Inhalation of Ultrafine Carbon Black-Induced Mitochondrial Dysfunction in Mouse Heart Through Changes in Acetylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure System
2.2. Animal Exposure
2.3. Cardiac Protein Isolation
2.4. Western Blotting
2.5. Immunoprecipitation
2.6. Blue Native Page (BN-PAGE)
2.7. ETC Complex Activity Assays
2.8. Biochemical Assays
2.9. Statistical Analysis
3. Results
3.1. Aerosol Characterization
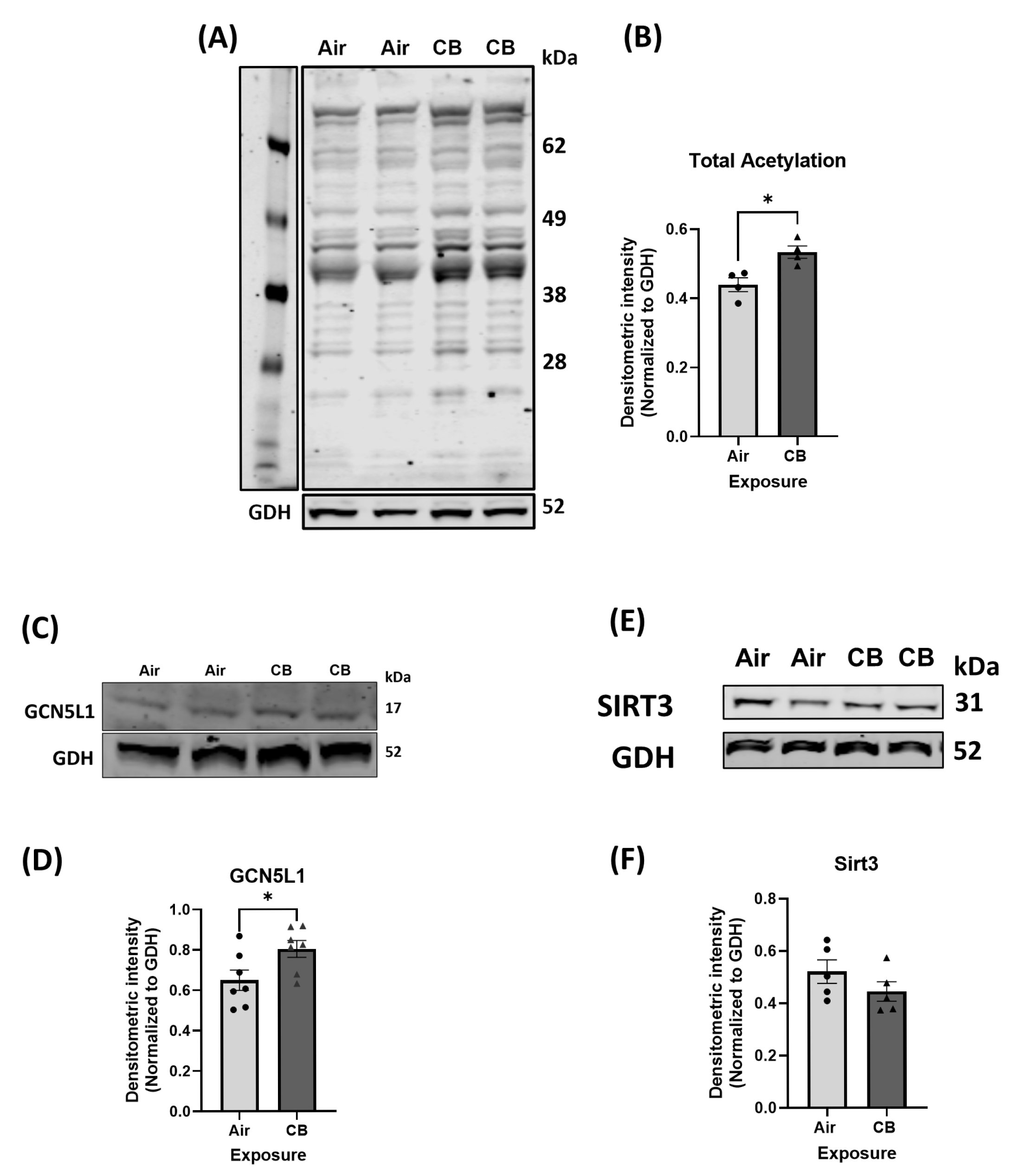
3.2. Repeated CB Exposure Increases Overall Cardiac Protein Acetylation and Induces a Pro-Acetylation Phenotype
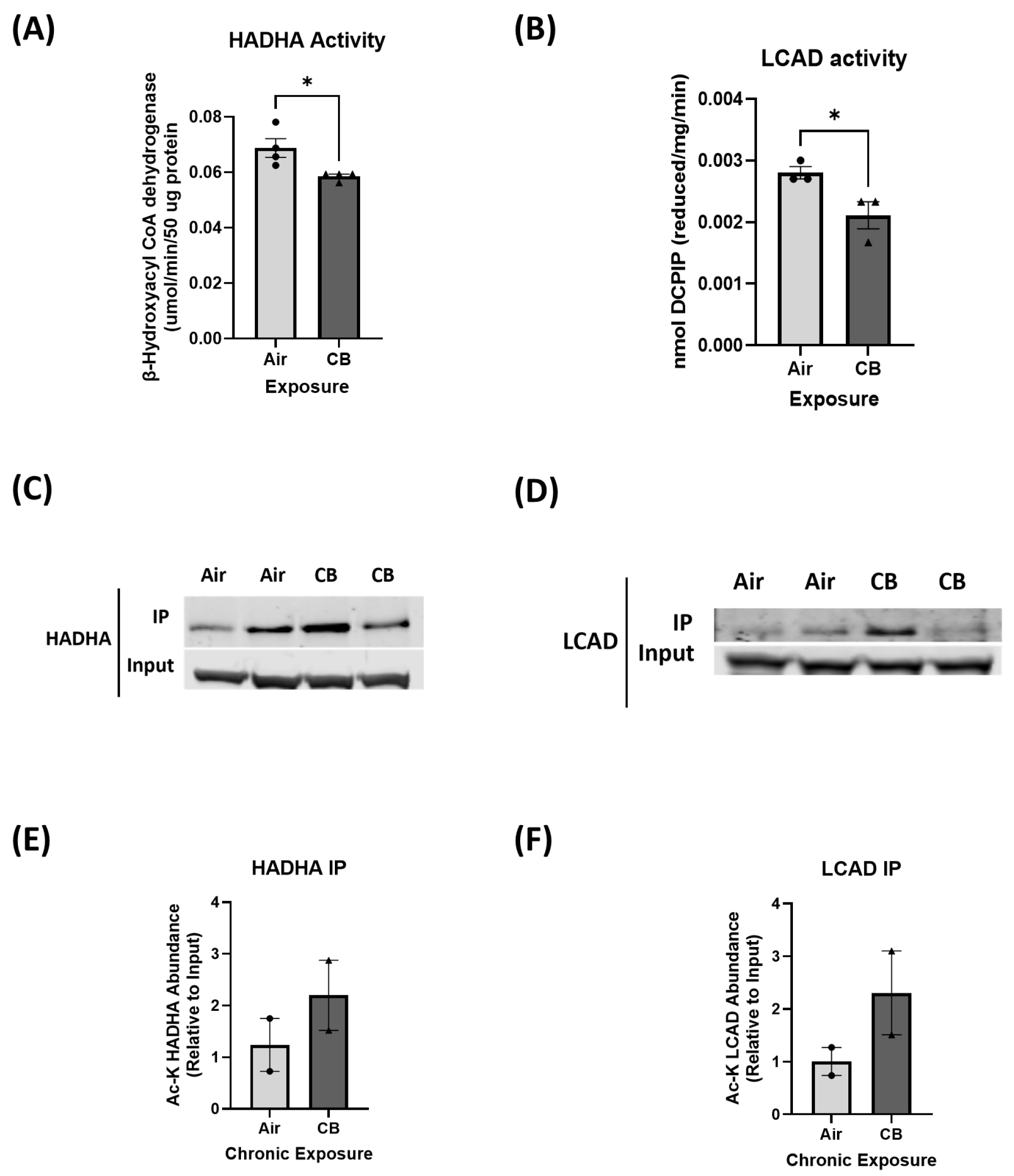
3.3. Hyperacetylation Induced by Repeated CB Exposure Leads to Decreased Fatty Acid Oxidation Enzyme Activity
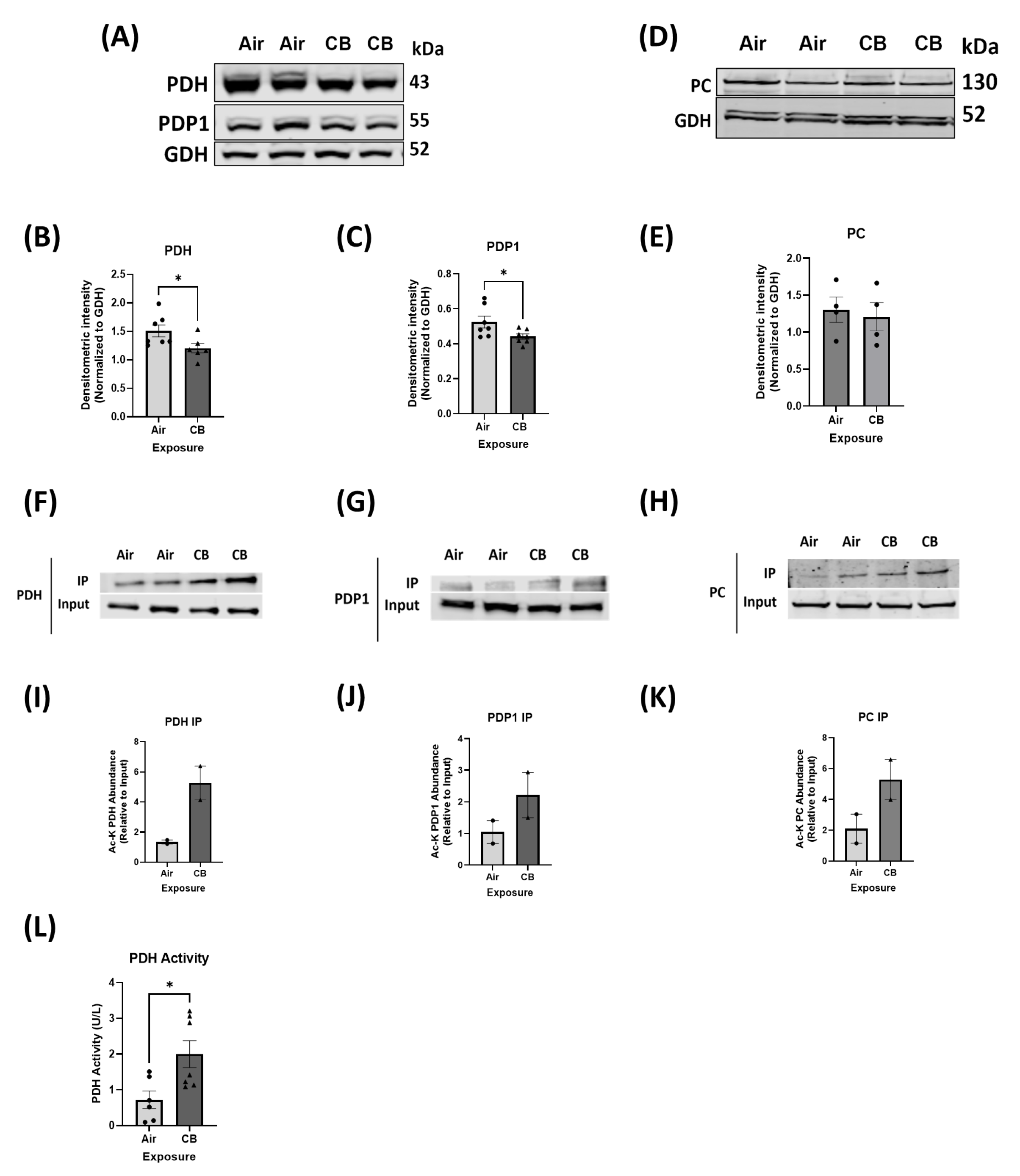
3.4. Hyperacetylation Induced by Repeated CB Exposure Leads to Increased Glucose Oxidation, Ensuring Metabolic Inflexibility
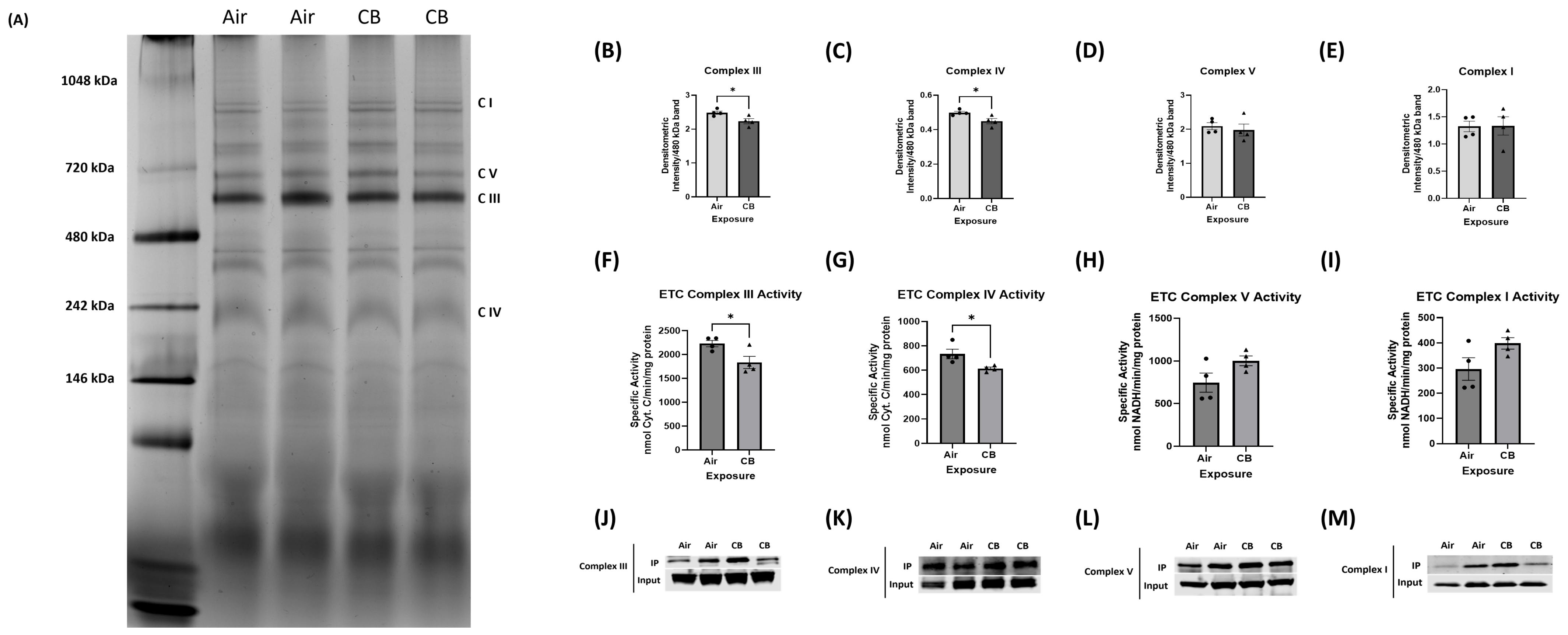
3.5. Mitochondrial Electron Transport Chain (ETC) Protein Content Remains Stable, but Complex Assembly and Activity Are Impaired with Repeated CB Exposure
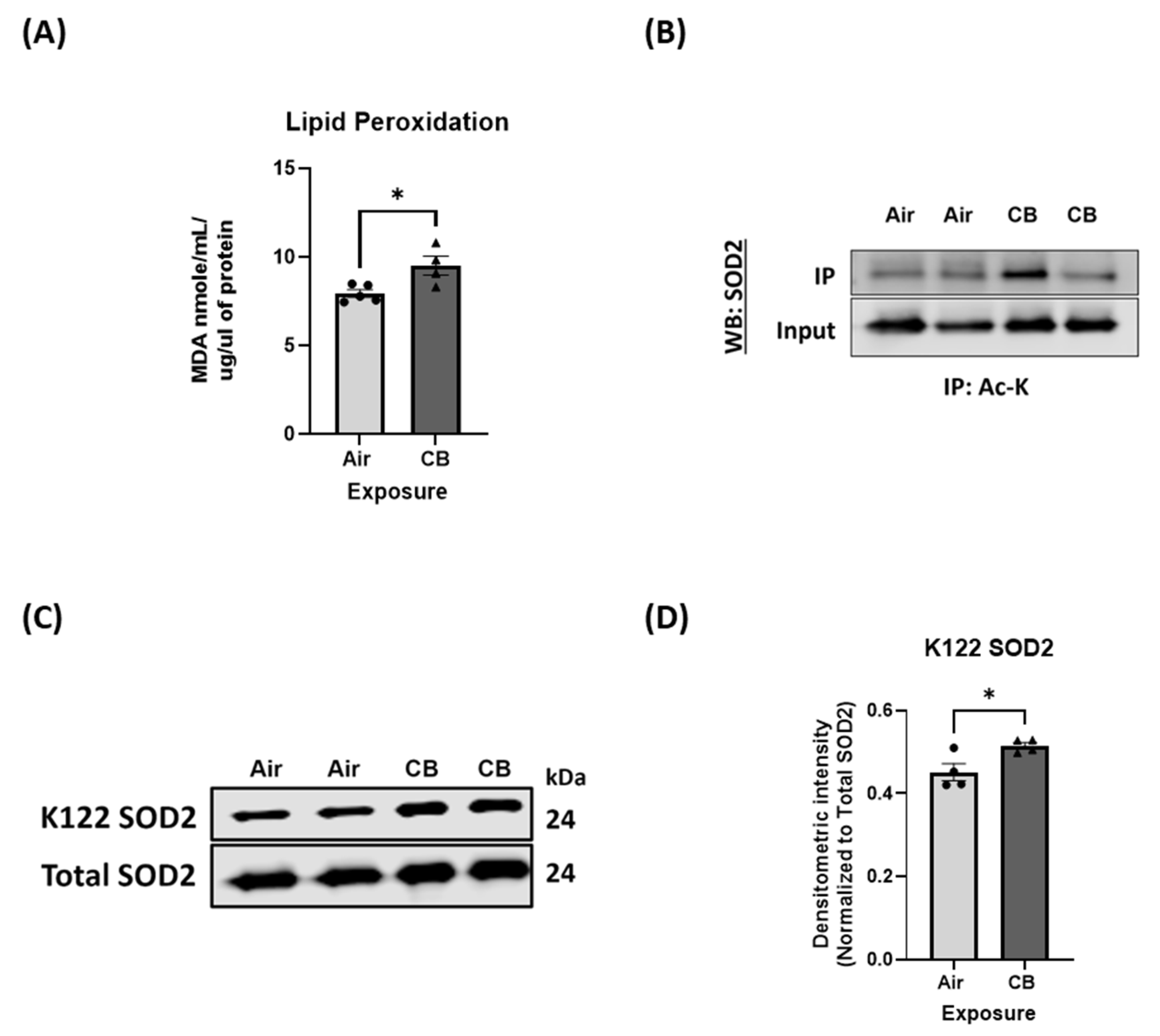
3.6. Mitochondrial Antioxidant Response Is Dysregulated Despite Unchanged Protein Expression with Repeated CB Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Gebrehiwot, T.T.; Lopez, A.D. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015 (vol 5, pg 691, 2017). Lancet Respir. Med. 2017, 5, 691. [Google Scholar]
- Collaborators, G.R.F. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Campen, M.J.; Buntz, J.; Lund, A.; Seagrave, J.; Vedal, S.; Mauderly, J.; McDonald, J. Vascular Effects of Vapor and Particulate Phases of Traffic-Related Air Pollution: Initial Results from the NPACT Initiative. Am. J. Respir. Crit. Care Med. 2009, A3150. [Google Scholar]
- Cleary, E.G.; Cifuentes, M.; Grinstein, G.; Brugge, D.; Shea, T.B. Association of low-level ozone with cognitive decline in older adults. J. Alzheimer’s Dis. 2017, 61, 67–78. [Google Scholar] [CrossRef]
- Hathaway, Q.A.; Majumder, N.; Goldsmith, W.T.; Kunovac, A.; Pinti, M.V.; Harkema, J.R.; Castranova, V.; Hollander, J.M.; Hussain, S. Transcriptomics of single dose and repeated carbon black and ozone inhalation co-exposure highlight progressive pulmonary mitochondrial dysfunction. Part. Fibre Toxicol. 2021, 18, 44. [Google Scholar] [CrossRef]
