Histological and Immunohistochemical Characteristics of Mechanically Processed Adipose Tissue: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Synthesis
2.6. Statistical Analysis
3. Results
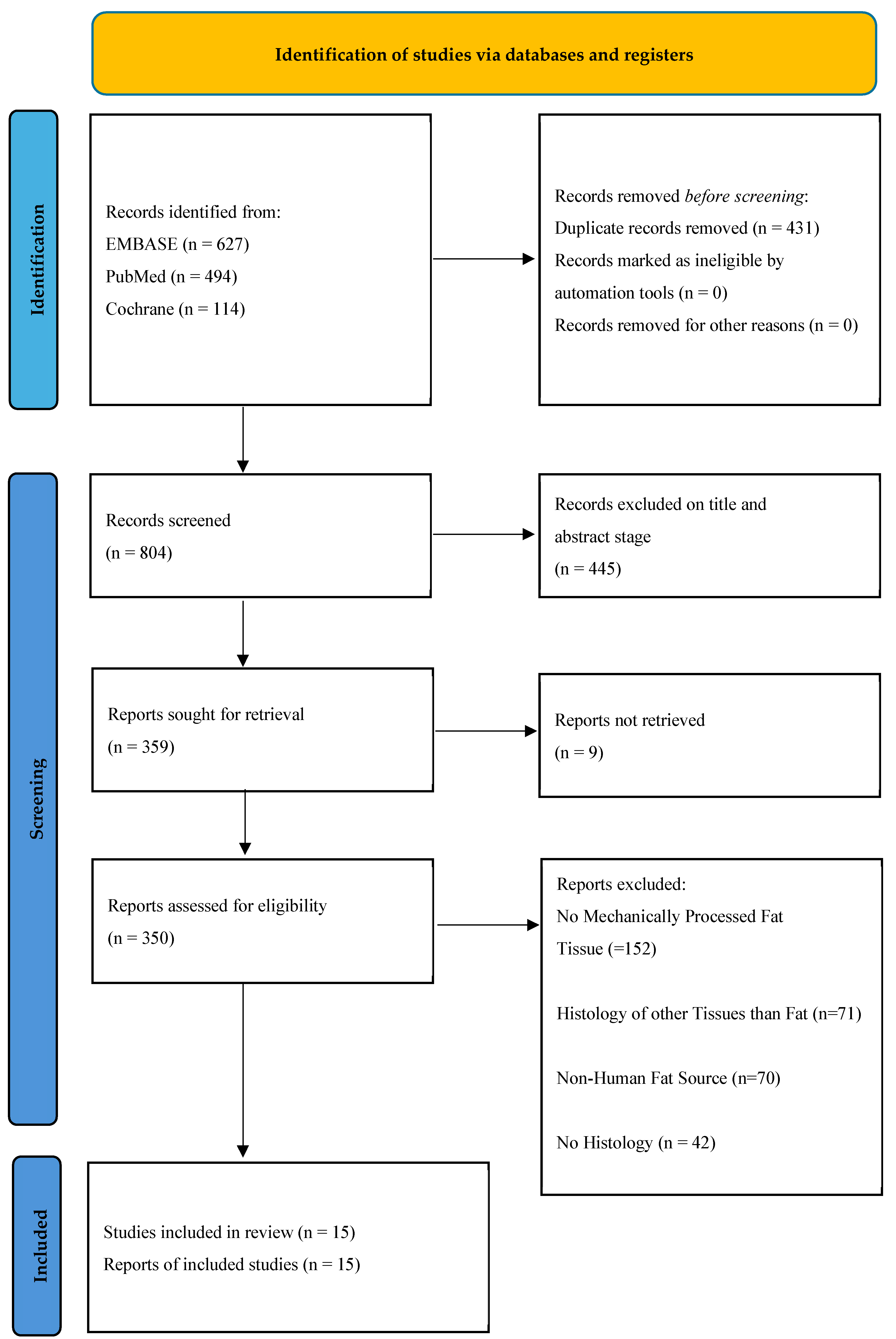
3.1. Study Selection
3.2. Study Characteristics
3.3. Histological Staining Techniques
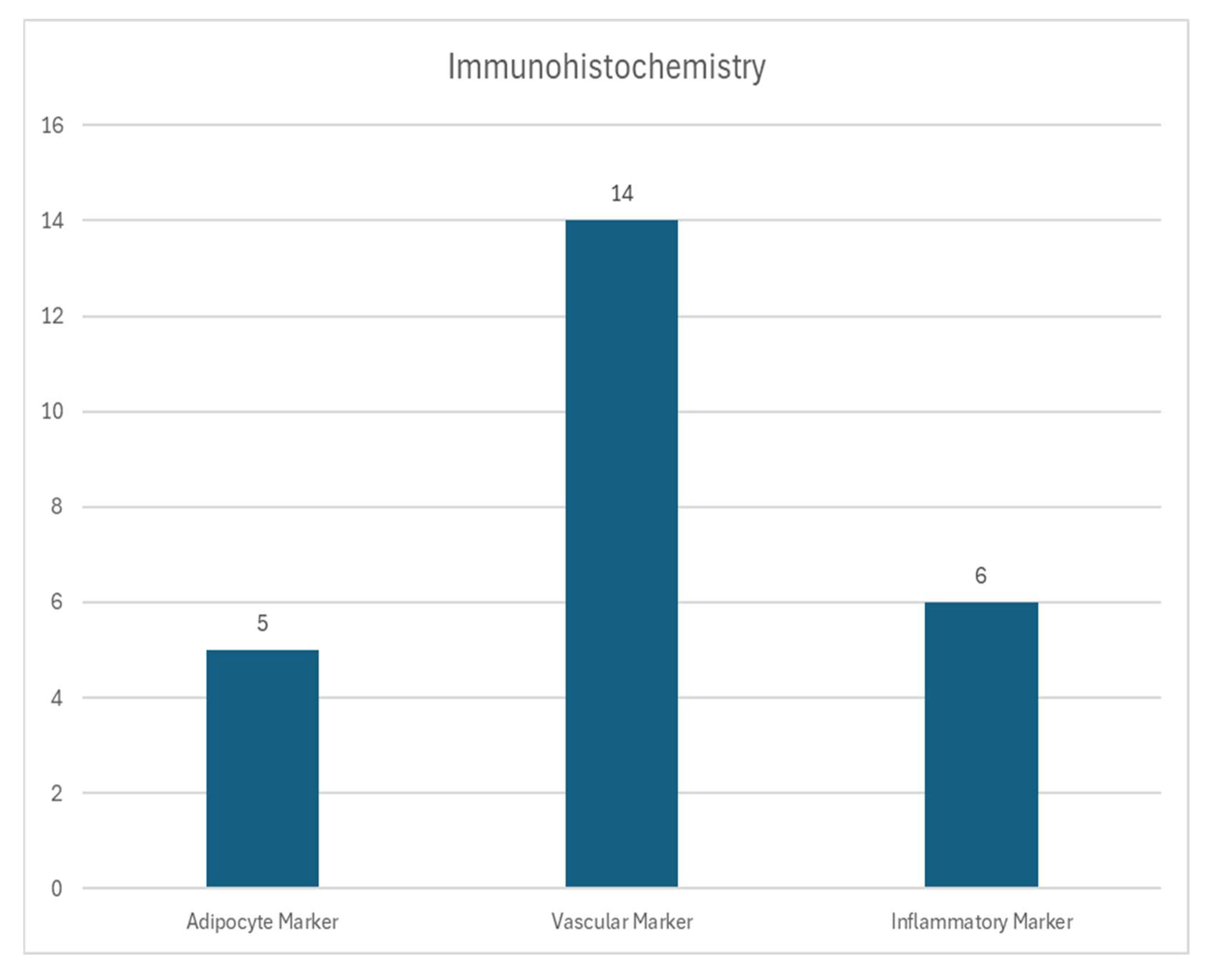
3.4. Immunohistochemical Analysis
3.5. In Vitro Protocols
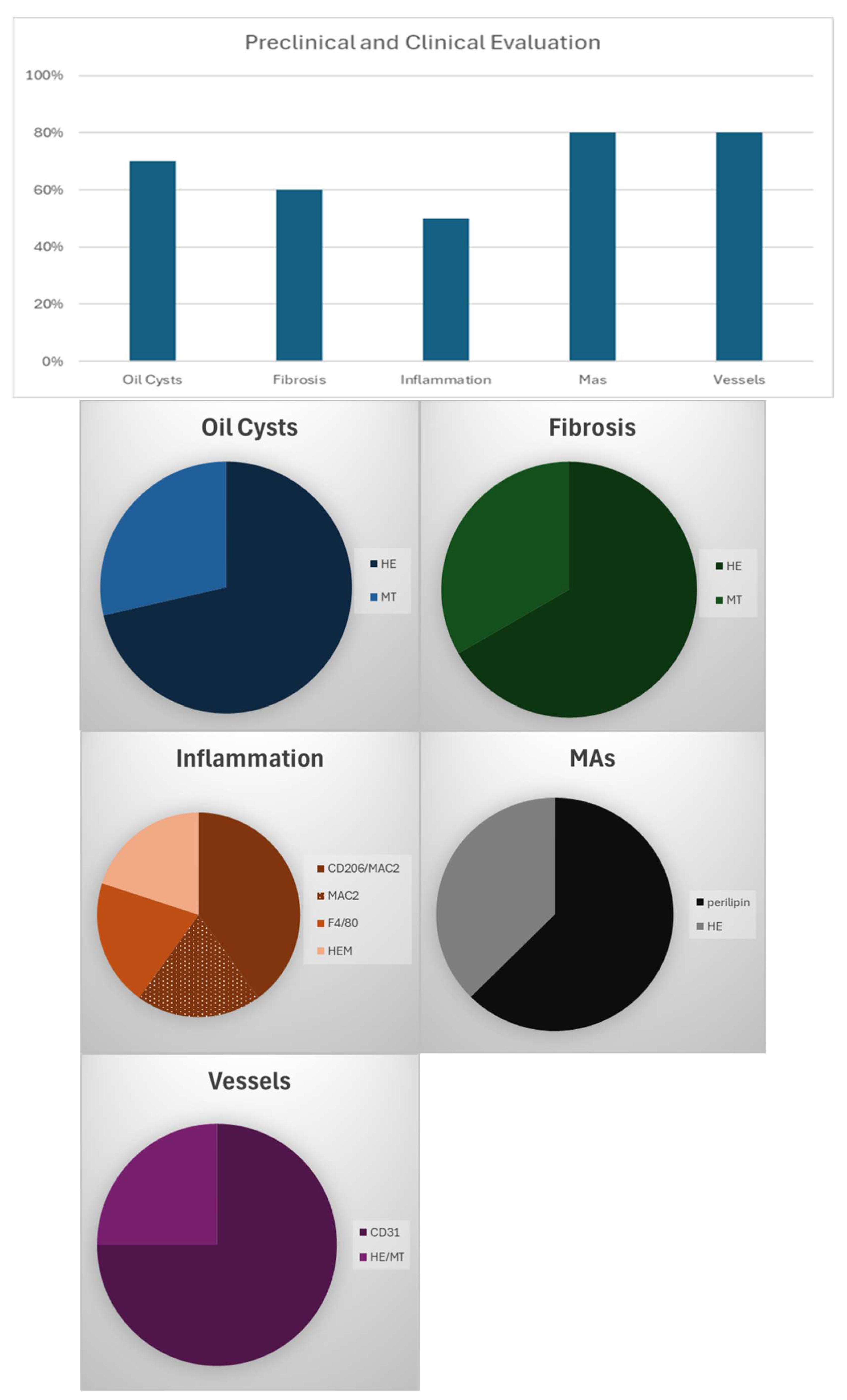
3.6. Preclinical/Clinical Protocols
3.7. Mechanical Processing Techniques
3.8. Methodological Variability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guerrerosantos, J. Autologous fat grafting for body contouring. Clin. Plast. Surg. 1996, 23, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Watanabe, Y.; Yamaguchi, S.; Noma, H.; Yamane, G.Y.; Abe, S.; Ide, Y. Use of the buccal fat pad as a pedicle graft. Bull. Tokyo Dent. Coll. 1996, 37, 161–165. [Google Scholar] [PubMed]
- Drommer, R.B.; Mende, U.; Krifka, F.J. Die freie Fetttransplantation im Gesichtsbereich. Hautarzt 1995, 46, 628–631. [Google Scholar] [CrossRef]
- Natali, S.; Screpis, D.; Patania, E.; De Berardinis, L.; Benoni, A.; Piovan, G.; Iacono, V.; Magnan, B.; Gigante, A.P.; Zorzi, C. Efficacy and Long-Term Outcomes of Intra-Articular Autologous Micro-Fragmented Adipose Tissue in Individuals with Glenohumeral Osteoarthritis: A 36-Month Follow-Up Study. J. Pers. Med. 2023, 13, 1309. [Google Scholar] [CrossRef]
- Froschauer, S.M.; Holzbauer, M.; Wenny, R.; Schmidt, M.; Huemer, G.M.; Kwasny, O.; Duscher, D. Autologous Fat Transplantation for Thumb Carpometacarpal Joint Osteoarthritis (Liparthroplasty): A Case Series with Two Years of Follow-UP. J. Clin. Med. 2020, 10, 113. [Google Scholar] [CrossRef]
- Weninger, P.; Feichtinger, X.; Steffel, C.; Kerschbaumer, C.; Duscher, D. Arthroscopy with Lipoaspirate and Plasma Infiltration Using Adipose-Derived Stem Cells Plus Platelet-Rich Plasma: Harvesting and Injection for Arthroscopic Treatment of Cartilage Defects of the Knee. Arthrosc. Tech. 2023, 12, e2265–e2271. [Google Scholar] [CrossRef]
