The Retinoid Tamibarotene Aggravates Skin Inflammation in a Model of Bullous Pemphigoid-like Epidermolysis Bullosa Acquisita
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antibody-Transfer Model of Bullous Pemphigoid (BP)-like Epidermolysis Bullosa Acquisita (EBA) and Treatment with Tamibarotene
2.3. Flow Cytometric Analyses
2.4. Isolation of Murine Bone-Marrow Neutrophils
2.5. Stimulation of Murine Bone-Marrow Neutrophils with Immune Complexes (ICs)
2.6. Chemotaxis Assay
2.7. Statistical Analyses
3. Results
3.1. Tamibarotene Aggravates BP-like EBA Markedly
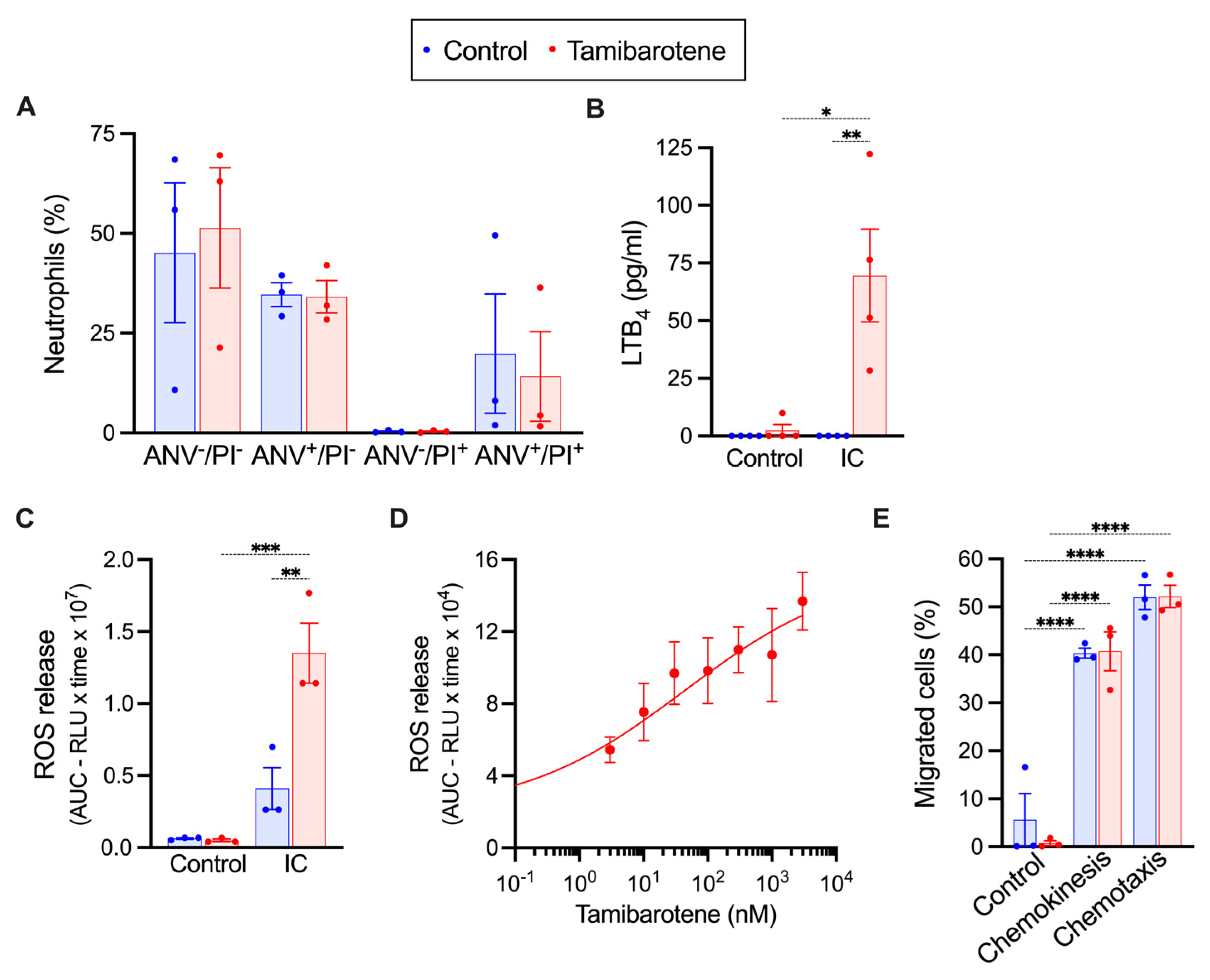
3.2. In Vitro Tamibarotene Preserves the Responsiveness of Neutrophils to ICs
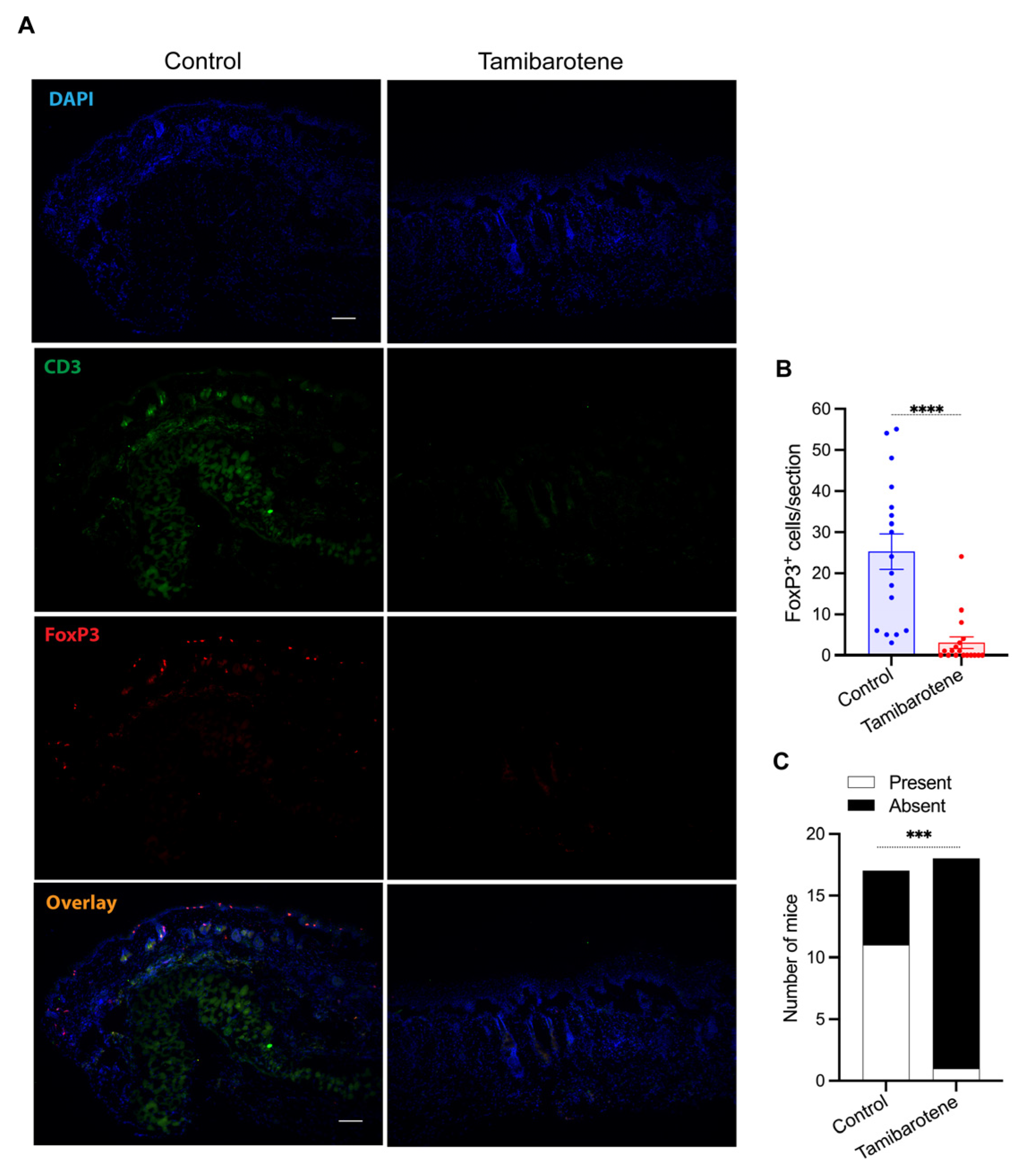
3.3. Tamibarotene Decreases the Recruitment of Regulatory T Cells (Tregs) into Lesional Skin Profoundly
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Bullous pemphigoid |
| EBA | Epidermolysis bullosa acquisita |
| IC | Immune complex |
References
- Li, L.; Qi, X.; Sun, W.; Abdel-Azim, H.; Lou, S.; Zhu, H.; Prasadarao, N.V.; Zhou, A.; Shimada, H.; Shudo, K.; et al. Am80-GCSF synergizes myeloid expansion and differentiation to generate functional neutrophils that reduce neutropenia-associated infection and mortality. EMBO Mol. Med. 2016, 8, 1340–1359. [Google Scholar] [CrossRef]
- Miwako, I.; Kagechika, H. Tamibarotene. Drugs Today 2007, 43, 563–568. [Google Scholar] [CrossRef]
- Miyabe, C.; Miyabe, Y.; Miura, N.N.; Takahashi, K.; Terashima, Y.; Toda, E.; Honda, F.; Morio, T.; Yamagata, N.; Ohno, N.; et al. Am80, a retinoic acid re-ceptor agonist, ameliorates murine vasculitis through the suppression of neutrophil migration and activation. Arthritis Rheum. 2013, 65, 503–512. [Google Scholar] [CrossRef]
- Klemann, C.; Raveney, B.J.; Klemann, A.K.; Ozawa, T.; von Horsten, S.; Shudo, K.; Oki, S.; Yamamura, T. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am. J. Pathol. 2009, 174, 2234–2245. [Google Scholar] [CrossRef]