- Majumder, N.; Kodali, V.; Velayutham, M.; Goldsmith, T.; Amedro, J.; Khramtsov, V.V.; Erdely, A.; Nurkiewicz, T.R.; Harkema, J.R.; Kelley, E.E. Aerosol physicochemical determinants of carbon black and ozone inhalation co-exposure induced pulmonary toxicity. Toxicol. Sci. 2023, 191, 61–78. [Google Scholar] [CrossRef]
- Hadley, M.B.; Vedanthan, R.; Fuster, V. Air pollution and cardiovascular disease: A window of opportunity. Nat. Rev. Cardiology 2018, 15, 193–194. [Google Scholar] [CrossRef]
- Niranjan, R.; Thakur, A.K. The toxicological mechanisms of environmental soot (black carbon) and carbon black: Focus on oxidative stress and inflammatory pathways. Front. Immunol. 2017, 8, 763. [Google Scholar] [CrossRef]
- Trembley, J.H.; So, S.W.; Nixon, J.P.; Bowdridge, E.C.; Garner, K.L.; Griffith, J.; Engles, K.J.; Batchelor, T.P.; Goldsmith, W.T.; Tomáška, J.M. Whole-body inhalation of nano-sized carbon black: A surrogate model of military burn pit exposure. BMC Res. Notes 2022, 15, 275. [Google Scholar] [CrossRef]
- Mazumder, M.H.H.; Gandhi, J.; Majumder, N.; Wang, L.; Cumming, R.I.; Stradtman, S.; Velayutham, M.; Hathaway, Q.A.; Shannahan, J.; Hu, G. Lung-gut axis of microbiome alterations following co-exposure to ultrafine carbon black and ozone. Part. Fibre Toxicol. 2023, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Majumder, N.; Velayutham, M.; Bitounis, D.; Kodali, V.K.; Mazumder, M.H.H.; Amedro, J.; Khramtsov, V.V.; Erdely, A.; Nurkiewicz, T.; Demokritou, P. Oxidized carbon black nanoparticles induce endothelial damage through CXC chemokine receptor 3-mediated pathway. Redox Biol. 2021, 47, 102161. [Google Scholar] [CrossRef] [PubMed]
- Majumder, N.; Goldsmith, W.T.; Kodali, V.K.; Velayutham, M.; Friend, S.A.; Khramtsov, V.V.; Nurkiewicz, T.R.; Erdely, A.; Zeidler-Erdely, P.C.; Castranova, V. Oxidant-induced epithelial alarmin pathway mediates lung inflammation and functional decline following ultrafine carbon and ozone inhalation co-exposure. Redox Biol. 2021, 46, 102092. [Google Scholar] [CrossRef]
- Colicino, E.; Power, M.C.; Cox, D.G.; Weisskopf, M.G.; Hou, L.; Alexeeff, S.E.; Sanchez-Guerra, M.; Vokonas, P.; Spiro, I.I.I.A.; Schwartz, J. Mitochondrial haplogroups modify the effect of black carbon on age-related cognitive impairment. Environ. Health 2014, 13, 42. [Google Scholar] [CrossRef]
