Cancer-Associated Fibroblasts Move and Interact More with Triple-Negative Breast Cancer Cells and Stimulate Their Proliferation in a Hyaluronan-Dependent Manner
Highlights
- Cancer-associated fibroblasts from Triple-negative breast cancer secrete high levels of hyaluronan, compared with normal breast fibroblasts, altering the tumour microenvironment.
- Hyaluronan production by Triple-negative breast cancer-associated fibroblasts enhances mixing of Triple-negative breast cancer cells with fibroblasts and promotes progression of this aggressive cancer type.
- Inhibition of the production of hyaluronan by Triple-negative breast cancer-associated fibroblasts is a potential future therapeutic target against Triple-negative breast cancer progression.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Western Blot
2.3. Traction Force Microscopy
2.4. Cell Migration Assay
2.5. Ex Vivo 2D Co-Culture Model of TNBC Cells and CAFs
2.6. Ex Vivo 3D Co-Culture Model of TNBC Cells and CAFs
2.7. Microscopy and Image Analysis
2.8. Flow Cytometry
2.9. Immunofluorescence Staining
2.10. Blebbistatin Treatment
2.11. Transient Knockdown of HAS2
2.12. Proliferation Assay
2.13. Two-Dimensional Co-Culture Viability
2.14. Generation of Fibroblast-Derived ECM
2.15. ECM Alignment and Coherency
2.16. Statistical Analysis
3. Results
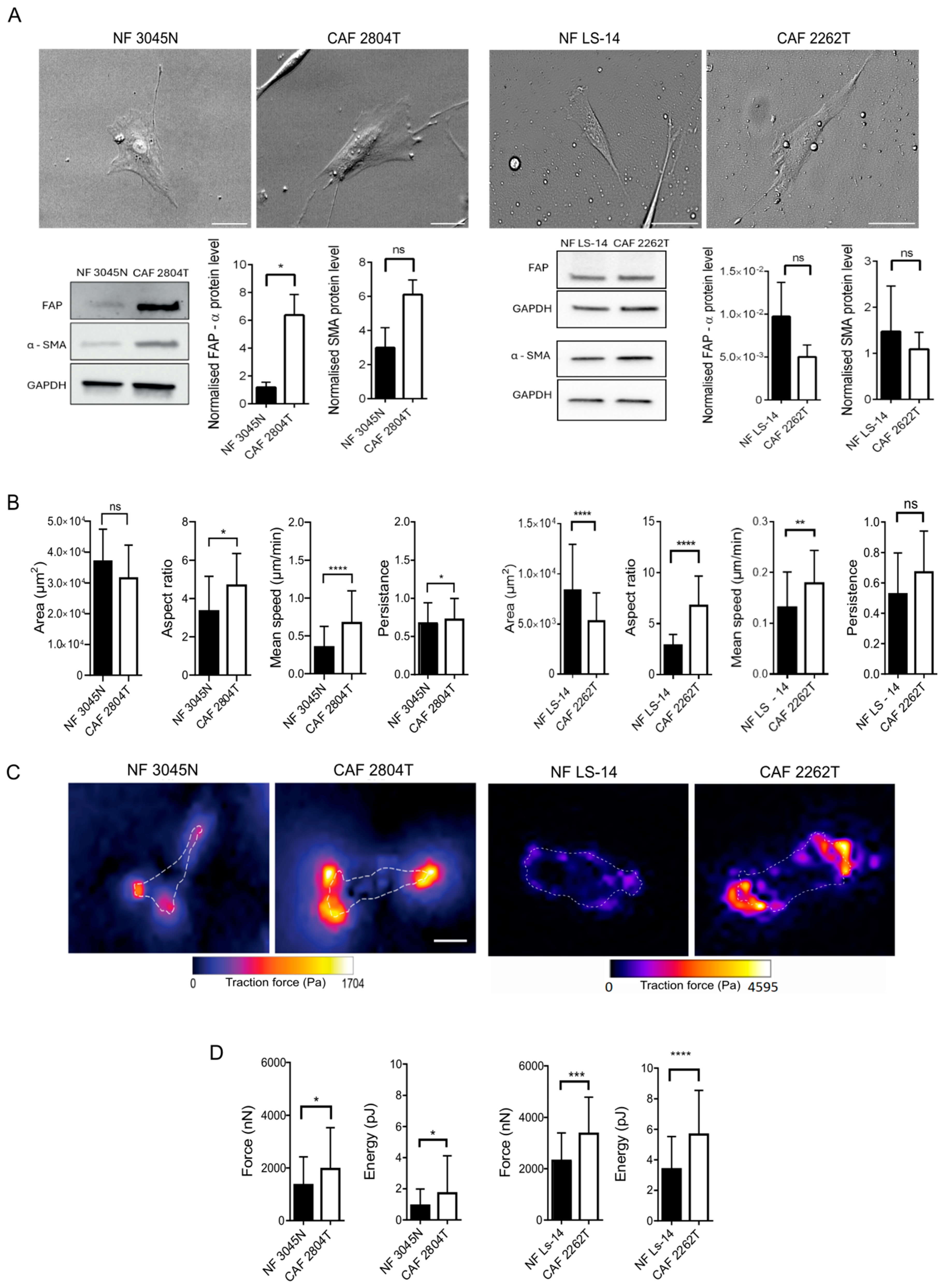
3.1. Triple-Negative Breast Cancer Fibroblasts Are More Elongated than Normal Breast Fibroblasts and Show Increased Cellular Contractile Force, Cell Migration Speed, and Persistence
3.2. TNBC CAF Co-Culture Reduces the Size of TNBC Cancer Cell Clusters in 2D
3.3. TNBC CAFs Mix More with Cancer Cells and Show Reduced Capacity to Suppress the Growth of Cancer Cells in 3D, Compared with NFs
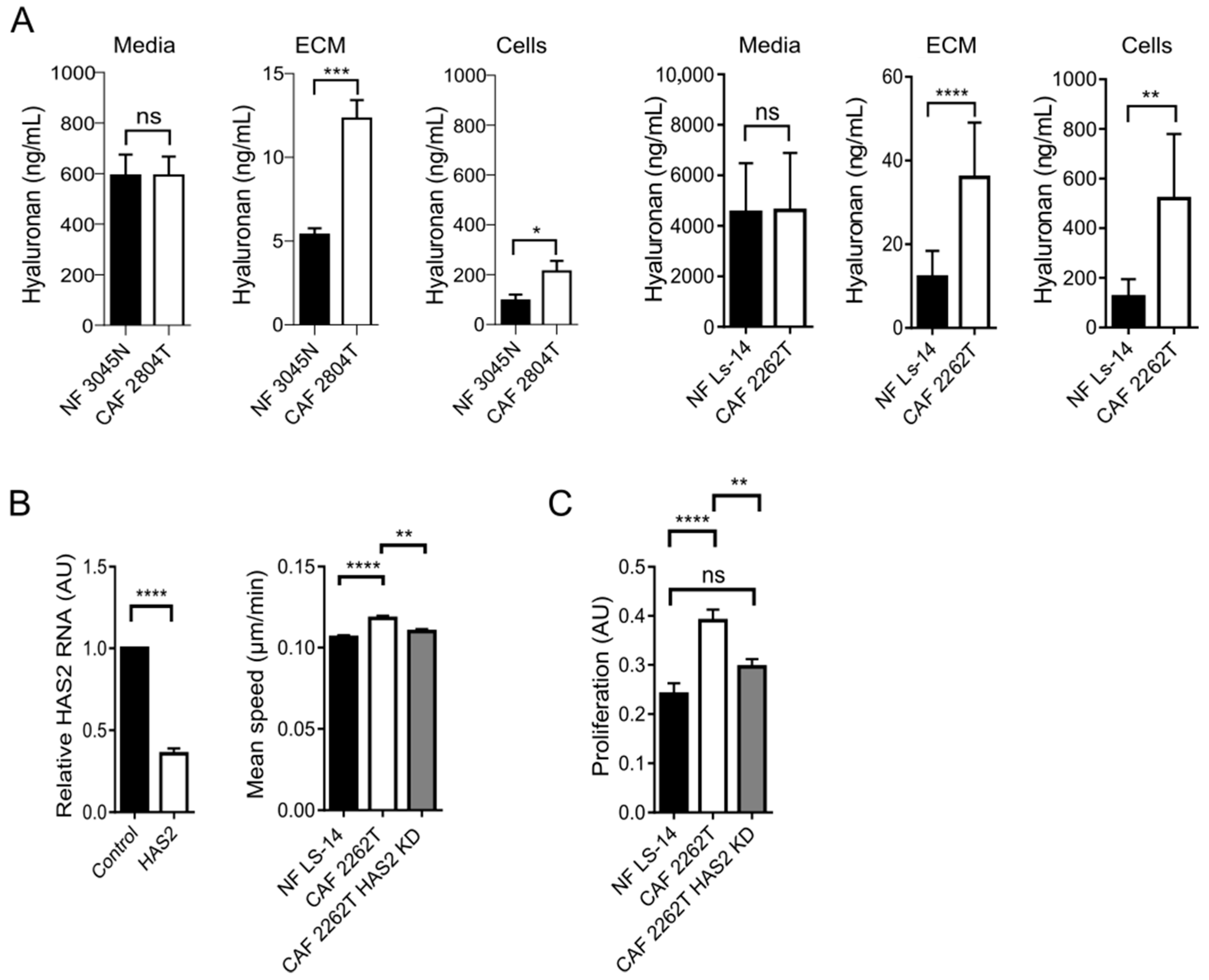
3.4. Increased Production and Deposition of Hyaluronan by TNBC CAFs Was Required for Cancer Cell Proliferation in 3D Spheroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Won, K.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S. Targeting CSC in a Most Aggressive Subtype of Breast Cancer TNBC. Adv. Exp. Med. Biol. 2019, 1152, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Kassam, F.; Enright, K.; Dent, R.; Dranitsaris, G.; Myers, J.; Flynn, C.; Fralick, M.; Kumar, R.; Clemons, M. Survival Outcomes for Patients with Metastatic Triple-Negative Breast Cancer: Implications for Clinical Practice and Trial Design. Clin. Breast Cancer 2009, 9, 29–33. [Google Scholar] [CrossRef] [PubMed]
