M1 Macrophages Are a Source of IL-1α: A Driver of Progesterone Metabolism and Myometrial Contraction
Abstract
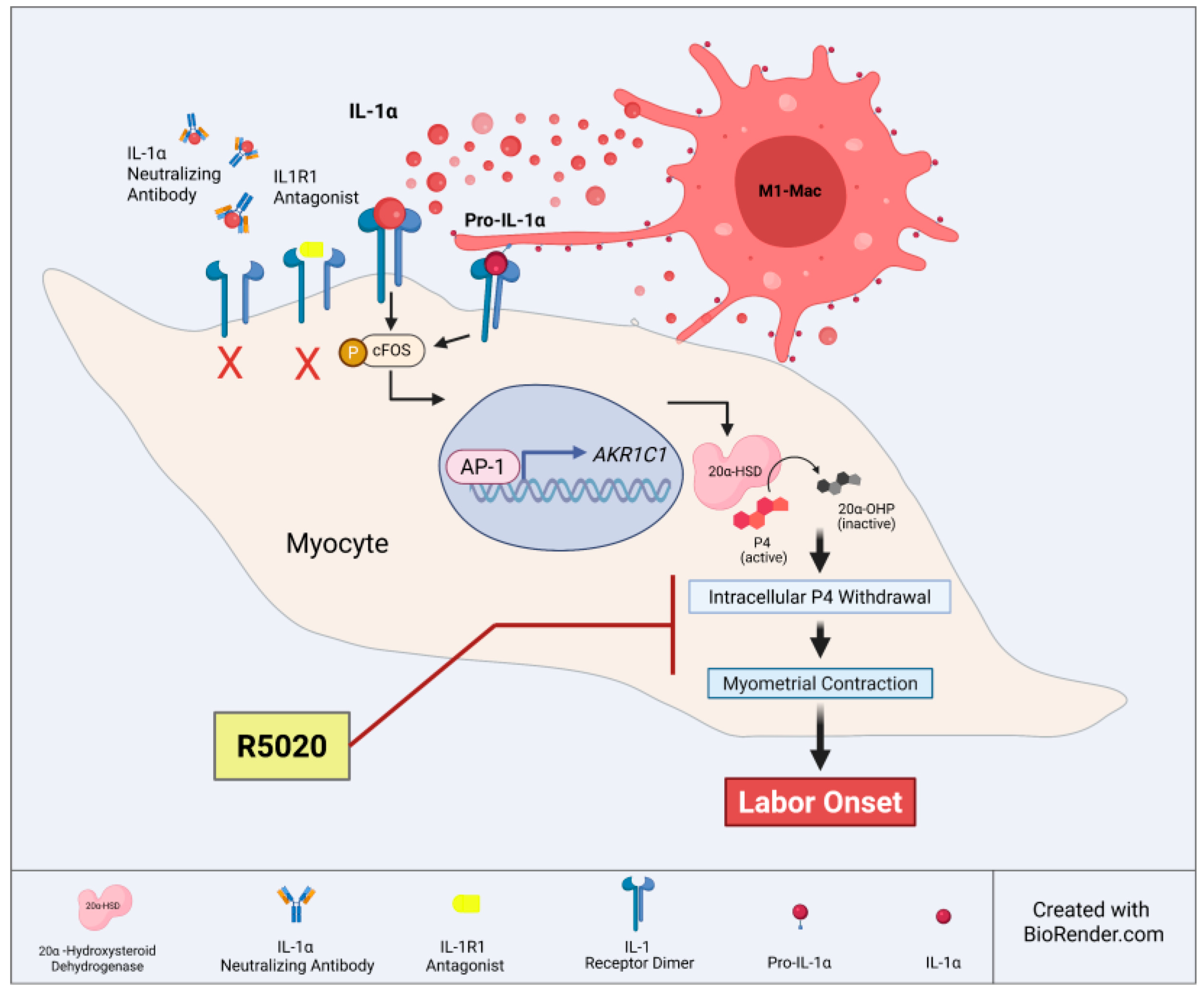
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
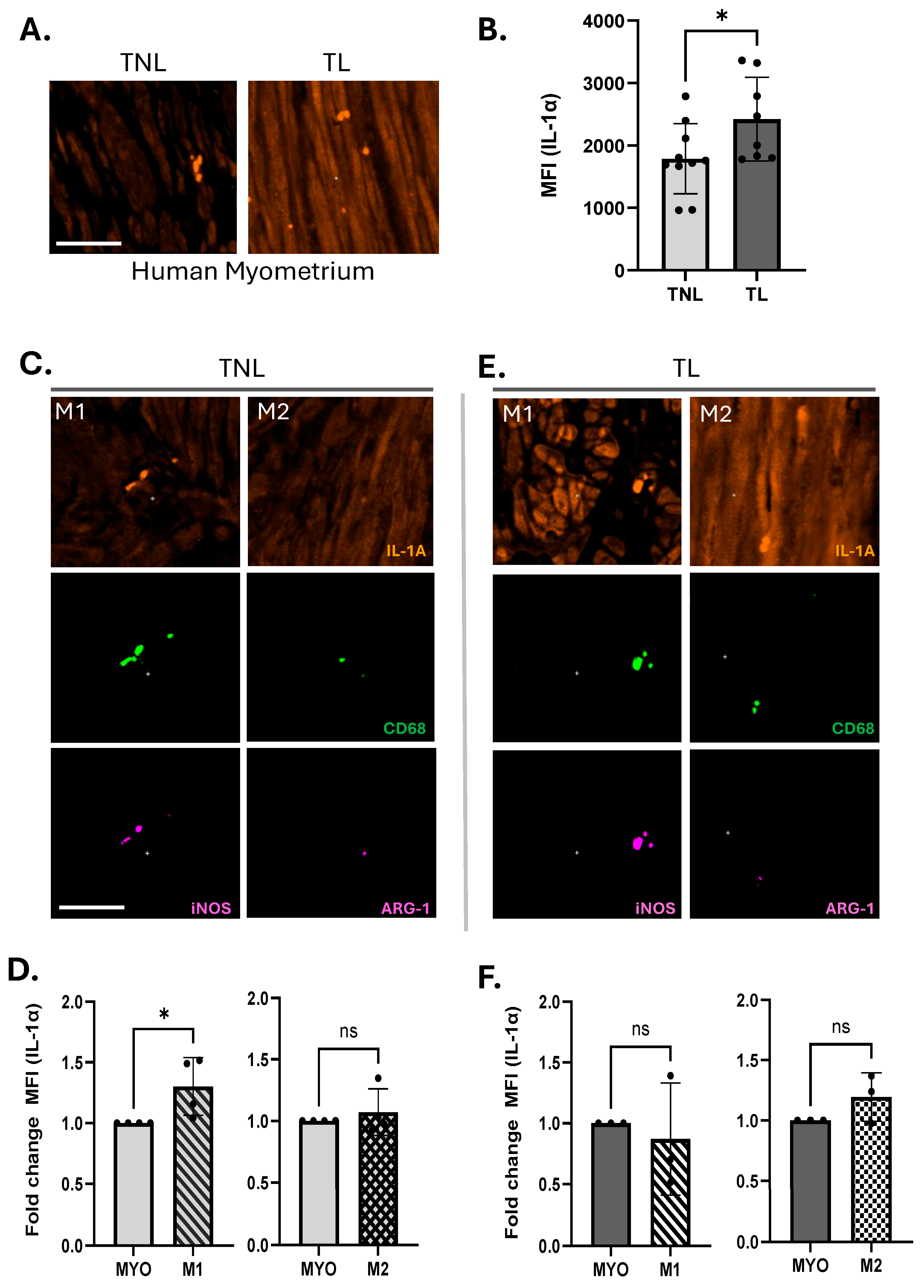
2.2. Immunohistochemistry of Human Myometrium
2.3. Primary Myometrial Cell Lines
2.4. Myometrial Cell Treatment Regimen
2.5. Peripheral Blood-Derived Monocytes
2.6. In Vitro Differentiation and Polarization of Macrophages
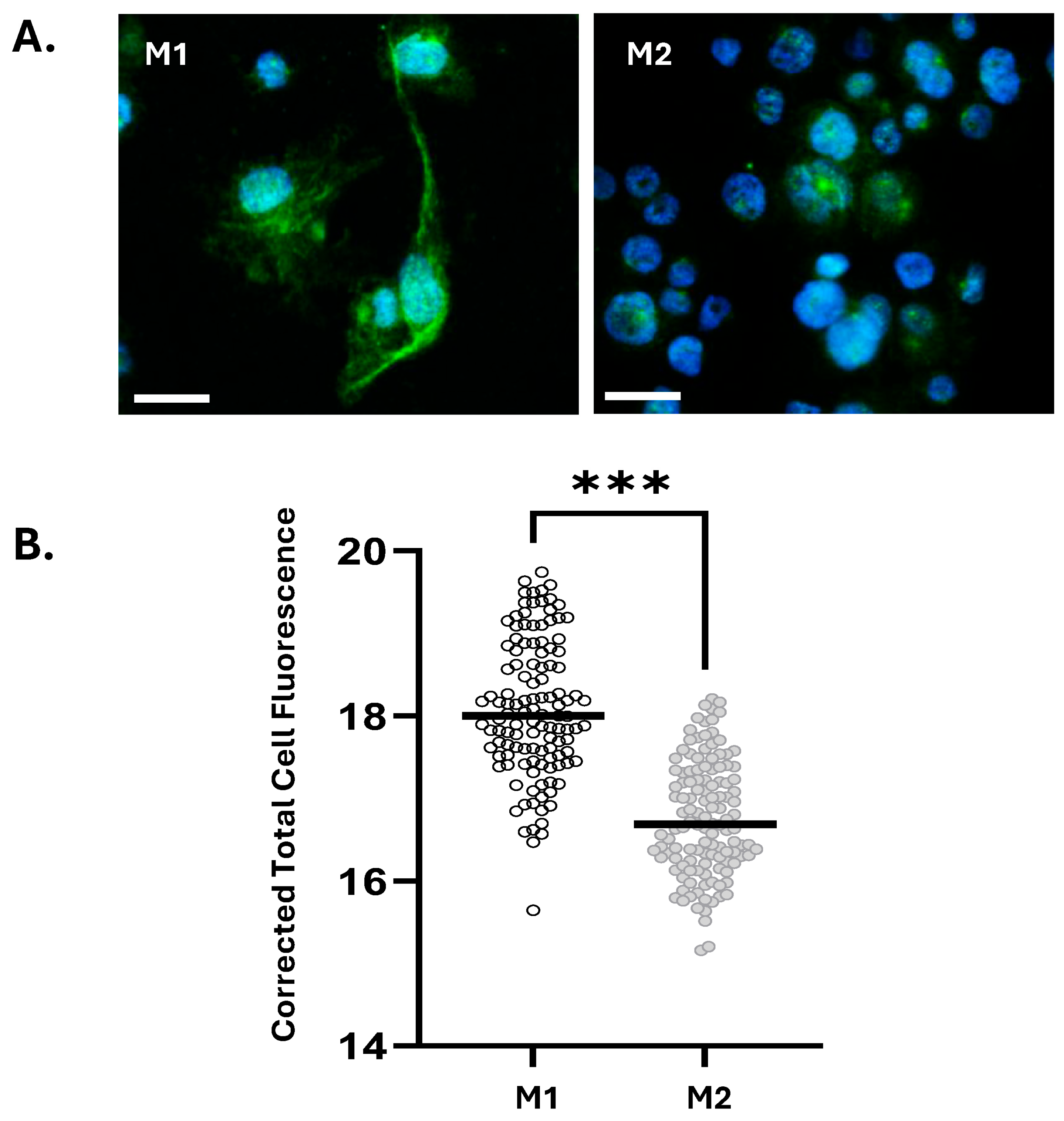
2.7. Immunocytochemistry (ICC)
2.8. Image Analysis
2.9. Protein Extraction
2.10. Immunoblotting
2.11. Real-Time PCR
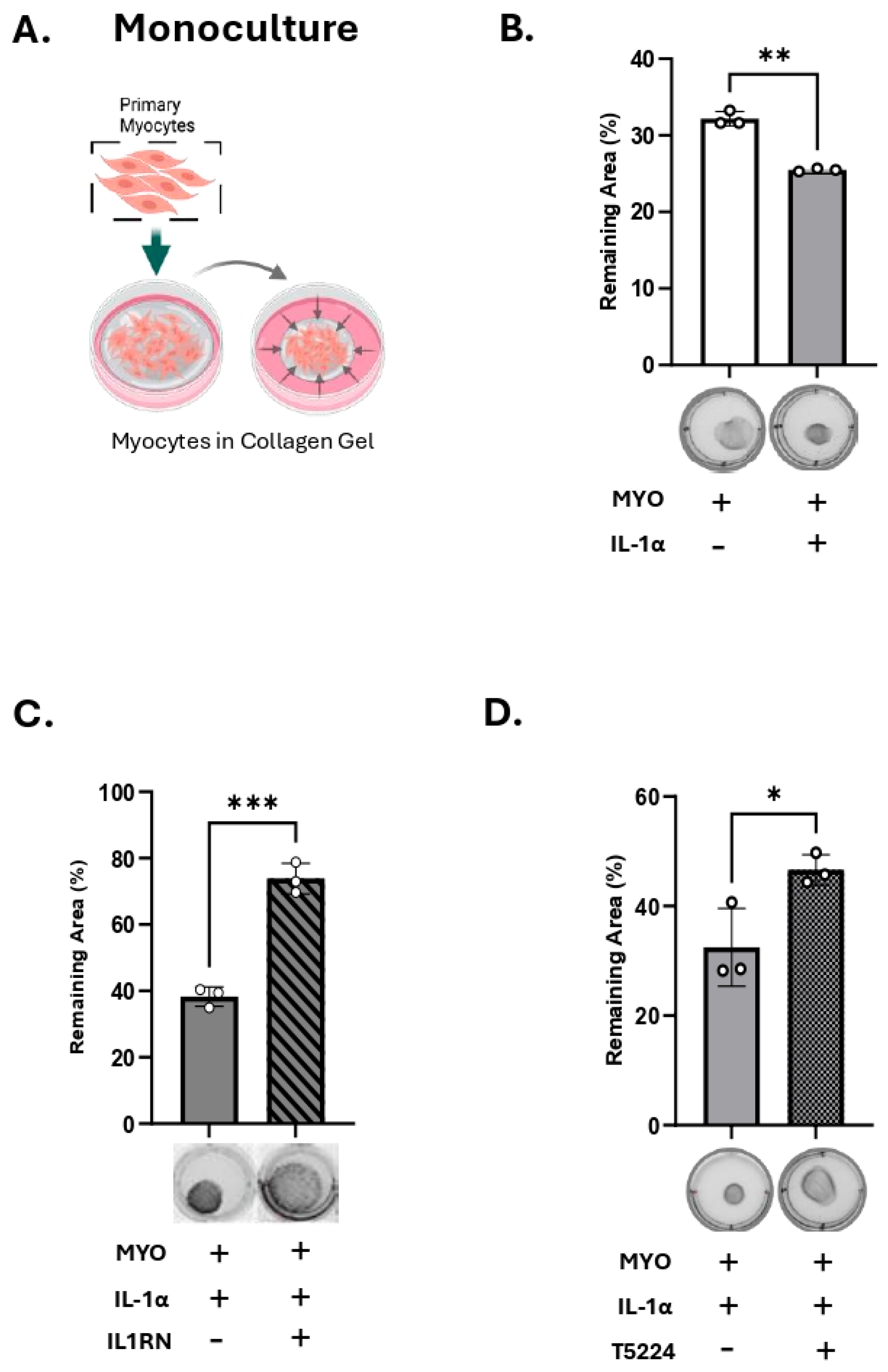
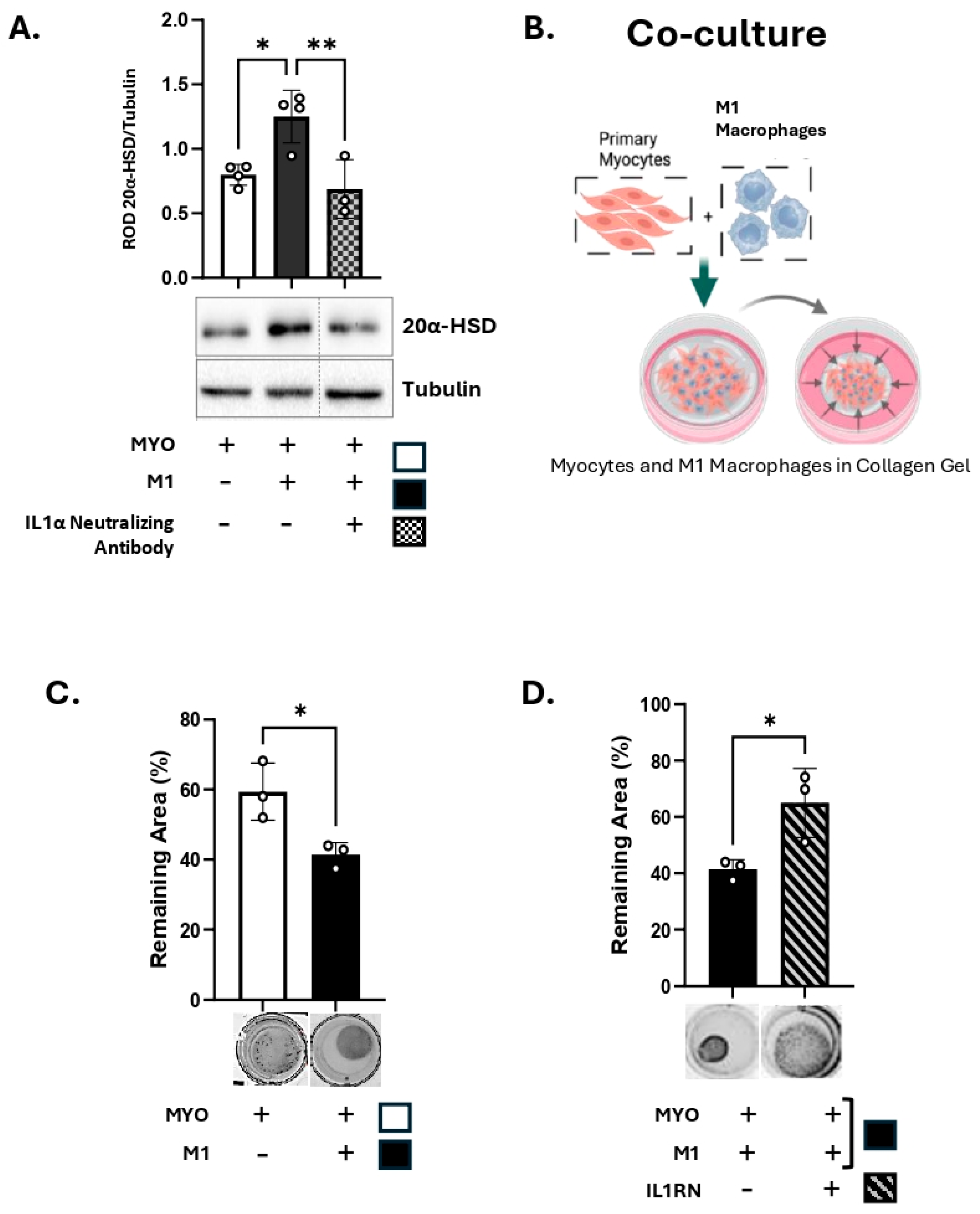
2.12. Collagen Gel Contraction Assay
2.13. Statistical Analysis
3. Results
3.1. M1 Macrophages Express Elevated Levels of IL-1α Compared to M2 Macrophages
3.2. IL-1α Induces 20a-HSD Levels and Contractility in the Myocytes and This Effect Is Mediated by AP-1 Transcription Factors
3.3. M1-Macrophages Induce 20α-HSD Protein Expression and Contractility of Human MYO
3.4. R5020 Blocks the Effect of IL-1α and M1-Macrophages on Myocyte Contractility
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120, 1337–1351. [Google Scholar] [CrossRef]
- Ward, R.M.; Beachy, J.C. Neonatal complications following preterm birth. BJOG 2003, 110 (Suppl. S20), 8–16. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Kim, C.J.; et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am. J. Reprod. Immunol. 2014, 71, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages infiltrate the human and rat decidua during term and preterm labor: Evidence that decidual inflammation precedes labor. Biol. Reprod. 2012, 86, 39. [Google Scholar] [CrossRef] [PubMed]
- Short, R.V. Blood progesterone levels in relation to parturition. J. Reprod. Fertil. 1960, 1, 61–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Csapo, A. Progesterone “block”. Am. J. Anat. 1956, 98, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Csapo, A.I.; Pohanka, O.; Kaihola, H.L. Progesterone deficiency and premature labour. Br. Med. J. 1974, 1, 137–140. [Google Scholar] [CrossRef]
