Rodent Models of Glaucoma: How Mice and Rats Can Help Human Vision Move Out of the Woods and Into the Light
Abstract
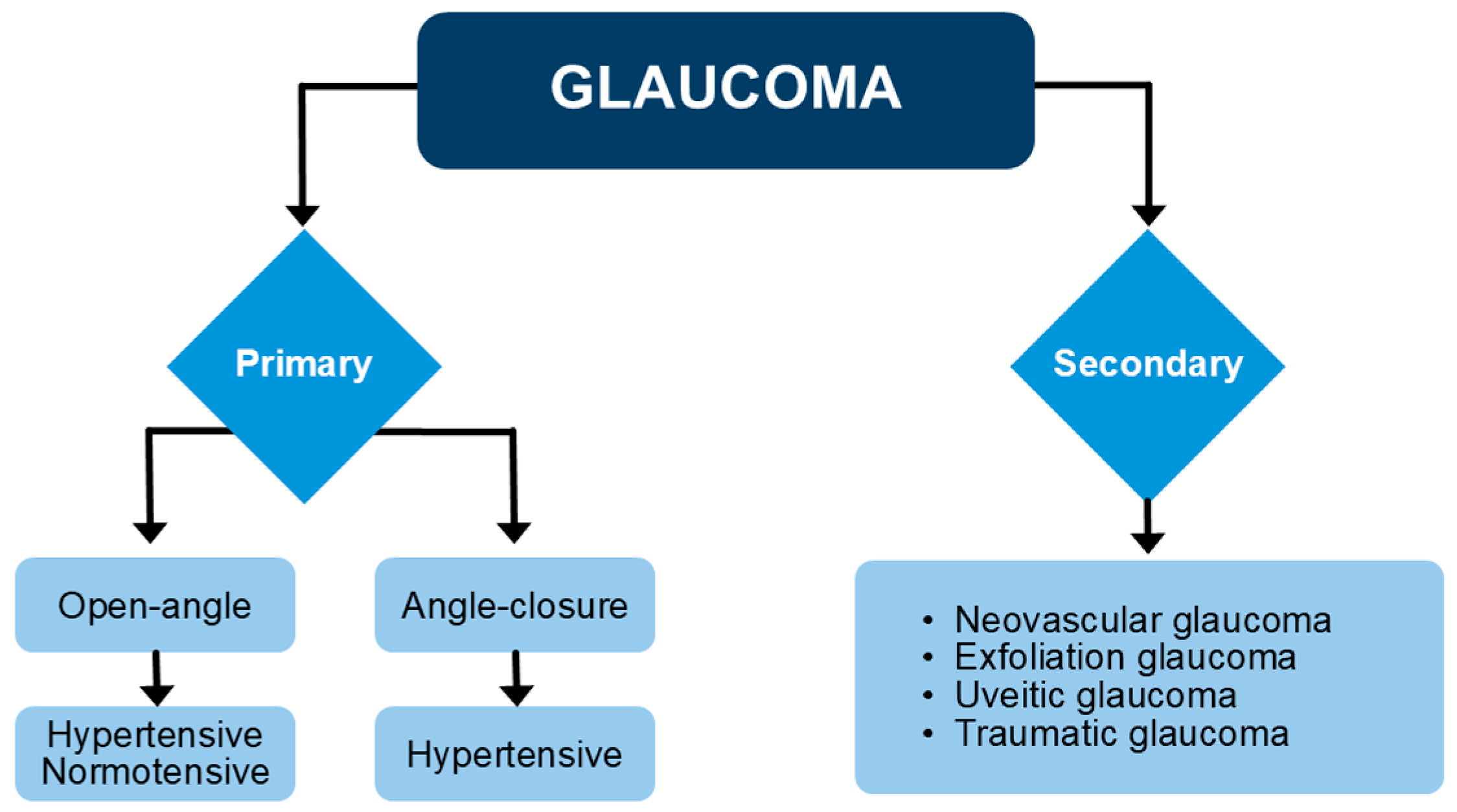
1. Introduction
2. Methods
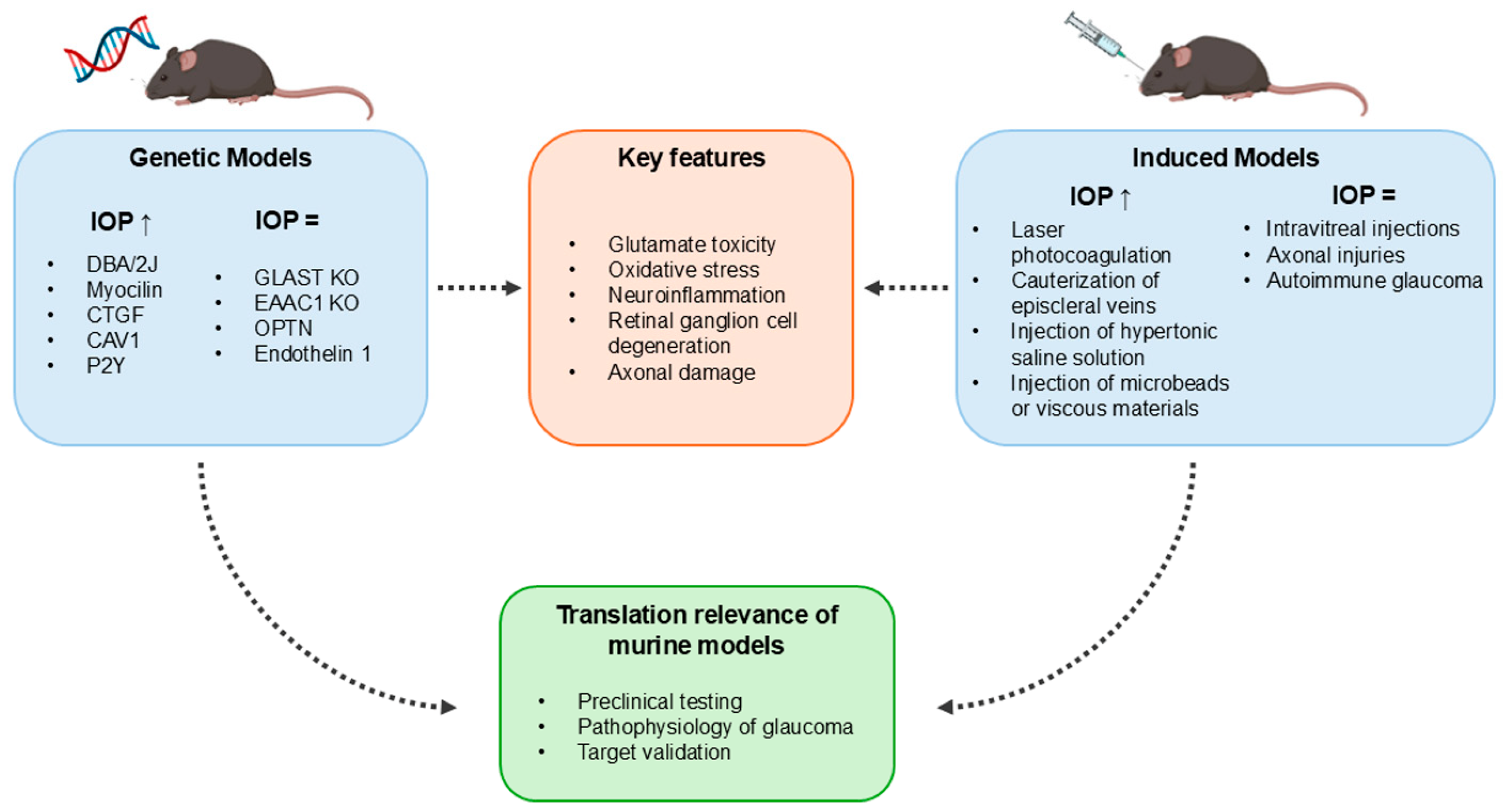
3. Genetic Models of Glaucoma
3.1. DBA/2J
3.2. Glutamate/Aspartate Transporter/Excitatory Amino Acid Carrier 1
3.3. Myocilin
3.4. Connective Tissue Growth Factor
3.5. Optineurin
3.6. Purinergic 2Y Receptors
3.7. Caveolin 1
3.8. Endothelin 1
4. Models of Induced Glaucoma
4.1. Laser Photocoagulation
4.2. Episcleral Vein Cauterization
4.3. Injection of Hypertonic Saline Solutions into Episcleral Veins
4.4. Injection of Microbeads or Viscous Materials into the Anterior Chamber
4.5. Intravitreal Injections of Excitotoxic Substances
4.6. Axonal Injuries
4.7. Other Experimental Models
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RGCs | Retinal ganglion cells; |
| NFL | Nerve fiber layer; |
| IOP | Intraocular pressure; |
| TM | Trabecular meshwork; |
| NTG | Normal tension glaucoma; |
| POAG | Primary open-angle glaucoma; |
| PACG | Primary angle-closure glaucoma; |
| GLAST | Glutamate/aspartate transporter; |
| EAAC1 | Excitatory amino acid carrier 1; |
| GSH | Glutathione; |
| KO | Knockout; |
| ECM | Extracellular matrix; |
| TGF-β | Transforming growth factor β; |
| CTGF | Connective tissue growth factor; |
| α-SMA | α smooth muscle actin; |
| GFAP | Glial fibrillary acidic protein; |
| OPL | Outer plexiform layer; |
| ONL | Outer nuclear layer; |
| P2Y | Purinergic 2Y receptors; |
| ATP | Adenosine triphosphate; |
| Cav1 | Caveolin 1; |
| ET-1 | Endothelin 1; |
| INL | Inner nuclear layer; |
| EVC | Episcleral vein cauterization; |
| NMDA | N-methyl-D-aspartate; |
| I/R | Ischemia/reperfusion; |
| GWAS | Genome-wide association studies. |
References
- Wang, X.; Sun, L.; Han, X.; Li, Z.; Xing, Y.; Chen, X.; Xi, R.; Sun, Y.; Wang, G.; Zhao, P. The molecular mechanisms underlying retinal ganglion cell apoptosis and optic nerve regeneration in glaucoma (Review). Int. J. Mol. Med. 2025, 55, 63. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Stuart, K.V.; de Vries, V.A.; Schuster, A.K.; Yu, Y.; van der Heide, F.C.T.; Delcourt, C.; Cougnard-Grégoire, A.; Schweitzer, C.; Brandl, C.; Zimmermann, M.A.; et al. Prevalence of Glaucoma in Europe and Projections to 2050: Findings from the European Eye Epidemiology Consortium. Ophthalmology 2025, 132, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Bhardwaj, A.; Yadav, A.; Dada, R.; Tanwar, M. Molecular genetics of primary open-angle glaucoma. Indian J. Ophthalmol. 2023, 71, 1739–1756. [Google Scholar] [CrossRef]
- Asrani, S.G.; McGlumphy, E.J.; Al-Aswad, L.A.; Chaya, C.J.; Lin, S.; Musch, D.C.; Pitha, I.; Robin, A.L.; Wirostko, B.; Johnson, T.V. The relationship between intraocular pressure and glaucoma: An evolving concept. Prog. Retin. Eye Res. 2024, 103, 101303. [Google Scholar] [CrossRef]
- Mueller, A.; Lam, I.; Kishor, K.; Lee, R.K.; Bhattacharya, S. Secondary glaucoma: Toward interventions based on molecular underpinnings. WIREs Mech. Dis. 2024, 16, e1628. [Google Scholar] [CrossRef]
- Gutiérrez Martín, L.C. Update on the diagnosis and treatment of normotensive glaucoma. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2023, 98, 344–350. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Zhang, Y.; Huang, S.; Zhong, Y. Differences and Similarities Between Primary Open Angle Glaucoma and Primary Angle-Closure Glaucoma. Eye Brain 2024, 16, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef] [PubMed]
- Casson, R.J.; Chidlow, G.; Wood, J.P.M.; Crowston, J.G.; Goldberg, I. Definition of glaucoma: Clinical and experimental concepts. Clin. Exp. Ophthalmol. 2012, 40, 341–349. [Google Scholar] [CrossRef]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental Models of Glaucoma: A Powerful Translational Tool for the Future Development of New Therapies for Glaucoma in Humans-A Review of the Literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef]
- John, S.W.; Smith, R.S.; Savinova, O.V.; Hawes, N.L.; Chang, B.; Turnbull, T.; Davisson, M.; Roderick, T.H.; Heckenlively, J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Opthalmol. Vis. Sci. 1998, 39, 951–962. [Google Scholar]
- Chang, B.; Smith, R.S.; Hawes, N.L.; Anderson, M.G.; Zabaleta, A.; Savinova, O.; Roderick, T.H.; Heckenlively, J.R.; Davisson, M.T.; John, J.W. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat. Genet. 1999, 21, 405–409. [Google Scholar] [CrossRef]
- Libby, R.T.; Anderson, M.G.; Pang, I.H.; Robinson, Z.H.; Savinova, O.V.; Cosma, I.M.; Snow, A.; Wilson, L.A.; Smith, R.S.; Clark, A.F.; et al. Inherited glaucoma in DBA/2J mice: Pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 2005, 22, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; va der Merwe, Y.; Sims, J.; Parra, C.; Ho, L.C.; Schuman, J.S.; Wollstein, G.; Lathrop, K.L.; Chan, K.C. Age-related Changes in Eye, Brain and Visuomotor Behavior in the DBA/2J Mouse Model of Chronic Glaucoma. Sci. Rep. 2018, 8, 4643. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Y.; Ten Hulzen, R.D.; Cameron, J.D.; Hodge, D.O.; Johnson, D.H. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am. J. Ophthalmol. 2003, 135, 794–799. [Google Scholar] [CrossRef]
- Anderson, M.G.; Libby, R.T.; Mao, M.; Cosma, I.M.; Wilson, L.A.; Smith, R.S.; John, S.W.M. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006, 4, 20. [Google Scholar] [CrossRef]
- Schlamp, C.L.; Li, Y.; Dietz, J.A.; Janssen, K.T.; Nickells, R.W. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006, 7, 66. [Google Scholar] [CrossRef]
- Ju, W.K.; Perkins, G.A.; Kim, K.Y.; Bastola, T.; Choi, W.Y.; Choi, S.H. Glaucomatous optic neuropathy: Mitochondrial dynamics, dysfunction and protection in retinal ganglion cells. Prog. Retin. Eye Res. 2023, 95, 101136. [Google Scholar] [CrossRef]
- Seitz, R.; Ohlmann, A.; Tamm, E.R. The role of Müller glia and microglia in glaucoma. Cell Tissue Res. 2013, 353, 339–345. [Google Scholar] [CrossRef]
- Chong, R.S.; Martin, K.R. Glial cell interactions and glaucoma. Curr. Opin. Ophthalmol. 2015, 26, 73–77. [Google Scholar] [CrossRef]
- Lye-Barthel, M.; Sun, D.; Jakobs, T.C. Morphology of astrocytes in a glaucomatous optic nerve. Investig. Opthalmol. Vis. Sci. 2013, 54, 909–917. [Google Scholar] [CrossRef]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Investig. 2011, 121, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.V.; Soto, I.; Kim, K.Y.; Bushong, E.A.; Oglesby, E.; Valiente-Soriano, F.J.; Yang, Z.; Davis, C.O.; Bedont, J.L.; Son, J.L.; et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. USA 2011, 108, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; MacNicoll, K.H.; Braine, C.E.; Soto, I.; Macalinao, D.G.; Sousa, G.L.; John, S.W.M. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol. Dis. 2014, 71, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Inman, D.M.; Horner, P.J. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia 2007, 55, 942–953. [Google Scholar] [CrossRef]
- Bosco, A.; Inman, D.M.; Steele, M.R.; Wu, G.; Soto, I.; Marsh-Armstrong, N.; Hubbard, W.C.; Clakins, D.J.; Horner, P.J.; Vetter, M.L. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Investig. Opthalmol. Vis. Sci. 2008, 49, 1437–1446. [Google Scholar] [CrossRef]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Models Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef]
- Amato, R.; Cammalleri, M.; Melecchi, A.; Bagnoli, P.; Porciatti, V. Natural History of Glaucoma Progression in the DBA/2J Model: Early Contribution of Müller Cell Gliosis. Cells 2023, 12, 1272. [Google Scholar] [CrossRef]
