Ovariectomy Induces Selective Alterations in Dura Mater Blood and Lymphatic Microvascular Network Architecture in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation and Image Acquisition
2.3. Image Processing and Microvascular Analysis
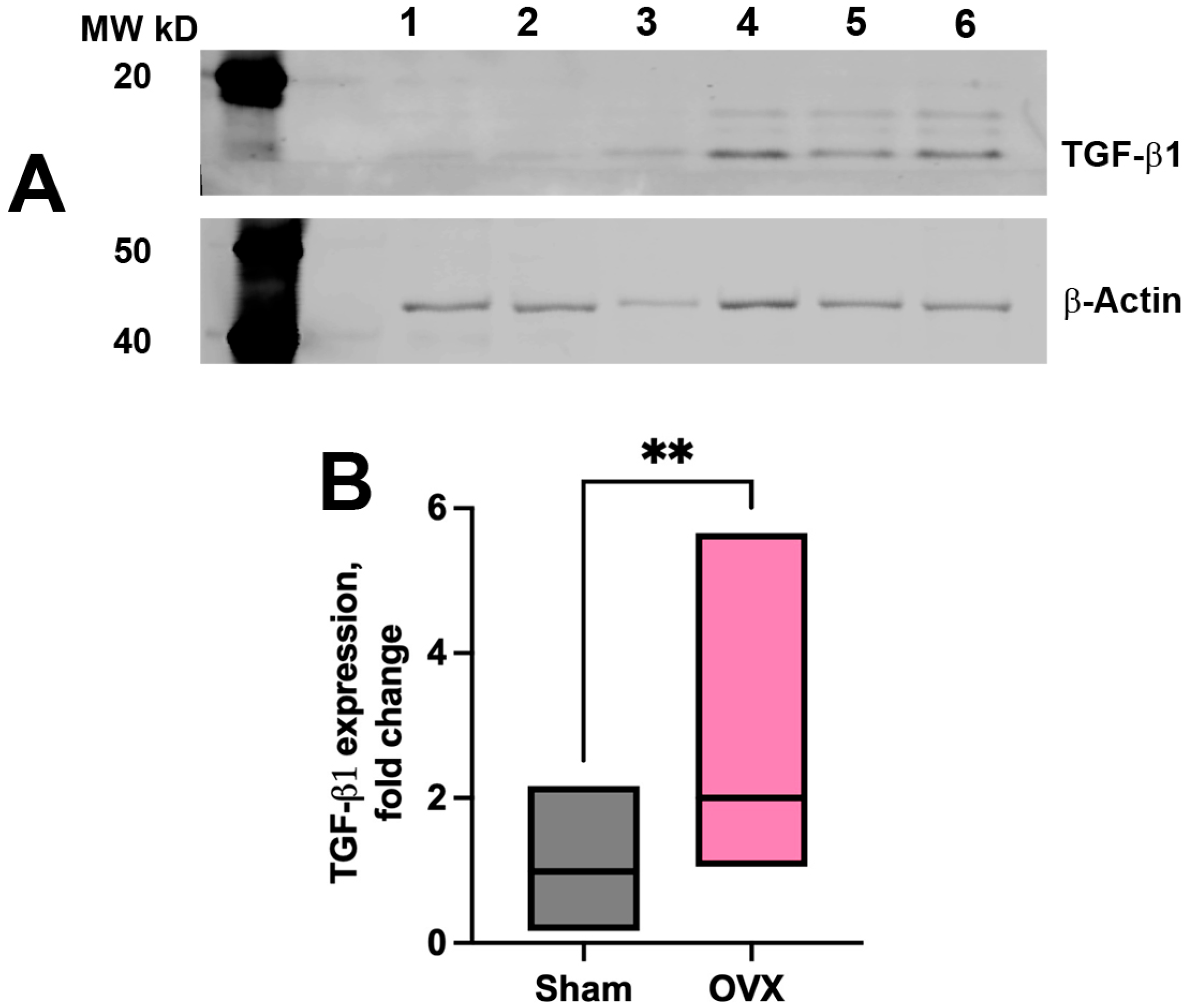
2.4. Western Blot (WB) Analysis
2.5. Statistical Analysis
3. Results
3.1. Ovariectomy Induces Selective Alterations in Microvascular Architecture
3.1.1. Vessel Length
3.1.2. Vessel Segment Length
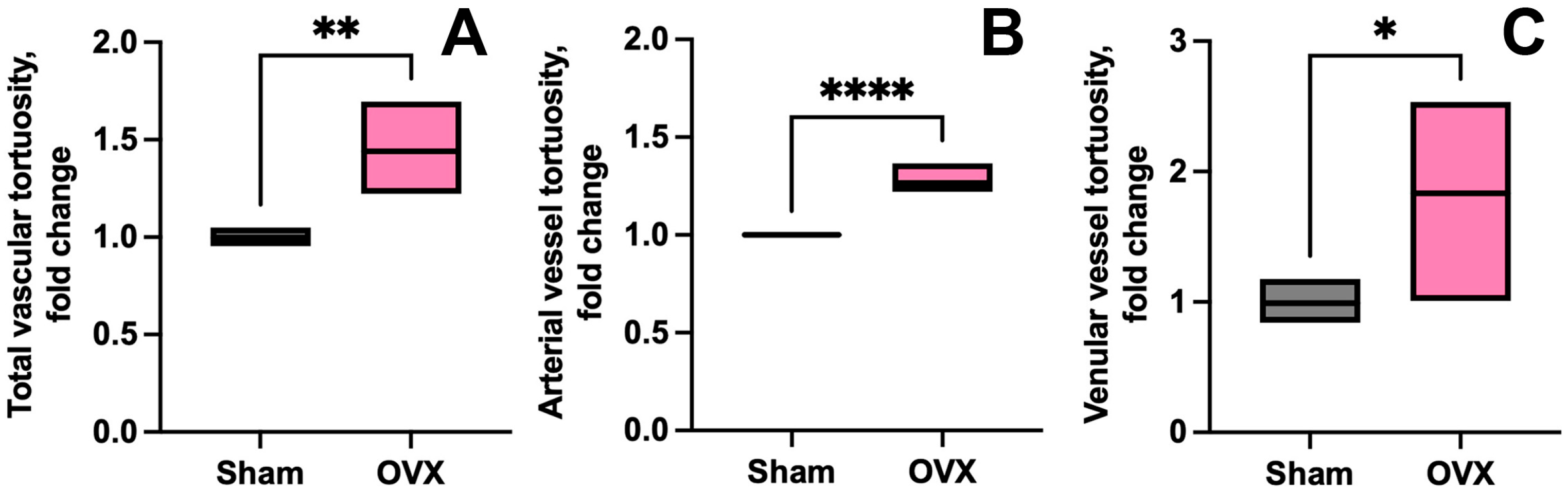
3.1.3. Vessel Tortuosity
3.2. Lymphatic Network Remodeling Within Coronal Suture-Associated Areas
3.3. Vascular and Tissue Stromal Alterations in α-SMA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brass, L.M. Estrogens and Stroke: Use of Oral Contraceptives and Postmenopausal Use of Estrogen: Current Recommendations. Curr. Treat. Opt. Neurol. 2004, 6, 459–467. [Google Scholar] [CrossRef]
- Lowe, G.D. Hormone replacement therapy and cardiovascular disease: Increased risks of venous thromboembolism and stroke, and no protection from coronary heart disease. J. Intern. Med. 2004, 256, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Baeza, I.; De Castro, N.; Giménez-Llort, L.; De la Fuente, M. Ovariectomy, a model of menopause in rodents, causes a premature aging of the nervous and immune systems. J. Neuroimmunol. 2010, 219, 90–99. [Google Scholar] [CrossRef]
- Arnal, J.F.; Fontaine, C.; Billon-Gales, A.; Favre, J.; Laurell, H.; Lenfant, F.; Gourdy, P. Estrogen receptors and endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Bake, S.; Sohrabji, F. 17beta-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology 2004, 145, 5471–5475. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Kaley, G. Gender-specific regulation of cardiovascular function: Estrogen as key player. Microcirculation 2004, 11, 9–38. [Google Scholar]
- Jia, K.; Luo, X.; Yi, J.; Zhang, C. Hormonal influence: Unraveling the impact of sex hormones on vascular smooth muscle cells. Biol. Res. 2024, 57, 61. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. Estrogen and the blood vessel wall. Curr. Opin. Cardiol. 1994, 9, 619–626. [Google Scholar] [CrossRef]
- Dehaini, H.; Fardoun, M.; Abou-Saleh, H.; El-Yazbi, A.; Eid, A.A.; Eid, A.H. Estrogen in vascular smooth muscle cells: A friend or a foe? Vasc. Pharmacol. 2018, 111, 15–21. [Google Scholar] [CrossRef]
- Ruehlmann, D.O.; Cannon, T.R.; Jacob, R.; Mann, G.E. Differential effects of steroids on vascular tone and smooth muscle cell Ca2+-homeostasis. Biochem. Soc. Trans. 1997, 25, 112S. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Karamanos, N.K. Estrogen receptor-mediated targeting of the extracellular matrix network in cancer. Semin. Cancer Biol. 2020, 62, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mangani, S.; Piperigkou, Z.; Koletsis, N.E.; Ioannou, P.; Karamanos, N.K. Estrogen receptors and extracellular matrix: The critical interplay in cancer development and progression. FEBS J. 2025, 292, 1558–1572. [Google Scholar] [CrossRef]