- Prantl, L.; Brix, E.; Kempa, S.; Felthaus, O.; Eigenberger, A.; Brébant, V.; Anker, A.; Strauss, C. Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study. Cells 2021, 10, 594. [Google Scholar] [CrossRef]
- Onorato, F.; Rucci, M.; Alessio-Mazzola, M.; Bistolfi, A.; Castagnoli, C.; Formica, M.; Ferracini, R. Autologous microfragmented adipose tissue treatment of knee osteoarthritis demonstrates effectiveness in 68% of patients at 4-year follow-up. Arch. Orthop. Trauma Surg. 2024, 144, 3925–3935. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, Q.; Li, S.; Liu, M.; Sun, H.; Li, L.; Han, K.; Liu, P. Intra-Articular Injection of Autologous Micro-Fragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: A Prospective Interventional Study. J. Pers. Med. 2023, 13, 504. [Google Scholar] [CrossRef]
- Eigenberger, A.; Felthaus, O.; Schratzenstaller, T.; Haerteis, S.; Utpatel, K.; Prantl, L. The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells. Cells 2022, 11, 2543. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Klein, S.; Limm, K.; Oefner, P.J.; Schratzenstaller, T.; Felthaus, O. Shear Force Processing of Lipoaspirates for Stem Cell Enrichment Does Not Affect Secretome of Human Cells Detected by Mass Spectrometry In Vitro. Plast. Reconstr. Surg. 2020, 146, 749e–758e. [Google Scholar] [CrossRef]
- Cicione, C.; Vadalà, G.; Di Giacomo, G.; Tilotta, V.; Ambrosio, L.; Russo, F.; Zampogna, B.; Cannata, F.; Papalia, R.; Denaro, V. Micro-fragmented and nanofat adipose tissue derivatives: In vitro qualitative and quantitative analysis. Front. Bioeng. Biotechnol. 2023, 11, 911600. [Google Scholar] [CrossRef]