- Amato, R.; Lazzara, F.; Chou, T.H.; Romano, G.L.; Cammalleri, M.; Dal Monte, M.; Casini, G.; Porciatti, V. Diabetes Exacerbates the Intraocular Pressure-Independent Retinal Ganglion Cells Degeneration in the DBA/2J Model of Glaucoma. Investig Opthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef]
- Ward, M.S.; Khoobehi, A.; Lavik, E.B.; Langer, R.; Young, M.J. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J. Pharm. Sci. 2007, 96, 558–568. [Google Scholar] [CrossRef]
- Zhong, L.; Bradley, J.; Schubert, W.; Ahmed, E.; Adamis, A.P.; Shima, D.T.; Robinson, G.S.; Ng, Y.S. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Investig. Opthalmol. Vis. Sci. 2007, 48, 1212–1218. [Google Scholar] [CrossRef]
- Zhou, X.; Li, F.; Ge, J.; Sarkisian Jr, S.R.; Tomita, H.; Zaharia, A.; Chodosh, J.; Cao, W. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev. Neurobiol. 2007, 67, 603–616. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Cardozo, B.H.; Foxworth, N.E.; John, S.W.M. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun. Integr. Biol. 2018, 11, e1356956. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Rossino, M.G.; Cammalleri, M.; Timperio, A.M.; Fanelli, G.; Dal Monte, M.; Pucci, L.; Casini, G. The Potential of Lisosan G as a Possible Treatment for Glaucoma. Front. Pharmacol. 2021, 12, 719951. [Google Scholar] [CrossRef]
- Turner, A.J.; Vander Wall, R.; Gupta, V.; Klistorner, A.; Graham, S.L. DBA/2J mouse model for experimental glaucoma: Pitfalls and problems. Clin. Exp. Ophthalmol. 2017, 45, 911–922. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Y. Characterization of intraocular pressure pattern and changes of retinal ganglion cells in DBA2J glaucoma mice. Int. J. Ophthalmol. 2016, 9, 211–217. [Google Scholar] [CrossRef]
- Harada, T.; Harada, C.; Nakamura, K.; Quah, H.M.A.; Okumura, A.; Namekata, K.; Saeki, T.; Aihara, M.; Yoshida, H.; Mitani, A.; et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Investig. 2007, 117, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.; Namekata, K.; Guo, X.; Yoshida, H.; Mitamura, Y.; Matsumoto, Y.; Tanaka, K.; Ichijo, H.; Harada, T. ASK1 deficiency attenuates neural cell death in GLAST-deficient mice, a model of normal tension glaucoma. Cell Death Differ. 2010, 17, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Harada, C.; Watanabe, M.; Inoue, Y.; Sakagawa, T.; Nakayama, N.; Sasaki, S.; Okuyama, S.; Watase, K.; Wada, K.; et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc. Natl. Acad. Sci. USA 1998, 95, 4663–4666. [Google Scholar] [CrossRef]
- Reichelt, W.; Stabel-Burow, J.; Pannicke, T.; Weichert, H.; Heinemann, U. The glutathione level of retinal Müller glial cells is dependent on the high-affinity sodium-dependent uptake of glutamate. Neuroscience 1997, 77, 1213–1224. [Google Scholar] [CrossRef]
- Gherghel, D.; Griffiths, H.R.; Hilton, E.J.; Cunliffe, I.A.; Hosking, S.L. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Investig. Opthalmol. Vis. Sci. 2005, 46, 877–883. [Google Scholar] [CrossRef]
- Gherghel, D.; Mroczkowska, S.; Qin, L. Reduction in blood glutathione levels occurs similarly in patients with primary-open angle or normal tension glaucoma. Investig. Opthalmol. Vis. Sci. 2013, 54, 3333–3339. [Google Scholar] [CrossRef]
- Kimura, A.; Guo, X.; Noro, T.; Harada, C.; Tanaka, K.; Namekata, K.; Harada, T. Valproic acid prevents retinal degeneration in a murine model of normal tension glaucoma. Neurosci. Lett. 2015, 588, 108–113. [Google Scholar] [CrossRef]
- Namekata, K.; Harada, C.; Guo, X.; Kikushima, K.; Kimura, A.; Fuse, N.; Mitamura, Y.; Kohyama, K.; Matsumoto, Y.; Tanaka, K.; et al. Interleukin-1 attenuates normal tension glaucoma-like retinal degeneration in EAAC1-deficient mice. Neurosci. Lett. 2009, 465, 160–164. [Google Scholar] [CrossRef]
- Okumichi, H.; Kanamoto, T.; Souchelnytskyi, N.; Tanimoto, S.; Tanaka, K.; Kiuchi, Y. Proteomic analyses of retina of excitatory amino acid carrier 1 deficient mice. Proteome Sci. 2007, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Semba, K.; Namekata, K.; Guo, X.; Harada, C.; Harada, T.; Mitamura, Y. Renin-angiotensin system regulates neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014, 5, e1333. [Google Scholar] [CrossRef]
- Dreyer, E.B.; Zurakowski, D.; Schumer, R.A.; Podos, S.M.; Lipton, S.A. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch. Ophthalmol. 1996, 114, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.A.; Baruah, S.; Zimmerman, M.B.; Khanna, C.L.; Weaver, Y.K.; Narkiewicz, J.; Waziri, R.; Gehrs, K.M.; Weigeinst, T.A.; Boldt, H.C.; et al. Vitreous amino acid concentrations in patients with glaucoma undergoing vitrectomy. Arch. Ophthalmol. 2003, 121, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Naskar, R.; Vorwerk, C.K.; Dreyer, E.B. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Investig. Opthalmol. Vis. Sci. 2000, 41, 1940–1944. [Google Scholar]
- Osborne, N.N. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009, 87, 450–454. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Namekata, K.; Aida, T.; Katou, S.; Takeda, T.; Harada, T.; Fuse, N. The Glaucoma Gene Research Group, Tanaka, K. EAAT1 variants associated with glaucoma. Biochem. Biophys. Res. Commun. 2020, 529, 943–949. [Google Scholar] [CrossRef]
- Dong, Z.; Shinmei, Y.; Dong, Y.; Inafuku, S.; Fukuhara, J.; Ando, R.; Kitaichi, N.; Kanda, A.; Tanaka, K.; Noda, K.; et al. Effect of geranylgeranylacetone on the protection of retinal ganglion cells in a mouse model of normal tension glaucoma. Heliyon 2016, 2, e00191. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Dong, Z.; Shinmei, Y.; Murata, M.; Kanda, A.; Noda, K.; Harada, T.; Ishida, S. Cytoprotective Effect of Astaxanthin in a Model of Normal Intraocular Pressure Glaucoma. J. Ophthalmol. 2020, 2020, 9539681. [Google Scholar] [CrossRef]
- Semba, K.; Namekata, K.; Kimura, A.; Harada, C.; Mitamura, Y.; Harada, T. Brimonidine prevents neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014, 5, e1341. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Namekata, K.; Azuchi, Y.; Kimura, A.; Guo, X.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine Ameliorates Neurodegeneration in a Mouse Model of Normal Tension Glaucoma. Investig. Opthalmol. Vis. Sci. 2015, 56, 5012–5019. [Google Scholar] [CrossRef] [PubMed]
- Akaiwa, K.; Namekata, K.; Azuchi, Y.; Guo, X.; Kimura, A.; Harada, C.; Mitamura, Y.; Harada, T. Edaravone suppresses retinal ganglion cell death in a mouse model of normal tension glaucoma. Cell Death Dis. 2017, 8, e2934. [Google Scholar] [CrossRef]
- Akaiwa, K.; Namekata, K.; Azuchi, Y.; Sano, H.; Guo, X.; Kimura, A.; Harada, C.; Mitamura, Y.; Harada, T. Topical Ripasudil Suppresses Retinal Ganglion Cell Death in a Mouse Model of Normal Tension Glaucoma. Investig. Opthalmol. Vis. Sci. 2018, 59, 2080–2089. [Google Scholar] [CrossRef]
- Sano, H.; Namekata, K.; Kimura, A.; Shitara, H.; Guo, X.; Harada, C.; Mitamura, Y.; Harada, T. Differential effects of N-acetylcysteine on retinal degeneration in two mouse models of normal tension glaucoma. Cell Death Dis. 2019, 10, 75. [Google Scholar] [CrossRef]