- Schummers, L.; Darling, E.K.; Dunn, S.; McGrail, K.; Gayowsky, A.; Law, M.R.; Laba, T.L.; Kaczorowski, J.; Norman, W.V. Abortion Safety and Use with Normally Prescribed Mifepristone in Canada. N. Engl. J. Med. 2022, 386, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, L.; Balendran, R.; Dorogin, A.; Mesiano, S.; Shynlova, O.; Lye, S.J. Pro-inflammatory signals induce 20alpha-HSD expression in myometrial cells: A key mechanism for local progesterone withdrawal. J. Cell Mol. Med. 2021, 25, 6773–6785. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Nadeem, L.; Dorogin, A.; Mesiano, S.; Lye, S.J. The selective progesterone receptor modulator-promegestone-delays term parturition and prevents systemic inflammation-mediated preterm birth in mice. Am. J. Obstet. Gynecol. 2022, 226, 249.e1–249.e21. [Google Scholar] [CrossRef]
- Nadeem, A.; Nadeem, L.; Lye, S.J.; Shynlova, O. Promegestone Prevents Lipopolysaccharide-Induced Cervical Remodeling in Pregnant Mice. Cells 2025, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Nguyen, T.; Lye, S.J. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J. Cell Mol. Med. 2013, 17, 311–324. [Google Scholar] [CrossRef]
- Shynlova, O.; Tsui, P.; Dorogin, A.; Lye, S.J. Monocyte Chemoattractant Protein-1 (CCL-2) Integrates Mechanical and Endocrine Signals That Mediate Term and Preterm Labor1. J. Immunol. 2008, 181, 1470–1479. [Google Scholar] [CrossRef]
- Mizoguchi, M.; Ishida, Y.; Nosaka, M.; Kimura, A.; Kuninaka, Y.; Yahata, T.; Nanjo, S.; Toujima, S.; Minami, S.; Ino, K.; et al. Prevention of lipopolysaccharide-induced preterm labor by the lack of CX3CL1-CX3CR1 interaction in mice. PLoS ONE 2018, 13, e0207085. [Google Scholar] [CrossRef]
- Shan, Y.; Shen, S.; Long, J.; Tang, Z.; Wu, C.; Ni, X. Term and Preterm Birth Initiation Is Associated with the Macrophages Shifting to M1 Polarization in Gestational Tissues in Mice. Biology 2022, 11, 1759. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Brough, D.; Le Feuvre, R.A.; Wheeler, R.D.; Solovyova, N.; Hilfiker, S.; Rothwell, N.J.; Verkhratsky, A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J. Immunol. 2003, 170, 3029–3036. [Google Scholar] [CrossRef]
