Modelling Osteoporosis in Pre-Clinical Research—Challenges, Trends and New Approaches
Highlights
- A comprehensive comparative analysis of current approaches to model osteoporosis, including in vivo, in vitro, and in silico systems.
- Identification of key limitations of current animal models and the emerging potential of advanced human-derived in vitro platforms.
- Advanced in vitro and organ-on-a-chip technologies are useful tools for bridging the translational gap between experimental research and clinical application.
- Integrating human-based and computational models may accelerate the development of more predictive, mechanism-driven therapeutic strategies.
Abstract
1. Introduction
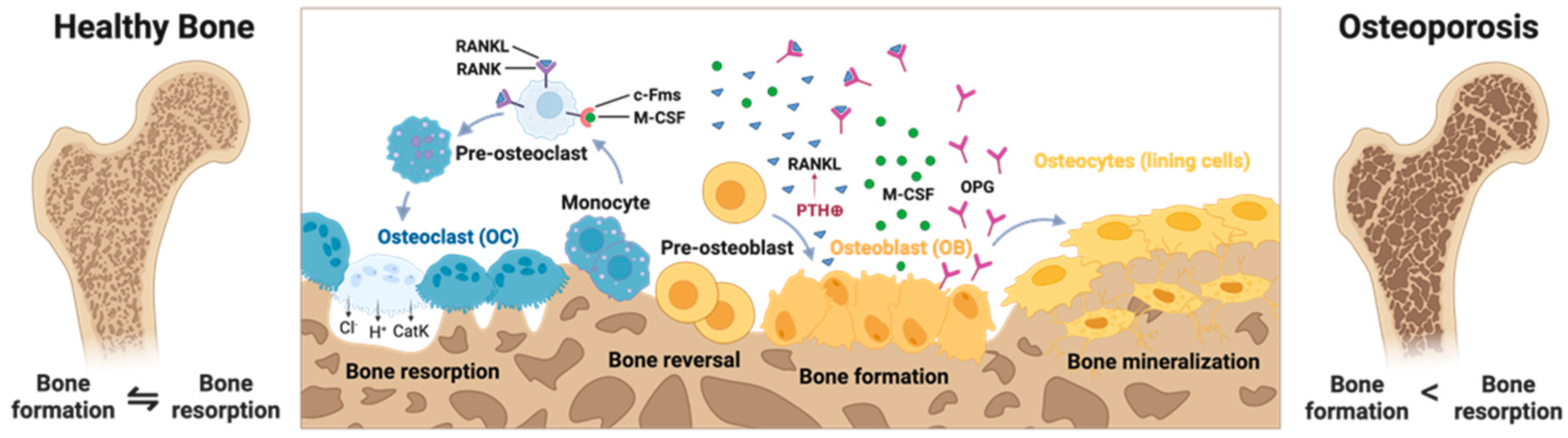
2. Modeling Osteoporosis In Vivo
2.1. In Vivo Models of Post-Menopausal Osteoporosis
2.2. In Vivo Models of Disuse Osteoporosis
2.3. In Vivo Models of Glucorticoid-Induced Osteoporosis
2.4. Limitations
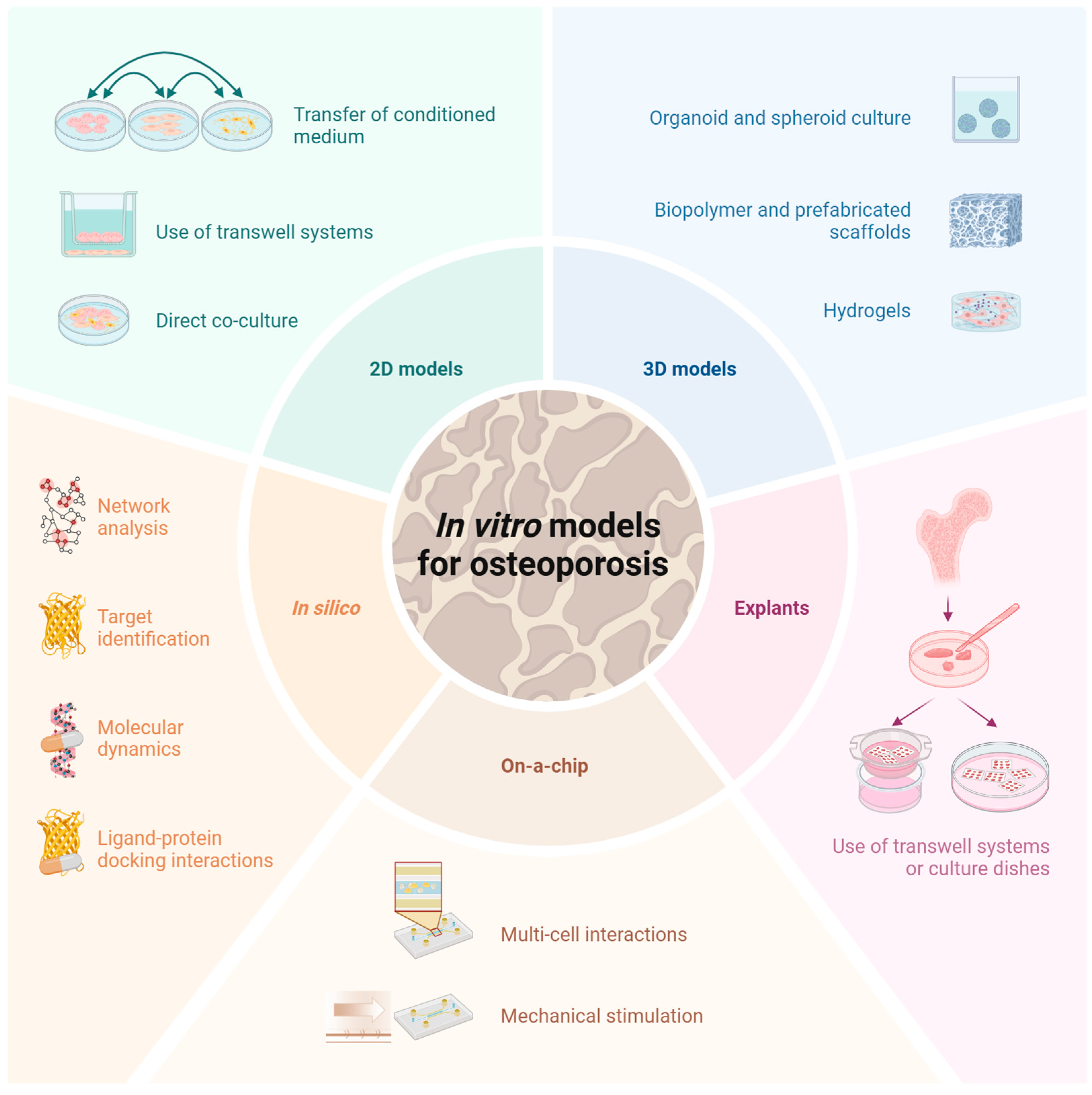
3. Modeling Osteoporosis In Vitro
3.1. Two-Dimensional In Vitro Models
3.2. Three-Dimensional In Vitro Models
3.3. Ex Vivo Models/Explants
3.4. On-a-Chip Models
3.5. In Silico Models
3.6. Ability to Resemble Osteoporosis
3.7. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Odén, A.; McCloskey, E.V.; Johansson, H.; Kanis, J.A. Assessing the Impact of Osteoporosis on the Burden of Hip Fractures. Calcif. Tissue Int. 2013, 92, 42–49. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos. Int. 2004, 15, 897–902. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation. 2025. Available online: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures (accessed on 14 August 2025).
- Bonjour, P.; Compston, J.; Dawson-Hughes, B.; Delmas, P.; Johansson, H.; Kanis, J.A.; Lau, E.; Lindsay, R.; Melton, J.; McClung, M.R.; et al. Assessment of Osteoporosis at the Primary Health Care Level; University of Sheffield: Sheffield, UK, 2007. [Google Scholar]
- Felsenberg, D.; Maurer, L. Osteoporose. Harrisons Innere Medizin. 20; Felsenberg, D., Ed.; ABW Wissenschaftsverlag: Berlin, Germany, 2020; pp. 3645–3678. [Google Scholar]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Boyadzhieva, Z.; Palmowski, A.; Buttgereit, F.; Hoff, P. Trabecular Bone Score in der Rheumatologie. Z. Für Rheumatol. 2023, 82, 672–677. [Google Scholar] [CrossRef]
- Al-Hashimi, L.; Klotsche, J.; Ohrndorf, S.; Gaber, T.; Hoff, P. Trabecular Bone Score Significantly Influences Treatment Decisions in Secondary Osteoporosis. J. Clin. Med. 2023, 12, 4147. [Google Scholar] [CrossRef]
- Morgan, E.F.; Gerstenfeld, L.C. Chapter 2—The bone organ system: Form and function. In Marcus and Feldman’s Osteoporosis, 5th ed.; Dempster, D.W., Cauley, J.A., Bouxsein, M.L., Cosman, F., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 15–35. [Google Scholar]
