A Focus on Inflammatory and Bacterial Biomarkers in Secondary Peritonitis
Abstract
1. Introduction
2. Tissue Organization of the Peritoneum
3. The Microbiome Role Within the Peritoneum
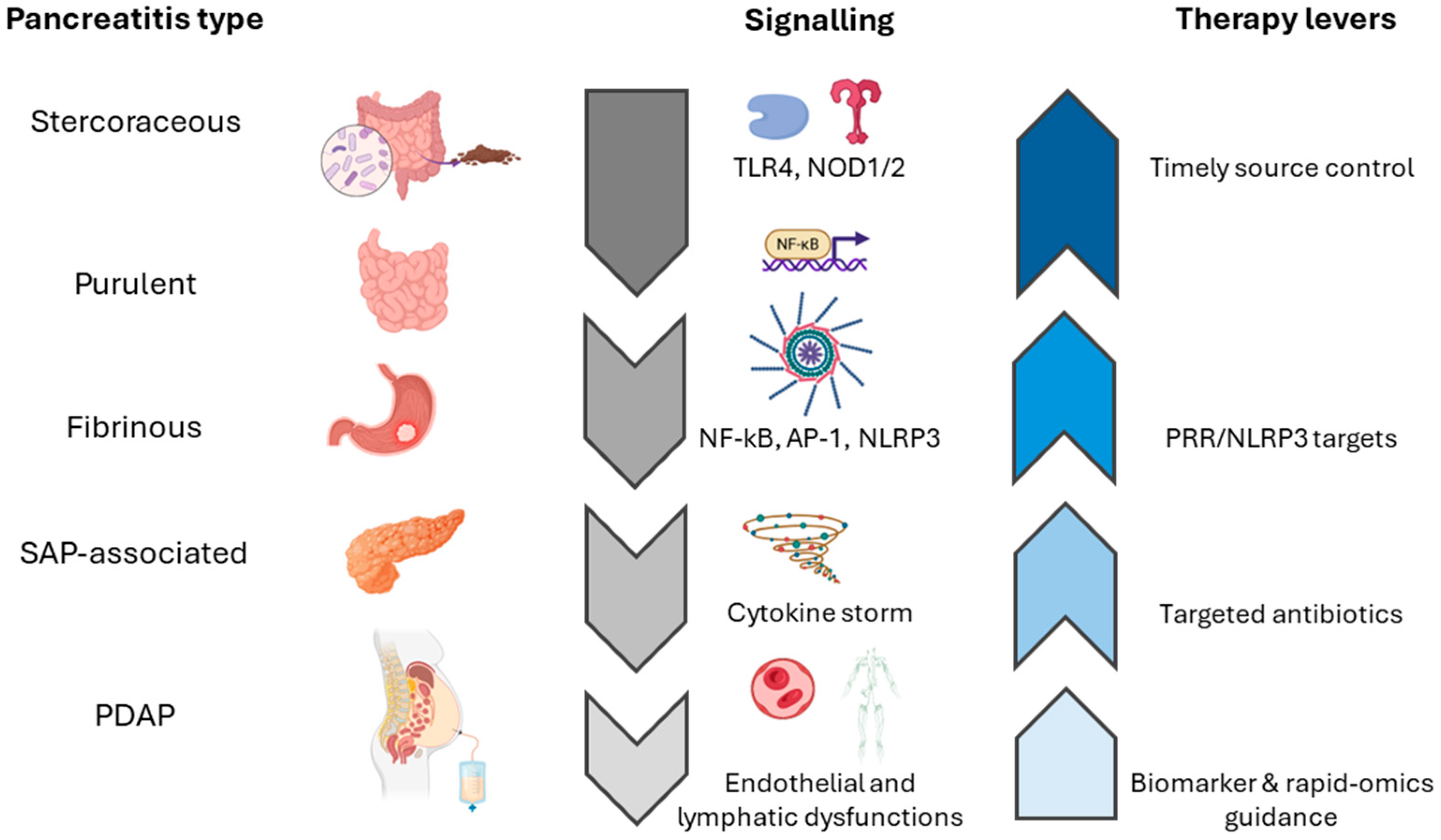
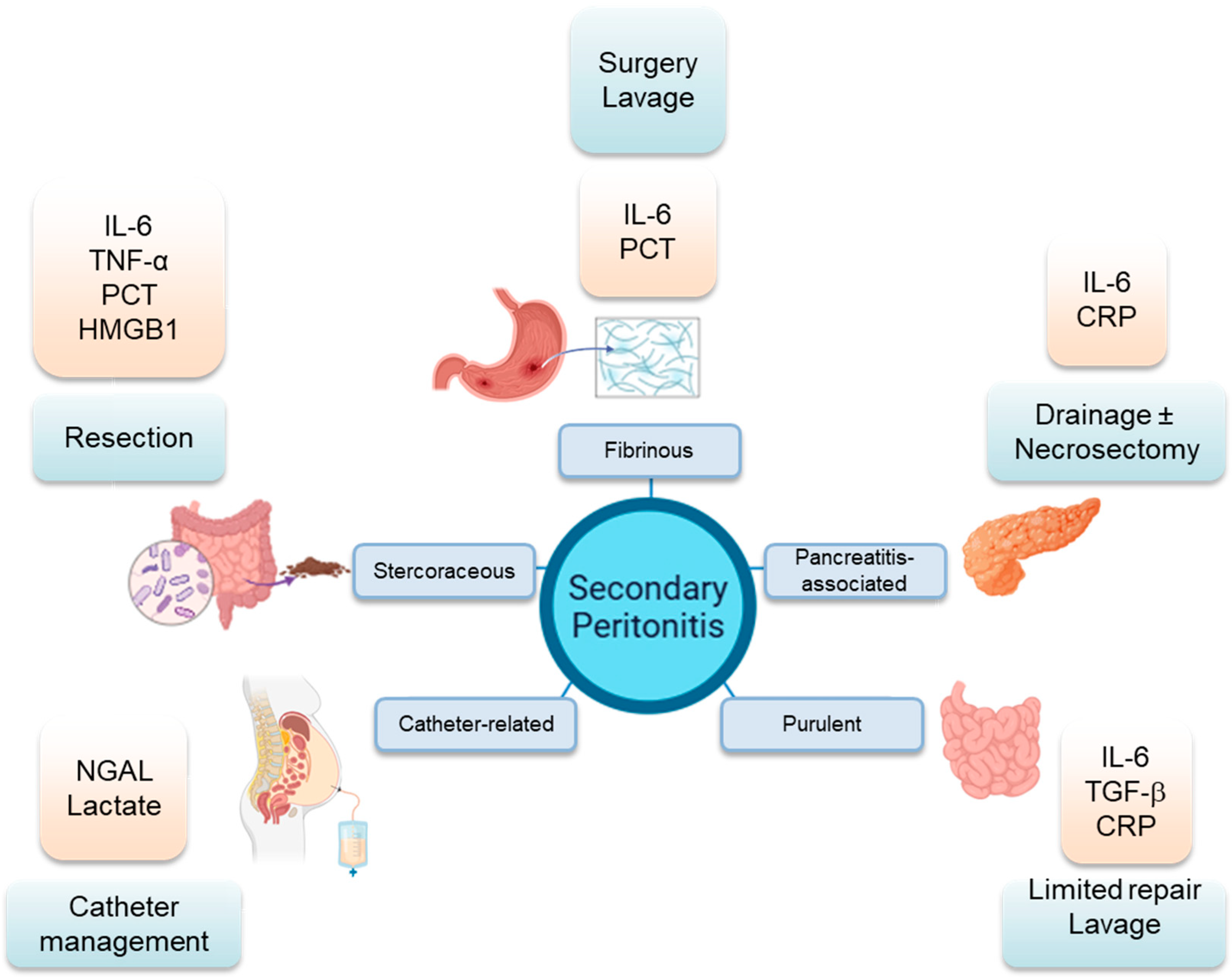
4. Clinical Variants of Secondary Peritonitis
4.1. Fibrinous Peritonitis
4.2. Purulent Peritonitis
4.3. Stercoraceous Peritonitis
4.4. Pancreatitis-Associated Chemical Peritonitis
4.5. Catheter-Related Peritonitis
4.6. Localized Peritonitis: Covered Bowel Perforation and Appendicitis
5. Pathophysiology Across Variants
5.1. Microbial Load and Pattern Recognition Receptors
5.2. Role of Neutrophils
5.3. Cytokine and Chemokine Profiles
5.4. Transcription Factor Signaling
5.5. Vascular and Lymphatic Dysfunction
6. Inflammatory Biomarkers
6.1. Biomarker Kinetics
6.2. microRNA Signature
7. Clinical and Surgical Management
8. Advanced Analytical Techniques
8.1. Organoids and Peritoneum-on-Chip Models
8.2. Omics Integration
8.3. Omics Identification as a Prompt Diagnostic Strategy Tool
9. Future Directions
10. Conclusions
11. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3′-UTR | three prime untranslated region |
| 5′-UTR | five prime untranslated region |
| AUC | area under the curve |
| CCL2 | chemokine (C-C motif) ligand 2 |
| CLP | cecal ligation and puncture |
| CXCL1 | chemokine (C-X-C motif) ligand 1 |
| ECM | extracellular matrix |
| ESBL | extended-spectrum beta-lactamase |
| EPS | encapsulating peritoneal sclerosis |
| G-CSF | granulocyte colony-stimulating factor |
| HC-IAI | health care-associated intra-abdominal infections |
| HMGB1 | high mobility group box 1 |
| ICU | intensive care unit |
| IKK | IκB kinase |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| LPS | lipopolysaccharide |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP-9 | matrix metalloproteinase-9 |
| MMT | mesothelial-to-mesenchymal transition |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NLR | neutrophil-to-lymphocyte ratio NGAL |
| NLRP3 | NLR family pyrin domain containing 3 |
| NOD1/2 | nucleotide-binding oligomerization domain-containing protein 1/2 |
| PCI | peritoneal contamination and infection |
| PCT | procalcitonin |
| PD | peritoneal dialysis |
| PDAP | peritoneal dialysis-associated peritonitis |
| PICU | pediatric intensive care unit |
| PLR | platelet-to-lymphocyte ratio |
| PUP | peptic ulcer perforation |
| RCT | randomized controlled trial |
| SAP | severe acute pancreatitis |
| SIRI | systemic immune-inflammation index |
| TGF-β | transforming growth factor β |
| TLR-4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor α |
References
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.C.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and Management of Complicated Intra-abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 133–164. [Google Scholar] [CrossRef]
- Huang, H.-B.; Peng, J.-M.; Weng, L.; Wang, C.-Y.; Jiang, W.; Du, B. Procalcitonin-guided antibiotic therapy in intensive care unit patients: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 114. [Google Scholar] [CrossRef]
- Yang, X.; Tong, Y.; Yan, H.; Ni, Z.; Qian, J.; Fang, W. High Intraperitoneal Interleukin-6 Levels Predict Peritonitis in Peritoneal Dialysis Patients: A Prospective Cohort Study. Am. J. Nephrol. 2018, 47, 317–324. [Google Scholar] [CrossRef]
- Cullaro, G.; Kim, G.; Pereira, M.R.; Brown, R.S.; Verna, E.C. Ascites Neutrophil Gelatinase-Associated Lipocalin Identifies Spontaneous Bacterial Peritonitis and Predicts Mortality in Hospitalized Patients with Cirrhosis. Dig. Dis. Sci. 2017, 62, 3487–3494. [Google Scholar] [CrossRef]
- Wang, S.S.; Lee, F.Y.; Chao, Y.; Chen, C.C.; Lin, H.Y.; Wu, S.L.; Lee, S.D. Clinical significance of complements in ascitic diseases: Elevated complement levels disapproving the liver disease origin. Proc. Natl. Sci. Counc. Repub. China Part B 1996, 20, 51–57. [Google Scholar]
- Bajaj, J.S.; Tandon, P.; O’Leary, J.G.; Wong, F.; Biggins, S.W.; Garcia-Tsao, G.; Kamath, P.S.; Maliakkal, B.; Fallon, M.B.; Lai, J.C.; et al. Outcomes in Patients with Cirrhosis on Primary Compared to Secondary Prophylaxis for Spontaneous Bacterial Peritonitis. Am. J. Gastroenterol. 2019, 114, 599–606. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Birnie, K.; Lansley, S.; Herrick, S.E.; Lim, C.-B.; Prãªle, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.M.; Barie, P.S.; Dellinger, E.P.; Forrester, J.D.; Duane, T.M.; Tessier, J.M.; Sawyer, R.G.; Cainzos, M.A.; Rasa, K.; Chipman, J.G.; et al. The Surgical Infection Society Guidelines on the Management of Intra-Abdominal Infection: 2024 Update. Surg. Infect. 2024, 25, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Altamirano, D.; Neira-Quezada, D.; Willches-Encalada, E.; Cabrera-Ordoñez, C.; Valdivieso-Espinoza, R.; Himmler, A.; Di Saverio, S. The influence of preoperative e intraoperative factors in predicting postoperative morbidity and mortality in perforated diverticulitis: A systematic review and meta-analysis. Updates Surg. 2024, 76, 397–409. [Google Scholar] [CrossRef]