- Romero, R.; Mazor, M.; Brandt, F.; Sepulveda, W.; Avila, C.; Cotton, D.B.; Dinarello, C.A. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am. J. Reprod. Immunol. 1992, 27, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Parvizi, S.T.; Oyarzun, E.; Mazor, M.; Wu, Y.K.; Avila, C.; Athanassiadis, A.P.; Mitchell, M.D. Amniotic fluid interleukin-1 in spontaneous labor at term. J. Reprod. Med. 1990, 35, 235–238. [Google Scholar]
- Heng, Y.J.; Liong, S.; Permezel, M.; Rice, G.E.; Di Quinzio, M.K.; Georgiou, H.M. The interplay of the interleukin 1 system in pregnancy and labor. Reprod. Sci. 2014, 21, 122–130. [Google Scholar] [CrossRef]
- Hirsch, E.; Filipovich, Y.; Mahendroo, M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am. J. Obstet. Gynecol. 2006, 194, 1334–1340. [Google Scholar] [CrossRef]
- Kobayashi, H. The entry of fetal and amniotic fluid components into the uterine vessel circulation leads to sterile inflammatory processes during parturition. Front. Immunol. 2012, 3, 321. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Grivel, J.C.; Tarca, A.L.; Chaemsaithong, P.; Xu, Z.; Fitzgerald, W.; Hassan, S.S.; Chaiworapongsa, T.; Margolis, L. Evidence of perturbations of the cytokine network in preterm labor. Am. J. Obstet. Gynecol. 2015, 213, 836.E1–836.E18. [Google Scholar] [CrossRef]
- Romero, R.; Mazor, M.; Tartakovsky, B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am. J. Obstet. Gynecol. 1991, 165, 969–971. [Google Scholar] [CrossRef]
- Motomura, K.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Xu, Y.; Galaz, J.; Slutsky, R.; Levenson, D.; Gomez-Lopez, N. The alarmin interleukin-1alpha causes preterm birth through the NLRP3 inflammasome. Mol. Hum. Reprod. 2020, 26, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Srikhajon, K.; Shynlova, O.; Preechapornprasert, A.; Chanrachakul, B.; Lye, S. A new role for monocytes in modulating myometrial inflammation during human labor. Biol. Reprod. 2014, 91, 10. [Google Scholar] [CrossRef] [PubMed]
- Chimal-Ramirez, G.K.; Espinoza-Sanchez, N.A.; Chavez-Sanchez, L.; Arriaga-Pizano, L.; Fuentes-Panana, E.M. Monocyte Differentiation towards Protumor Activity Does Not Correlate with M1 or M2 Phenotypes. J. Immunol. Res. 2016, 2016, 6031486. [Google Scholar] [CrossRef]