- Tresguerres, F.G.F.; Torres, J.; López, Q.; Hernández, G.; Vega, J.A.; Tresguerres, I.F. The osteocyte: A multifunctional cell within the bone. Ann. Anat. 2020, 227, 31563568, Erratum in Anat. Anz. 2020, 230, 151510. [Google Scholar] [CrossRef]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2021, 9, 770143. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, X.; Wan, Y. Minireview: Nuclear receptor regulation of osteoclast and bone remodeling. Mol. Endocrinol. 2015, 29, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Marcus, R. Chapter 48—Osteoporosis Associated with Illnesses and Medications. In Osteoporosis, 4th ed.; Marcus, R., Feldman, D., Dempster, D.W., Luckey, M., Cauley, J.A., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 1173–1190. [Google Scholar]
- Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Nagy, E.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; El-Husseini, A. Secondary Osteoporosis and Metabolic Bone Diseases. J. Clin. Med. 2022, 11, 2382. [Google Scholar] [CrossRef]
- Mirza, F.; Canalis, E. Management of endocrine disease: Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Legrand, E.; Hedde, C.; Gallois, Y.; Degasne, I.; Boux de Casson, F.; Mathieu, E.; Baslé, M.F.; Chappard, D.; Audran, M. Osteoporosis in men: A potential role for the sex hormone binding globulin. Bone 2001, 29, 90–95. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Delmas, P.D. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 2000, 355, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Geusens, P.P.M.M.; Boonen, S. Osteoporosis and the Growth Hormone-Insulin-Like Growth Factor Axis. Horm. Res. 2004, 58 (Suppl. S3), 49–55. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Palmowski, A.; Bond, M.; Adami, G.; Dejaco, C. Osteoporosis and fracture risk are multifactorial in patients with inflammatory rheumatic diseases. Nat. Rev. Rheumatol. 2024, 20, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, A.; Boyadzhieva, Z.; Nielsen, S.M.; Muche, B.; Hermann, S.; Boers, M.; Bliddal, H.; Christensen, R.; Wiebe, E.; Buttgereit, F. Sex and age do not modify the association between glucocorticoids and bone mineral density in patients with rheumatoid arthritis: A cross-sectional study. Arthritis Res. Ther. 2023, 25, 98. [Google Scholar] [CrossRef]
- Ilias, I.; Milionis, C.; Zoumakis, E. An Overview of Glucocorticoid-Induced Osteoporosis South Dartmouth. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278968/ (accessed on 14 August 2025).
- Van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Zhang, B.; Cooper, C. Use of Oral Corticosteroids and Risk of Fractures. J. Bone Miner. Res. 2000, 15, 993–1000. [Google Scholar] [CrossRef]