- Ohyanagi, N.; Ishido, M.; Suzuki, F.; Kaneko, K.; Kubota, T.; Miyasaka, N.; Nanki, T. Retinoid ameliorates ex-perimental autoimmune myositis, with modulation of Th cell differentiation and antibody production in vivo. Arthritis Rheum. 2009, 60, 3118–3127. [Google Scholar] [CrossRef]
- Naskar, D.; Teng, F.; Felix, K.M.; Bradley, C.P.; Wu, H.J. Synthetic Retinoid AM80 Ameliorates Lung and Arthritic Autoimmune Responses by Inhibiting T Follicular Helper and Th17 Cell Responses. J. Immunol. 2017, 198, 1855–1864. [Google Scholar] [CrossRef]
- Kwok, S.K.; Park, M.K.; Cho, M.L.; Oh, H.J.; Park, E.M.; Lee, D.G.; Lee, J.; Kim, H.-Y.; Park, S.-H. Retinoic acid attenuates rheumatoid inflammation in mice. J. Immunol. 2012, 189, 1062–1071. [Google Scholar] [CrossRef]
- Keino, H.; Watanabe, T.; Sato, Y.; Okada, A.A. Oral administration of retinoic acid recep-tor-alpha/beta-specific ligand Am80 suppresses experimental autoimmune uveoretinitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1548–1556. [Google Scholar] [CrossRef]
- Nishimori, H.; Maeda, Y.; Teshima, T.; Sugiyama, H.; Kobayashi, K.; Yamasuji, Y.; Kadohisa, S.; Uryu, H.; Taekuchi, K.; Tanaka, T.; et al. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by down-regulating Th1 and Th17. Blood 2012, 119, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Asano, Y.; Akamata, K.; Noda, S.; Taniguchi, T.; Takahashi, T.; Ichimura, Y.; Shudo, K.; Sato, S.; Kadono, T. Tamibarotene Ameliorates Bleomycin-Induced Dermal Fibrosis by Modulating Phenotypes of Fibroblasts, Endothelial Cells, and Immune Cells. J. Investig. Dermatol. 2016, 136, 387–398. [Google Scholar] [CrossRef]
- Watanabe, H.; Bi, J.; Murata, R.; Fujimura, R.; Nishida, K.; Imafuku, T.; Nakamura, Y.; Maeda, H.; Mukunoki, A.; Taeko, T.; et al. A synthetic retinoic acid receptor agonist Am80 ameliorates renal fibrosis via inducing the production of alpha-1-acid glycoprotein. Sci. Rep. 2020, 10, 11424. [Google Scholar] [CrossRef]
- Sadik, C.D.; Schmidt, E. Resolution in bullous pemphigoid. Semin. Immunopathol. 2019, 41, 645–654. [Google Scholar] [CrossRef]