- Gao, X.; Xu, H.; Shang, J.; Yuan, L.; Zhang, Y.; Wang, L.; Zhang, W.; Luan, X.; Hu, G.; Chu, H. Ozonized carbon black induces mitochondrial dysfunction and DNA damage. Environ. Toxicol. 2017, 32, 944–955. [Google Scholar] [CrossRef]
- Jia, X.; Hao, Y.; Guo, X. Ultrafine carbon black disturbs heart rate variability in mice. Toxicology letters 2012, 211, 274–280. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Ivanova, E.A.; Sobenin, I.A.; Yet, S.-F.; Orekhov, A.N. The role of mitochondria in cardiovascular diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef]
- Shang, Y.; Xue, W.; Kong, J.; Chen, Y.; Qiu, X.; An, X.; Li, Y.; Wang, H.; An, J. Ultrafine black carbon caused mitochondrial oxidative stress, mitochondrial dysfunction and mitophagy in SH-SY5Y cells. Sci. Total Environ. 2022, 813, 151899. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Su, B.; Gao, H.; Ren, M.; Lin, Y.; Shen, H. A role of ROS-dependent defects in mitochondrial dynamic and autophagy in carbon black nanoparticle-mediated myocardial cell damage. Free. Radic. Biol. Med. 2024, 220, 249–261. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef]
- Neely, J.R.; Morgan, H.E. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu. Rev. Physiol. 1974, 36, 413–459. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Belke, D.D.; Gamble, J.; Toshiyuki, I.; Schönekess, B.O. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1994, 1213, 263–276. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef]
- Anderson, K.A.; Hirschey, M.D. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012, 52, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.N.; Finkel, T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb. Perspect. Biol. 2012, 4, a013102. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Zhang, M.; Manning, J.R.; Guimarães, D.A.; Stoner, M.W.; O’Doherty, R.M.; Shiva, S.; Scott, I. Acetylation of mitochondrial proteins by GCN5L1 promotes enhanced fatty acid oxidation in the heart. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H265–H274. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Crawford, J.M.; Mullen, W.E.; Jacques, A.; Stoner, M.W.; Scott, I.; Thapa, D. Cardiomyocyte-specific deletion of GCN5L1 reduces lysine acetylation and attenuates diastolic dysfunction in aged mice by improving cardiac fatty acid oxidation. Biochem. J. 2024, 481, 423–436. [Google Scholar] [CrossRef]
