Hsp60-Bearing Exosomes in Helicobacter pylori-Induced Gastric Tumorigenesis: A Pathomorphological and Therapeutical Overview
Highlights
- This review identifies Hsp60-bearing extracellular vesicles (EVs)—both bacterial (GroEL in H. pylori OMVs) and host-derived (human exosomes)—as central mediators of the infection–inflammation–cancer axis in gastric mucosa.
- It also introduces the novel concept of the muco-microbiotic layer as a fifth, functionally distinct layer of the gastric wall, serving as the key site for vesicle-mediated host–microbe interactions.
- Recognizing Hsp60-bearing EVs as active pathogenic and immunomodulatory agents opens new diagnostic and therapeutic avenues, including EV-based biomarkers and Hsp60-targeted interventions.
- The proposed framework not only redefines gastric wall morphology but also provides a new conceptual and experimental basis for studying early events in H. pylori-associated gastric carcinogenesis.
Abstract
1. Introduction
2. The Muco-Microbiotic Layer: A Novel Morpho-Functional Framework
3. H. pylori GroEL and Host Hsp60 in Vesicle-Mediated Pathogenicity
4. Gastric Epithelial Cell Stress and Exosomal Hsp60
5. Vesicular Crosstalk and Molecular Mimicry
6. Pathomorphological and Immunological Implications of EV Crosstalks
7. Diagnostic/Therapeutic Insights and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamun, T.I.; Younus, S.; Rahman, H. Gastric cancer—Epidemiology, modifiable and non-modifiable risk factors, challenges and opportunities: An updated review. Cancer Treat. Res. Commun. 2024, 41, 100845. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’MOrain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Logan, R.P.H.; Walker, M.M. ABC of the upper gastrointestinal tract: Epidemiology and diagnosis of Helicobacter pylori infection. BMJ 2001, 323, 920–922. [Google Scholar] [CrossRef]
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.M.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori World Gastroenterology Organization Global Guideline. J. Clin. Gastroenterol. 2022, 57, 111–126. [Google Scholar] [CrossRef]
- Matysiak-Budnik, T.; Mégraud, F. Helicobacter pylori infection and gastric cancer. Eur. J. Cancer 2006, 42, 708–716. [Google Scholar] [CrossRef]
- Manna, O.M.; Caruso Bavisotto, C.; Gratie, M.I.; Damiani, P.; Bonaventura, G.; Cappello, F.; Tomasello, G.; D’andrea, V. Targeting Helicobacter pylori Through the “Muco-Microbiotic Layer” Lens: The Challenge of Probiotics and Microbiota Nanovesicles. Nutrients 2025, 17, 569. [Google Scholar] [CrossRef]
- Manna, O.M.; Caruso Bavisotto, C.; Gratie, M.I.; Damiani, P.; Tomasello, G.; Cappello, F. The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases’ Pathogenesis. Int. J. Mol. Sci. 2025, 26, 5065. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Merendino, A.M.; Bucchieri, F.; Campanella, C.; Marcianò, V.; Ribbene, A.; David, S.; Zummo, G.; Burgio, G.; Corona, D.F.V.; De Macario, E.C.; et al. Hsp60 Is Actively Secreted by Human Tumor Cells. PLoS ONE 2010, 5, e9247. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Wang, W.; Pu, N.; Liu, L. Epithelial-mesenchymal transition orchestrates tumor microenvironment: Current perceptions and challenges. J. Transl. Med. 2025, 23, 1–18. [Google Scholar] [CrossRef]