- Shahi, R.; Siddiqui, N.H.; Khan, I.A.; Bashar, A. Etiologies and Outcomes Following Duodenal Perforation in Acute Peritonitis: A Systematic Review. Cureus 2024, 16, e74707. [Google Scholar] [CrossRef]
- Luțenco, V.; Beznea, A.; Mihailov, R.; Țocu, G.; Luțenco, V.; Mihailov, O.M.; Patriciu, M.; Pascaru, G.; Baroiu, L. Literature Review of Prognostic Factors in Secondary Generalized Peritonitis. Life 2025, 15, 880. [Google Scholar] [CrossRef]
- Luo, S.; Lin, H.; Wu, C.; Zhu, L.; Hua, Q.; Weng, Y.; Wang, L.; Fan, X.; Zhao, K.-B.; Liu, G.; et al. Cholinergic macrophages promote the resolution of peritoneal inflammation. Proc. Natl. Acad. Sci. USA 2024, 121, e2402143121. [Google Scholar] [CrossRef]
- Hatanaka, K.; Ito, T.; Madokoro, Y.; Kamikokuryo, C.; Niiyama, S.; Yamada, S.; Maruyama, I.; Kakihana, Y. Circulating Syndecan-1 as a Predictor of Persistent Thrombocytopenia and Lethal Outcome: A Population Study of Patients with Suspected Sepsis Requiring Intensive Care. Front. Cardiovasc. Med. 2021, 8, 730553. [Google Scholar] [CrossRef]
- Yao, V.; Platell, C.; Hall, J.C. Role of peritoneal mesothelial cells in peritonitis. Br. J. Surg. 2003, 90, 1187–1194. [Google Scholar] [CrossRef]
- Grari, O.; Ezrari, S.; El Yandouzi, I.; Benaissa, E.; Ben Lahlou, Y.; Lahmer, M.; Saddari, A.; Elouennass, M.; Maleb, A. A comprehensive review on biofilm-associated infections: Mechanisms, diagnostic challenges, and innovative therapeutic strategies. Microbe 2025, 8, 100436. [Google Scholar] [CrossRef]
- Simões-Silva, L.; Araujo, R.; Pestana, M.; Soares-Silva, I.; Sampaio-Maia, B. Peritoneal Microbiome in End-Stage Renal Disease Patients and the Impact of Peritoneal Dialysis Therapy. Microorganisms 2020, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, M.; Cui, S.; Wang, L.; Li, Y.; Li, Y.; Jiang, C. Gut microbiome and peritoneal inflammation: A new perspective on peritoneal dialysis-associated peritonitis. Ren. Fail. 2025, 47, 2542532. [Google Scholar] [CrossRef]
- Porcari, S.; Mullish, B.H.; Asnicar, F.; Ng, S.C.; Zhao, L.; Hansen, R.; O’Toole, P.W.; Raes, J.; Hold, G.; Putignani, L.; et al. International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol. Hepatol. 2025, 10, 154–167. [Google Scholar] [CrossRef] [PubMed]
- He, P.; He, L.-J.; Huang, C.; Hu, J.-P.; Sun, S.-R. Neutrophil-to-Lymphocyte Ratio and Treatment Failure in Peritoneal Dialysis-Associated Peritonitis. Front. Med. 2021, 8, 699502. [Google Scholar] [CrossRef]
- Liu, Y. Diagnostic value of NLR, PLR and SIRI in peritoneal dialysis-associated peritonitis. Am. J. Transl. Res. 2025, 17, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-S.; Lin, O.S.; Chen, Y.-Y.; Hwang, K.-L.; Soon, M.-S.; Keeffe, E.B. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J. Hepatol. 2001, 34, 215–221. [Google Scholar] [CrossRef]
- Pawar, R.D.; Shih, J.A.; Balaji, L.; Grossestreuer, A.V.; Patel, P.V.; Hansen, C.K.; Donnino, M.W.; Moskowitz, A. Variation in SOFA (Sequential Organ Failure Assessment) Score Performance in Different Infectious States. J. Intensive Care Med. 2021, 36, 1217–1222. [Google Scholar] [CrossRef]
- Boey, J.; Wong, J.; Ong, G.B. A Prospective Study of Operative Risk Factors in Perforated Duodenal Ulcers. Ann. Surg. 1982, 195, 265–269. [Google Scholar] [CrossRef]
- Møller, M.H.; Engebjerg, M.C.; Adamsen, S.; Bendix, J.; Thomsen, R.W. The Peptic Ulcer Perforation (PULP) score: A predictor of mortality following peptic ulcer perforation. A cohort study. Acta Anaesthesiol. Scand. 2012, 56, 655–662. [Google Scholar] [CrossRef]
- Muralidhar, V.A.; Madhu, C.P.; Sudhir, S.; Srinivasarangan, M. Efficacy of Mannheim Peritonitis Index (MPI) Score in Patients with Secondary Peritonitis. J. Clin. Diagn. Res. 2014, 8, NC01–NC03. [Google Scholar] [CrossRef]
- Ramteke, H.; Deshpande, S.G.; Bhoyar, R. The Role of the Mannheim Peritonitis Index for Predicting Outcomes in Patients with Perforation Peritonitis in a Rural Hospital in India. Cureus 2023, 15, e36620. [Google Scholar] [CrossRef]
- Mokart, D.; Boutaba, M.; Servan, L.; Bertrand, B.; Baldesi, O.; Lefebvre, L.; Gonzalez, F.; Bisbal, M.; Pastene, B.; Duclos, G.; et al. Empirical antifungal therapy for health care-associated intra-abdominal infection: A retrospective, multicentre and comparative study. Ann. Intensive Care 2024, 14, 98. [Google Scholar] [CrossRef]
- Sartelli, M.; Chichom-Mefire, A.; Labricciosa, F.M.; Hardcastle, T.; Abu-Zidan, F.M.; Adesunkanmi, A.K.; Ansaloni, L.; Bala, M.; Balogh, Z.J.; Beltrán, M.A.; et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J. Emerg. Surg. 2017, 12, 29. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K.; Calandra, T.F.; Edwards, J.E.; Filler, S.G.; Fisher, J.F.; Kullberg, B.-J.; Zeichner, L.O.; et al. Clinical Practice Guidelines for the Management Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Mira, J.P.; Gangneux, J.P.; Leroy, O.; Lortholary, O. A multicentre study of antifungal strategies and outcome of Candida spp. peritonitis in intensive-care units. Clin. Microbiol. Infect. 2011, 17, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, K.; Clarke, S.; Moore, L.S.P.; Hughes, S. Bacterial peritonitis in paediatric appendicitis; microbial epidemiology and antimicrobial management. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 45. [Google Scholar] [CrossRef]