- Namekata, K.; Kimura, A.; Kawamura, K.; Guo, X.; Harada, C.; Tanaka, K.; Harada, T. Dock3 attenuates neural cell death due to NMDA neurotoxicity and oxidative stress in a mouse model of normal tension glaucoma. Cell Death Differ. 2013, 20, 1250–1256. [Google Scholar] [CrossRef]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a gene that causes primary open angle glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Grover, A. Myocilin-associated Glaucoma: A Historical Perspective and Recent Research Progress. Mol. Vis. 2021, 27, 480–493. [Google Scholar]
- Wang, H.; Li, M.; Zhang, Z.; Xue, H.; Chen, X.; Ji, Y. Physiological function of myocilin and its role in the pathogenesis of glaucoma in the trabecular meshwork (Review). Int. J. Mol. Med. 2019, 43, 671–681. [Google Scholar] [CrossRef]
- Senatorov, V.; Malyuokova, I.; Fariss, R.; Wawrousek, E.F.; Swaminathan, S.; Sharan, S.K.; Tomarev, S. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J. Neurosci. 2006, 26, 11903–11914. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Aleard, W.L.M. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef]
- Kim, B.S.; Savinova, O.V.; Reedy, M.V.; Martin, J.; Lun, Y.; Gan, L.; Smith, R.S.; Tomarev, S.I.; John, S.W.; Johnson, R.L. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol. Cell. Biol. 2001, 21, 7707–7713. [Google Scholar] [CrossRef]
- Gould, D.B.; Miceli-Libby, L.; Savinova, O.V.; Torrado, M.; Tomarev, S.I.; Smith, R.S.; John, S.W.M. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol. Cell. Biol. 2004, 24, 9019–9025. [Google Scholar] [CrossRef] [PubMed]
- Resch, Z.T.; Fautsch, M.P. Glaucoma-associated myocilin: A better understanding but much more to learn. Exp. Eye Res. 2009, 88, 704–712. [Google Scholar] [CrossRef]
- Gould, D.B.; Reedy, M.; Wilson, L.A.; Smith, R.S.; Johnson, R.L.; John, S.W.M. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol. Cell. Biol. 2006, 26, 8427–8436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Grinchuk, O.; Tomarev, S.I. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Investig. Opthalmol. Vis. Sci. 2008, 49, 1932–1939. [Google Scholar] [CrossRef]
- Shepard, A.R.; Jacobson, N.; Millar, J.C.; Pang, I.H.; Steely, H.T.; Searby, C.C.; Sheffield, V.C.; Stone, E.M.; Clark, A.F. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum. Mol. Genet. 2007, 16, 609–617. [Google Scholar] [CrossRef]
- Zode, G.S.; Kuehn, M.H.; Nishimura, D.Y.; Searby, C.C.; Mohan, K.; Grozdanic, S.D.; Bugge, K.; Anderson, M.G.; Clark, A.F.; Stone, E.M.; et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Investig. 2011, 121, 3542–3553. [Google Scholar] [CrossRef]
- McDowell, C.M.; Luan, T.; Zhang, Z.; Putliwala, T.; Wordinger, R.J.; Millar, J.C.; John, S.W.M.; Pang, I.H.; Clark, A.F. Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice. Exp. Eye Res. 2012, 100, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wu, S.; Yan, X.; Liu, Q.; Lin, D.; Zhang, J.; Wang, N. Human Pro370Leu Mutant Myocilin Induces the Phenotype of Open-Angle Glaucoma in Transgenic Mice. Cell. Mol. Neurobiol. 2023, 43, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wu, S.; Liu, Q.; Cheng, Y.; Zhang, J.; Wang, N. Myocilin Gene Mutation Induced Autophagy Activation Causes Dysfunction of Trabecular Meshwork Cells. Front. Cell Dev. Biol. 2022, 10, 900777. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.K.; Nakaya, N.; Abu-Asab, M.; Tomarev, S.I. Mutated myocilin and heterozygous Sod2 deficiency act synergistically in a mouse model of open-angle glaucoma. Hum. Mol. Genet. 2015, 24, 3322–3334. [Google Scholar] [CrossRef] [PubMed]
- Kaipa, B.R.; Kasetti, R.; Sundaresan, Y.; Li, L.; Yacoub, S.; Millar, J.C.; Cho, W.; Skowronska-Krawczyk, D.; Maddineni, P.; Palczewski, K.; et al. Impaired axonal transport contributes to neurodegeneration in a Cre-inducible mouse model of myocilin-associated glaucoma. JCI Insight 2025, 10, e188710. [Google Scholar] [CrossRef]
- Vernazza, S.; Tirendi, S.; Bassi, A.M.; Traverso, C.E.; Saccà, S.C. Neuroinflammation in Primary Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3172. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Li, J.; Chan, W.F.; Tripathi, B.J. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp. Eye Res. 1994, 59, 723–727. [Google Scholar] [CrossRef]
- Ozcan, A.A.; Ozdemir, N.; Canataroglu, A. The aqueous levels of TGF-beta2 in patients with glaucoma. Int. Ophthalmol. 2004, 25, 19–22. [Google Scholar] [CrossRef]
- Agarwal, P.; Daher, A.M.; Agarwal, R. Aqueous humor TGF-β2 levels in patients with open-angle glaucoma: A meta-analysis. Mol. Vis. 2015, 21, 612–620. [Google Scholar]
- Wordinger, R.J.; Sharma, T.; Clark, A.F. The role of TGF-β2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J. Ocul. Pharmacol. Ther. 2014, 30, 154–162. [Google Scholar] [CrossRef]
- Junglas, B.; Yu, A.H.L.; Welge-Lüssen, U.; Tamm, E.R.; Fuchshofer, R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp. Eye Res. 2009, 88, 1065–1075. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I. Genetic rodent models of glaucoma in representing disease phenotype and insights into the pathogenesis. Mol. Asp. Med. 2023, 94, 101228. [Google Scholar] [CrossRef] [PubMed]
- Van Setten, G.B.; Blalock, T.D.; Grotendorst, G.; Schultz, G.S. Detection of connective tissue growth factor in human aqueous humor. Ophthal. Res. 2002, 34, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Tomarev, S.I.; Wistow, G.; Raymond, V.; Dubois, S.; Malyukova, I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Investig. Opthalmol. Vis. Sci. 2003, 44, 2588–2596. [Google Scholar] [CrossRef]
- Van Setten, G.B.; Trost, A.; Schrödl, F.; Kaser-Eichberger, A.; Bogner, B.; van Setten, M.; Heindl, L.M.; Grabner, G.; Reitsamer, H.A. Immunohistochemical Detection of CTGF in the Human Eye. Curr. Eye Res. 2016, 41, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Junglas, B.; Kuespert, S.; Seleem, A.A.; Struller, T.; Ullmann, S.; Bösl, M.; Bosserhoff, A.; Köstler, J.; Wagner, R.; Tamm, E.R.; et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 2012, 180, 2386–2403. [Google Scholar] [CrossRef]
- Reinehr, S.; Koch, D.; Weiss, M.; Froemel, F.; Voss, C.; Dick, H.B.; Fuchshofer, R.; Joachim, S.C. Loss of retinal ganglion cells in a new genetic mouse model for primary open-angle glaucoma. J. Cell. Mol. Med. 2019, 23, 5497–5507. [Google Scholar] [CrossRef]
- Weiss, M.; Reinehr, S.; Mueller-Buehl, A.M.; Doerner, J.D.; Fuchshofer, R.; Stute, G.; Dick, H.B.; Joachim, S.C. Activation of Apoptosis in a βB1-CTGF Transgenic Mouse Model. Int. J. Mol. Sci. 2021, 22, 1997. [Google Scholar] [CrossRef]
- Loo, Y.; Chan, A.S.Y.; Khor, C.C.; Aung, T.; Wang, Z. Rodent genetically modified models of glaucoma. Mol. Asp. Med. 2024, 95, 101229. [Google Scholar] [CrossRef]
- Rezaie, T.; Child, A.; Hitchings, R.; Brice, G.; Miller, L.; Coca-Prados, M.; Héon, E.; Krupin, T.; Ricth, R.; Kreutzer, D.; et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002, 295, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Alward, W.L.M.; Kwon, Y.H.; Kawase, K.; Craig, J.E.; Hayreh, S.S.; Johnson, A.T.; Khanna, C.L.; Yamamoto, T.; Mackey, D.A.; Roos, B.R.; et al. Evaluation of optineurin sequence variations in 1,048 patients with open-angle glaucoma. Am. J. Ophthalmol. 2003, 136, 904–910. [Google Scholar] [CrossRef]
- Aung, T.; Ebenezer, N.D.; Brice, G.; Child, A.H.; Prescott, Q.; Lehmann, O.J.; Hitchings, R.A.; Bhattacharya, S.S. Prevalence of optineurin sequence variants in adult primary open angle glaucoma: Implications for diagnostic testing. J. Med. Genet. 2003, 40, e101. [Google Scholar] [CrossRef]
- Ryan, T.A.; Tumbarello, D.A. Optineurin: A Coordinator of Membrane-Associated Cargo Trafficking and Autophagy. Front. Immunol. 2018, 9, 1024. [Google Scholar] [CrossRef]