- Fan, P.; Fang, M.; Li, J.; Solari, M.G.; Wu, D.; Tan, W.; Wang, Y.; Yang, X.; Lei, S. A Novel Fat Making Strategy With Adipose-Derived Progenitor Cell-Enriched Fat Improves Fat Graft Survival. Aesthet. Surg. J. 2021, 41, NP1228–NP1236. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen Fzhou Zhang, Y.; Tan Pching Li, Q.; Cheng, C. Concentrated ultrasound-processed fat (CUPF): More than a mechanically emulsified graft. J. Plast. Reconstr. Aesthet. Surg. 2023, 83, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Viganò, M.; Torretta, E.; Perucca Orfei, C.; Colombini, A.; Tremolada, C.; Gelfi, C.; de Girolamo, L. Characterization of Microfragmented Adipose Tissue Architecture, Mesenchymal Stromal Cell Content and Release of Paracrine Mediators. J. Clin. Med. 2022, 11, 2231. [Google Scholar] [CrossRef]
- Säljö, K.; Orrhult, L.S.; Apelgren, P.; Markstedt, K.; Kölby, L.; Gatenholm, P. Successful engraftment, vascularization, and In vivo survival of 3D-bioprinted human lipoaspirate-derived adipose tissue. Bioprinting 2020, 17, e00065. [Google Scholar] [CrossRef]
- Sesé, B.; Sanmartín, J.M.; Ortega, B.; Llull, R. Human Stromal Cell Aggregates Concentrate Adipose Tissue Constitutive Cell Population by In Vitro DNA Quantification Analysis. Plast. Reconstr. Surg. 2020, 146, 1285–1293. [Google Scholar] [CrossRef]
- Tran, V.V.T.; Hong, K.Y.; Jin, X.; Chang, H. Histological Comparison of Nanofat and Lipoconcentrate: Enhanced Effects of Lipoconcentrate on Adipogenesis and Angiogenesis. Aesthetic Plast. Surg. 2024, 48, 752–763. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Gostelie, O.F.E.; Vonk, L.A.; De Bruijn, J.J.; Van Der Lei, B.; Harmsen, M.C.; Stevens, H.P. Fractionation of Adipose Tissue Procedure With a Disposable One-Hole Fractionator. Aesthet. Surg. J. 2020, 40, NP194–NP201. [Google Scholar] [CrossRef]
- Wu, W.; Bi, X.; Zhao, J.; Lin, Z.; Lu, F.; Dong, Z.; Li, Y. Ultra-condensed Fat: A Novel Fat Product for Volume Augmentation. Aesthetic Plast. Surg. 2023, 47, 2074–2083. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, J.; Zhang, P.; Liao, Y.; Yuan, Y.; Dong, Z.; Lu, F. Adipose Stromal Vascular Fraction Gel Grafting: A New Method for Tissue Volumization and Rejuvenation. Dermatol. Surg. 2018, 44, 1278–1286. [Google Scholar] [CrossRef]