- Sadik, C.D.; Miyabe, Y.; Sezin, T.; Luster, A.D. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin. Immunol. 2018, 37, 21–29. [Google Scholar] [CrossRef]
- Sezin, T.; Krajewski, M.; Wutkowski, A.; Mousavi, S.; Chakievska, L.; Bieber, K.; Ludwig, R.J.; Dahlke, M.; Rades, D.; Schulze, F.S.; et al. The Leukotriene B(4) and its Receptor BLT1 Act as Critical Drivers of Neutrophil Recruitment in Murine Bullous Pemphigoid-Like Epidermolysis Bullosa Acquisita. J. Investig. Dermatol. 2017, 137, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Rashid, H.; Hammers, C.M.; Diercks, G.F.H.; Weidinger, A.; Beissert, S.; Schauer, F.; Fettiplace, J.; Thaci, D.; Ngai, Y.; et al. Evaluation of Nomacopan for Treatment of Bullous Pemphigoid: A Phase 2a Nonrandomized Controlled Trial. JAMA Dermatol. 2022, 158, 641–649. [Google Scholar] [CrossRef]
- Sezin, T.; Murthy, S.; Attah, C.; Seutter, M.; Holtsche, M.M.; Hammers, C.M.; Schmidt, E.; Meshrkey, F.; Mousavi, S.; Zillikens, D.; et al. Dual inhibition of complement factor 5 and leukotriene B4 synergistically suppresses murine pemphigoid disease. JCI Insight 2019, 4, e128239. [Google Scholar] [CrossRef]
- Sitaru, C.; Mihai, S.; Otto, C.; Chiriac, M.T.; Hausser, I.; Dotterweich, B.; Saito, H.; Rose, C.; Ishiko, A.; Zillikens, D. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J. Clin. Investig. 2005, 115, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, M.T.; Roesler, J.; Sindrilaru, A.; Scharffetter-Kochanek, K.; Zillikens, D.; Sitaru, C. NADPH oxidase is required for neutrophil-dependent autoantibody-induced tissue damage. J. Pathol. 2007, 212, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Shimanovich, I.; Mihai, S.; Oostingh, G.J.; Ilenchuk, T.T.; Brocker, E.B.; Opdenakker, G.; Zillikens, D.; Sitaru, C. Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J. Pathol. 2004, 204, 519–527. [Google Scholar] [CrossRef]
- Bieber, K.; Sun, S.; Witte, M.; Kasprick, A.; Beltsiou, F.; Behnen, M.; Laskay, T.; Schulze, F.S.; Pipi, E.; Reichhelm, N.; et al. Regulatory T Cells Suppress Inflammation and Blistering in Pemphigoid Diseases. Front. Immunol. 2017, 8, 1628. [Google Scholar] [CrossRef]