- Kroeber, M.; Ohlmann, A.; Russell, P.; Tamm, E.R. Transgenic studies on the role of optineurin in the mouse eye. Exp. Eye Res. 2006, 82, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.F.; Fan, B.J.; Lam, D.S.C.; Lee, W.S.; Tam, P.O.S.; Chua, J.K.H.; Tham, C.C.Y.; Lai, J.S.M.; Fan, D.S.P.; Pang, C.P. Different optineurin mutation pattern in primary open-angle glaucoma. Investig. Opthalmol. Vis. Sci. 2003, 44, 3880–3884. [Google Scholar] [CrossRef]
- Fuse, N.; Takahashi, K.; Akiyama, H.; Nakazawa, T.; Seimiya, M.; Kuwahara, S.; Tamai, M. Molecular genetic analysis of optineurin gene for primary open-angle and normal tension glaucoma in the Japanese population. J. Glaucoma 2004, 13, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Rezaie, T.; Okada, K.; Viswanathan, A.C.; Child, A.H.; Brice, G.; Bhattacharya, S.S.; Lehmann, O.J.; Sarfarazi, M.; Hitchings, R.A. Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Investig. Opthalmol. Vis. Sci. 2005, 46, 2816–2822. [Google Scholar] [CrossRef]
- Hauser, M.A.; Figueiredo Sena, D.; Flor, J.; Walter, J.; Auguste, J.; Larocque-Abramson, K.; Graham, F.; Delbono, E.; Haines, J.L.; Pericak-Vance, M.A.; et al. Distribution of optineurin sequence variations in an ethnically diverse population of low-tension glaucoma patients from the United States. J. Glaucoma 2006, 15, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.L.; Akahori, M.; Obazawa, M.; Minami, M.; Noda, T.; Nakaya, N.; Tomarev, S.; Kawase, K.; Yamamoto, T.; Noda, S.; et al. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum. Mol. Genet. 2010, 19, 2606–2615. [Google Scholar] [CrossRef]
- Tseng, H.C.; Riday, T.T.; McKee, C.; Braine, C.E.; Bomze, H.; Barak, I.; Marean-Reardon, C.; John, S.W.M.; Philpot, B.D.; Ehlers, M.D. Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol. Aging 2015, 36, 2201–2212. [Google Scholar] [CrossRef]
- Mut-Arbona, P.; Sperlágh, B. P2 receptor-mediated signaling in the physiological and pathological brain: From development to aging and disease. Neuropharmacology 2023, 233, 109541. [Google Scholar] [CrossRef]
- Mitchell, C.H.; Carré, D.A.; McGlinn, A.M.; Stone, R.A.; Civan, M.M. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 7174–7178. [Google Scholar] [CrossRef]
- Gomes, P.; Srinivas, S.P.; Van Driessche, W.; Vereecke, J.; Himpens, B. ATP release through connexin hemichannels in corneal endothelial cells. Investig. Opthalmol. Vis. Sci. 2005, 46, 1208–1218. [Google Scholar] [CrossRef]
- Crooke, A.; Guzmán-Aranguez, A.; Peral, A.; Abdurrahman, M.K.A.; Pintor, J. Nucleotides in ocular secretions: Their role in ocular physiology. Pharmacol. Ther. 2008, 119, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Reigada, D.; Lu, W.; Zhang, M.; Mitchell, C.H. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience 2008, 157, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Li, G.; Qiu, J.; Challa, P.; Epstein, D.L.; Gonzalez, P. Extracellular release of ATP mediated by cyclic mechanical stress leads to mobilization of AA in trabecular meshwork cells. Investig. Opthalmol. Vis. Sci. 2009, 50, 5805–5810. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Lim, J.C.; Lu, W.; Beckel, J.M.; Macarak, E.J.; Laties, A.M.; Mitchell, C.H. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J. Physiol. 2012, 590, 2285–2304. [Google Scholar] [CrossRef]
- Guzmán-Aranguez, A.; Santano, C.; Martin-Gil, A.; Fonseca, B.; Pintor, J. Nucleotides in the eye: Focus on functional aspects and therapeutic perspectives. J. Pharmacol. Exp. Ther. 2013, 345, 331–341. [Google Scholar] [CrossRef]
- Beckel, J.M.; Argall, A.J.; Lim, J.C.; Xia, J.; Lu, W.; Coffey, E.E.; Macarak, E.J.; Shahidullah, M.; Delamere, N.A.; Zode, G.S.; et al. Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: A mechanism for purinergic involvement in chronic strain. Glia 2014, 62, 1486–1501. [Google Scholar] [CrossRef]
- Martin-Gil, A.; Perez de Lara, M.J.; Crooke, A.; Santana, C.; Peral, A.; Pintor, J. Silencing of P2Y(2) receptors reduces intraocular pressure in New Zealand rabbits. Br. J. Pharmacol. 2012, 165, 1163–1172. [Google Scholar] [CrossRef]
- Pintor, J.; Sánchez-Nogueiro, J.; Irazu, M.; Mediero, A.; Peláez, T.; Peral, A. Immunolocalisation of P2Y receptors in the rat eye. Purinergic Signal 2004, 1, 83–90. [Google Scholar] [CrossRef]
- Soto, D.; Pintor, J.; Peral, A.; Gual, A.; Gasull, X. Effects of dinucleoside polyphosphates on trabecular meshwork cells and aqueous humor outflow facility. J. Pharmacol. Exp. Ther. 2005, 314, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Markovskaya, A.; Crooke, A.; Guzmán-Aranguez, A.; Peral, A.; Ziganshin, A.U.; Pintor, J. Hypotensive effect of UDP on intraocular pressure in rabbits. Eur. J. Pharmacol. 2008, 579, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Eliahu, S.; Martín-Gil, A.; Perez de Lara, M.J.; Pintor, J.; Camden, J.; Weisman, G.A.; Lecka, J.; Sévigny, J.; Fischer, B. 2-MeS-beta,gamma-CCl2-ATP is a potent agent for reducing intraocular pressure. J. Med. Chem. 2010, 53, 3305–3319. [Google Scholar] [CrossRef]
- Li, A.; Zhang, X.; Zheng, D.; Ge, J.; Laties, A.M.; Mitchell, C.H. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp. Eye Res. 2011, 93, 528–533. [Google Scholar] [CrossRef]
- Hamada, K.; Shinozaki, Y.; Namekata, K.; Matsumoto, M.; Ohno, N.; Segawa, T.; Kashiwagi, K.; Harada, T.; Koizumi, S. Loss of P2Y1 receptors triggers glaucoma-like pathology in mice. Br. J. Pharmacol. 2021, 178, 4552–4571. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Kashiwagi, K.; Namekata, K.; Takeda, A.; Ohno, N.; Robaye, B.; Harada, T.; Iwata, T.; Koizumi, S. Purinergic dysregulation causes hypertensive glaucoma-like optic neuropathy. JCI Insight 2017, 2, e93456. [Google Scholar] [CrossRef] [PubMed]
- Loomis, S.J.; Kang, J.H.; Weinreb, R.N.; Yaspan, B.L.; Cooke Bailey, J.N.; Gaasterland, D.; Gaasterland, T.; Lee, R.K.; Lichter, P.R.; Budenz, D.L.; et al. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology 2014, 121, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Ozel, A.B.; Moroi, S.E.; Reed, D.M.; Nika, M.; Schmidt, C.M.; Akbari, S.; Scott, K.; Rozsa, F.; Pawar, H.; Musch, D.C.; et al. Genome-wide association study and meta-analysis of intraocular pressure. Hum. Genet. 2014, 133, 41–57. [Google Scholar] [CrossRef]
- Gu, X.; Reagan, A.M.; McClellan, M.E.; Elliott, M.H. Caveolins and caveolae in ocular physiology and pathophysiology. Prog. Retin. Eye Res. 2017, 56, 84–106. [Google Scholar] [CrossRef]
- Enyong, E.N.; Gurley, J.M.; De Ieso, M.L.; Stamer, W.D.; Elliott, M.H. Caveolar and non-Caveolar Caveolin-1 in ocular homeostasis and disease. Prog. Retin. Eye Res. 2022, 91, 101094. [Google Scholar] [CrossRef]
- Patel, H.H.; Murray, F.; Insel, P.A. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 359–391. [Google Scholar] [CrossRef]
- Joshi, B.; Bastiani, M.; Strugnell, S.S.; Boscher, C.; Parton, R.G.; Nabi, I.R. Phosphocaveolin-1 is a mechanotransducer that induces caveola biogenesis via Egr1 transcriptional regulation. J. Cell Biol. 2012, 199, 425–435. [Google Scholar] [CrossRef]
- Lo, H.P.; Nixon, S.J.; Hall, T.E.; Cowling, B.S.; Ferguson, C.; Morgan, G.P.; Schieber, N.L.; Fernandez-Rojo, M.A.; Bastiani, M.; Floetenmeyer, M.; et al. The caveolin-cavin system plays a conserved and critical role in mechanoprotection of skeletal muscle. J. Cell Biol. 2015, 210, 833–849. [Google Scholar] [CrossRef]