- Abo Alrob, O.; Lopaschuk, G.D. Role of CoA and acetyl-CoA in regulating cardiac fatty acid and glucose oxidation. Biochem. Soc. Trans. 2014, 42, 1043–1051. [Google Scholar] [CrossRef]
- Fernandes, J.; Weddle, A.; Kinter, C.S.; Humphries, K.M.; Mather, T.; Szweda, L.I.; Kinter, M. Lysine acetylation activates mitochondrial aconitase in the heart. Biochemistry 2015, 54, 4008–4018. [Google Scholar] [CrossRef] [PubMed]
- Baeza, J.; Smallegan, M.J.; Denu, J.M. Site-specific reactivity of nonenzymatic lysine acetylation. ACS Chem. Biol. 2015, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.L.; Martin, O.J.; Lai, L.; Riley, N.M.; Richards, A.L.; Vega, R.B.; Leone, T.C.; Pagliarini, D.J.; Muoio, D.M.; Bedi, K.C., Jr. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2016, 1, e84897. [Google Scholar] [CrossRef]
- Thapa, D.; Manning, J.R.; Stoner, M.W.; Zhang, M.; Xie, B.; Scott, I. Cardiomyocyte-specific deletion of GCN5L1 in mice restricts mitochondrial protein hyperacetylation in response to a high fat diet. Sci. Rep. 2020, 10, 10665. [Google Scholar] [CrossRef]
- Baseler, W.A.; Dabkowski, E.R.; Jagannathan, R.; Thapa, D.; Nichols, C.E.; Shepherd, D.L.; Croston, T.L.; Powell, M.; Razunguzwa, T.T.; Lewis, S.E. Reversal of mitochondrial proteomic loss in Type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R553–R565. [Google Scholar] [CrossRef]
- Croston, T.L.; Thapa, D.; Holden, A.A.; Tveter, K.J.; Lewis, S.E.; Shepherd, D.L.; Nichols, C.E.; Long, D.M.; Olfert, I.M.; Jagannathan, R. Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H54–H65. [Google Scholar] [CrossRef]
- Barrientos, A.; Fontanesi, F.; Díaz, F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. 2009, 63, 19.3.1–19.3.14. [Google Scholar] [CrossRef]
- Hathaway, Q.A.; Nichols, C.E.; Shepherd, D.L.; Stapleton, P.A.; McLaughlin, S.L.; Stricker, J.C.; Rellick, S.L.; Pinti, M.V.; Abukabda, A.B.; McBride, C.R. Maternal-engineered nanomaterial exposure disrupts progeny cardiac function and bioenergetics. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H446–H458. [Google Scholar] [CrossRef]
- Alrob, O.A.; Sankaralingam, S.; Ma, C.; Wagg, C.S.; Fillmore, N.; Jaswal, J.S.; Sack, M.N.; Lehner, R.; Gupta, M.P.; Michelakis, E.D.; et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc. Res. 2014, 103, 485–497. [Google Scholar] [CrossRef]