- Yu, Q.; Cai, Y.; Huang, H.; Wang, Z.; Xu, P.; Wang, X.; Zhang, L.; Zhang, W.; Li, W. Co-Transplantation of Nanofat Enhances Neovascularization and Fat Graft Survival in Nude Mice. Aesthet. Surg. J. 2018, 38, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Zhou, T.; Yao, Y.; Dong, Z.; Lu, F. Improved Long-Term Volume Retention of Stromal Vascular Fraction Gel Grafting with Enhanced Angiogenesis and Adipogenesis. Plast. Reconstr. Surg. 2018, 141, 676e–686e. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, B.; Wang, L.; Fu, Z.; Hu, J.; Liu, Y.; Wang, J.; He, Y. Rapid printing of 3D porous scaffolds for breast reconstruction. Bio-Des. Manuf. 2023, 6, 691–703. [Google Scholar] [CrossRef]
- Zhu, H.; Quan, Y.; Wang, J.; Jiang, S.; Lu, F.; Cai, J.; Liao, Y.M. Improving Low-Density Fat by Condensing Cellular and Collagen Content through a Mechanical Process: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2021, 148, 1029–1039. [Google Scholar] [CrossRef]
- Uguten, M.; van der Sluis, N.; Vriend, L.; Coert, J.H.; Harmsen, M.C.; van der Lei, B.; van Dongen, J.A. Comparing mechanical and enzymatic isolation procedures to isolate adipose-derived stromal vascular fraction: A systematic review. Wound Repair. Regen. 2024, 32, 1008–1021. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef]
- Schipper, J.; Van Laarhoven, C.; Schepers, R.; Tuin, A.; Harmsen, M.; Spijkervet, F.; Jansma, J.; van Dongen, J.A. Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction. Bioengineering 2023, 10, 1175. [Google Scholar] [CrossRef]
- Schimanski, T.; Loucas, R.; Loucas, M.; Felthaus, O.; Brébant, V.; Klein, S.; Anker, A.; Frank, K.; Siegmund, A.; Pagani, A.; et al. Histology and Immunohistochemistry of Adipose Tissue: A Scoping Review on Staining Methods and Their Informative Value. Cells 2025, 14, 898. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Tuin, A.J.; Harmsen, M.C.; Van Der Lei, B.; Stevens, H.P. The Difference between Stromal Vascular Fraction Isolation and Fat Emulsification: A Crucial Role for Centrifugation. Plast. Reconstr. Surg. 2020, 145, 232e–233e. [Google Scholar] [CrossRef]