- Shiratori, H.; Feinweber, C.; Luckhardt, S.; Linke, B.; Resch, E.; Geisslinger, G.; Weigert, A.; Parnham, M.J. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol. Immunol. 2017, 88, 58–68. [Google Scholar] [CrossRef]
- Dallot, E.; Pouchelet, M.; Gouhier, N.; Cabrol, D.; Ferré, F.o.; Breuiller-Fouché, M. Contraction of Cultured Human Uterine Smooth Muscle Cells after Stimulation with Endothelin-1. Biol. Reprod. 2003, 68, 937–942. [Google Scholar] [CrossRef][Green Version]
- Fleenor, D.L.; Pang, I.-H.; Clark, A.F. Involvement of AP-1 in Interleukin-1α–Stimulated MMP-3 Expression in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3494–3501. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K.; Mallers, T.M.; Larsen, B.; Kwak-Kim, J.; Chaouat, G.; Gilman-Sachs, A.; Beaman, K.D. V-ATPase upregulation during early pregnancy: A possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 2012, 143, 713–725. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Lee, Y.H.; Shynlova, O.; Lye, S.J. Stretch-induced human myometrial cytokines enhance immune cell recruitment via endothelial activation. Cell Mol. Immunol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Tsao, F.Y.; Wu, M.Y.; Chang, Y.L.; Wu, C.T.; Ho, H.N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J. Formos. Med. Assoc. 2018, 117, 204–211. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Gilman-Sachs, A.; Chaouat, G.; Beaman, K.D. Placental ATPase expression is a link between multiple causes of spontaneous abortion in mice. Biol. Reprod. 2011, 85, 626–634. [Google Scholar] [CrossRef]
- Diamond, A.K.; Sweet, L.M.; Oppenheimer, K.H.; Bradley, D.F.; Phillippe, M. Modulation of monocyte chemotactic protein-1 expression during lipopolysaccharide-induced preterm delivery in the pregnant mouse. Reprod. Sci. 2007, 14, 548–559. [Google Scholar] [CrossRef]
- Xu, Y.; Romero, R.; Miller, D.; Kadam, L.; Mial, T.N.; Plazyo, O.; Garcia-Flores, V.; Hassan, S.S.; Xu, Z.; Tarca, A.L.; et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J. Immunol. 2016, 196, 2476–2491. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Liu, Z.; Romero, R.; Pique-Regi, R.; Xu, Y.; Miller, D.; Levenson, D.; Galaz, J.; Winters, A.D.; Farias-Jofre, M.; et al. Homeostatic Macrophages Prevent Preterm Birth and Improve Neonatal Outcomes by Mitigating In Utero Sterile Inflammation in Mice. J. Immunol. 