- Cappello, F.; Saguto, D.; Burgio, S.; Paladino, L.; Bucchieri, F. Does Intestine Morphology Still Have Secrets to Reveal? A Proposal about the “Ghost” Layer of the Bowel. Appl. Biosci. 2022, 1, 95–100. [Google Scholar] [CrossRef]
- Fucarino, A.; Burgio, S.; Paladino, L.; Caruso Bavisotto, C.; Pitruzzella, A.; Bucchieri, F.; Cappello, F. The Microbiota Is Not an Organ: Introducing the Muco-Microbiotic Layer as a Novel Morphofunctional Structure. Anatomia 2022, 1, 186–203. [Google Scholar] [CrossRef]
- Li, J.; Liao, T.; Chua, E.G.; Zhang, M.; Shen, Y.; Song, X.; Marshall, B.J.; Benghezal, M.; Tang, H.; Li, H. Helicobacter pylori Outer Membrane Vesicles: Biogenesis, Composition, and Biological Functions. Int. J. Biol. Sci. 2024, 20, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Luo, H.; Xie, Y.; Cao, H.; Mao, L.; Liu, T.; Yue, Y.; Qian, H. Extracellular vesicles in Helicobacter pylori-mediated diseases: Mechanisms and therapeutic potential. Cell Commun. Signal. 2025, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Mao, L.; Yao, Y. Helicobacter pylori outer membrane vesicles and infected cell exosomes: New players in host immune modulation and pathogenesis. Front. Immunol. 2024, 15, 1512935. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- González, M.F.; Díaz, P.; Sandoval-Bórquez, A.; Herrera, D.; Quest, A.F.G. Helicobacter pylori Outer Membrane Vesicles and Extracellular Vesicles from Helicobacter pylori-Infected Cells in Gastric Disease Development. Int. J. Mol. Sci. 2021, 22, 4823. [Google Scholar] [CrossRef]
- Zhang, G.; Ducatelle, R.; Pasmans, F.; D’Herde, K.; Huang, L.; Smet, A.; Haesebrouck, F.; Flahou, B. Effects of Helicobacter suis γ- Glutamyl Transpeptidase on Lymphocytes: Modulation by Glutamine and Glutathione Supplementation and Outer Membrane Vesicles as a Putative Delivery Route of the Enzyme. PLoS ONE 2013, 8, e77966. [Google Scholar] [CrossRef][Green Version]
- Paquet, M.F.; Charette, S.J. Co-purification of the GroEL chaperone during outer membrane vesicle purification: Insights from Aeromonas salmonicida subsp. salmonicida. Microbiology 2025, 171, 001558. [Google Scholar] [CrossRef]
- Zhu, D.; Fan, Y.; Wang, X.; Li, P.; Huang, Y.; Jiao, J.; Zhao, C.; Li, Y.; Wang, S.; Du, X. Characterization of Molecular Chaperone GroEL as a Potential Virulence Factor in Cronobacter sakazakii. Foods 2023, 12, 3404. [Google Scholar] [CrossRef]