- Thieme, M.; Bieber, K.; Sezin, T.; Wannick, M.; Gupta, Y.; Kalies, K.; Ludwig, R.J.; Mousavi, S.; Zillikens, D.; Sadik, C.D. The Sphingosine-1-Phosphate Receptor Modulator Fingolimod Aggravates Murine Epidermolysis Bullosa Acquisita. J. Investig. Dermatol. 2019, 139, 2381–2384.e3. [Google Scholar] [CrossRef]
- Kibsgaard, L.; Bay, B.; Deleuran, M.; Vestergaard, C. A retrospective consecutive case-series study on the effect of systemic treatment, length of admission time, and co-morbidities in 98 bullous pemphigoid patients admitted to a tertiary centre. Acta Derm. Venereol. 2015, 95, 307–311. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Sadik, C.D.; Bieber, K.; Ibrahim, S.M.; Manz, R.A.; Schmidt, E.; Zillikens, D.; Ludwig, R.J. Epidermolysis Bullosa Acquisita: From Pathophysiology to Novel Therapeutic Options. J. Investig. Dermatol. 2016, 136, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Schmidt, E.; Zillikens, D.; Hashimoto, T. Recent progresses and perspectives in autoimmune bullous diseases. J. Allergy Clin. Immunol. 2020, 145, 1145–1147. [Google Scholar] [CrossRef]
- Murthy, S.; Patzelt, S.; Kunstner, A.; Busch, H.; Schmidt, E.; Sadik, C.D. Intravenous Ig Ameliorates Disease in a Murine Model of Anti-Laminin 332 Mucous Membrane Pemphigoid. J. Investig. Dermatol. 2024, 144, 2671–2681.e1. [Google Scholar] [CrossRef]
- Hirose, M.; Tiburzy, B.; Ishii, N.; Pipi, E.; Wende, S.; Rentz, E.; Nimmerjahn, F.; Zillikens, D.; Manz, R.A.; Ludwig, R.J.; et al. Effects of intravenous immunoglobulins on mice with experimental epidermolysis bullosa acquisita. J. Investig. Dermatol. 2015, 135, 768–775. [Google Scholar] [CrossRef]
- Sezin, T.; Ferreiros, N.; Jennrich, M.; Ochirbold, K.; Seutter, M.; Attah, C.; Mousavi, S.; Zillikens, D.; Geisslinger, G.; Sadik, C.D. 12/15-Lipoxygenase choreographs the resolution of IgG-mediated skin inflammation. J. Autoimmun. 2020, 115, 102528. [Google Scholar] [CrossRef]