- E Skinner, K.; Haiderali, A.; Huang, M.; Schwartzberg, L.S. Real-World Effectiveness Outcomes in Patients Diagnosed with Metastatic Triple-Negative Breast Cancer. Futur. Oncol. 2020, 17, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Belli, P.; Bufi, E.; Asunis, A.M.; Ferra, E.; Bitti, G.T. Association between sonographic appearances of breast cancers and their histopathologic features and biomarkers. J. Clin. Ultrasound 2015, 44, 26–33. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, H.; Zhang, S.-D.; Cheng, X.-Y.; Zheng, S.-M.; Sun, Y.-H.; Zhang, D.-W.; Jiang, Y.; Tian, J.-W. Mammographic and clinicopathological features of triple-negative breast cancer. Br. J. Radiol. 2014, 87, 20130496. [Google Scholar] [CrossRef]
- Wojcinski, S.; Soliman, A.A.; Schmidt, J.; Makowski, L.; Degenhardt, F.; Hillemanns, P. Sonographic Features of Triple-Negative and Non-Triple-Negative Breast Cancer. J. Ultrasound Med. 2012, 31, 1531–1541. [Google Scholar] [CrossRef]
- Moorman, A.; Vink, R.; Heijmans, H.; van der Palen, J.; Kouwenhoven, E. The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 307–313. [Google Scholar] [CrossRef]
- Kramer, C.J.H.; Vangangelt, K.M.H.; van Pelt, G.W.; Dekker, T.J.A.; Tollenaar, R.A.E.M.; Mesker, W.E. The prognostic value of tumour–stroma ratio in primary breast cancer with special attention to triple-negative tumours: A review. Breast Cancer Res. Treat. 2018, 173, 55–64. [Google Scholar] [CrossRef]
- Millar, E.K.; Browne, L.H.; Beretov, J.; Lee, K.; Lynch, J.; Swarbrick, A.; Graham, P.H. Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer. Cancers 2020, 12, 3749. [Google Scholar] [CrossRef]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Pogoda, K.; Bucki, R.; Byfield, F.J.; Cruz, K.; Lee, T.; Marcinkiewicz, C.; Janmey, P.A. Soft Substrates Containing Hyaluronan Mimic the Effects of Increased Stiffness on Morphology, Motility, and Proliferation of Glioma Cells. Biomacromolecules 2017, 18, 3040–3051. [Google Scholar] [CrossRef] [PubMed]
- Donelan, W.; Dominguez-Gutierrez, P.R.; Kusmartsev, S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor-associated immune suppression. Front. Immunol. 2022, 13, 971278. [Google Scholar] [CrossRef] [PubMed]
- Karalis, T.; Skandalis, S.S. Hyaluronan network: A driving force in cancer progression. Am. J. Physiol. Physiol. 2022, 323, C145–C158. [Google Scholar] [CrossRef]