- Palacios, E.; Lobos-González, L.; Guerrero, S.; Kogan, M.J.; Shao, B.; Heinecke, J.W.; Quest, A.F.G.; Leyton, L.; Valenzuela-Valderrama, M. Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa B activation and cause neuronal damage in vivo in a murine model. J. Neuroinflammation 2023, 20, 1–22. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, M.; Pu, X.; Zheng, H.; Ning, X.; Tu, Y.; Xu, C.; Zhang, D.; Liu, C.; Xie, J. GroEL triggers NLRP3 inflammasome activation through the TLR/NF-κB p-p65 axis in human periodontal ligament stem cells. Acta Biochim. et Biophys. Sin. 2024, 56, 1340–1351. [Google Scholar] [CrossRef]
- Fourie, K.R.; Wilson, H.L. Understanding GroEL and DnaK Stress Response Proteins as Antigens for Bacterial Diseases. Vaccines 2020, 8, 773. [Google Scholar] [CrossRef]
- Cappello, F.; Conway De Macario, E.; Di Felice, V.; Zummo, G.; Macario, A.J.L. Chlamydia trachomatis Infection and Anti-Hsp60 Immunity: The Two Sides of the Coin. PLoS Pathog. 2009, 5, e1000552. [Google Scholar] [CrossRef]
- Jones, K.R.; Whitmire, J.M.; Merrell, D.S. A Tale of Two Toxins: Helicobacter pylori CagA and VacA Modulate Host Pathways that Impact Disease. Front. Microbiol. 2010, 1, 6955. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, N.C.; Monzo, P.; Kaddai, V.; Doye, A.; Ricci, V.; Boquet, P. Helicobacter pylori VacA Cytotoxin: A Probe for a Clathrin-independent and Cdc42-dependent Pinocytic Pathway Routed to Late Endosomes. Mol. Biol. Cell 2005, 16, 4852–4866. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.M.; Cappello, F.; Conway de Macario, E.; Macario, A.J.L.; Gammazza, A.M. Hsp60 in inflammation and autoimmunity. In The Multitasking Molecular Chaperone Hsp60: Structure, Function, and Impact on Health and Disease, 1st ed.; Cappello, F., Conway de Macario, E., Macario, A.J.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 77–98. [Google Scholar]
- Planesse, C.; Nativel, B.; Iwema, T.; Gasque, P.; Silva, C.R.-D.; Viranaïcken, W. Recombinant human HSP60 produced in ClearColi™ BL21(DE3) does not activate the NFκB pathway. Cytokine 2015, 73, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2006, 81, 1–5. [Google Scholar] [CrossRef]
- Campanella, C.; Rappa, F.; Sciumè, C.; Gammazza, A.M.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Bavisotto, C.C.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef]
- Kumar, S.; O’mAlley, J.; Chaudhary, A.K.; Inigo, J.R.; Yadav, N.; Kumar, R.; Chandra, D. Hsp60 and IL-8 axis promotes apoptosis resistance in cancer. Br. J. Cancer 2019, 121, 934–943. [Google Scholar] [CrossRef]
- Fais, S.; O’dRiscoll, L.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Carvalho, J.; da Silva, A.C.; Del Portillo, H.; El Andaloussi, S.; et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef]
- Singh, M.K.; Shin, Y.; Han, S.; Ha, J.; Tiwari, P.K.; Kim, S.S.; Kang, I. Molecular Chaperonin HSP60: Current Understanding and Future Prospects. Int. J. Mol. Sci. 2024, 25, 5483. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Mallegol, J.; Bevilacqua, C.; Candalh, C.; Brugière, S.; Tomaskovic-Crook, E.; Heath, J.K.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 2003, 52, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen presentation by extracellular vesicles from professional antigen-presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef]