2024, 213, 1620–1634. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhang, M.; Cui, D.; Yang, H. The pathologic changes of human placental macrophages in women with hyperglycemia in pregnancy. Placenta 2022, 130, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sisino, G.; Bouckenooghe, T.; Aurientis, S.; Fontaine, P.; Storme, L.; Vambergue, A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochim. Biophys. Acta 2013, 1832, 1959–1968. [Google Scholar] [CrossRef]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef]
- Boriboonhirunsarn, D.; Tanpong, S. Rate of Spontaneous Preterm Delivery Between Pregnant Women with and Without Gestational Diabetes. Cureus 2023, 15, e34565. [Google Scholar] [CrossRef]
- Köck, K.; Köck, F.; Klein, K.; Bancher-Todesca, D.; Helmer, H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J. Matern. Fetal Neonatal Med. 2010, 23, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Dudley, D.J.; Collmer, D.; Mitchell, M.D.; Trautman, M.S. Inflammatory cytokine mRNA in human gestational tissues: Implications for term and preterm labor. J. Soc. Gynecol. Investig. 1996, 3, 328–335. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal 2010, 3, cm1. [Google Scholar] [CrossRef]
- Niu, J.; Li, Z.; Peng, B.; Chiao, P.J. Identification of an autoregulatory feedback pathway involving interleukin-1alpha in induction of constitutive NF-kappaB activation in pancreatic cancer cells. J. Biol. Chem. 2004, 279, 16452–16462. [Google Scholar] [CrossRef]
- McCarthy, D.A.; Ranganathan, A.; Subbaram, S.; Flaherty, N.L.; Patel, N.; Trebak, M.; Hempel, N.; Melendez, J.A. Redox-control of the alarmin, Interleukin-1α. Redox Biol. 2013, 1, 218–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rider, P.; Kaplanov, I.; Romzova, M.; Bernardis, L.; Braiman, A.; Voronov, E.; Apte, R.N. The transcription of the alarmin cytokine interleukin-1 alpha is controlled by hypoxia inducible factors 1 and 2 alpha in hypoxic cells. Front. Immunol. 2012, 3, 290. [Google Scholar] [CrossRef] [PubMed]
- Freigang, S.; Ampenberger, F.; Weiss, A.; Kanneganti, T.-D.; Iwakura, Y.; Hersberger, M.; Kopf, M. Fatty acid–induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 2013, 14, 1045–1053. [Google Scholar] [CrossRef]