- Schilf, P.; Schmitz, M.; Derenda-Hell, A.; Thieme, M.; Bremer, T.; Vaeth, M.; Zillikens, D.; Sadik, C.D. Inhibition of Glucose Metabolism Abrogates the Effector Phase of Bullous Pemphigoid-Like Epidermolysis Bullosa Acquisita. J. Investig. Dermatol. 2021, 141, 1646–1655.e3. [Google Scholar] [CrossRef]
- Murthy, S.; Schilf, P.; Patzelt, S.; Thieme, M.; Becker, M.; Kroger, L.; Bremer, T.; Derenda-Hell, A.; Knebel, L.; Fagiani, F.; et al. Dapsone Suppresses Disease in Preclinical Murine Models of Pemphigoid Diseases. J. Investig. Dermatol. 2021, 141, 2587–2595.e2. [Google Scholar] [CrossRef]
- Olbrich, H.; Sadik, C.D. Emerging drugs for the treatment of bullous pemphigoid: What’s new on the horizon? Expert Opin. Emerg. Drugs 2025. [Google Scholar] [CrossRef]
- Ding, W.; Shimada, H.; Li, L.; Mittal, R.; Zhang, X.; Shudo, K.; He, Q.; Prasadarao, N.V.; Wu, L. Retinoid agonist Am80-enhanced neutrophil bactericidal activity arising from granulopoiesis in vitro and in a neutropenic mouse model. Blood 2013, 121, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Erkelens, M.N.; Mebius, R.E. Retinoic Acid and Immune Homeostasis: A Balancing Act. Trends Immunol. 2017, 38, 168–180. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yokota, A.; Ohoka, Y.; Kagechika, H.; Kato, C.; Song, S.Y.; Iwata, M. Efficient induction of CCR9 on T cells requires coactivation of retinoic acid receptors and retinoid X receptors (RXRs): Exaggerated T Cell homing to the intestine by RXR activation with organotins. J. Immunol. 2010, 185, 5289–5299. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yokota-Nakatsuma, A.; Ohoka, Y.; Kagechika, H.; Kato, C.; Song, S.Y.; Iwata, M. Retinoid X receptor agonists modulate Foxp3(+) regulatory T cell and Th17 cell differentiation with differential dependence on retinoic acid receptor activation. J. Immunol. 2013, 191, 3725–3733. [Google Scholar] [CrossRef]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef]