- Burgio, S.; de Macario, E.C.; Macario, A.J.; Cappello, F. SARS-CoV-2 in patients with cancer: Possible role of mimicry of human molecules by viral proteins and the resulting anti-cancer immunity. Cell Stress Chaperon- 2021, 26, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Pitruzzella, A.; Burgio, S.; Presti, P.L.; Ingrao, S.; Fucarino, A.; Bucchieri, F.; Cabibi, D.; Cappello, F.; de Macario, E.C.; Macario, A.J.L.; et al. Hsp60 Quantification in Human Gastric Mucosa Shows Differences between Pathologies with Various Degrees of Proliferation and Malignancy Grade. Appl. Sci. 2021, 11, 3582. [Google Scholar] [CrossRef]
- Weiss, C.; Jebara, F.; Nisemblat, S.; Azem, A. Dynamic Complexes in the Chaperonin-Mediated Protein Folding Cycle. Front. Mol. Biosci. 2016, 3, 80. [Google Scholar] [CrossRef]
- Vilasi, S.; Bulone, D.; Bavisotto, C.C.; Campanella, C.; Gammazza, A.M.; Biagio, P.L.S.; Cappello, F.; de Macario, E.C.; Macario, A.J.L. Chaperonin of Group I: Oligomeric Spectrum and Biochemical and Biological Implications. Front. Mol. Biosci. 2018, 4, 99. [Google Scholar] [CrossRef]
- Quintana, F.J.; Mimran, A.; Carmi, P.; Mor, F.; Cohen, I.R. HSP60 as a Target of Anti-Ergotypic Regulatory T Cells. PLoS ONE 2008, 3, e4026. [Google Scholar] [CrossRef]
- David, S.; Vitale, A.; Fucarino, A.; Scalia, F.; Vergilio, G.; Conway de Macario, E.; Macario, A.; Caruso Bavisotto, C.; Pitruzzella, A. The Challenging Riddle about the Janus-Type Solution. Appl. Sci. 2021, 11, 1175. [Google Scholar] [CrossRef]
- Khor, B.; Snow, M.; Herrman, E.; Ray, N.; Mansukhani, K.; Patel, K.A.; Said-Al-Naief, N.; Maier, T.; Machida, C.A. Interconnections between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance between Systemic Health and Disease. Microorganisms 2021, 9, 496. [Google Scholar] [CrossRef]
- Liu, K.S.-H.; Wong, I.O.-L.; Leung, W.K. Helicobacter pylori associated gastric intestinal metaplasia: Treatment and surveillance. World, J. Gastroenterol. 2016, 22, 1311–1320. [Google Scholar] [CrossRef]
- González, M.F.; Burgos-Ravanal, R.; Shao, B.; Heinecke, J.; Valenzuela-Valderrama, M.; Corvalán, A.H.; Quest, A.F.G. Extracellular vesicles from gastric epithelial GES-1 cells infected with Helicobacter pylori promote changes in recipient cells associated with malignancy. Front. Oncol. 2022, 12, 962920. [Google Scholar] [CrossRef]
- Wang, S.-N.; Yun, T.; Zhu, C.-Y.; Li, P.; Ge, D.-F.; Li, S.-L.; Wang, Y.-K. Immune cell changes in Helicobacter pylori infection-induced glandular epithelial cell damage of the gastric mucosa. Ann. Med. 2024, 56, 2425072. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, H.; Qiu, D.; Wang, N.; Wang, Y.; Wen, T.; Wang, J.; Zhu, H. The proteomics analysis of extracellular vesicles revealed the possible function of heat shock protein 60 in Helicobacter pylori infection. Cancer Cell Int. 2023, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Farinha, P.; Gascoyne, R.D. Helicobacter pylori and MALT Lymphoma. Gastroenterology 2005, 128, 1579–1605. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Logozzi, M.; Campanella, C.; Bavisotto, C.C.; Marcilla, A.; Properzi, F.; Fais, S. Exosome levels in human body fluids: A tumor marker by themselves? Eur. J. Pharm. Sci. 2017, 96, 93–98. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Cappello, F.; Macario, A.J.; Conway de Macario, E.; Logozzi, M.; Fais, S.; Campanella, C. Exosomal HSP60: A potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev. Mol. Diagn. 2017, 17, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Zocco, D.; Ferruzzi, P.; Cappello, F.; Kuo, W.P.; Fais, S. Extracellular Vesicles as Shuttles of Tumor Biomarkers and Anti-Tumor Drugs. Front. Oncol. 2014, 4, 267. [Google Scholar] [CrossRef]
- Cappello, F.; Marino Gammazza, A.; Piccionello, A.P.; Campanella, C.; Pace, A.; de Macario, E.C.; Macario, A.J. Hsp60 chaperonopathies and chaperonotherapy: Targets and agents. Expert Opin. Ther. Targets 2013, 18, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Lemos, F.F.B.; de Castro, C.T.; Calmon, M.S.; Luz, M.S.; Pinheiro, S.L.R.; dos Santos, C.F.S.M.; Santos, G.L.C.; Marques, H.S.; Delgado, H.A.; Teixeira, K.N.; et al. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J. Gastroenterol. 2023, 29, 2202–2221. [Google Scholar] [CrossRef] [PubMed]
- Shatila, M. Helicobacter pylori infection negatively affects response of gastric cancer to immunotherapy. Ann. Gastroenterol. 2025, 38, 262–269. [Google Scholar] [CrossRef] [PubMed]
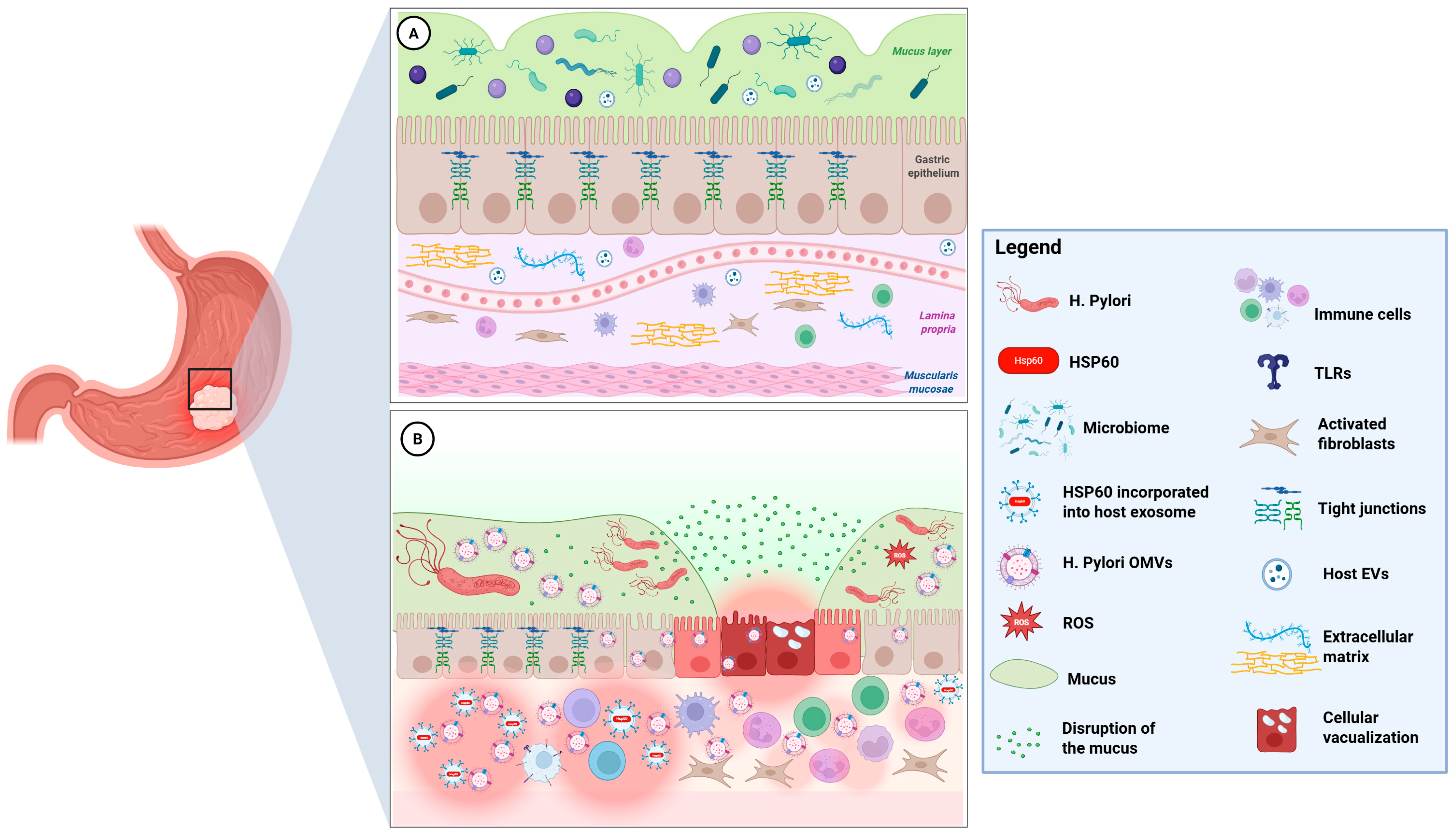
| Pathogenic Mechanisms | Description | References |
|---|---|---|
| Muco-Microbiotic Disruption | H. pylori modify the mucus layer by altering mucin expression, neutralizing acidity with urease, and adhering to epithelial cells. | [7,12,13] |
| OMVs-Mediated Pathogenesis | OMVs play a critical role in the virulence and immune evasion of Helicobacter pylori. They carry various virulence factors, including CagA, VacA, urease subunits, and adhesins, as well as chaperones like GroEL, which is a bacterial equivalent of Hsp60. Furthermore, OMVs contribute to the modulation of epithelial cell function and transformation. | [14,15,16,17,18,26,27] |
| GroEL–TLR Activation | GroEL can be recognized by Toll-like receptors TLR4 and TLR2 found on epithelial and immune cells. This recognition activates the NF-κB- and MAPK-signaling pathways, leading to the production of pro-inflammatory cytokines, such as IL-8 and TNF-α. These cytokines may promote neutrophil recruitment and contribute to chronic mucosal inflammation. Additionally, GroEL’s interaction with TLR4 and TLR2 can activate NLRP3 inflammasomes, further driving the production of chemokines and cytokines. | [23,24] |