- Albrecht, K.; Huscher, D.; Buttgereit, F.; Aringer, M.; Hoese, G.; Ochs, W.; Thiele, K.; Zink, A. Long-term glucocorticoid treatment in patients with polymyalgia rheumatica, giant cell arteritis, or both diseases: Results from a national rheumatology database. Rheumatol. Int. 2018, 38, 569–577. [Google Scholar] [CrossRef]
- Canalis, E.; Delany, A.M. Mechanisms of glucocorticoid action in bone. Ann. N. Y. Acad. Sci. 2002, 966, 73–81. [Google Scholar] [CrossRef]
- Wiebe, E.; Huscher, D.; Schaumburg, D.; Palmowski, A.; Hermann, S.; Buttgereit, T.; Biesen, R.; Burmester, G.-R.; Palmowski, Y.; Boers, M.; et al. Optimising both disease control and glucocorticoid dosing is essential for bone protection in patients with rheumatic disease. Ann. Rheum. Dis. 2022, 81, 1313–1322. [Google Scholar] [CrossRef]
- Palmowski, A.; Wiebe, E.; Muche, B.; Hermann, S.; Dejaco, C.; Matteson, E.L.; Buttgereit, F. Glucocorticoids Are Not Associated with Bone Mineral Density in Patients with Polymyalgia Rheumatica, Giant Cell Arteritis and Other Vasculitides—Cross-Sectional Baseline Analysis of the Prospective Rh-GIOP Cohort. Cells 2022, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Fassio, A.; Rossini, M.; Benini, C.; Pistillo, F.; Viapiana, O.; Bertelle, D.; Gatti, D. Bone Loss in Inflammatory Rheumatic Musculoskeletal Disease Patients Treated With Low-Dose Glucocorticoids and Prevention by Anti-Osteoporosis Medications. Arthritis Rheumatol. 2023, 75, 1762–1769. [Google Scholar] [CrossRef]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2022, 110, 592–604. [Google Scholar] [CrossRef]
- Inderjeeth, C.A.; Inderjeeth, D.C. The use of anabolic agents in the treatment of osteoporosis: A clinical update. Curr. Opin. Endocrinol. Diabetes Obes. 2024, 31, 157–163. [Google Scholar] [CrossRef]
- Hansen, K.E.; Mortezavi, M.; Nagy, E.; Wang, C.; Connell, C.A.; Radi, Z.; Litman, H.J.; Adami, G.; Rossini, M. Fracture in clinical studies of tofacitinib in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221142346. [Google Scholar] [CrossRef]
- Gaber, T.; Brinkman, A.C.K.; Pienczikowski, J.; Diesing, K.; Damerau, A.; Pfeiffenberger, M.; Lang, A.; Ohrndorf, S.; Burmester, G.-R.; Buttgereit, F.; et al. Impact of Janus Kinase Inhibition with Tofacitinib on Fundamental Processes of Bone Healing. Int. J. Mol. Sci. 2020, 21, 865. [Google Scholar] [CrossRef]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef]
- Dachverband Osteologie, e.V. Prophylaxe, Diagnostik und Therapie der OSTEOPOROSE bei Postmenopausalen Frauen und bei Männern ab dem 50. Lebensjahr Leitlinie des Dachverbands der Deutschsprachigen Wissenschaftlichen Osteologischen Gesellschaften e.V. 2023. Available online: https://leitlinien.dv-osteologie.org/wp-content/uploads/2024/02/DVO-Leitlinie-zur-Diagnostik-und-Therapie-der-Osteoporose-Version-2.1.-2023-002.pdf (accessed on 14 August 2025).
