.; Eckardt, K.; Cramer, A.; Horrighs, A.; Arner, P.; Eckel, J. Chemerin Is a Novel Adipocyte-Derived Factor Inducing Insulin Resistance in Primary Human Skeletal Muscle Cells. Diabetes 2009, 58, 2731–2740. [Google Scholar] [CrossRef]
- Ernst, M.C.; Issa, M.; Goralski, K.B.; Sinal, C.J. Chemerin Exacerbates Glucose Intolerance in Mouse Models of Obesity and Diabetes. Endocrinology 2010, 151, 1998–2007. [Google Scholar] [CrossRef]
- Coimbra, S.; Brandão Proença, J.; Santos-Silva, A.; Neuparth, M.J. Adiponectin, Leptin, and Chemerin in Elderly Patients with Type 2 Diabetes Mellitus: A Close Linkage with Obesity and Length of the Disease. Biomed. Res. Int. 2014, 2014, 701915. [Google Scholar] [CrossRef]
- Aronis, K.N.; Sahin-Efe, A.; Chamberland, J.P.; Spiro, A.; Vokonas, P.; Mantzoros, C.S. Chemerin Levels as Predictor of Acute Coronary Events: A Case–Control Study Nested within the Veterans Affairs Normative Aging Study. Metabolism 2014, 63, 760–766. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The Hormone Resistin Links Obesity to Diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, R.; Momiyama, Y.; Kato, R.; Taniguchi, H.; Ogura, M.; Ayaori, M.; Nakamura, H.; Ohsuzu, F. Associations between Serum Resistin Levels and Insulin Resistance, Inflammation, and Coronary Artery Disease. J. Am. Coll. Cardiol. 2005, 46, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A Reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef]
- Krizanac, M.; Mass Sanchez, P.B.; Weiskirchen, R.; Asimakopoulos, A. A Scoping Review on Lipocalin-2 and Its Role in Non-Alcoholic Steatohepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 2865. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Zhao, X.; Dong, G.; Li, C. Diagnostic and Prognostic Value of Neutrophil Gelatinase-Associated Lipocalin, Matrix Metalloproteinase-9, and Tissue Inhibitor of Matrix Metalloproteinases-1 for Sepsis in the Emergency Department: An Observational Study. Crit. Care 2014, 18, 634. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Scotece, M.; Conde, J.; Gómez, R.; Lois, A.; Pino, J.; Gómez-Reino, J.J.; Lago, F.; Mobasheri, A.; Gualillo, O. The Potential of Lipocalin-2/NGAL as Biomarker for Inflammatory and Metabolic Diseases. Biomarkers 2015, 20, 565–571. [Google Scholar] [CrossRef]
- Daoud, M.S.; Alshareef, F.H.; Alnaami, A.M.; Amer, O.E.; Hussain, S.D.; Al-Daghri, N.M. Prospective Changes in Lipocalin-2 and Adipocytokines among Adults with Obesity. Sci. Rep. 2025, 15, 28794. [Google Scholar] [CrossRef]
- Lögdberg, L.; Wester, L. Immunocalins: A Lipocalin Subfamily That Modulates Immune and Inflammatory Responses. Biochim. Biophys. Acta 2000, 1482, 284–297. [Google Scholar] [CrossRef]
- Auguet, T.; Quintero, Y.; Terra, X.; Martínez, S.; Lucas, A.; Pellitero, S.; Aguilar, C.; Hernández, M.; del Castillo, D.; Richart, C. Upregulation of Lipocalin 2 in Adipose Tissues of Severely Obese Women: Positive Relationship with Proinflammatory Cytokines. Obesity 2011, 19, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Law, I.K.M.; Xu, A.; Lam, K.S.L.; Berger, T.; Mak, T.W.; Vanhoutte, P.M.; Liu, J.T.C.; Sweeney, G.; Zhou, M.; Yang, B.; et al. Lipocalin-2 Deficiency Attenuates Insulin Resistance Associated With Aging and Obesity. Diabetes 2010, 59, 872–882. [Google Scholar] [CrossRef]
- Bauer, C.; Smith, C.; Vogrin, S.; Palmer, A.S.; Woessner, M.; Landen, S.; Jacques, M.; Byrnes, E.; Eynon, N.; Sim, M.; et al. Circulating Lipocalin-2 across the Adult Lifespan. JBMR Plus 2025, 9, ziae162. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Lee, J.K.; Shin, O.S. Aging and the Immune System: The Impact of Immunosenescence on Viral Infection, Immunity and Vaccine Immunogenicity. Immune Netw. 2019, 19, e37. [Google Scholar] [CrossRef] [PubMed]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and Age-Related Diseases: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, L.; López-Hoyos, M.; Muñoz-Cacho, P.; Martínez-Taboada, V.M. Aging Is Associated with Circulating Cytokine Dysregulation. Cell. Immunol. 2012, 273, 124–132. [Google Scholar] [CrossRef]