- Gupta, S.; Zingade, A.; Baviskar, M.; Vakil, R.B. Efficacy of the Mannheim Peritonitis Index (MPI) in Predicting Postoperative Outcomes in Patients with Perforation Peritonitis. Cureus 2025, 17, e83193. [Google Scholar] [CrossRef]
- Tominaga, G.T.; Staudenmayer, K.L.; Shafi, S.; Schuster, K.M.; Savage, S.A.; Ross, S.; Muskat, P.; Mowery, N.T.; Miller, P.; Inaba, K.; et al. The American Association for the Surgery of Trauma grading scale for 16 emergency general surgery conditions. J. Trauma Acute Care Surg. 2016, 81, 593–602. [Google Scholar] [CrossRef]
- Subedi, R.P.; Kumar, N.; Karn, S.; Arunkumar, V.; Raj, N.; Maheshwari, P.; Singh, D.; Edem, S.; Das, A.; Huda, F.; et al. Prognostic value of the combination of serial APACHE II with serum lactate for predicting post-operative mortality in gastrointestinal perforation peritonitis: A prospective cohort study. BMC Surg. 2025, 25, 374. [Google Scholar] [CrossRef]
- Bauhofer, A.; Huttel, M.; Lorenz, W.; Sessler, D.I.; Torossian, A. Differential effects of antibiotics in combination with G-CSF on survival and polymorphonuclear granulocyte cell functions in septic rats. BMC Infect. Dis. 2008, 8, 55. [Google Scholar] [CrossRef]
- Dumitrascu, C.O.; Gherghe, M.; Costache, M.; Cretu, B.; Cirstoiu, C. The Role of Serum and Peritoneal Biomarkers in Predicting Sepsis and Septic Multiorgan Failure in Patients with Secondary Peritonitis. Cureus 2023, 15, e41724. [Google Scholar] [CrossRef]
- Horiuchi, A. Evaluation of prognostic factors and scoring system in colonic perforation. World J. Gastroenterol. 2007, 13, 3228. [Google Scholar] [CrossRef]
- Souza, L.J.; Coelho, A.M.M.; Sampietre, S.N.; Martins, J.O.; Cunha, J.E.M.; Machado, M.C.C. Anti-Inflammatory Effects of Peritoneal Lavage in Acute Pancreatitis. Pancreas 2010, 39, 1180–1184. [Google Scholar] [CrossRef]
- Hong, X.-X.; Wang, H.-Y.; Yang, J.-M.; Lin, B.-F.; Min, Q.-Q.; Liang, Y.-Z.; Huang, P.-D.; Zhong, Z.-Y.; Guo, S.-J.; Huang, B.; et al. Systemic injury caused by taurocholate-induced severe acute pancreatitis in rats. Exp. Ther. Med. 2022, 24, 468. [Google Scholar] [CrossRef]
- Xiao, Z.; Wilson, C.; Robertson, H.L.; Roberts, D.J.; Ball, C.G.; Jenne, C.N.; Kirkpatrick, A.W. Inflammatory mediators in intra-abdominal sepsis or injury—A scoping review. Crit. Care 2015, 19, 373. [Google Scholar] [CrossRef]
- Silva-Vaz, P.; Abrantes, A.M.; Castelo-Branco, M.; Gouveia, A.; Botelho, M.F.; Tralhão, J.G. Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 2794. [Google Scholar] [CrossRef]
- Tian, H.; Chen, L.; Wu, X.; Li, F.; Ma, Y.; Cai, Y.; Song, S. Infectious Complications in Severe Acute Pancreatitis: Pathogens, Drug Resistance, and Status of Nosocomial Infection in a University-Affiliated Teaching Hospital. Dig. Dis. Sci. 2020, 65, 2079–2088. [Google Scholar] [CrossRef]
- Møller, M.H.; Adamsen, S.; Thomsen, R.W.; Møller, A.M. Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation. Br. J. Surg. 2011, 98, 802–810. [Google Scholar] [CrossRef]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Tabak, Y.; Conwell, D.L.; Banks, P.A. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 2008, 57, 1698–1703. [Google Scholar] [CrossRef]
- Papachristou, G.I.; Muddana, V.; Yadav, D.; O’Connell, M.; Sanders, M.K.; Slivka, A.; Whitcomb, D.C. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI Scores in Predicting Organ Failure, Complications, and Mortality in Acute Pancreatitis. Am. J. Gastroenterol. 2010, 105, 435–441. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chow, K.M.; Cho, Y.; Fan, S.; Figueiredo, A.E.; Harris, T.; Kanjanabuch, T.; Kim, Y.-L.; Madero, M.; Malyszko, J.; et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2022, 42, 110–153. [Google Scholar] [CrossRef]
- Al Sahlawi, M.; Bargman, J.M.; Perl, J. Peritoneal Dialysis–Associated Peritonitis: Suggestions for Management and Mistakes to Avoid. Kidney Med. 2020, 2, 467–475. [Google Scholar] [CrossRef]
- Akiyama, M.; Kamei, K.; Nishi, K.; Kaneda, T.; Inoki, Y.; Osaka, K.; Sato, M.; Ogura, M.; Ito, S. Frequency and prognosis of peritoneal dialysis-associated peritonitis in children. Clin. Exp. Nephrol. 2024, 28, 692–700. [Google Scholar] [CrossRef]
- Kim, Y.; Gu, C.; Kim, H.U.; Lee, S.Y. Current status of pan-genome analysis for pathogenic bacteria. Curr. Opin. Biotechnol. 2020, 63, 54–62. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Li, L.; Hu, M.; Chen, L.; Xu, B.; Song, Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. 2020, 40, 301–312. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Cirocchi, R.; Randolph, J.J.; Binda, G.A.; Gioia, S.; Henry, B.M.; Tomaszewski, K.A.; Allegritti, M.; Arezzo, A.; Marzaioli, R.; Ruscelli, P. Is the outpatient management of acute diverticulitis safe and effective? A systematic review and meta-analysis. Tech. Coloproctology 2019, 23, 87–100. [Google Scholar] [CrossRef]