- Thapa, D.; Wu, K.; Stoner, M.W.; Xie, B.; Zhang, M.; Manning, J.R.; Lu, Z.; Li, J.H.; Chen, Y.; Gucek, M.; et al. The protein acetylase GCN5L1 modulates hepatic fatty acid oxidation activity via acetylation of the mitochondrial beta-oxidation enzyme HADHA. J. Biol. Chem. 2018, 293, 17676–17684. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.W. Systemic and cardiovascular effects of airway injury and inflammation: Ultrafine particle exposure in humans. Environ. Health Perspect. 2001, 109, 529–532. [Google Scholar] [PubMed]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six US cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef]
- Puett, R.C.; Hart, J.E.; Yanosky, J.D.; Paciorek, C.; Schwartz, J.; Suh, H.; Speizer, F.E.; Laden, F. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ. Health Perspect. 2009, 117, 1697–1701. [Google Scholar] [CrossRef]
- Weichenthal, S.; Villeneuve, P.J.; Burnett, R.T.; Van Donkelaar, A.; Martin, R.V.; Jones, R.R.; DellaValle, C.T.; Sandler, D.P.; Ward, M.H.; Hoppin, J.A. Long-term exposure to fine particulate matter: Association with nonaccidental and cardiovascular mortality in the agricultural health study cohort. Environ. Health Perspect. 2014, 122, 609–615. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health Part C 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Park, E.-J.; Yi, J.; Chung, K.-H.; Ryu, D.-Y.; Choi, J.; Park, K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef]
- Kunovac, A.; Hathaway, Q.A.; Pinti, M.V.; Taylor, A.D.; Hollander, J.M. Cardiovascular adaptations to particle inhalation exposure: Molecular mechanisms of the toxicology. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H282–H305. [Google Scholar] [CrossRef]
- Büchner, N.; Ale-Agha, N.; Jakob, S.; Sydlik, U.; Kunze, K.; Unfried, K.; Altschmied, J.; Haendeler, J. Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp. Gerontol. 2013, 48, 8–16. [Google Scholar] [CrossRef]
- Hornstein, T.; Spannbrucker, T.; Unfried, K. Combustion-derived carbon nanoparticles cause delayed apoptosis in neutrophil-like HL-60 cells in vitro and in primed human neutrophilic granulocytes ex vivo. Part. Fibre Toxicol. 2025, 22, 6. [Google Scholar] [CrossRef]
- Boland, S.; Hussain, S.; Baeza-Squiban, A. Carbon black and titanium dioxide nanoparticles induce distinct molecular mechanisms of toxicity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2014, 6, 641–652. [Google Scholar] [CrossRef]