| CagA/VacA Signaling | Delivery of CagA and VacA via OMVs can result in changes to the cytoskeleton, disruption of tight junctions, vacuolization, and mitochondrial dysfunction. These alterations may promote cell proliferation and increase resistance to apoptosis. | [26,27] |
| Exosomal Hsp60 Release | Oxidative, inflammatory, and bacterial stress increases Hsp60 levels in human gastric epithelial cells. Typically located in the mitochondria, Hsp60 moves to the plasma membrane under stress, is packaged into multivesicular bodies, and is secreted as exosomes, leading to elevated extracellular Hsp60 levels. | [10,28,29,30,31,32,33] |
| Molecular Mimicry | The release of Hsp60 from human exosomes in pre-neoplastic or dysplastic cells may facilitate immune recognition through molecular mimicry. This process could potentially lead to autoimmune reactions involving T cells or antibodies against gastric cells, which might enhance immune surveillance but could also contribute to tissue damage and carcinogenesis. | [25,34,35,36,37] |
| Chronic Inflammatory Loop | The production of OMVs by H. pylori is a crucial mechanism for its virulence and ability to evade the immune system. In response to this, the release of Hsp60 via human exosomes activates innate immune receptors such as TLR4 on macrophages and dendritic cells. This activation leads to an increased secretion of pro-inflammatory cytokines, including IL-6, IL-8, and TNF-α. This process sustains chronic inflammation typical of H. pylori-infected mucosa. | [14,15,16,23,24,30,33] |
| MALT Lymphoid Induction | The presence of GroEL in H. pylori OMVs contributes to a low-grade inflammatory state, marked by lymphoplasmacytic infiltration, neutrophil accumulation, and lymphoid follicle formation, which is associated with MALT lymphoma development. | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gratie, M.I.; Manna, O.M.; Accomando, S.; Tomasello, G.; Cappello, F.; Fucarino, A. Hsp60-Bearing Exosomes in Helicobacter pylori-Induced Gastric Tumorigenesis: A Pathomorphological and Therapeutical Overview. Cells 2025, 14, 1652. https://doi.org/10.3390/cells14211652
Gratie MI, Manna OM, Accomando S, Tomasello G, Cappello F, Fucarino A. Hsp60-Bearing Exosomes in Helicobacter pylori-Induced Gastric Tumorigenesis: A Pathomorphological and Therapeutical Overview. Cells. 2025; 14(21):1652. https://doi.org/10.3390/cells14211652
Chicago/Turabian StyleGratie, Melania Ionelia, Olga Maria Manna, Salvatore Accomando, Giovanni Tomasello, Francesco Cappello, and Alberto Fucarino. 2025. "Hsp60-Bearing Exosomes in Helicobacter pylori-Induced Gastric Tumorigenesis: A Pathomorphological and Therapeutical Overview" Cells 14, no. 21: 1652. https://doi.org/10.3390/cells14211652
APA StyleGratie, M. I., Manna, O. M., Accomando, S., Tomasello, G., Cappello, F., & Fucarino, A. (2025). Hsp60-Bearing Exosomes in Helicobacter pylori-Induced Gastric Tumorigenesis: A Pathomorphological and Therapeutical Overview. Cells, 14(21), 1652. https://doi.org/10.3390/cells14211652