- Arshad, S.A.; Murphy, P.; Gould, J.C. Management of Perforated Peptic Ulcer. JAMA Surg. 2025, 160, 450. [Google Scholar] [CrossRef]
- Mäkelä, J.T.; Klintrup, K.; Takala, H.; Rautio, T. The role of C-reactive protein in prediction of the severity of acute diverticulitis in an emergency unit. Scand. J. Gastroenterol. 2015, 50, 536–541. [Google Scholar] [CrossRef]
- Palacios Huatuco, R.M.; Pantoja Pachajoa, D.A.; Bruera, N.; Pinsak, A.E.; Llahi, F.; Doniquian, A.M.; Alvarez, F.A.; Parodi, M. Neutrophil-to-lymphocyte ratio as a predictor of complicated acute diverticulitis: A retrospective cohort study. Ann. Med. Surg. 2021, 63, 102128. [Google Scholar] [CrossRef]
- Gaurav, K.; Kumar, K.; Kumar, K.; Kamal, A.K.; Mehta, M.K.; Soy, H.; Bhagat, R. Effectiveness of Mannheim’s Peritonitis Index in Patients with Peritonitis Secondary to Hollow Viscus Perforation in a Tertiary Care Hospital in Jharkhand, India. Cureus 2024, 16, e59631. [Google Scholar] [CrossRef]
- Zhou, H.; Coveney, A.P.; Wu, M.; Huang, J.; Blankson, S.; Zhao, H.; O’Leary, D.P.; Bai, Z.; Li, Y.; Redmond, H.P.; et al. Activation of Both TLR and NOD Signaling Confers Host Innate Immunity-Mediated Protection Against Microbial Infection. Front. Immunol. 2019, 9, 3082. [Google Scholar] [CrossRef]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, J.H.; Chung, D.H. NOD2-mediated Suppression of CD55 on Neutrophils Enhances C5a Generation During Polymicrobial Sepsis. PLoS Pathog. 2013, 9, e1003351. [Google Scholar] [CrossRef]
- Srdić, T.; Đurašević, S.; Lakić, I.; Ružičić, A.; Vujović, P.; Jevđović, T.; Dakić, T.; Đorđević, J.; Tosti, T.; Glumac, S.; et al. From Molecular Mechanisms to Clinical Therapy: Understanding Sepsis-Induced Multiple Organ Dysfunction. Int. J. Mol. Sci. 2024, 25, 7770. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Rimessi, A.; Bezzerri, V.; Patergnani, S.; Marchi, S.; Cabrini, G.; Pinton, P. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 2015, 6, 6201. [Google Scholar] [CrossRef]
- Bezzerri, V.; Borgatti, M.; Finotti, A.; Tamanini, A.; Gambari, R.; Cabrini, G. Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. J. Immunol. 2011, 187, 6069–6081. [Google Scholar] [CrossRef]
- Topley, N.; Mackenzie, R.K.; Williams, J.D. Macrophages and Mesothelial Cells in Bacterial Peritonitis. Immunobiology 1996, 195, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Dunn, D.L.; Barke, R.A.; Knight, N.B.; Humphrey, E.W.; Simmons, R.L. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect. Immun. 1985, 49, 257–264. [Google Scholar] [CrossRef]
- Broche, F.; Tellado, J.M. Defense mechanisms of the peritoneal cavity. Curr. Opin. Crit. Care 2001, 7, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Al-Banna, N.A.; Pavlovic, D.; Gründling, M.; Zhou, J.; Kelly, M.; Whynot, S.; Hung, O.; Johnston, B.; Issekutz, T.B.; Kern, H.; et al. Impact of antibiotics on the microcirculation in local and systemic inflammation. Clin. Hemorheol. Microcirc. 2013, 53, 155–169. [Google Scholar] [CrossRef]
- Lin, H.C.; Wang, C.H.; Liu, C.Y.; Yu, C.T.; Kuo, H.P. Erythromycin inhibits β2-integrins (CD11b/CD18) expression, interleukin-8 release and intracellular oxidative metabolism in neutrophils. Respir. Med. 2000, 94, 654–660. [Google Scholar] [CrossRef]
- Weng, T.-C.; Chen, C.-C.; Toh, H.-S.; Tang, H.-J. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J. Microbiol. Immunol. Infect. 2011, 44, 418–423. [Google Scholar] [CrossRef]
- Chang, C.K.; Llanes, S.; Schumer, W. Effect of Dexamethasone on NF-kB Activation, Tumor Necrosis Factor Formation, and Glucose Dyshomeostasis in Septic Rats. J. Surg. Res. 1997, 72, 141–145. [Google Scholar] [CrossRef]
- Shimazui, T.; Matsumura, Y.; Nakada, T.A.; Oda, S. Serum levels of interleukin-6 may predict organ dysfunction earlier than SOFA score. Acute Med. Surg. 2017, 4, 255–261. [Google Scholar] [CrossRef]
- Varga, N.-I.; Bagiu, I.C.; Vulcanescu, D.D.; Lazureanu, V.; Turaiche, M.; Rosca, O.; Bota, A.V.; Horhat, F.G. IL-6 Baseline Values and Dynamic Changes in Predicting Sepsis Mortality: A Systematic Review and Meta-Analysis. Biomolecules 2025, 15, 407. [Google Scholar] [CrossRef]
- Faes, S.; Hübner, M.; Demartines, N.; Hahnloser, D. Cytokine clearance in serum and peritoneal fluid of patients undergoing damage control surgery with abdominal negative pressure therapy for abdominal sepsis. Pleura Peritoneum 2021, 6, 31–38. [Google Scholar] [CrossRef]
- Fröhlich, D.; Eiber, R.M.; Jochum, M.; Billing, A. Perioperative Pattern of Peritoneal Interleukin 8, Tumour Necrosis Factor-α, and Granulocyte Elastase Release in Human Secondary Peritonitis. Cytokine 1997, 9, 288–292. [Google Scholar] [CrossRef]
- Coldewey, S.M.; Rogazzo, M.; Collino, M.; Patel, N.S.A.; Thiemermann, C. Inhibition of IκB kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Dis. Models Mech. 2013, 6, 1031–1042. [Google Scholar] [CrossRef]