- Eigenberger, A.; Felthaus, O.; Bartsch, A.; Schimanski, T.; Utpatel, K.; Prantl, L. The Influence of Sedimentation on the Composition of the Lipoaspirate and the Effects on Further Mechanical Processing. Cells 2025, 14, 601. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I. The 2011 Oxford CEBM Levels of Evidence (Introductory Document). Oxford Centre for Evidence-Based Medicine. 2011. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 2 April 2025).
- Paiva Barbosa, V.; Bastos Silveira, B.; Amorim Dos Santos, J.; Monteiro, M.M.; Coletta, R.D.; De Luca Canto, G.; Stefani, C.M.; Guerra, E.N.S. Critical appraisal tools used in systematic reviews of in vitro cell culture studies: A methodological study. Res. Synth. Methods 2023, 14, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Pulsfort, A.K.; Wolter, T.P.; Pallua, N. The Effect of Centrifugal Forces on Viability of Adipocytes in Centrifuged Lipoaspirates. Ann. Plast. Surg. 2011, 66, 292–295. [Google Scholar] [CrossRef]
- Ramaut, L.; Moonen, L.; Laeremans, T.; Aerts, J.L.; Geeroms, M.; Hamdi, M. Push-Through Filtration of Emulsified Adipose Tissue Over a 500-µm Mesh Significantly Reduces the Amount of Stromal Vascular Fraction and Mesenchymal Stem Cells. Aesthet. Surg. J. 2023, 43, NP696–NP703. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Zinger, G.; Gronovich, Y.; Lotan, A.M.; Sharon-Gabbay, R. Pilot Study for Isolation of Stromal Vascular Fraction with Collagenase Using an Automated Processing System. Int. J. Mol. Sci. 2024, 25, 7148. [Google Scholar] [CrossRef]
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Study Type | Preclinical and experimental studies with histology of fat tissue which underwent mechanical processing | Case reports, editorials, commentaries, reviews without original data, systematic reviews or meta-analyses |