- Milan-Mattos, J.C.; Anibal, F.F.; Perseguini, N.M.; Minatel, V.; Rehder-Santos, P.; Castro, C.A.; Vasilceac, F.A.; Mattiello, S.M.; Faccioli, L.H.; Catai, A.M. Effects of Natural Aging and Gender on Pro-Inflammatory Markers. Braz. J. Med. Biol. Res. 2019, 52, e8392. [Google Scholar] [CrossRef]
- Silva, R.C.M.C. The Dichotomic Role of Cytokines in Aging. Biogerontology 2024, 26, 17. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Ageing and Obesity Shared Patterns: From Molecular Pathogenesis to Epigenetics. Diseases 2021, 9, 87. [Google Scholar] [CrossRef]
- Lisko, I.; Tiainen, K.; Stenholm, S.; Luukkaala, T.; Hurme, M.; Lehtimäki, T.; Hervonen, A.; Jylhä, M. Inflammation, Adiposity, and Mortality in the Oldest Old. Rejuvenation Res. 2012, 15, 445–452. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Wang, Y.; Ma, C.; Zhao, Q.; Yin, H.; Li, L.; Wang, D.; Huang, Y.; Zhao, Y.; et al. Interleukin-6 Promotes Visceral Adipose Tissue Accumulation during Aging via Inhibiting Fat Lipolysis. Int. Immunopharmacol. 2024, 132, 111906. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Mooney, R.A. Counterpoint: Interleukin-6 Does Not Have a Beneficial Role in Insulin Sensitivity and Glucose Homeostasis. J. Appl. Physiol. 2007, 102, 816–818, discussion 818–819. [Google Scholar] [CrossRef]
- Gruver, A.; Hudson, L.; Sempowski, G. Immunosenescence of Ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Rane, S.; Das, R.; Faulkner, M.; Gund, R.; Kandpal, U.; Lewis, V.; Mattoo, H.; Prabhu, S.; Ranganathan, V.; et al. T Cell Ageing: Effects of Age on Development, Survival & Function. Indian J. Med. Res. 2013, 138, 595. [Google Scholar]
- Lapice, E.; Maione, S.; Patti, L.; Cipriano, P.; Rivellese, A.A.; Riccardi, G.; Vaccaro, O. Abdominal Adiposity Is Associated With Elevated C-Reactive Protein Independent of BMI in Healthy Nonobese People. Diabetes Care 2009, 32, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.P.; Shah, T.; Hingorani, A.D.; Danesh, J.; Pepys, M.B. C-Reactive Protein and Coronary Heart Disease: A Critical Review. J. Intern. Med. 2008, 264, 295–314. [Google Scholar] [CrossRef]
- Hutchinson, W.L.; Koenig, W.; Fröhlich, M.; Sund, M.; Lowe, G.D.; Pepys, M.B. Immunoradiometric Assay of Circulating C-Reactive Protein: Age-Related Values in the Adult General Population. Clin. Chem. 2000, 46, 934–938. [Google Scholar] [CrossRef]
- Pasceri, V.; Willerson, J.T.; Yeh, E.T. Direct Proinflammatory Effect of C-Reactive Protein on Human Endothelial Cells. Circulation 2000, 102, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- Kittel-Schneider, S.; Kaspar, M.; Berliner, D.; Weber, H.; Deckert, J.; Ertl, G.; Störk, S.; Angermann, C.; Reif, A. CRP Genetic Variants Are Associated with Mortality and Depressive Symptoms in Chronic Heart Failure Patients. Brain Behav. Immun. 2018, 71, 133–141. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Xue, W.; Fan, Z.; Li, L.; Lu, J.; Zhai, Y.; Zhao, J. The Chemokine System and Its Role in Obesity. J. Cell. Physiol. 2019, 234, 3336–3346. [Google Scholar] [CrossRef]
- He, W.; Wang, H.; Yang, G.; Zhu, L.; Liu, X. The Role of Chemokines in Obesity and Exercise-Induced Weight Loss. Biomolecules 2024, 14, 1121. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, Q. Macrophage Recruitment in Obese Adipose Tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Inouye, K.E.; Shi, H.; Howard, J.K.; Daly, C.H.; Lord, G.M.; Rollins, B.J.; Flier, J.S. Absence of CC Chemokine Ligand 2 Does Not Limit Obesity-Associated Infiltration of Macrophages into Adipose Tissue. Diabetes 2007, 56, 2242–2250. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, Y.-J.; Choi, I.; Kim, M.; Sung, J.-S. CXCL16/CXCR6 Axis in Adipocytes Differentiated from Human Adipose Derived Mesenchymal Stem Cells Regulates Macrophage Polarization. Cells 2021, 10, 3410. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Yoon, J.H.; Ghim, J.; Yea, K.; Song, P.; Park, S.; Lee, A.; Hong, C.-P.; Jang, M.S.; et al. CXCL12 Secreted from Adipose Tissue Recruits Macrophages and Induces Insulin Resistance in Mice. Diabetologia 2014, 57, 1456–1465. [Google Scholar] [CrossRef]