- Yamanaka, K.; Dimitroff, C.J.; Fuhlbrigge, R.C.; Kakeda, M.; Kurokawa, I.; Mizutani, H.; Kupper, T.S. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. J. Allergy Clin. Immunol. 2008, 121, 148–157.e3. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Sigmundsdottir, H.; Pan, J.; Debes, G.F.; Alt, C.; Habtezion, A.; Soler, D.; Butcher, E.C. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007, 8, 285–293. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Tukaj, S.; Bieber, K.; Witte, M.; Ghorbanalipoor, S.; Schmidt, E.; Zillikens, D.; Ludwig, R.J.; Kasperkiewicz, M. Calcitriol Treatment Ameliorates Inflammation and Blistering in Mouse Models of Epidermolysis Bullosa Acquisita. J. Investig. Dermatol. 2018, 138, 301–309. [Google Scholar] [CrossRef]
- Antiga, E.; Quaglino, P.; Volpi, W.; Pierini, I.; Del Bianco, E.; Bianchi, B.; Novelli, M.; Savoia, P.; Bernengo, M.G.; Fabbri, P.; et al. Regulatory T cells in skin lesions and blood of patients with bullous pemphigoid. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 222–230. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thieme, M.; Schilf, P.; Murthy, S.; Gonther, S.; Hammers, C.M.; Heine, G.; Sadik, C.D. The Retinoid Tamibarotene Aggravates Skin Inflammation in a Model of Bullous Pemphigoid-like Epidermolysis Bullosa Acquisita. Cells 2025, 14, 1661. https://doi.org/10.3390/cells14211661
Thieme M, Schilf P, Murthy S, Gonther S, Hammers CM, Heine G, Sadik CD. The Retinoid Tamibarotene Aggravates Skin Inflammation in a Model of Bullous Pemphigoid-like Epidermolysis Bullosa Acquisita. Cells. 2025; 14(21):1661. https://doi.org/10.3390/cells14211661
Chicago/Turabian StyleThieme, Markus, Paul Schilf, Sripriya Murthy, Sina Gonther, Christoph M. Hammers, Guido Heine, and Christian D. Sadik. 2025. "The Retinoid Tamibarotene Aggravates Skin Inflammation in a Model of Bullous Pemphigoid-like Epidermolysis Bullosa Acquisita" Cells 14, no. 21: 1661. https://doi.org/10.3390/cells14211661
APA StyleThieme, M., Schilf, P., Murthy, S., Gonther, S., Hammers, C. M., Heine, G., & Sadik, C. D. (2025). The Retinoid Tamibarotene Aggravates Skin Inflammation in a Model of Bullous Pemphigoid-like Epidermolysis Bullosa Acquisita. Cells, 14(21), 1661. https://doi.org/10.3390/cells14211661