- Karousou, E.; Parnigoni, A.; Moretto, P.; Passi, A.; Viola, M.; Vigetti, D. Hyaluronan in the Cancer Cells Microenvironment. Cancers 2023, 15, 798. [Google Scholar] [CrossRef]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Tolg, C.; Messam, B.J.-A.; McCarthy, J.B.; Nelson, A.C.; Turley, E.A. Hyaluronan Functions in Wound Repair That Are Captured to Fuel Breast Cancer Progression. Biomolecules 2021, 11, 1551. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Wang, Y.; Jin, J.; Xie, X.; Zhang, J. Hyaluronic acid predicts poor prognosis in breast cancer patients. Medicine 2020, 99, e20438. [Google Scholar] [CrossRef]
- Parnigoni, A.; Moretto, P.; Viola, M.; Karousou, E.; Passi, A.; Vigetti, D. Effects of Hyaluronan on Breast Cancer Aggressiveness. Cancers 2023, 15, 3813. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Lee, S.; Kumar, S. Cofilin is required for polarization of tension in stress fiber networks during migration. J. Cell Sci. 2020, 133, jcs243873. [Google Scholar] [CrossRef] [PubMed]
- Martiel, J.-L.; Leal, A.; Kurzawa, L.; Balland, M.; Wang, I.; Vignaud, T.; Tseng, Q.; Théry, M. Measurement of cell traction forces with ImageJ. Methods Cell Biol. 2015, 125, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Warrington, S.J.; López-Guajardo, A.; Al Hennawi, K.; Cook, S.L.; Griffith, Z.D.J.; Symmes, D.; Zhang, T.; Qu, Z.; Xu, Y.; et al. ALD-R491 regulates vimentin filament stability and solubility, cell contractile force, cell migration speed and directionality. Front. Cell Dev. Biol. 2022, 10, 926283. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Kushida, M.; Higashi, K.; Itoh, J.; Higashiyama, R.; Hong, Y.Y.; Kawada, N.; Namikawa, K.; Kiyama, H.; Bou-Gharios, G.; et al. Cell type-specific intervention of transforming growth factor beta/Smad signaling suppresses collagen gene expression and hepatic fibrosis in mice. Gastroenterology 2005, 129, 259–268. [Google Scholar] [CrossRef]
- Gad, A.K.B.; Rönnlund, D.; Widengren, J.; Aspenström, P. Analysis of Rho GTPase-induced localization of nanoscale adhesions using fluorescence nanoscopy. Methods Mol. Biol. 2014, 1120, 339–357. [Google Scholar] [CrossRef]
- Fonck, E.; Feigl, G.G.; Fasel, J.; Sage, D.; Unser, M.; Rüfenacht, D.A.; Stergiopulos, N. Effect of Aging on Elastin Functionality in Human Cerebral Arteries. Stroke 2009, 40, 2552–2556. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2452–2459. [Google Scholar] [CrossRef]
- Wang, Y.; Lauer, M.E.; Anand, S.; Mack, J.A.; Maytin, E.V. Hyaluronan Synthase 2 Protects Skin Fibroblasts against Apoptosis Induced by Environmental Stress. J. Biol. Chem. 2014, 289, 32253–32265. [Google Scholar] [CrossRef]