- Rougier, J.-P.; Moullier, P.; Piedagnel, R.; Ronco, P.M.; with The Technical Assistance of Sophie, G. Hyperosmolality suppresses but TGFβ1 increases MMP9 in human peritoneal mesothelial cells. Kidney Int. 1997, 51, 337–347. [Google Scholar] [CrossRef]
- Shi, Q.; Le, X.; Abbruzzese, J.L.; Wang, B.; Mujaida, N.; Matsushima, K.; Huang, S.; Xiong, Q.; Xie, K. Cooperation Between Transcription Factor AP-1 and NF-kappa B in the Induction of Interleukin-8 in Human Pancreatic Adenocarcinoma Cells by Hypoxia. J. Interferon Cytokine Res. 1999, 19, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-C.; Huang, J.-W.; Shyu, R.-S.; Yen, C.-J.; Shiao, C.-H.; Chiang, C.-K.; Hu, R.-H.; Tsai, T.-J. Fibrin-Induced Epithelial-to-Mesenchymal Transition of Peritoneal Mesothelial Cells as a Mechanism of Peritoneal Fibrosis: Effects of Pentoxifylline. PLoS ONE 2012, 7, e44765. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A High Admission Syndecan-1 Level, A Marker of Endothelial Glycocalyx Degradation, Is Associated with Inflammation, Protein C Depletion, Fibrinolysis, and Increased Mortality in Trauma Patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Dixon, J.B. Differential transport function of lymphatic vessels in the rat tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imaging. Front. Physiol. 2013, 4, 215. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, M.S.; Winkler, M.S.; Strunden, M.S.; Izbicki, J.R.; Schoen, G.; Greiwe, G.; Pinnschmidt, H.O.; Poppe, A.; Saugel, B.; Daum, G.; et al. Syndecan-1 as a Biomarker for Sepsis Survival After Major Abdominal Surgery. Biomark. Med. 2018, 12, 119–127. [Google Scholar] [CrossRef]
- Matthaiou, D.K.; Ntani, G.; Kontogiorgi, M.; Poulakou, G.; Armaganidis, A.; Dimopoulos, G. An ESICM systematic review and meta-analysis of procalcitonin-guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med. 2012, 38, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Sakuraya, M.; Sugimoto, H.; Takahashi, N.; Kano, K.-I.; Yoshimura, J.; Egi, M.; Kondo, Y. Benefits and Harms of Procalcitonin- or C-Reactive Protein-Guided Antimicrobial Discontinuation in Critically Ill Adults with Sepsis: A Systematic Review and Network Meta-Analysis. Crit. Care Med. 2024, 52, e522–e534. [Google Scholar] [CrossRef]
- Wejnaruemarn, S.; Susantitaphong, P.; Komolmit, P.; Treeprasertsuk, S.; Thanapirom, K. Procalcitonin and presepsin for detecting bacterial infection and spontaneous bacterial peritonitis in cirrhosis: A systematic review and meta-analysis. World J. Gastroenterol. 2025, 31, 99506. [Google Scholar] [CrossRef]
- Zugel, N. Circulating Mediators and Organ Function in Patients Undergoing Planned Relaparotomy vs Conventional Surgical Therapy in Severe Secondary Peritonitis. Arch. Surg. 2002, 137, 590–599. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, R.; Hijazi, M.M.; Alali, F.; Hamad, A.; Bushra, A.; Mirow, L.; Siepmann, T. Diagnostic Accuracy of MMP-8 and IL-6-Based Point-of-Care Testing to Detect Peritoneal Dialysis-Related Peritonitis: A Single-Center Experience. Diagnostics 2024, 14, 1113. [Google Scholar] [CrossRef]
- Htay, H.; Choo, J.C.J.; Huang, D.H.; Jayaballa, M.; Johnson, D.W.; Koniman, R.; Oei, E.L.; Suai, T.C.; Wu, S.Y.; Foo, M.W.Y. Rapid point-of-care test for diagnosis of peritonitis in peritoneal dialysis patients. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2024, 44, 413–418. [Google Scholar] [CrossRef]
- Milić, L.; Grigorov, I.; Krstić, S.; Ćeranić, M.S.; Jovanović, B.; Stevanović, J.; Peško, P. Serum Level of HMGB1 Protein and Inflammatory Markers in Patients with Secondary Peritonitis: Time Course and the Association with Clinical Status. J. Med. Biochem. 2017, 36, 44–53. [Google Scholar] [CrossRef]
- Paul, V.; Tridente, A.; Kaur, P.; Mahmood, M.; Mellors, R.; Raithatha, A. Critically ill patients with faecal peritonitis: A 5-year review in a tertiary centre. Crit. Care 2015, 19, P374. [Google Scholar] [CrossRef]
- Watanabe, H.; Rana, M.; Son, M.; Chiu, P.Y.; Fei-Bloom, Y.; Choi, K.; Diamond, B.; Sherry, B. Single cell RNA-seq reveals cellular and transcriptional heterogeneity in the splenic CD11b+Ly6Chigh monocyte population expanded in sepsis-surviving mice. Mol. Med. 2024, 30, 202. [Google Scholar] [CrossRef]
- Martino, F.K.; Filippi, I.; Giavarina, D.; Kaushik, M.; Rodighiero, M.P.; Crepaldi, C.; Teixeira, C.; Nadal, A.F.; Rosner, M.H.; Ronco, C. Neutrophil Gelatinase-Associated Lipocalin in the Early Diagnosis of Peritonitis: The Case of Neutrophil Gelatinase-Associated Lipocalin; S. Karger AG: Basel, Switzerland, 2012; pp. 258–263. [Google Scholar]
- Virzi, G.M.; Mattiotti, M.; Milan Manani, S.; Gnappi, M.; Tantillo, I.; Corradi, V.; De Cal, M.; Giuliani, A.; Carta, M.; Giavarina, D.; et al. Neutrophil Gelatinase-Associated Lipocalin in Peritoneal Dialysis-Related Peritonitis: Correlation with White Blood Cells over Time and a Possible Role as the Outcome Predictor. Blood Purif. 2024, 53, 316–324. [Google Scholar] [CrossRef]