| Population | All human studies | All animal studies |
| Intervention | Whole tissue | Studies focusing only on stem cells of the fat (SVF) and their histology |
| Publication Date | All studies available (2013–2024) | - |
| Language | Published in English | Non-English language studies |
| Peer-reviewed | Studies must be published in peer-reviewed journals | Non-peer-reviewed articles |
| Study Author | Year | OHAT Score | Study Design | Cohort Size | Number of Samples | Time of Explantation | Staining Method | |
|---|---|---|---|---|---|---|---|---|
| Processed | Not Processed | |||||||
| Cicione et al. [12] | 2023 | Level 1 | Experimental | 9♀, 9♂ 63 ± 11 y | 6 MAT, 6 NAT | 6 LA | - | HE, Anti-PCNA, Anti-CD31, Anti-CD34, Anti-COLL1 |
| Eigenberger et al. [10] | 2022 | Level 1 | Experimental | 11♀; 25–62 y; BMI: 29.6 ± 5.2 kg/m2 | 11 CELT; 11 CELTPLUS | 11 S | - | HE |
| Fan et al. [13] | 2021 | Level 1 | preclinical experimental | ♀; 20–55Y; BMI: 21–31 kg/m2 | 10 AERF | 10 CF | 10 weeks | HE, Anti-CD31, Anti-perilipin |
| He et al. [14] | 2023 | Level 2 | Preclinical experimental | 6♀; 20–30 y | 6 NF; 6 CUPF | 6 MF; 6 C | 4 weeks | HE, Anti-Ki67, Anti-CD31 |
| Ragni et al. [15] | 2022 | Level 1 | Experimental | 4♀, 3♂ 44 ± 6 y | - | - | - | HE, Anti-CD31, Anti-CD90, Anti-CD146 |
| Säljö et al. [16] | 2020 | Level 2 | Preclinical experimental | 2♀ | 9 LAT | 3, 7, or 30 days | HE, Anti-CD31, Anti-CD90 | |
| Sesé et al. [17] | 2020 | Level 1 | Experimental | 6♀; 18–45 y; BMI: 21.5 ± 2 kg/m2 | 6 SCA | 6 LA | - | HE, Masson Trichrome |
| Tran et al. [18] | 2024 | Level 1 | Preclinical experimental | 1♀; 47 y | 5 NF; 5 LC | 5 CF | 4 weeks | HE, Anti-CD31, Anti-F4/80, Anti-perilipin |
| Van Dongen et al. [19] | 2020 | Level 2 | Experimental | 3 | 3 tSVF | 3 LA | - | Masson Trichrome Anti-perilipin, Anti-αSMA |