- Heldin, P.; Basu, K.; Olofsson, B.; Porsch, H.; Kozlova, I.; Kahata, K. Deregulation of hyaluronan synthesis, degradation and binding promotes breast cancer. J. Biochem. 2013, 154, 395–408. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Barbazan, J.; Pérez-González, C.; Gómez-González, M.; Dedenon, M.; Richon, S.; Latorre, E.; Serra, M.; Mariani, P.; Descroix, S.; Sens, P.; et al. Cancer-associated fibroblasts actively compress cancer cells and modulate mechanotransduction. Nat. Commun. 2023, 14, 6966. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef]
- Conti, S.; Kato, T.; Park, D.; Sahai, E.; Trepat, X.; Labernadie, A. CAFs and Cancer Cells Co-Migration in 3D Spheroid Invasion Assay. In The Epithelial-to Mesenchymal Transition; Humana: New York, NY, USA, 2020; pp. 243–256. [Google Scholar] [CrossRef]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef]
- Goetz, J.G.; Minguet, S.; Navarro-Lérida, I.; Lazcano, J.J.; Samaniego, R.; Calvo, E.; Tello, M.; Osteso-Ibáñez, T.; Pellinen, T.; Echarri, A.; et al. Biomechanical Remodeling of the Microenvironment by Stromal Caveolin-1 Favors Tumor Invasion and Metastasis. Cell 2011, 146, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Kraning-Rush, C.M.; Califano, J.P.; A. Reinhart-King, C. Cellular Traction Stresses Increase with Increasing Metastatic Potential. PLoS ONE 2012, 7, e32572. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Ågren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.-M. Hyaluronan in Peritumoral Stroma and Malignant Cells Associates with Breast Cancer Spreading and Predicts Survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hämäläinen, K.; Tammi, M.; Tammi, R.; Kosma, V.; Oikari, S.; Auvinen, P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef]
- Anttila, M.A.; Tammi, R.H.; Tammi, M.I.; Syrjänen, K.J.; Saarikoski, S.V.; Kosma, V.M. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000, 60, 150–155. [Google Scholar] [PubMed]
- Llaneza, A.; Vizoso, F.; Rodríguez, J.C.; Raigoso, P.; García-Muñiz, J.L.; Allende, M.T.; García-Morán, M. Hyaluronic acid as prognostic marker in resectable colorectal cancer. Br. J. Surg. 2000, 87, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Tahkola, K.; Ahtiainen, M.; Mecklin, J.-P.; Kellokumpu, I.; Laukkarinen, J.; Tammi, M.; Tammi, R.; Väyrynen, J.P.; Böhm, J. Stromal hyaluronan accumulation is associated with low immune response and poor prognosis in pancreatic cancer. Sci. Rep. 2021, 11, 12216. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Firsanov, D.; Tombline, G.; Ning, H.; Ablaeva, J.; Seluanov, A.; Gorbunova, V. Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties. Nat. Commun. 2020, 11, 2376. [Google Scholar] [CrossRef]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef]