- Yang, N.; Yang, K.; Pan, S.; He, Q.; Jin, J. Progress in the application of the neutrophil-to-lymphocyte ratio in dialysis-related complications. Ren. Fail. 2023, 45, 2259996. [Google Scholar] [CrossRef]
- Brook, A.C.; Jenkins, R.H.; Clayton, A.; Kift-Morgan, A.; Raby, A.-C.; Shephard, A.P.; Mariotti, B.; Cuff, S.M.; Bazzoni, F.; Bowen, T.; et al. Neutrophil-derived miR-223 as local biomarker of bacterial peritonitis. Sci. Rep. 2019, 9, 10136. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Fang, J.-Y.; Song, A.H.; Deng, H.; Li, P.; Huang, Z.-H.; Ji, O.-Y.; Ge, X.-L.; Zhu, T.-Y.; Liu, Y.-L. Peritoneal dialysis effluent-derived exosomal miR-432-5p: An assessment tool for peritoneal dialysis efficacy. Ann. Transl. Med. 2022, 10, 242. [Google Scholar] [CrossRef]
- Pupelis, G.; Drozdova, N.; Mukans, M.; Malbrain, M.L. Serum procalcitonin is a sensitive marker for septic shock and mortality in secondary peritonitis. Anestezjol. Intensywna Ter. 2014, 46, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Maseda, E.; Suarez-De-La-Rica, A.; Anillo, V.; Tamayo, E.; García-Bernedo, C.A.; Ramasco, F.; Villagran, M.-J.; Maggi, G.; Gimenez, M.-J.; Aguilar, L.; et al. Procalcitonin-guided therapy may reduce length of antibiotic treatment in intensive care unit patients with secondary peritonitis: A multicenter retrospective study. J. Crit. Care 2015, 30, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Van Ruler, O.; Lamme, B.; De Vos, R.; Obertop, H.; Reitsma, J.B.; Boermeester, M.A. Decision Making for Relaparotomy in Secondary Peritonitis. Dig. Surg. 2008, 25, 339–346. [Google Scholar] [CrossRef]
- Van Ruler, O.; Mahler, C.W.; Boer, K.R.; Reuland, E.A.; Gooszen, H.G.; Opmeer, B.C.; De Graaf, P.W.; Lamme, B.; Gerhards, M.F.; Steller, E.P.; et al. Comparison of On-Demand vs Planned Relaparotomy Strategy in Patients with Severe Peritonitis. JAMA 2007, 298, 865. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef]
- Saadh, M.J.; Saeed, T.N.; Alfarttoosi, K.H.; Sanghvi, G.; Roopashree, R.; Thakur, V.; Lakshmi, L.; Kubaev, A.; Taher, W.M.; Alwan, M.; et al. Exosomes and MicroRNAs: Key modulators of macrophage polarization in sepsis pathophysiology. Eur. J. Med. Res. 2025, 30, 298. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Roberts, D.J.; Faris, P.D.; Ball, C.G.; Kubes, P.; Tiruta, C.; Xiao, Z.; Holodinsky, J.K.; McBeth, P.B.; Doig, C.J.; et al. Active Negative Pressure Peritoneal Therapy After Abbreviated Laparotomy. Ann. Surg. 2015, 262, 38–46. [Google Scholar] [CrossRef]
- Sartelli, M.; Barie, P.; Agnoletti, V.; Al-Hasan, M.N.; Ansaloni, L.; Biffl, W.; Buonomo, L.; Blot, S.; Cheadle, W.G.; Coimbra, R.; et al. Intra-abdominal infections survival guide: A position statement by the Global Alliance for Infections in Surgery. World J. Emerg. Surg. 2024, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Tascini, C.; Coccolini, F.; Dellai, F.; Ansaloni, L.; Antonelli, M.; Bartoletti, M.; Bassetti, M.; Boncagni, F.; Carlini, M.; et al. Management of intra-abdominal infections: Recommendations by the Italian council for the optimization of antimicrobial use. World J. Emerg. Surg. 2024, 19, 23. [Google Scholar] [CrossRef]
- Ozen, C.; Karasoy, D.; Yalcinkaya, A.; Pedersen, S.H.; Fagerberg, S.K.; Hindersson, P.; Leutscher, P.D.C.; Holte, K. Evaluation of procalcitonin versus conventional inflammatory biomarkers for clinical severity grading in patients with intra-abdominal infection. Langenbeck’s Arch. Surg. 2025, 410, 93. [Google Scholar] [CrossRef] [PubMed]
- Vibhash, C.; Choudhury, S.R.; Maheshwari, A.; Sarin, Y.K.; Sharma, S.; Singh, R. Profile of Serum Inflammatory Biomarkers in Children with Peritonitis and their Role in Predicting the Severity and Outcome. J. Indian Assoc. Pediatr. Surg. 2025, 30, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.A.; Chow, A.W.; Edwards, M.S.; Humphries, R.; Tamma, P.D.; Abrahamian, F.M.; Bessesen, M.; Dellinger, E.P.; Goldstein, E.; Hayden, M.K.; et al. 2024 Clinical Practice Guideline Update by the Infectious Diseases Society of America on Complicated Intra-abdominal Infections: Risk Assessment, Diagnostic Imaging, and Microbiological Evaluation in Adults, Children, and Pregnant People. Clin. Infect. Dis. 2024, 79, S81–S87. [Google Scholar] [CrossRef]
- Dark, P.; Hossain, A.; McAuley, D.F.; Brealey, D.; Carlson, G.; Clayton, J.C.; Felton, T.W.; Ghuman, B.K.; Gordon, A.C.; Hellyer, T.P.; et al. Biomarker-Guided Antibiotic Duration for Hospitalized Patients with Suspected Sepsis. JAMA 2025, 333, 682. [Google Scholar] [CrossRef]
- Gupta, S.; Klompas, M.; Rhee, C. Reassessing Procalcitonin-Guided Antibiotic Therapy in Critically Ill Patients with Sepsis: Lessons From the ADAPT-Sepsis Trial. Clin. Infect. Dis. 2025, ciaf336. [Google Scholar] [CrossRef]
- Ozdemir, Y.E.; Ensaroglu, E.; Akkaya, S.; Cizmeci, Z.; Kart Yasar, K. Clinical Outcomes and Microbiological Profiles of Patients with Culture-Confirmed Peritonitis. Infect. Dis. Clin. Microbiol. 2025, 7, 88–96. [Google Scholar] [CrossRef]