- Ethier, C.R. The inner wall of Schlemm’s canal. Exp. Eye Res. 2002, 74, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.H.; Ashpole, N.E.; Gu, X.; Herrnberger, L.; McClellan, M.E.; Griffith, G.L.; Reagan, A.M.; Boyce, T.M.; Tanito, M.; Tamm, E.R.; et al. Caveolin-1 modulates intraocular pressure: Implications for caveolae mechanoprotection in glaucoma. Sci. Rep. 2016, 6, 37127. [Google Scholar] [CrossRef]
- De Ieso, M.L.; Kuhn, M.; Bernatchez, P.; Elliott, M.H.; Stamer, W.D. A Role of Caveolae in Trabecular Meshwork Mechanosensing and Contractile Tone. Front. Cell Dev. Biol. 2022, 10, 855097. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, D.; Galindo-Romero, C.; Gupta, V.; Agudo-Barriuso, M.; Gupta, V.B.; Graham, S.L.; Chitranshi, N. Signalling pathways and cell death mechanisms in glaucoma: Insights into the molecular pathophysiology. Mol. Asp. Med. 2023, 94, 101216. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Gupta, V.; Chitranshi, N.; Moustardas, P.; Ranjbaran, R.; Graham, S.L. Molecular Mechanisms of Glaucoma Pathogenesis with Implications to Caveolin Adaptor Protein and Caveolin-Shp2 Axis. Aging Dis. 2024, 15, 2051–2068. [Google Scholar] [CrossRef]
- Banecki, K.M.R.M.; Dora, K.A. Endothelin-1 in Health and Disease. Int. J. Mol. Sci. 2023, 24, 11295. [Google Scholar] [CrossRef]
- Salvatore, S.; Vingolo, E.M. Endothelin-1 role in human eye: A review. J. Ophthalmol. 2010, 2010, 354645. [Google Scholar] [CrossRef] [PubMed]
- Holló, G.; Lakatos, P.; Vargha, P. Immediate increase in aqueous humour endothelin 1 concentration and intra-ocular pressure after argon laser trabeculoplasty in the rabbit. Ophthalmologica 2000, 214, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Krishnamoorthy, R.R.; Prasanna, G.; Narayan, S.; Clark, A.; Yorio, T. Dexamethasone regulates endothelin-1 and endothelin receptors in human non-pigmented ciliary epithelial (HNPE) cells. Exp. Eye Res. 2003, 76, 261–272. [Google Scholar] [CrossRef]
- Cellini, M.; Versura, P.; Zamparini, E.; Bendo, E.; Campos, E.C. Effects of endothelin-1 and flunarizine on human trabecular meshwork cell contraction. Exp. Biol. Med. 2006, 231, 1081–1084. [Google Scholar]
- Thieme, H.; Schimmat, C.; Münzer, G.; Boxberger, M.; Fromm, M.; Pfeiffer, N.; Rosenthal, R. Endothelin antagonism: Effects of FP receptor agonists prostaglandin F2alpha and fluprostenol on trabecular meshwork contractility. Investig. Opthalmol. Vis. Sci. 2006, 47, 938–945. [Google Scholar] [CrossRef]
- Renieri, G.; Choritz, L.; Rosenthal, R.; Meissner, S.; Pfeiffer, N.; Thieme, H. Effects of endothelin-1 on calcium-independent contraction of bovine trabecular meshwork. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1107–1115. [Google Scholar] [CrossRef]
- Rosenthal, R.; Froom, M. Endothelin antagonism as an active principle for glaucoma therapy. Br. J. Pharmacol. 2011, 162, 806–816. [Google Scholar] [CrossRef]
- Zhou, E.H.; Paolucci, M.; Dryja, T.P.; Manley, T.; Xiang, C.; Rice, D.S.; Prasanna, G.; Chen, A. A Compact Whole-Eye Perfusion System to Evaluate Pharmacologic Responses of Outflow Facility. Investig. Opthalmol. Vis. Sci. 2017, 58, 2991–3003. [Google Scholar] [CrossRef]
- Sugiyama, T.; Moriya, S.; Oku, H.; Azuma, I. Association of endothelin-1 with normal tension glaucoma: Clinical and fundamental studies. Surv. Ophthalmol. 1995, 39 (Suppl. S1), S49–S56. [Google Scholar] [CrossRef] [PubMed]
- Cellini, M.; Possati, G.L.; Profazio, V.; Sbrocca, M.; Caramazza, N.; Caramazza, R. Color Doppler imaging and plasma levels of endothelin-1 in low-tension glaucoma. Acta Ophthalmol. Scand. Suppl. 1997, 224, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Noske, W.; Hensen, J.; Wiederholt, M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 551–552. [Google Scholar] [CrossRef]
- Tezel, G.; Kass, M.A.; Kolker, A.E.; Becker, B.; Wax, M.B. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J. Glaucoma 1997, 6, 83–89. [Google Scholar] [CrossRef]
- Yorio, T.; Krishnamoorthy, R.; Prasanna, G. Endothelin: Is it a contributor to glaucoma pathophysiology? J. Glaucoma 2002, 11, 259–270. [Google Scholar] [CrossRef]
- Prasanna, G.; Narayan, S.; Krishnamoorthy, R.R.; Yorio, T. Eyeing endothelins: A cellular perspective. Mol. Cell. Biochem. 2003, 253, 71–88. [Google Scholar] [CrossRef]
- Leung, J.W.C.; Ho, M.C.Y.; Lo, A.C.Y.; Chung, S.S.M.; Chung, S.K. Endothelial cell-specific over-expression of endothelin-1 leads to more severe cerebral damage following transient middle cerebral artery occlusion. J. Cardiovasc. Pharmacol. 2004, 44 (Suppl. S1), S293–S300. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.S.; Zhang, X.; Feng, Q.; Lo, A.C.Y.; Chung, S.K.; So, K.F. Progressive retinal degeneration in transgenic mice with overexpression of endothelin-1 in vascular endothelial cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 4842–4851. [Google Scholar] [CrossRef]
- Ramírez, J.M.; Salobrar-García, E.; de Hoz, R.; Salazar, J.J.; Matamoros, J.A.; Sánchez-Puebla, L.; López-Cuenca, I.; Fernández-Albarral, J.A.; Ramírez, A.I. Laser-Induced Ocular Hypertension in a Mouse Model of Glaucoma. Methods Mol. Biol. 2023, 2708, 49–56. [Google Scholar] [CrossRef]
- Bugara, K.; Pacwa, A.; Smedowski, A. Molecular pathways in experimental glaucoma models. Front. Neurosci. 2024, 18, 1363170. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Schumer, R.A.; Serle, J.B.; Podos, S.M. A comparison of argon laser and diode laser photocoagulation of the trabecular meshwork to produce the glaucoma monkey model. J. Glaucoma 1998, 7, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, F.; Aihara, M.; Mackey, M.R.; Lindsey, J.D.; Weinreb, R.N. Optic nerve damage in experimental mouse ocular hypertension. Investig. Opthalmol. Vis. Sci. 2003, 44, 4321–4330. [Google Scholar] [CrossRef]
- Biermann, J.; van Oterendorp, C.; Stoykow, C.; Volz, C.; Jehle, T.; Boehringer, D.; Lagrèze, W.A. Evaluation of intraocular pressure elevation in a modified laser-induced glaucoma rat model. Exp. Eye Res. 2012, 104, 7–14. [Google Scholar] [CrossRef]
- Ji, J.; Chang, P.; Pennesi, M.E.; Yang, Z.; Zhang, J.; Li, D.; Wu, S.M.; Gross, R.L. Effects of elevated intraocular pressure on mouse retinal ganglion cells. Vis. Res. 2005, 45, 169–179. [Google Scholar] [CrossRef]
- WoldeMussie, E.; Ruiz, G.; Wijono, M.; Wheeler, L.A. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Investig. Opthalmol. Vis. Sci. 2001, 42, 2849–2855. [Google Scholar]
- Biswas, S.; Wan, K.H. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef]
- Shimizu, S.; Honjo, M.; Sugimoto, K.; Okamoto, M.; Aihara, M. Effect of pigmentation intensity of trabecular meshwork cells on mechanisms of micropulse laser trabeculoplasty. Sci. Rep. 2022, 12, 10535. [Google Scholar] [CrossRef] [PubMed]
- Grozdanic, S.D.; Kwon, Y.H.; Sakaguchi, D.S.; Kardon, R.H.; Sonea, I.M. Functional evaluation of retina and optic nerve in the rat model of chronic ocular hypertension. Exp. Eye Res. 2004, 79, 75–83. [Google Scholar] [CrossRef]
- Morrison, J.C.; Moore, C.G.; Deppmeier, L.M.; Gold, B.G.; Meshul, C.K.; Johnson, E.C. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997, 64, 85–96. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.; Liu, H.; Feng, Y.; Wang, X.; Herb, M.; Ji, H.; Wagner, S.; Vogt, J.; Prokosch, V. HOCPCA Exerts Neuroprotection on Retinal Ganglion Cells by Binding to CaMKIIα and Modulating Oxidative Stress and Neuroinflammation in Experimental Glaucoma. Neurosci. Bull. 2025, 41, 1329–1346. [Google Scholar] [CrossRef]
- Vecino, E.; Urcola, H.; Bayon, A.; Sharma, S.C. Ocular Hypertension/Glaucoma in Minipigs: Episcleral Veins Cauterization and Microbead Occlusion Methods. Methods Mol. Biol. 2018, 1695, 41–48. [Google Scholar] [CrossRef]