- Tonge, J.J.; Notley, S.V.; Dunning, M.J.; López-Guajardo, A.; Medcalf, J.D.; Heldin, P.; Panoutsos, G.; Gad, A.K.B. Hyaluronan nanoscale clustering and Hyaluronan synthase 2 expression are linked to the invasion of child fibroblasts and infantile fibrosarcoma in vitro and in vivo. Sci. Rep. 2022, 12, 19835. [Google Scholar] [CrossRef]
- Kozlova, I.; Ruusala, A.; Voytyuk, O.; Skandalis, S.S.; Heldin, P. IQGAP1 regulates hyaluronan-mediated fibroblast motility and proliferation. Cell Signal. 2012, 24, 1856–1862. [Google Scholar] [CrossRef]
- Albeiroti, S.; Soroosh, A.; de la Motte, C.A. Hyaluronan’s Role in Fibrosis: A Pathogenic Factor or a Passive Player? BioMed Res. Int. 2015, 2015, 790203. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.-Y.; Macfarlane, S.C.; Gómez Torijano, A.; Kim, H.R.; Rosier, M.; Dobra, K.; Ottewell, P.D.; Gad, A.K.B. Cancer-Associated Fibroblasts Move and Interact More with Triple-Negative Breast Cancer Cells and Stimulate Their Proliferation in a Hyaluronan-Dependent Manner. Cells 2025, 14, 1663. https://doi.org/10.3390/cells14211663
Hou S-Y, Macfarlane SC, Gómez Torijano A, Kim HR, Rosier M, Dobra K, Ottewell PD, Gad AKB. Cancer-Associated Fibroblasts Move and Interact More with Triple-Negative Breast Cancer Cells and Stimulate Their Proliferation in a Hyaluronan-Dependent Manner. Cells. 2025; 14(21):1663. https://doi.org/10.3390/cells14211663
Chicago/Turabian StyleHou, Sz-Ying, Sarah C. Macfarlane, Ariadna Gómez Torijano, Hyejeong Rosemary Kim, Marieke Rosier, Katalin Dobra, Penelope D. Ottewell, and Annica K. B. Gad. 2025. "Cancer-Associated Fibroblasts Move and Interact More with Triple-Negative Breast Cancer Cells and Stimulate Their Proliferation in a Hyaluronan-Dependent Manner" Cells 14, no. 21: 1663. https://doi.org/10.3390/cells14211663
APA StyleHou, S.-Y., Macfarlane, S. C., Gómez Torijano, A., Kim, H. R., Rosier, M., Dobra, K., Ottewell, P. D., & Gad, A. K. B. (2025). Cancer-Associated Fibroblasts Move and Interact More with Triple-Negative Breast Cancer Cells and Stimulate Their Proliferation in a Hyaluronan-Dependent Manner. Cells, 14(21), 1663. https://doi.org/10.3390/cells14211663