- Itoh, Y.; Hayashi, H.; Miyazawa, K.; Kojima, S.; Akahoshi, T.; Onozaki, K. 17β-Estradiol Induces IL-1α Gene Expression in Rheumatoid Fibroblast-Like Synovial Cells through Estrogen Receptor α (ERα) and Augmentation of Transcriptional Activity of Sp1 by Dissociating Histone Deacetylase 2 from ERα1. J. Immunol. 2007, 178, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Bakouche, O.; Brown, D.C.; Lachman, L.B. Subcellular localization of human monocyte interleukin 1: Evidence for an inactive precursor molecule and a possible mechanism for IL 1 release. J. Immunol. 1987, 138, 4249–4255. [Google Scholar] [CrossRef]
- Menon, R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front. Immunol. 2014, 5, 567. [Google Scholar] [CrossRef]
- Schmiedecke, S.S.; Estrada, S.M.; Burd, I.; Napolitano, P.G.; Ieronimakis, N.M. 12: Evidence for the crucial role of estrogen signaling with preterm labor and perinatal neuroinflammation. Am. J. Obstet. Gynecol. 2019, 220, S10–S11. [Google Scholar] [CrossRef]
- Challis, J.R.G. Mechanism of parturition and preterm labor. Obstet. Gynecol. Surv. 2000, 55, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Lamacchia, C.; Rodriguez, E.; Palmer, G.; Gabay, C. Endogenous IL-1α is a chromatin-associated protein in mouse macrophages. Cytokine 2013, 63, 135–144. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Beller, D.I.; Mizel, S.B.; Unanue, E.R. Identification of a membrane-associated interleukin 1 in macrophages. Proc. Natl. Acad. Sci. USA 1985, 82, 1204–1208. [Google Scholar] [CrossRef]
- Chan, J.N.E.; Humphry, M.; Kitt, L.; Krzyzanska, D.; Filbey, K.J.; Bennett, M.R.; Clarke, M.C.H. Cell surface IL-1alpha trafficking is specifically inhibited by interferon-gamma, and associates with the membrane via IL-1R2 and GPI anchors. Eur. J. Immunol. 2020, 50, 1663–1675. [Google Scholar] [CrossRef]
- Brody, D.T.; Durum, S.K. Membrane IL-1: IL-1 alpha precursor binds to the plasma membrane via a lectin-like interaction. J. Immunol. 1989, 143, 1183–1187. [Google Scholar] [CrossRef]