- Shareef, S.R.; Garcia-Valenzuela, E.; Salierno, A.; Walsh, J.; Sharma, S.C. Chronic ocular hypertension following episcleral venous occlusion in rats. Exp. Eye Res. 1995, 61, 379–382. [Google Scholar] [CrossRef]
- Garcia-Valenzuela, E.; Shareef, S.; Walsh, J.; Sharma, S.C. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp. Eye Res. 1995, 61, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, A.H.; Sawada, A.; Becker, B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc. Natl. Acad. Sci. USA 1999, 96, 9944–9948. [Google Scholar] [CrossRef]
- Urcola, J.H.; Hernández, M.; Vecino, E. Three experimental glaucoma models in rats: Comparison of the effects of intraocular pressure elevation on retinal ganglion cell size and death. Exp. Eye Res. 2006, 83, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Nickells, R.W.; Kerrigan, L.A.; Pease, M.E.; Thibault, D.J.; Zack, D.J. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Investig. Opthalmol. Vis. Sci. 1995, 36, 774–786. [Google Scholar]
- Johansson, J.O. Inhibition and recovery of retrograde axoplasmic transport in rat optic nerve during and after elevated IOP in vivo. Exp. Eye Res. 1988, 46, 223–227. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, Y.; Chen, Q.; Xu, J.; Sarunic, M.V.; Saragovi, U.H.; Zhuo, Y. Validation of glaucoma-like features in the rat episcleral vein cauterization model. Chin. Med. J. 2014, 127, 359–364. [Google Scholar] [CrossRef]
- Ruiz-Ederra, J.; Verkman, A.S. Mouse model of sustained elevation in intraocular pressure produced by episcleral vein occlusion. Exp. Eye Res. 2006, 82, 879–884. [Google Scholar] [CrossRef]
- Laquis, S.; Chaudhary, P.; Sharma, S.C. The patterns of retinal ganglion cell death in hypertensive eyes. Brain Res. 1998, 784, 100–104. [Google Scholar] [CrossRef]
- Sawada, A.; Neufeld, A.H. Confirmation of the rat model of chronic, moderately elevated intraocular pressure. Exp. Eye Res. 1999, 69, 525–531. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yoshitomi, T.; Zorumski, C.F.; Izumi, Y. Experimentally Induced Mammalian Models of Glaucoma. Biomed. Res. Int. 2015, 2015, 281214. [Google Scholar] [CrossRef]
- Fedorchak, M.V.; Conner, I.P.; Medina, C.A.; Wingard, J.B.; Schuman, J.S.; Little, S.R. 28-day intraocular pressure reduction with a single dose of brimonidine tartrate-loaded microspheres. Exp. Eye Res. 2014, 125, 210–216. [Google Scholar] [CrossRef]
- Morrison, J.C.; Johnson, E.; Cepurna, W.O. Rat models for glaucoma research. Prog. Brain Res. 2008, 173, 285–301. [Google Scholar] [CrossRef]
- Morrison, J.C.; Cepurna, W.O.; Johnson, E.C. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Exp. Eye Res. 2015, 141, 23–32. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- Fortune, B.; Bui, B.V.; Morrison, J.C.; Johnson, E.C.; Dong, J.; Cepurna, W.O.; Jia, L.; Barber, S.; Cioffi, G.A. Selective ganglion cell functional loss in rats with experimental glaucoma. Investig. Opthalmol. Vis. Sci. 2004, 45, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Johnson, E.C.; Cepurna, W.O. Hypertonic Saline Injection Model of Experimental Glaucoma in Rats. Methods Mol. Biol. 2018, 1695, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Aloe, L.; Centofanti, M.; Parisi, V.; Báo, S.N.; Mantelli, F.; Colafrancesco, V.; Manni, G.L.; Bucci, M.G.; Bonini, S.; et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA 2009, 106, 13469–13474. [Google Scholar] [CrossRef]
- Mickevičius, K.; Paulauskas, T.; Čėsna, R.; Bijeikis, S.; Dragašius, M.; Urbanavičiūtė, J.; Ragauskas, S.; Kalesnykas, G. Comprehensive characterization of the rat magnetic microbead model using longitudinal monitoring of structural and functional parameters. Exp. Eye Res. 2025, 261, 110651. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, C. Establishment of an experimental glaucoma animal model: A comparison of microbead injection with or without hydroxypropyl methylcellulose. Exp. Ther. Med. 2017, 14, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Tian, F.; Glynn, C.; Tsekov, S.; Huang, S.; Zhou, S.; He, Z.; Rao, S.; Wang, Q. Biologically Driven In Vivo Occlusion Design Provides a Reliable Experimental Glaucoma Model. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cone, F.E.; Steinhart, M.R.; Oglesby, E.N.; Kalesnykas, G.; Pease, M.E.; Quigley, H.A. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp. Eye Res. 2012, 99, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Samsel, P.A.; Kisiswa, L.; Erichsen, J.T.; Cross, S.D.; Morgan, J.E. A novel method for the induction of experimental glaucoma using magnetic microspheres. Investig. Opthalmol. Vis. Sci. 2011, 52, 1671–1675. [Google Scholar] [CrossRef]
- Bunker, S.; Holeniewska, J.; Vijay, S.; Dahlmann-Noor, A.; Khaw, P.; Ng, Y.S.; Shima, D.; Foxton, R. Experimental glaucoma induced by ocular injection of magnetic microspheres. J. Vis. Exp. 2015, 96, 52400. [Google Scholar] [CrossRef]
- Rombaut, A.; Brautaset, R.; Williams, P.A.; Tribble, J.R. Intravitreal injection of the Galectin-3 inhibitor TD139 provides neuroprotection in a rat model of ocular hypertensive glaucoma. Mol. Brain 2024, 17, 84. [Google Scholar] [CrossRef]
- Chen, H.; Wei, X.; Cho, K.S.; Chen, G.; Sappington, R.; Calkins, D.J.; Chen, D.F. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Investig. Opthalmol. Vis. Sci. 2011, 52, 36–44. [Google Scholar] [CrossRef]
- Benozzi, J.; Nahuman, L.P.; Campanelli, J.L.; Rosenstein, R.E. Effect of hyaluronic acid on intraocular pressure in rats. Investig. Opthalmol. Vis. Sci. 2002, 43, 2196–2200. [Google Scholar]
- Zhu, M.D.; Cai, F.Y. Development of experimental chronic intraocular hypertension in the rabbit. Aust. N. Z. J. Ophthalmol. 1992, 20, 225–234. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Pezzino, S.; Corsaro, R.; Pescosolido, N.; Bagnoli, P.; Rusciano, D. Hypotensive Effect of Nanomicellar Formulation of Melatonin and Agomelatine in a Rat Model: Significance for Glaucoma Therapy. Diagnostics 2020, 10, 138. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Amato, R.; Pezzino, S.; Corsaro, R.; Bagnoli, P.; Rusciano, D. A Topical Formulation of Melatoninergic Compounds Exerts Strong Hypotensive and Neuroprotective Effects in a Rat Model of Hypertensive Glaucoma. Int. J. Mol. Sci. 2020, 21, 9267. [Google Scholar] [CrossRef]
- Amato, R.; Canovai, A.; Melecchi, A.; Maci, S.; Quintela, F.; Fonseca, B.A.; Cammalleri, M.; Dal Monte, M. Efficacy of a Spearmint (Mentha spicata L.) Extract as Nutritional Support in a Rat Model of Hypertensive Glaucoma. Transl. Vis. Sci. Technol. 2023, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, M.; Dal Monte, M.; Amato, R.; Bagnoli, P.; Rusciano, D. A Dietary Combination of Forskolin with Homotaurine, Spearmint and B Vitamins Protects Injured Retinal Ganglion Cells in a Rodent Model of Hypertensive Glaucoma. Nutrients 2020, 12, 1189. [Google Scholar] [CrossRef]
- Melecchi, A.; Amato, R.; Dal Monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Restored retinal physiology after administration of niacin with citicoline in a mouse model of hypertensive glaucoma. Front. Med. 2023, 10, 1230941. [Google Scholar] [CrossRef]
- Lucchesi, M.; Marracci, S.; Amato, R.; Lapi, D.; Santana-Garrido, Á.; Espinosa-Martín, P.; Vázquez, C.M.; Mate, A.; Dal Monte, M. The Anti-Inflammatory and Antioxidant Properties of Acebuche Oil Exert a Retinoprotective Effect in a Murine Model of High-Tension Glaucoma. Nutrients 2024, 16, 409. [Google Scholar] [CrossRef]