- Zakrzewicz, A.; Secomb, T.W.; Pries, A.R. Angioadaptation: Keeping the vascular system in shape. Physiology 2002, 17, 197–201. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Abraha, T.W.; Turk, J.R.; Rubin, L.J.; Huxley, V.H.; Glinsky, V.V. Microvascular network remodeling in dura mater of ovariectomized pigs: Role for angiopoietin-1 in estrogen-dependent control of vascular stability. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1131–H1137. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Huxley, V.H.; Glinskii, V.V.; Rubin, L.J.; Glinsky, V.V. Pulsed estrogen therapy prevents post-OVX porcine dura mater microvascular network weakening via a PDGF-BB-dependent mechanism. PLoS ONE 2013, 8, e82900. [Google Scholar]
- Glinskii, O.V.; Huxley, V.H.; Glinsky, V.V. Estrogen-Dependent Changes in Dura Mater Microvasculature Add New Insights to the Pathogenesis of Headache. Front. Neurol. 2017, 8, 549. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessel. Nature 2015, 523, 337–341, Erratum in Nature 2016, 533, 278. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Drieu, A.; Mamuladze, T.; de Lima, K.A.; Dykstra, T.; Wall, M.; Papadopoulos, Z.; Kanamori, M.; Salvador, A.F.; Baker, W.; et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 2021, 184, 1000–1016.e27. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, Y.; Li, Y.; Xia, C.; Chen, Z.; Chen, Y.; Feng, H. Meningeal lymphatic drainage: Novel insights into central nervous system disease. Signal Transduct. Target. Ther. 2025, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Glinskii, O.V.; Glinsky, V.V.; Xie, L.; Bunyak, F.; Glinskii, V.V.; Sinha, S.; Gupta, S.; Iozzo, R.V.; Mohan, R.R. Corneal injury is associated with stromal and vascular alterations within cranial dura mater. PLoS ONE 2023, 18, e0284082. [Google Scholar] [CrossRef] [PubMed]
- Glinskii, O.V.; Huxley, V.H.; Xie, L.; Bunyak, F.; Palaniappan, K.; Glinsky, V.V. Complex Non-sinus-associated Pachymeningeal Lymphatic Structures: Interrelationship With Blood Microvasculature. Front. Physiol. 2019, 10, 1364. [Google Scholar] [CrossRef]
- Kassim, Y.M.; Glinskii, O.V.; Glinsky, V.V.; Huxley, V.H.; Palaniappan, K. Patch-Based Semantic Segmentation for Detecting Arterioles and Venules in Epifluorescence Imagery. In Proceedings of the IEEE Applied Imagery Pattern Recognition Workshop (AIPR), Washington, DC, USA, 9–11 October 2018. [Google Scholar]
- Wang, Y.Y.; Glinskii, O.V.; Bunyak, F.; Palaniappan, K. Ensemble of Deep Learning Cascades for Segmentation of Blood Vessels in Confocal Microscopy Images. In Proceedings of the IEEE Applied Imagery Pattern Recognition Workshop, Washington, DC, USA, 12–14 October 2021. [Google Scholar]