- Lopez, T.E.; Zhang, H.; Bouysse, E.; Neiers, F.; Ye, X.Y.; Garrido, C.; Wendremaire, M.; Lirussi, F. A pivotal role for the IL-1beta and the inflammasome in preterm labor. Sci. Rep. 2024, 14, 4234. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.A.; Yi, L.; Skomorovska-Prokvolit, Y.; Patel, B.; Amini, P.; Tan, H.; Mesiano, S. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology 2017, 158, 158–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doring, B.; Shynlova, O.; Tsui, P.; Eckardt, D.; Janssen-Bienhold, U.; Hofmann, F.; Feil, S.; Feil, R.; Lye, S.J.; Willecke, K. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J. Cell Sci. 2006, 119, 1715–1722. [Google Scholar] [CrossRef]
- Mesiano, S. Progesterone withdrawal and parturition. J. Steroid Biochem. Mol. Biol. 2022, 224, 106177. [Google Scholar] [CrossRef]
- Raynaud, J.P.; Ojasoo, T. Promegestone, a new progestin. J. Gynecol. Obstet. Biol. Reprod. 1983, 12, 697–710. [Google Scholar]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8 (Suppl. S1), 3–63. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.A.; Heuerman, A.C.; Custer, M.; Dobyns, A.E.; Strilaeff, R.; Stutz, K.N.; Cooperrider, J.; Elsissy, J.G.; Yellon, S.M. Progesterone Receptor-Mediated Actions Regulate Remodeling of the Cervix in Preparation for Preterm Parturition. Reprod. Sci. 2016, 23, 1473–1483. [Google Scholar] [CrossRef]
- Kuon, R.J.; Garfield, R.E. Actions of progestins for the inhibition of cervical ripening and uterine contractions to prevent preterm birth. Facts Views Vis. Obgyn 2012, 4, 110–119. [Google Scholar]
- Winneker, R.C.; Bitran, D.; Zhang, Z. The preclinical biology of a new potent and selective progestin: Trimegestone. Steroids 2003, 68, 915–920. [Google Scholar] [CrossRef]
- Anonymous. Trimegestone. Drugs R D 1999, 1, 228–229. [Google Scholar] [CrossRef]
- Kuon, R.J.; Shi, S.Q.; Maul, H.; Sohn, C.; Balducci, J.; Maner, W.L.; Garfield, R.E. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am. J. Obstet. Gynecol. 2010, 202, 455.e1–455.e9. [Google Scholar] [CrossRef]
- Shi, S.Q.; Maner, W.L.; Mackay, L.B.; Garfield, R.E. Identification of term and preterm labor in rats using artificial neural networks on uterine electromyography signals. Am. J. Obstet. Gynecol. 2008, 198, 235.e1–235.e4. [Google Scholar] [CrossRef]