- Kimura, A.; Noro, T.; Harada, T. Role of animal models in glaucoma research. Neural Regen. Res. 2020, 15, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Reinehr, S.; Deppe, L.; Strubbe, A.; Kluge, N.; Dick, H.B.; Joachim, S.C. Glaucoma Animal Models beyond Chronic IOP Increase. Int. J. Mol. Sci. 2024, 25, 906. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Fleishaker, M.; Ivanov, D. Molecular mechanisms of NMDA excitotoxicity in the retina. Sci. Rep. 2023, 13, 18471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schlamp, C.L.; Poulsen, G.L.; Jackson, M.W.; Griep, A.E.; Nickells, N.W. p53 regulates apoptotic retinal ganglion cell death induced by N-methyl-D-aspartate. Mol. Vis. 2002, 8, 341–350. [Google Scholar]
- Vorwerk, C.K.; Lipton, S.A.; Zurakowski, D.; Hyman, B.T.; Sabel, B.A.; Dreyer, E.B. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Investig. Opthalmol. Vis. Sci. 1996, 37, 1618–1624. [Google Scholar]
- Georgiou, A.L.; Guo, L.; Cordeiro, M.F.; Salt, T.E. Changes in NMDA receptor contribution to synaptic transmission in the brain in a rat model of glaucoma. Neurobiol. Dis. 2010, 39, 344–351. [Google Scholar] [CrossRef]
- Lambuk, L.; Jafri, A.J.A.; Iezhitsa, I.; Agarwal, R.; Bakar, N.S.; Agarwal, P.; Abdullah, A.; Ismail, N.M. Dose-dependent effects of NMDA on retinal and optic nerve morphology in rats. Int. J. Ophthalmol. 2019, 12, 746–753. [Google Scholar] [CrossRef]
- Schuettauf, F.; Naskar, R.; Vorwerk, C.K.; Zurakowski, D.; Dreyer, E.B. Ganglion cell loss after optic nerve crush mediated through AMPA-kainate and NMDA receptors. Investig. Opthalmol. Vis. Sci. 2000, 41, 4313–4316. [Google Scholar]
- Chidlow, G.; Osborne, N.N. Rat retinal ganglion cell loss caused by kainate, NMDA and ischemia correlates with a reduction in mRNA and protein of Thy-1 and neurofilament light. Brain Res. 2003, 963, 298–306. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.M.; Lam, T.T.; Caprioli, J. Hyperthermic pre-conditioning protects retinal neurons from N-methyl-D-aspartate (NMDA)-induced apoptosis in rat. Brain Res. 2003, 970, 119–130. [Google Scholar] [CrossRef]
- Schuettauf, F.; Vorwerk, C.; Naskar, R.; Orlin, A.; Quinto, K.; Zurakowski, D.; Dejneka, N.S.; Klein, R.L.; Meyer, E.M.; Bennett, J. Adeno-associated viruses containing bFGF or BDNF are neuroprotective against excitotoxicity. Curr. Eye Res. 2004, 29, 379–386. [Google Scholar] [CrossRef]
- Ma, J.; Yu, W.; Wang, Y.; Cao, G.; Cai, S.; Chen, X.; Yan, N.; Yuan, Y.; Zeng, H.; Fleenor, D.L.; et al. Neuroprotective effects of C-type natriuretic peptide on rat retinal ganglion cells. Investig. Opthalmol. Vis. Sci. 2010, 51, 3544–3553. [Google Scholar] [CrossRef]
- Bui, B.V.; Fortune, B. Ganglion cell contributions to the rat full-field electroretinogram. J. Physiol. 2004, 555 Pt 1, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Casson, R.J.; Chidlow, G.; Wood, J.P.; Vidal-Sanz, M.; Osborne, N.N. The effect of retinal ganglion cell injury on light-induced photoreceptor degeneration. Investig. Opthalmol. Vis. Sci. 2004, 45, 685–693. [Google Scholar] [CrossRef]
- Teister, J.; Anders, F.; Beck, S.; Funke, S.; von Pein, H.; Prokosch, V.; Pfeiffer, N.; Grus, F. Decelerated neurodegeneration after intravitreal injection of α-synuclein antibodies in a glaucoma animal model. Sci. Rep. 2017, 7, 6260. [Google Scholar] [CrossRef]
- Lamirande, P.; Gaffney, E.A.; Gertz, M.; Maini, P.K.; Crawshaw, J.R.; Caruso, A. A First-Passage Model of Intravitreal Drug Delivery and Residence Time-Influence of Ocular Geometry, Individual Variability, and Injection Location. Investig. Opthalmol. Vis. Sci. 2024, 65, 21. [Google Scholar] [CrossRef]
- Opere, C.A.; Heruye, S.; Njie-Mbye, Y.F.; Ohia, S.E.; Sharif, N.A. Regulation of Excitatory Amino Acid Transmission in the Retina: Studies on Neuroprotection. J. Ocul. Pharmacol. Ther. 2018, 34, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Bou Ghanem, G.O.; Wareham, L.K.; Calkins, D.J. Addressing neurodegeneration in glaucoma: Mechanisms, challenges, and treatments. Prog. Retin. Eye Res. 2024, 100, 101261. [Google Scholar] [CrossRef]
- Locri, F.; Cammalleri, M.; Dal Monte, M.; Rusciano, D.; Bagnoli, P. Protective Efficacy of a Dietary Supplement Based on Forskolin, Homotaurine, Spearmint Extract, and Group B Vitamins in a Mouse Model of Optic Nerve Injury. Nutrients 2019, 11, 2931. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Ruan, Y.W.; Ren, C.R.; Cui, Q.; So, K.F. Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen. Res. 2014, 9, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Pitha, I.; Du, L.; Nguyen, T.D.; Quigley, H. IOP and glaucoma damage: The essential role of optic nerve head and retinal mechanosensors. Prog. Retin. Eye Res. 2024, 99, 101232. [Google Scholar] [CrossRef]
- Maddineni, P.; Sundaresan, Y.; Zode, G. Mouse Model of Glucocorticoid-Induced Glaucoma. Methods Mol. Biol. 2025, 2858, 131–141. [Google Scholar] [CrossRef]
- Mesripour, A.; Asghari-Varzaneh, M.; Safaeian, L. An overview of animal models induced by glucocorticoids. Physiol. Pharmacol. 2023, 27, 211–233. [Google Scholar] [CrossRef]
- Withlock, N.A.; McKnight, B.; Corcoran, K.N.; Rodriguez, R.A.; Rice, D.S. Increased intraocular pressure in mice treated with dexamethasone. Investig. Opthalmol. Vis. Sci. 2010, 51, 6496–6503. [Google Scholar] [CrossRef]
- Overby, D.R.; Bertrand, J.; Tektas, O.Y.; Boussommier-Calleja, A.; Schicht, M.; Ethier, C.R.; Woodward, D.F.; Stamer, W.D.; Lütjen-Drecoll, E. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Investig. Opthalmol. Vis. Sci. 2014, 55, 4922–4933. [Google Scholar] [CrossRef]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.; Deutsch, E.R.; Tang, H.M.; Danias, J. Triamcinolone acetonide decreases outflow facility in C57BL/6 mouse eyes. Investig. Opthalmol. Vis. Sci. 2013, 54, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Song, D.Y.; Kim, S.H. Effect of Strain on Rodent Glaucoma Models: Magnetic Bead Injection Versus Hydrogel Injection Versus Circumlimbal Suture. Transl. Vis. Sci. Technol. 2022, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.H.Y.; Zhao, D.; Bui, B.V.; Millar, C.J.; Nguyen, C.T.O. Increased episcleral venous pressure in a mouse model of circumlimbal suture induced ocular hypertension. Exp. Eye Res. 2021, 202, 108348. [Google Scholar] [CrossRef]
- Reinehr, S.; Reinhard, J.; Wiemann, S.; Hesse, K.; Voss, C.; Gandej, M.; Dick, H.B.; Faissner, A.; Joachim, S.C. Transfer of the Experimental Autoimmune Glaucoma Model from Rats to Mice-New Options to Study Glaucoma Disease. Int. J. Mol. Sci. 2019, 20, 2563. [Google Scholar] [CrossRef]
- Reinher, S.; Wulf, J.; Theile, J.; Schulte, K.K.; Peters, M.; Fuchshofer, R.; Dick, H.B.; Joachim, S.C. In a novel autoimmune and high-pressure glaucoma model a complex immune response is induced. Front. Immunol. 2024, 15, 1296178. [Google Scholar] [CrossRef]
- Joachim, S.C.; Mondon, C.; Gramlich, O.W.; Grus, F.H.; Dick, H.B. Apoptotic retinal ganglion cell death in an autoimmune glaucoma model is accompanied by antibody depositions. J. Mol. Neurosci. 2014, 52, 216–224. [Google Scholar] [CrossRef]
- Qing, K.X.; Lo, A.C.Y.; Lu, S.; Zhou, Y.; Yang, D.; Yang, D. Integrated bioinformatics analysis of retinal ischemia/reperfusion injury in rats with potential key genes. BMC Genom. 2024, 25, 367. [Google Scholar] [CrossRef] [PubMed]