| Wu et al. [20] | 2023 | Level 1 | Preclinical experimental | 16–60 y BMI: 18.3–31.5 kg/m2 | 24 UCF | 24 CF | 3, 7, 14, 30, 60, or 90 days | HE, Anti-CD31, Anti-perilipin, Anti-galectin 3, Anti-CD206 |
| Yao et al. [21] | 2013 | Level 2 | Clinical experimental | 92♀, 34♂ 34.7 ± 9.3 y BMI: 23.4 ± 2.1 kg/m2 | 1 SFVG | - | 12 months | HE |
| Yu et al. [22] | 2018 | Level 1 | Preclinical experimental | 5♀; 25–38 y | 10 Nano100; 10 Nano200 | 10 MF | 12 weeks | HE, Masson Trichrome, Anti-CD31 |
| Zhang et al. [23] | 2018 | Level 1 | Preclinical experimental | 11♀; 33.4 ± 6.3 y; BMI: 23.2 ± 1.9 kg/m2 | 63 SVFG | 63 CF | 1, 3, 5, 7, 11, 15, 30, 60, or 90 days | HE, Masson Trichrome, Anti-perilipin, Anti-Mac2, Anti-CD206, Anti-HLA |
| Zhao et al. [24] | 2023 | Level 2 | Preclinical experimental | - | 2 SVFG | - | 14 and 28 days | HE |
| Zhu et al. [25] | 2021 | Level 1 | Preclinical experimental | 8♀ | 6 CLDF | 6 CF, 6 HDF, 6 LDF | 3 months | HE, Anti-Mac2, Anti-perilipin, |
| Study Author | Protocol Maneuver |
|---|---|
| Cicione et al. [12] | MAT: Lipogems© processing kit; NAT: C: 1200× g 3 min; emulsification with 90° stopcock; C: 1200× g 3 min |
| Eigenberger et al. [10] | CELT: C: 1600× g 2 min CELT PLUS: C: 1600× g 2 min; emulsification with 90° stopcock; C: 1600× g 2 min |
| Fan et al. [13] | AERF: Extracorporal cutting device with 1500–1600 rpm; F: 100 mesh |
| He et al. [14] | NF: rinsed; emulsified with Luer-Lock connector; C: 300× g 3 min CUPF: ultrasonic disruption for 15–20 s and 30 W; C: 1200× g 5 min |
| Ragni et al. [15] | µFAT: Lipogems© processing kit 120 TM and 240 TM |
| Säljö et al. [16] | LAT: Lipogems© processing kit |
| Sesé et al. [17] | SCA: C: 1200× g 3 min; emulsification with 90° stopcock; C: 800× g 10 min |
| Tran et al. [18] | NF: F: Nylon cloth 0.5 mm pores; emulsification with 2.4 mm and 1.2 mm connector; F: Nylon cloth 0.5 mm pores LC: C: 1200× g 3 min; emulsification with 2.4-mm and 1.2-mm connector; C: 1200× g 3 min |
| Van Dongen et al. [19] | tSVF: 1-hole and 3-hole fractionator Tulip Medical Products with centrifugation before and after |