- Moreno, B.; Hueso, L.; Ortega, R.; Benito, E.; Martínez-Hervas, S.; Peiro, M.; Civera, M.; Sanz, M.-J.; Piqueras, L.; Real, J.T. Association of Chemokines IP-10/CXCL10 and I-TAC/CXCL11 with Insulin Resistance and Enhance Leukocyte Endothelial Arrest in Obesity. Microvasc. Res. 2022, 139, 104254. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.M.T.L.; Lopes, L.R.; de Paula Costa, G.; Figueiredo, V.P.; Shrestha, D.; Batista, A.P.; Nicolato, R.L.d.C.; de Oliveira, F.L.P.; Gomes, J.A.S.; Talvani, A. CXCL-16, IL-17, and Bone Morphogenetic Protein 2 (BMP-2) Are Associated with Overweight and Obesity Conditions in Middle-Aged and Elderly Women. Immun. Ageing 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, H.d.L.; Almeida, C.d.S.; Abramo, C.; Grunewald, S.T.F.; Levy, R.A.; Teixeira, H.C. CCL2, CXCL8, CXCL9 and CXCL10 Serum Levels Increase with Age but Are Not Altered by Treatment with Hydroxychloroquine in Patients with Osteoarthritis of the Knees. Int. J. Rheum. Dis. 2017, 20, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-Rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An Inflammatory Aging Clock (iAge) Based on Deep Learning Tracks Multimorbidity, Immunosenescence, Frailty and Cardiovascular Aging. Nat. Aging 2021, 1, 598–615. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; De Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards Frailty Biomarkers: Candidates from Genes and Pathways Regulated in Aging and Age-Related Diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Chaudhary, J.K.; Danga, A.K.; Kumari, A.; Bhardwaj, A.; Rath, P.C. Role of Chemokines in Aging and Age-Related Diseases. Mech. Ageing Dev. 2025, 223, 112009. [Google Scholar] [CrossRef]
- Romacho, T.; Sánchez-Ferrer, C.F.; Peiró, C. Visfatin/Nampt: An Adipokine with Cardiovascular Impact. Mediators Inflamm. 2013, 2013, 946427. [Google Scholar] [CrossRef] [PubMed]
- Skoczylas, A. The role of visfatin in the pathophysiology of human. Wiad. Lek. 2009, 62, 190–196. [Google Scholar] [PubMed]
| Biomarker | Site of Production | Association with Obesity and Aging | Clinical Implications | Ref. |
|---|---|---|---|---|
| Visfatin | Mainly VAT (adipocytes and macrophages) |  in obesity levels; in obesity levels;  with age in obese nondiabetic subjects; associated with VAT, BMI, and body fat; conflicting data on correlation with fat distribution. with age in obese nondiabetic subjects; associated with VAT, BMI, and body fat; conflicting data on correlation with fat distribution. | Potential marker for cardiometabolic risk; role in elderly requires further study. | [25,26,27,28,29,30,31,32] |
| Leptin | Primarily WAT; also, BAT, placenta, other tissues |  in obesity and with increased fat mass in elderly; leptin resistance common in obesity and aging; higher in sarcopenic visceral obesity; AL ratio is a better marker for metabolic syndrome. in obesity and with increased fat mass in elderly; leptin resistance common in obesity and aging; higher in sarcopenic visceral obesity; AL ratio is a better marker for metabolic syndrome. | Marker for MetS and cardiometabolic risk; AL ratio useful for risk stratification and monitoring interventions. | [33,34,35,36,37,38,39,40,41,42,43,44] |
| RBP4 | Adipose tissue, liver, macrophages |  in IR states (obesity, metabolic syndrome, T2DM); in elderly, not always correlated with adiposity; may be associated with atherosclerosis and IR in obese elderly. in IR states (obesity, metabolic syndrome, T2DM); in elderly, not always correlated with adiposity; may be associated with atherosclerosis and IR in obese elderly. | Potential marker for atherosclerosis and IR; more studies needed in elderly. | [45,46,47,48,49,50] |