- Colombo, G.; Aloisio, E.; Panteghini, M. Laboratory investigation of peritoneal fluids: An updated practical approach based on the available evidence. J. Clin. Pathol. 2024, 77, 579–585. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Tsai, I.T.; Lai, C.-H.; Chen, C.-P.; Chen, C.C.; Hsu, Y.-C. Time to positivity of Klebsiella pneumoniae in blood cultures as prognostic marker in patients with intra-abdominal infection: A retrospective study. Virulence 2024, 15, 2329397. [Google Scholar] [CrossRef]
- Karjagin, J.; Lefeuvre, S.; Oselin, K.; Kipper, K.; Marchand, S.; Tikkerberi, A.; Starkopf, J.; Couet, W.; Sawchuk, R. Pharmacokinetics of Meropenem Determined by Microdialysis in the Peritoneal Fluid of Patients with Severe Peritonitis Associated with Septic Shock. Clin. Pharmacol. Ther. 2008, 83, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Milan Manani, S.; Virzì, G.M.; Marcello, M.; Zanella, M. Neutrophil gelatinase-associated lipocalin dipstick test in peritoneal dialysis patients with peritonitis. Clin. Kidney J. 2022, 15, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef]
- Kaur, N.; Gupta, M.K.; Minocha, V.R. Early Enteral Feeding by Nasoenteric Tubes in Patients with Perforation Peritonitis. World J. Surg. 2005, 29, 1023–1027. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef]
- Joshi, N.; Kopec, A.K.; Towery, K.; Williams, K.J.; Luyendyk, J.P. The Antifibrinolytic Drug Tranexamic Acid Reduces Liver Injury and Fibrosis in a Mouse Model of Chronic Bile Duct Injury. J. Pharmacol. Exp. Ther. 2014, 349, 383–392. [Google Scholar] [CrossRef]
- De Vitis, E.; La Pesa, V.; Gervaso, F.; Romano, A.; Quattrini, A.; Gigli, G.; Moroni, L.; Polini, A. A microfabricated multi-compartment device for neuron and Schwann cell differentiation. Sci. Rep. 2021, 11, 7019. [Google Scholar] [CrossRef]
- Polini, A.; Del Mercato, L.L.; Barra, A.; Zhang, Y.S.; Calabi, F.; Gigli, G. Towards the development of human immune-system-on-a-chip platforms. Drug Discov. Today 2019, 24, 517–525. [Google Scholar] [CrossRef]
- Ibrahim, L.I.; Hajal, C.; Offeddu, G.S.; Gillrie, M.R.; Kamm, R.D. Omentum-on-a-chip: A multicellular, vascularized microfluidic model of the human peritoneum for the study of ovarian cancer metastases. Biomaterials 2022, 288, 121728. [Google Scholar] [CrossRef]
- Holl, M.; Becker, L.; Keller, A.-L.; Feuerer, N.; Marzi, J.; Carvajal Berrio, D.A.; Jakubowski, P.; Neis, F.; Pauluschke-Fröhlich, J.; Brucker, S.Y.; et al. Laparoscopic Peritoneal Wash Cytology-Derived Primary Human Mesothelial Cells for In Vitro Cell Culture and Simulation of Human Peritoneum. Biomedicines 2021, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Burnham, P.; Chen, F.; Cheng, A.P.; Srivatana, V.; Zhang, L.T.; Edusei, E.; Albakry, S.; Botticelli, B.; Guo, X.; Renaghan, A.; et al. Peritoneal Effluent Cell-Free DNA Sequencing in Peritoneal Dialysis Patients with and Without Peritonitis. Kidney Med. 2022, 4, 100383. [Google Scholar] [CrossRef]
- Ye, P.; Xie, C.; Wu, C.; Yu, C.; Chen, Y.; Liang, Z.; Chen, Y.; Chen, Q.; Kong, Y. The application of metagenomic next-generation sequencing for detection of pathogens from dialysis effluent in peritoneal dialysis–associated peritonitis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2022, 42, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, Y.; Xia, Y.; Shao, Q.; Zhao, M.; Xu, P.; Tang, T.; Liu, J.; Jin, B.; Liu, S.; et al. Clinical efficacy of metagenomic next-generation sequencing for the detection of pathogens in peritoneal dialysis-related peritonitis: A prospective cohort study. Eur. J. Med. Res. 2025, 30, 198. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Virgin, H.W. Kingdom-Agnostic Metagenomics and the Importance of Complete Characterization of Enteric Microbial Communities. Gastroenterology 2014, 146, 1459–1469. [Google Scholar] [CrossRef]
- Marzano, V.; Mancinelli, L.; Bracaglia, G.; Del Chierico, F.; Vernocchi, P.; Di Girolamo, F.; Garrone, S.; Tchidjou Kuekou, H.; D’Argenio, P.; Dallapiccola, B.; et al. “Omic” investigations of protozoa and worms for a deeper understanding of the human gut “parasitome”. PLoS Negl. Trop. Dis. 2017, 11, e0005916. [Google Scholar] [CrossRef] [PubMed]
- Zwicky, S.N.; Mordasini, L.; Spari, D.; Yilmaz, B.; Beldi, G. Bacterial translocation to mesenteric lymph nodes fueling surgical site infections: Evidence, technical challenges and future directions. J. Transl. Med. 2025, 23, 866. [Google Scholar] [CrossRef]
- Putignani, L.; Gasbarrini, A.; Dallapiccola, B. Potential of multiomics technology in precision medicine. Curr. Opin. Gastroenterol. 2019, 35, 491–498. [Google Scholar] [CrossRef]