| Wu et al. [20] | UCF: C: 1200× g 3 min; Agitate and Rest 5 min |
| Yao et al. [21] | SVFG: C: 1200× g 3 min; emulsification with stopcock; C: 1200× g 3 min |
| Yu et al. [22] | NF: F: Nylon cloth 0.5 mm pores; emulsification with female-to-female Luer-Lock; F: Nylon cloth 0.5-mm pores |
| Zhang et al. [23] | SVFG: C: 1200× g 3 min; emulsification with stopcock; F: 500 mym; C: 2000× g 3 min |
| Zhao et al. [24] | SVFG: emulsification with stopcock; C: 2000× g 3 min |
| Zhu et al. [25] | CLDF: C: 1200× g 3 min; upper half emulsified with female-to-female Luer-Lock; C: 1600× g 3 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schimanski, T.; Loucas, R.; Loucas, M.; Brébant, V.; Anker, A.; Klein, S.; Diesch, S.T.; Pagani, A.; Prantl, L. Histological and Immunohistochemical Characteristics of Mechanically Processed Adipose Tissue: A Systematic Review and Meta-Analysis. Cells 2025, 14, 1664. https://doi.org/10.3390/cells14211664
Schimanski T, Loucas R, Loucas M, Brébant V, Anker A, Klein S, Diesch ST, Pagani A, Prantl L. Histological and Immunohistochemical Characteristics of Mechanically Processed Adipose Tissue: A Systematic Review and Meta-Analysis. Cells. 2025; 14(21):1664. https://doi.org/10.3390/cells14211664
Chicago/Turabian StyleSchimanski, Tom, Rafael Loucas, Marios Loucas, Vanessa Brébant, Alexandra Anker, Silvan Klein, Sophia Theresa Diesch, Andrea Pagani, and Lukas Prantl. 2025. "Histological and Immunohistochemical Characteristics of Mechanically Processed Adipose Tissue: A Systematic Review and Meta-Analysis" Cells 14, no. 21: 1664. https://doi.org/10.3390/cells14211664
APA StyleSchimanski, T., Loucas, R., Loucas, M., Brébant, V., Anker, A., Klein, S., Diesch, S. T., Pagani, A., & Prantl, L. (2025). Histological and Immunohistochemical Characteristics of Mechanically Processed Adipose Tissue: A Systematic Review and Meta-Analysis. Cells, 14(21), 1664. https://doi.org/10.3390/cells14211664