| Chemerin | Adipose tissue |  in overweight, prediabetes, T2DM; in elderly, levels increase with age and are independent of BMI; may reflect adipocyte dysfunction with aging. in overweight, prediabetes, T2DM; in elderly, levels increase with age and are independent of BMI; may reflect adipocyte dysfunction with aging. | Possible marker for metabolic dysfunction in elderly; further studies needed for clinical use. | [51,52,53,54] |
| Resistin | Monocytes, macrophages (humans) |  with age and in inflammaging; associated with insulin resistance and atherosclerosis in elderly; lower in offspring of centenarians with better metabolic health. with age and in inflammaging; associated with insulin resistance and atherosclerosis in elderly; lower in offspring of centenarians with better metabolic health. | Potential biomarker for age-related chronic disease risk and metabolic health. | [55,56,57] |
| Lipocalin 2 (LCN2) | Adipocytes, neutrophils, liver, kidney |  in obesity and with age; correlates with resistin and adiponectin; associated with body fat, glucose, HOMA-IR; role in aging not fully clarified. in obesity and with age; correlates with resistin and adiponectin; associated with body fat, glucose, HOMA-IR; role in aging not fully clarified. | May serve as a biomarker for obesity-related metabolic disorders; possible therapeutic target for age-related disease prevention. | [58,59,60,61,62,63,64,65] |
| TNF-α, IL-1β, IL-6 | Adipose tissue macrophages, immune cells |  in obesity and aging; promote adipose dysfunction, sarcopenia, frailty; IL-6 correlates with visceral fat in elderly; dual roles (pro- and anti-inflammatory effects). in obesity and aging; promote adipose dysfunction, sarcopenia, frailty; IL-6 correlates with visceral fat in elderly; dual roles (pro- and anti-inflammatory effects). | Risk factors for CVD, diabetes, sarcopenia, frailty; potential targets for anti-inflammatory interventions. | [66,67,68,69,70,71,72,73,74,75,76,77] |
| CRP | Liver (in response to IL-6 and IL-1β) |  with adiposity and age; higher in elderly, especially women; associated with CV risk; genetic variants linked to mortality in heart failure patients. with adiposity and age; higher in elderly, especially women; associated with CV risk; genetic variants linked to mortality in heart failure patients. | Established biomarker for inflammation and CVD risk; useful for risk stratification in elderly obese individuals. | [78,79,80,81,82,83,84] |
| Chemokines (e.g., CXCL16, CCL2, CXCL10, CXCL9) | Adipose tissue, immune cells, endothelial cells |  in obesity and aging; associated with increased CVD risk, insulin resistance, and frailty; some (e.g., CXCL16, CCL2) increase with age and adiposity. in obesity and aging; associated with increased CVD risk, insulin resistance, and frailty; some (e.g., CXCL16, CCL2) increase with age and adiposity. | Potential early markers for frailty, CVD, and metabolic dysfunction; possible therapeutic targets. | [85,86,87,88,89,90,91,92,93,94,95,96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamvakou, G.; Theodorakis, N.; Anagnostou, D.; Kreouzi, M.; Rallidis, L.S.; Katsi, V.; Simou, E.; Archontakis, S.; Skalis, G.; Hitas, C.; et al. Obesity-Related Inflammatory Biomarkers in the Elderly Population. Cells 2025, 14, 1733. https://doi.org/10.3390/cells14211733
Vamvakou G, Theodorakis N, Anagnostou D, Kreouzi M, Rallidis LS, Katsi V, Simou E, Archontakis S, Skalis G, Hitas C, et al. Obesity-Related Inflammatory Biomarkers in the Elderly Population. Cells. 2025; 14(21):1733. https://doi.org/10.3390/cells14211733
Chicago/Turabian StyleVamvakou, Georgia, Nikolaos Theodorakis, Dimitris Anagnostou, Magdalini Kreouzi, Loukianos S. Rallidis, Vasiliki Katsi, Effie Simou, Stefanos Archontakis, George Skalis, Christos Hitas, and et al. 2025. "Obesity-Related Inflammatory Biomarkers in the Elderly Population" Cells 14, no. 21: 1733. https://doi.org/10.3390/cells14211733
APA StyleVamvakou, G., Theodorakis, N., Anagnostou, D., Kreouzi, M., Rallidis, L. S., Katsi, V., Simou, E., Archontakis, S., Skalis, G., Hitas, C., Toutouzas, K., & Nikolaou, M. (2025). Obesity-Related Inflammatory Biomarkers in the Elderly Population. Cells, 14(21), 1733. https://doi.org/10.3390/cells14211733