- Yellon, S.M.; Dobyns, A.E.; Beck, H.L.; Kurtzman, J.T.; Garfield, R.E.; Kirby, M.A. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS ONE 2013, 8, e81340. [Google Scholar] [CrossRef] [PubMed]
- Neilson, J.P. Mifepristone for induction of labour. Cochrane Database Syst. Rev. 2000, 4, Cd002865. [Google Scholar] [CrossRef]
- Thong, K.J.; Baird, D.T. Induction of abortion with mifepristone and misoprostol in early pregnancy. Br. J. Obstet. Gynaecol. 1992, 99, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Tribe, R.M.; Moriarty, P.; Dalrymple, A.; Hassoni, A.A.; Poston, L. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol. Reprod. 2003, 68, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, D.C.; Karagiannidis, L.K.; Paltoglou, G.; Margeli, A.; Kaparos, G.; Valsamakis, G.; Chrousos, G.P.; Creatsas, G.; Mastorakos, G. Pulsatile interleukin-6 leads CRH secretion and is associated with myometrial contractility during the active phase of term human labor. J. Clin. Endocrinol. Metab. 2013, 98, 4105–4112. [Google Scholar] [CrossRef] [PubMed][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, L.; Librach, M.; Boros-Rausch, A.; Matthews, B.; Aguiar-Cabeza, E.; Shynlova, O.; Lye, S.J. M1 Macrophages Are a Source of IL-1α: A Driver of Progesterone Metabolism and Myometrial Contraction. Cells 2025, 14, 1692. https://doi.org/10.3390/cells14211692
Nadeem L, Librach M, Boros-Rausch A, Matthews B, Aguiar-Cabeza E, Shynlova O, Lye SJ. M1 Macrophages Are a Source of IL-1α: A Driver of Progesterone Metabolism and Myometrial Contraction. Cells. 2025; 14(21):1692. https://doi.org/10.3390/cells14211692
Chicago/Turabian StyleNadeem, Lubna, Maxwell Librach, Adam Boros-Rausch, Benjamin Matthews, Eduardo Aguiar-Cabeza, Oksana Shynlova, and Stephen James Lye. 2025. "M1 Macrophages Are a Source of IL-1α: A Driver of Progesterone Metabolism and Myometrial Contraction" Cells 14, no. 21: 1692. https://doi.org/10.3390/cells14211692
APA StyleNadeem, L., Librach, M., Boros-Rausch, A., Matthews, B., Aguiar-Cabeza, E., Shynlova, O., & Lye, S. J. (2025). M1 Macrophages Are a Source of IL-1α: A Driver of Progesterone Metabolism and Myometrial Contraction. Cells, 14(21), 1692. https://doi.org/10.3390/cells14211692