- Palmhof, M.; Frank, V.; Rappard, P.; Kortenhorn, E.; Demuth, J.; Biert, N.; Stute, G.; Dick, H.B.; Joachim, S.C. From Ganglion Cell to Photoreceptor Layer: Timeline of Deterioration in a Rat Ischemia/Reperfusion Model. Front. Cell. Neurosci. 2019, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Plaice, G.B.; Geddes, A.; Botfield, H.F.; Hill, L.J.; Masood, I. Understanding Factors Contributing to Glaucoma in Populations of African Descent. J. Clin. Transl. Ophthalmol. 2024, 2, 155–170. [Google Scholar] [CrossRef]
- Singh, N.; Kizhatil, K.; Duraikannu, D.; Choquet, H.; Nair, K.S. Structural framework to address variant-gene relationship in primary open-angle glaucoma. Vis. Res. 2025, 226, 108505. [Google Scholar] [CrossRef] [PubMed]
| Class | Medications | Mechanism of Action |
|---|---|---|
| α2-adrenergic agonists | Brimonidine Apraclonidine | Reduction in aqueous humor production Increase in aqueous humor outflow |
| β-adrenergic antagonists | Timolol Carteolol Levobunolol Metipranolol Betaxolol | Reduction in aqueous humor production |
| Cholinergic agonists | Pilocarpine Carbachol | Increase in aqueous humor outflow |
| Carbonic anhydrase inhibitors | Dorzolamide Brinzolamide Acetazolamide Methazolamide Dichlorphenamide | Reduction in aqueous humor production |
| Prostaglandin analogs | Latanoprost Travoprost Bimatoprost Tafluprost Unoprostone Latanoprostene bunod | Increase in aqueous humor outflow |
| Rho kinase inhibitors | Netarsudil | Reduction in aqueous humor production Increase in aqueous humor outflow Reduction in episcleral venous pression |
| Hyperosmotic agents | Glycerol Mannitol Isosorbide | Reduction in aqueous humor volume by moving water out of the eye and into the blood (these medications are used for the short-term management of acute glaucoma) |
| Model | Glaucoma Phenotype | IOP | RGC Loss | Structural Alterations | Onset | Notes |
|---|---|---|---|---|---|---|
| DBA/2J | Pigment dispersion glaucoma | High | Progressive and severe | Pigment dispersion, angle closure, optic nerve atrophy | 6–9 months | Widely used; spontaneous degeneration; inter-individual variability |
| GLAST KO | NTG | Normal | Significant and early | RGC degeneration, oxidative stress, glial activation | ~3 months | Mimics retinal damage from oxidative stress and glial dysfunction |
| EAAC1 KO | NTG | Normal | Early and progressive | Early RGC loss, oxidative damage | ~3 months | Glutamate excitoxicity model; no IOP elevation |
| MYOC Y437H | Juvenile-onset POAG | High | Axonal degeneration | TM dysfunction, ER stress, intracellular myocilin aggregates | 2–4 months (variable) | Humanized knock-in mutation; relevant for genetic studies |
| βB1-CTGF | POAG | High | Axonopathy and RGC apoptosis | α-SMA increase, altered TM cytoskeleton, glial activation | 1 month | Lens-specific CTGF secretion; ECM and TM remodeling |
| AAv-CTGF | POAG | High from day 7 post-injection | Axonal loss by 2 months | α-SMA increase in TM, cytoskeletal reorganization, altered TM ultrastructure | Days to weeks after injection | Acute, inducible model |
| OPTN | NTG | Normal | Progressive | Axonal damage, retinal thinning | 4–6 months | Mimics OPTN mutation-associated NTG |
| P2Y | POAG | High | Progressive | Axonal degeneration, retinal thinning | Variable | Highlights role of purinergic signaling |
| Cav1 | POAG | High | Progressive | Loss of caveolae in Schlemm’s canal/TM, reduced aqueous outflow | Variable | Biomechanical dysfunction |
| ET-1 | NTG | Normal | Progressive | Vascular changes, optic nerve degeneration; retinal thinning | ~12 months | Vascular contribution to NTG |
| Model | Glaucoma Phenotype | IOP | RGC Loss | Structural Alterations | Onset | Notes |
|---|---|---|---|---|---|---|
| Laser photocoagulation | IOP-dependent | High | Progressive | TM damage; Optic nerve degeneration; inflammation | Rapid onset (days) | Mimics human high-tension glaucoma; requires specialized technique |
| Episcleral vein cauterization | IOP-dependent | High | Variable, comparable between rats/mice | Outflow resistance increase; axonal and optic nerve damage | Days to weeks | Reproducible; technically challenging in mice |
| Hypertonic saline injection | IOP-dependent | High | Progressive | TM and SM sclerosis | Weeks to months | Valuable for biomechanical and chronic IOP studies; technically demanding in mice |
| Microbead/viscous material injection | IOP-dependent | High | RGC loss (~25–38%) | Physical TM blockage | Days to weeks | Adjustable IOP elevation; low inflammation; repeated injections may be needed |
| Intravitreal excitotoxins | IOP-independent | Normal | Acute, dose-dependent | Direct RGC injury via excitotoxicity | Immediate | NTG model; may also affect retinal neurons other than RGC |
| Axonal injury (crush/transection) | IOP-independent | Normal | Rapid, severe | Direct axonal damage; Wallerian degeneration | Immediate | Useful for neurodegeneration and regeneration studies |
| Steroid-induced methods | IOP-dependent | Moderately elevated | Gradual, variable | TM function alteration; optic nerve degeneration | Weeks | Different modality of drug administration; high variability |
| Circumlimbal suture | IOP-dependent | High | Progressive | Outflow resistance increase; optic nerve degeneration | Weeks to months | Sustained IOP elevation |
| Experimental autoimmune glaucoma | IOP-independent | Normal | Progressive | Immune-mediated RGC degeneration; microglial activation | Days to weeks | Mimics autoimmune glaucoma pathogenesis |
| Ischemia–reperfusion injury | IOP-dependent | High but transient | Rapid | Retinal cell degeneration | Days | Acute model of ischemic damage; not chronic glaucoma |
| IOP Levels | Model | Type of Human Glaucoma Mimicked | RGC Damage | Onset |
|---|---|---|---|---|
| High | AAv-CTGF | POAG | Progressive | Days to weeks |
| BB1-CTGF | 1 month | |||
| MYOC Y437H | 2–4 months | |||
| P2Y | 4–6 months | |||
| Cav1 | variable | |||
| Laser photocoagulation | Secondary | Days | ||
| EVC | Days to weeks | |||
| Microbead/viscous material injection | ||||
| Hypertonic saline injection | Weeks to months | |||
| Circumlimbal suture | ||||
| DBA2J | 6–9 months | |||
| High but transient | I/R injury | Rapid | Days | |
| Moderately high | Steroid-induced methods | Progressive | Weeks | |
| Normal | Intravitreal cytotoxin | NTG | Acute | Immediate |
| Experimental autoimmune glaucoma | Progressive | Days to weeks | ||
| Axonal injury | Weeks to months | |||
| GLAST KO | About 3 months | |||
| EAAC1 KO | About 3 months | |||
| OPTN | 4–6 months | |||
| ET-1 | About 12 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Marsico, L.; Sturlese Verduri, A.; Marracci, S.; Amato, R.; Dal Monte, M. Rodent Models of Glaucoma: How Mice and Rats Can Help Human Vision Move Out of the Woods and Into the Light. Cells 2025, 14, 1648. https://doi.org/10.3390/cells14211648
Di Marsico L, Sturlese Verduri A, Marracci S, Amato R, Dal Monte M. Rodent Models of Glaucoma: How Mice and Rats Can Help Human Vision Move Out of the Woods and Into the Light. Cells. 2025; 14(21):1648. https://doi.org/10.3390/cells14211648
Chicago/Turabian StyleDi Marsico, Lorenza, Arianna Sturlese Verduri, Silvia Marracci, Rosario Amato, and Massimo Dal Monte. 2025. "Rodent Models of Glaucoma: How Mice and Rats Can Help Human Vision Move Out of the Woods and Into the Light" Cells 14, no. 21: 1648. https://doi.org/10.3390/cells14211648
APA StyleDi Marsico, L., Sturlese Verduri, A., Marracci, S., Amato, R., & Dal Monte, M. (2025). Rodent Models of Glaucoma: How Mice and Rats Can Help Human Vision Move Out of the Woods and Into the Light. Cells, 14(21), 1648. https://doi.org/10.3390/cells14211648