- Lee, J.Y.; Park, C.; Cho, Y.P.; Lee, E.; Kim, H.; Kim, P.; Yun, S.H.; Yoon, Y.-S. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 2010, 122, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Ricci, S.; Degan, D.; Carolei, A. Migraine in women: The role of hormones and their impact on vascular diseases. J. Headache Pain 2012, 13, 177–189. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.M.C.S.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.W.; Pries, A.R. The microcirculation: Physiology at the mesoscale. J. Physiol. 2011, 589, 1047–1052. [Google Scholar] [CrossRef]
- Den Uil, C.A.; Lagrand, W.K.; van der Ent, M.; Jewbali, L.S.; Cheng, J.M.; Spronk, P.E.; Simoons, M.L. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2010, 31, 3032–3039. [Google Scholar] [CrossRef]
- Trzeciak, S.; McCoy, J.V.; Dellinger, R.P.; Arnold, R.C.; Rizzuto, M.; Abate, N.L.; Shapiro, N.I.; Parrillo, J.E.; Hollenberg, S.M.; on behalf of the Microcirculatory Alterations in Resuscitation and Shock (MARS) investigators. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensiv. Care Med. 2008, 34, 2210–2217. [Google Scholar]
- De Backer, D.; Ortiz, J.A.; Salgado, D. Coupling microcirculation to systemic hemodynamics. Curr. Opin. Crit. Care 2010, 16, 250–254. [Google Scholar] [CrossRef]
- De Backer, D.; Donadello, K.; Sakr, Y.; Ospina-Tascon, G.; Salgado, D.; Scolletta, S.; Vincent, J.-L. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit. Care Med. 2013, 41, 791–799. [Google Scholar]
- White, E.R. Estrogen and vascular function. Vasc. Pharmacol. 2002, 38, 73–80. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- Koh, K. Effects of estrogen on the vascular wall: Vasomotor function and inflammation. Cardiovasc. Res. 2002, 55, 714–726. [Google Scholar] [CrossRef]
- Kim-Schulze, S.; McGowan, K.A.; Hubchak, S.C.; Cid, M.C.; Martin, M.B.; Kleinman, H.K.; Greene, G.L.; Schnaper, H.W. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation 1996, 94, 1402–1407. [Google Scholar] [CrossRef]
- Russell, K.S.; Haynes, M.P.; Sinha, D.; Clerisme, E.; Bender, J.R. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 5930–5935. [Google Scholar] [CrossRef]
- Li, L.; Haynes, M.P.; Bender, J.R. Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4807–4812. [Google Scholar] [CrossRef]
- An, D.; Chung-Wah-Cheong, J.; Yu, D.-Y.; Balaratnasingam, C. Alpha-Smooth Muscle Actin Expression and Parafoveal Blood Flow Pathways Are Altered in Preclinical Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2022, 63, 8. [Google Scholar] [CrossRef] [PubMed]