- Gutor, S.S.; Salinas, R.I.; Nichols, D.S.; Bazzano, J.M.; Han, W.; Gokey, J.J.; Vasiukov, G.; West, J.D.; Newcomb, D.C.; Dikalova, A.E. Repetitive sulfur dioxide exposure in mice models post-deployment respiratory syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 326, L539–L550. [Google Scholar] [CrossRef]
- Dikalov, S.; Itani, H.; Richmond, B.; Arslanbaeva, L.; Vergeade, A.; Rahman, S.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monogr. Eval. Carcinog. Risks Hum. 2008, 97, 3.
- Husain, M.; Kyjovska, Z.O.; Bourdon-Lacombe, J.; Saber, A.T.; Jensen, K.A.; Jacobsen, N.R.; Williams, A.; Wallin, H.; Halappanavar, S.; Vogel, U. Carbon black nanoparticles induce biphasic gene expression changes associated with inflammatory responses in the lungs of C57BL/6 mice following a single intratracheal instillation. Toxicol. Appl. Pharmacol. 2015, 289, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Hougaard, K.S.; Boisen, A.M.Z.; Jacobsen, N.R.; Jensen, K.A.; Møller, P.; Brunborg, G.; Gutzkow, K.B.; Andersen, O.; Loft, S. Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: Effects on liver DNA strand breaks in dams and offspring. Nanotoxicology 2012, 6, 486–500. [Google Scholar] [CrossRef]
- Borm, P.J.; Cakmak, G.; Jermann, E.; Weishaupt, C.; Kempers, P.; van Schooten, F.J.; Oberdorster, G.; Schins, R.P. Formation of PAH-DNA adducts after in vivo and vitro exposure of rats and lung cells to different commercial carbon blacks. Toxicol. Appl. Pharmacol. 2005, 205, 157–167. [Google Scholar] [CrossRef]
- Bourdon, J.A.; Saber, A.T.; Jacobsen, N.R.; Jensen, K.A.; Madsen, A.M.; Lamson, J.S.; Wallin, H.; Moller, P.; Loft, S.; Yauk, C.L.; et al. Carbon black nanoparticle instillation induces sustained inflammation and genotoxicity in mouse lung and liver. Part. Fibre Toxicol. 2012, 9, 5. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; Saber, A.T.; White, P.; Moller, P.; Pojana, G.; Vogel, U.; Loft, S.; Gingerich, J.; Soper, L.; Douglas, G.R.; et al. Increased mutant frequency by carbon black, but not quartz, in the lacZ and cII transgenes of muta mouse lung epithelial cells. Env. Mol Mutagen 2007, 48, 451–461. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; White, P.A.; Gingerich, J.; Moller, P.; Saber, A.T.; Douglas, G.R.; Vogel, U.; Wallin, H. Mutation spectrum in FE1-MUTA(TM) Mouse lung epithelial cells exposed to nanoparticulate carbon black. Env. Mol. Mutagen. 