- Putignani, L.; Dallapiccola, B. Foodomics as part of the host-microbiota-exposome interplay. J. Proteom. 2016, 147, 3–20. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Sun, Y.; Zhou, W. Transcriptomics Changes in the Peritoneum of Mice with Lipopolysaccharide-Induced Peritonitis. Int. J. Mol. Sci. 2021, 22, 13008. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, G.; Dong, W.; He, P.; Liu, W.; Wu, Y.; Liang, H.; Wen, F.; Yu, F.; Yin, Y.; et al. Single-cell sequencing reveals peritoneal environment and insights into fibrosis in CAPD patients. iScience 2023, 26, 106336. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Moroni, L. The convergence of high-tech emerging technologies into the next stage of organ-on-a-chips. Biomater. Biosyst. 2021, 1, 100012. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Xu, Z.; Liu, Y.; Ren, H.; Li, Z.; Chen, C.; Chen, K.; Wu, X.; Ren, J. Melt electrowriting-printed peritoneal scaffold prevents peritoneal adhesion and facilitates peritoneal repair. Int. J. Bioprinting 2023, 9, 682. [Google Scholar] [CrossRef]
| Feature | Fibrinous | Purulent | Stercoraceous | Pancreatitis- Associated | Catheter-Related in Peritoneal Dialysis |
|---|---|---|---|---|---|
| Primary contaminant | Gastric acid, acidic chyme, digestive enzymes | Bile, small bowel content, limited fecal, urine | Gross fecal content | Pancreatic enzymes | Biofilm bacteria |
| Microbe type | Enteric polymicrobial communities | Anaerobes: Bacteroides fragilis, Clostridium perfringens, Fusobacterium spp.; aerobes: Escherichia coli, Klebsiella spp., Enterobacter spp., Pseudomonas aeruginosa | Facultative anaerobes: Enterobacteriaceae; anaerobes: Bacteroides fragilis, Clostridium spp., Fusobacterium spp.; Prevotella spp. | Initially absent followed by presence of opportunistic bacteria: Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli | Staphylococcus epidermidis, Pseudomonas aeruginosa, Streptococcus spp. |
| Inflammatory condition | Variable | Localized, Mild Systemic | Diffuse, Mild-to-Severe Systemic | Protease-driven, Localized | Chronic, low-grade |
| Key biomarkers | PCT, IL-6 | IL-6, TGF-β, CRP | IL-6, TNF-α, PCT, HMGB1 | IL-6, CRP, serum amylase | NGAL, effluent lactate |
| Surgical approach | Laparoscopic repair, lavage | Limited repair/lavage | Resection, possible open abdomen | Drainage ± necrosectomy | Catheter salvage vs. removal |
| Prognosis | Variable, timing critical | Variable, generally favorable | High mortality | Moderate, worsens if infected | Technique failure common |
| Biomarker | Source | Diagnostic Utility | Prognostic Utility | 2025 Update |
|---|---|---|---|---|
| PCT | Serum | PCT for SBP diagnosis in cirrhosis shows 76% sensitivity and 87% specificity [83] | PCT-guided antibiotic discontinuation reduces antibiotic duration by 1.9 days without increasing mortality or relapse risk [84] | Meta-analysis of 5 RCTs shows 15% reduction in antibiotic days without mortality increase [85]. |
| IL-6 | Serum, peritoneal fluid | Early rise (<6 h) correlates with contamination load [86] | Dialysate IL-6 as a predictor of peritonitis in patients on peritoneal dialysis | IL-6 as biomarker of PDAP severity [87,88] |
| HMGB1 | Serum | Reflects cellular necrosis; higher in stercoraceous cases [89,90] | Associated with ICU length of stay [40] | scRNA-seq reveals HMGB1+ macrophage subset [91] |
| NGAL | Peritoneal fluid | Detects PD-associated infection earlier than culture [92] | In PD distinguishes cases of peritonitis from non-infected cases with high sensitivity and specificity, preceding microbiological confirmation [93] | Concentrations >250 ng/mL double the risk of treatment failure in peritonitis-associated catheter removal [93] |
| NLR | Peripheral Blood | Simple, low-cost severity marker [94] | NLR shows an incremental relationship with the risk of treatment failure (OR 1.82) [19] | Use of NLR, PLR, and SIRI levels for enhancing PDAP diagnostic accuracy [19,20,94] |
| miR-233 | Peritoneal fluid | Severity marker in PD | Predicts catheter loss | Correlates with NGAL levels [95] |
| miR-432-5p | Peritoneal fluid exosomes | biomarker of impaired fluid and sodium removal in PD | Predictive of disease severity | miR-432-5p inhibits α-ENaC expression in mesothelial cells [96]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezzerri, V.; Putignani, L.; Mantuano, E.; Polini, A.; Navarini, L.; Vomero, M.; Corberi, E.; Miacci, V.; Papuc, P.E.; Schiavone, V.; et al. A Focus on Inflammatory and Bacterial Biomarkers in Secondary Peritonitis. Cells 2025, 14, 1653. https://doi.org/10.3390/cells14211653
Bezzerri V, Putignani L, Mantuano E, Polini A, Navarini L, Vomero M, Corberi E, Miacci V, Papuc PE, Schiavone V, et al. A Focus on Inflammatory and Bacterial Biomarkers in Secondary Peritonitis. Cells. 2025; 14(21):1653. https://doi.org/10.3390/cells14211653
Chicago/Turabian StyleBezzerri, Valentino, Lorenza Putignani, Elisabetta Mantuano, Alessandro Polini, Luca Navarini, Marta Vomero, Erika Corberi, Valentina Miacci, Paula Elena Papuc, Vincenzo Schiavone, and et al. 2025. "A Focus on Inflammatory and Bacterial Biomarkers in Secondary Peritonitis" Cells 14, no. 21: 1653. https://doi.org/10.3390/cells14211653
APA StyleBezzerri, V., Putignani, L., Mantuano, E., Polini, A., Navarini, L., Vomero, M., Corberi, E., Miacci, V., Papuc, P. E., Schiavone, V., & Costa, G. (2025). A Focus on Inflammatory and Bacterial Biomarkers in Secondary Peritonitis. Cells, 14(21), 1653. https://doi.org/10.3390/cells14211653