- Hutter-Schmid, B.; Humpel, C. Alpha-Smooth Muscle Actin mRNA and Protein Are Increased in Isolated Brain Vessel Extracts of Alzheimer Mice. Pharmacology 2016, 98, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Győri, F.; Mészáros, Á.; Krecsmarik, M.; Molnár, K.; Balta, C.; Hermenean, A.; Farkas, A.E.; Krizbai, I.A.; Wilhelm, I. Expression of alpha smooth muscle actin decreases with ageing and increases upon lumen obstruction in mouse brain pericytes. GeroScience 2025, 47, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Garoffolo, G.; Pesce, M. Vascular dysfunction and pathology: Focus on mechanical forces. Vasc. Biol. 2021, 3, R69–R75. [Google Scholar] [CrossRef]
- Yu, H.; Clarke, M.C.; Figg, N.; Littlewood, T.D.; Bennett, M.R. Smooth muscle cell apoptosis promotes vessel remodeling and repair via activation of cell migration, proliferation, and collagen synthesis. Arter. Thromb. Vasc. Biol. 2011, 31, 2402–2409. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Ramos-Mondragón, R.; Galindo, A.C.; Avila, G. Role of TGF-β on cardiac structural and electrical remodeling. Vasc. Health Risk Manag. 2008, 4, 1289–1300. [Google Scholar] [PubMed]
- Wang, D.; Zhao, Y.; Zhou, Y.; Yang, S.; Xiao, X.; Feng, L. Angiogenesis—An Emerging Role in Organ Fibrosis. Int. J. Mol. Sci. 2023, 24, 14123. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Lazaropoulos, M.P.; Elrod, J.W. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ. Res. 2020, 127, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, J.; Qin, J.; Chen, R.; Yi, Z. Correction to: TGF-beta-induced alpha-SMA expression is mediated by C/EBPbeta acetylation in human alveolar epithelial cells. Mol. Med. 2021, 27, 75. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor-b in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glinskii, O.V.; Toubal, I.E.; Xie, L.; Sinha, S.; Palaniappan, K.; Glinsky, V.V. Ovariectomy Induces Selective Alterations in Dura Mater Blood and Lymphatic Microvascular Network Architecture in Mice. Cells 2025, 14, 1647. https://doi.org/10.3390/cells14211647
Glinskii OV, Toubal IE, Xie L, Sinha S, Palaniappan K, Glinsky VV. Ovariectomy Induces Selective Alterations in Dura Mater Blood and Lymphatic Microvascular Network Architecture in Mice. Cells. 2025; 14(21):1647. https://doi.org/10.3390/cells14211647
Chicago/Turabian StyleGlinskii, Olga V., Imad Eddine Toubal, Leike Xie, Sunilima Sinha, Kannappan Palaniappan, and Vladislav V. Glinsky. 2025. "Ovariectomy Induces Selective Alterations in Dura Mater Blood and Lymphatic Microvascular Network Architecture in Mice" Cells 14, no. 21: 1647. https://doi.org/10.3390/cells14211647
APA StyleGlinskii, O. V., Toubal, I. E., Xie, L., Sinha, S., Palaniappan, K., & Glinsky, V. V. (2025). Ovariectomy Induces Selective Alterations in Dura Mater Blood and Lymphatic Microvascular Network Architecture in Mice. Cells, 14(21), 1647. https://doi.org/10.3390/cells14211647