2011, 52, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kyjovska, Z.O.; Jacobsen, N.R.; Saber, A.T.; Bengtson, S.; Jackson, P.; Wallin, H.; Vogel, U. DNA damage following pulmonary exposure by instillation to low doses of carbon black (Printex 90) nanoparticles in mice. Env. Mol. Mutagen. 2015, 56, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, H.; Wang, G.; Ren, H. Imbalance of lysine acetylation contributes to the pathogenesis of Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 7182. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Xu, Z.; Tzan, K.; Zhong, M.; Wang, A.; Lippmann, M.; Chen, L.-C.; Rajagopalan, S.; Sun, Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol. Sci. 2011, 124, 88–98. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef]
- Ahn, B.H.; Kim, H.S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef]
- Scott, I.; Webster, B.R.; Li, J.H.; Sack, M.N. Identification of a molecular component of the mitochondrial acetyltransferase programme: A novel role for GCN5L1. Biochem. J. 2012, 443, 655–661. [Google Scholar] [CrossRef]
- Dikalova, A.; Fehrenbach, D.; Mayorov, V.; Panov, A.; Ao, M.; Lantier, L.; Amarnath, V.; Lopez, M.G.; Billings, F.T., IV; Sack, M.N. Mitochondrial CypD acetylation promotes endothelial dysfunction and hypertension. Circ. Res. 2024, 134, 1451–1464. [Google Scholar] [CrossRef]
- Genova, M.L.; Lenaz, G. The interplay between respiratory supercomplexes and ROS in aging. Antioxid. Redox Signal. 2015, 23, 208–238. [Google Scholar] [CrossRef]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodríguez-Hernández, M. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Hoppel, C.L. Mitochondrial metabolism in aging heart. Circ. Res. 2016, 118, 1593–1611. [Google Scholar] [CrossRef]
- Dorn, G.W. Mitochondrial dynamism and heart disease: Changing shape and shaping change. EMBO Mol. Med. 2015, 7, 865–877. [Google Scholar] [CrossRef]
- Vazquez, E.J.; Berthiaume, J.M.; Kamath, V.; Achike, O.; Buchanan, E.; Montano, M.M.; Chandler, M.P.; Miyagi, M.; Rosca, M.G. Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc. Res. 2015, 107, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.; Arroum, T.; Wan, J.; Pavelich, L.; Bell, J.; Morse, P.T.; Lee, I.; Grossman, L.I.; Sanderson, T.H.; Malek, M.H. Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease–implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer. Redox Biol. 2024, 103426. [Google Scholar] [CrossRef] [PubMed]
- Morse, P.T.; Arroum, T.; Wan, J.; Pham, L.; Vaishnav, A.; Bell, J.; Pavelich, L.; Malek, M.H.; Sanderson, T.H.; Edwards, B.F. Phosphorylations and acetylations of cytochrome c control mitochondrial respiration, mitochondrial membrane potential, energy, ROS, and apoptosis. Cells 2024, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free. Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leung, M.C.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a target of environmental toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef]
- Finley, L.W.; Haigis, M.C. Metabolic regulation by SIRT3: Implications for tumorigenesis. Trends Mol. Med. 2012, 18, 516–523. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef]
- Manning, J.R.; Thapa, D.; Zhang, M.; Stoner, M.W.; Traba, J.; McTiernan, C.F.; Corey, C.; Shiva, S.; Sack, M.N.; Scott, I. Cardiac-specific deletion of GCN5L1 restricts recovery from ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2019, 129, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- Thapa, D.; Bugga, P.; Mushala, B.A.; Manning, J.R.; Stoner, M.W.; McMahon, B.; Zeng, X.; Cantrell, P.S.; Yates, N.; Xie, B. GCN5L1 impairs diastolic function in mice exposed to a high fat diet by restricting cardiac pyruvate oxidation. Physiol. Rep. 2022, 10, e15415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, R.; Stewart, J.E.; Mullen, W.E.; Lin, D.; Hussain, S.; Thapa, D. Inhalation of Ultrafine Carbon Black-Induced Mitochondrial Dysfunction in Mouse Heart Through Changes in Acetylation. Cells 2025, 14, 1728. https://doi.org/10.3390/cells14211728
Islam R, Stewart JE, Mullen WE, Lin D, Hussain S, Thapa D. Inhalation of Ultrafine Carbon Black-Induced Mitochondrial Dysfunction in Mouse Heart Through Changes in Acetylation. Cells. 2025; 14(21):1728. https://doi.org/10.3390/cells14211728
Chicago/Turabian StyleIslam, Rahatul, Jackson E. Stewart, William E. Mullen, Dena Lin, Salik Hussain, and Dharendra Thapa. 2025. "Inhalation of Ultrafine Carbon Black-Induced Mitochondrial Dysfunction in Mouse Heart Through Changes in Acetylation" Cells 14, no. 21: 1728. https://doi.org/10.3390/cells14211728
APA StyleIslam, R., Stewart, J. E., Mullen, W. E., Lin, D., Hussain, S., & Thapa, D. (2025). Inhalation of Ultrafine Carbon Black-Induced Mitochondrial Dysfunction in Mouse Heart Through Changes in Acetylation. Cells, 14(21), 1728. https://doi.org/10.3390/cells14211728