- Loddenkemper, K.; Bohl, N.; Perka, C.; Burmester, G.-R.; Buttgereit, F. Correlation of different bone markers with bone density in patients with rheumatic diseases on glucocorticoid therapy. Rheumatol. Int. 2006, 26, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar]
- Gasparini, S.J.; Weber, M.C.; Henneicke, H.; Kim, S.; Zhou, H.; Seibel, M.J. Continuous corticosterone delivery via the drinking water or pellet implantation: A comparative study in mice. Steroids 2016, 116, 76–82. [Google Scholar] [CrossRef]
- Li, J.; Geng, J.; Lin, T.; Cai, M.; Sun, Y. A mouse model of disuse osteoporosis based on a movable noninvasive 3D-printed unloading device. J. Orthop. Translat. 2022, 33, 1–12. [Google Scholar] [CrossRef]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; van Rietbergen, B. Bone remodelling in humans is load-driven but not lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef]
- Oheim, R.; Simon, M.J.K.; Steiner, M.; Vettorazzi, E.; Barvencik, F.; Ignatius, A.; Amling, M.; Clarke, I.J.; Pogoda, P.; Beil, F.T. Sheep model for osteoporosis: The effects of peripheral hormone therapy on centrally induced systemic bone loss in an osteoporotic sheep model. Injury 2017, 48, 841–848. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Chapter 39—Animal Models for Osteoporosis. In Osteoporosis, 4th ed.; Marcus, R., Feldman, D., Dempster, D.W., Luckey, M., Cauley, J.A., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 939–961. [Google Scholar]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Burr, D.B. Basic biomechanical measurements of bone: A tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.; Toumi, H.; Lespessailles, E. Animal Model for Glucocorticoid Induced Osteoporosis: A Systematic Review from 2011 to 2021. Int. J. Mol. Sci. 2021, 23, 377. [Google Scholar] [CrossRef]
- FDA Osteoporosis: Nonclinical Evaluation of Drugs Intended for Treatment Guidance for Industry. 2019. Available online: https://www.fda.gov/media/129899/download (accessed on 14 August 2025).
- Brent, M.B. Pharmaceutical treatment of bone loss: From animal models and drug development to future treatment strategies. Pharmacol. Ther. 2023, 244, 108383. [Google Scholar] [CrossRef]
- Yılmaz, D.; Mathavan, N.; Wehrle, E.; Kuhn, G.A.; Müller, R. Mouse models of accelerated aging in musculoskeletal research for assessing frailty, sarcopenia, and osteoporosis—A review. Ageing Res. Rev. 2024, 93, 102118. [Google Scholar] [CrossRef]
- Wang, L.T.; Chen, L.R.; Chen, K.H. Hormone-Related and Drug-Induced Osteoporosis: A Cellular and Molecular Overview. Int. J. Mol. Sci. 2023, 24, 5814. [Google Scholar] [CrossRef]
- Nalvarte, I.; Antonson, P. Estrogen Receptor Knockout Mice and Their Effects on Fertility. Receptors 2023, 2, 116–126. [Google Scholar] [CrossRef]
- Kõks, S.; Dogan, S.; Tuna, B.G.; González-Navarro, H.; Potter, P.; Vandenbroucke, R.E. Mouse models of ageing and their relevance to disease. Mech. Ageing Dev. 2016, 160, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.C.; Giorgi, M.; Oliviero, S.; Wang, N.; Boudiffa, M.; Dall’Ara, E. The longitudinal effects of ovariectomy on the morphometric, densitometric and mechanical properties in the murine tibia: A comparison between two mouse strains. Bone 2019, 127, 260–270. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [PubMed]
- Shao, B.; Fu, X.; Yu, Y.; Yang, D. Regulatory effects of miRNA-181a on FasL expression in bone marrow mesenchymal stem cells and its effect on CD4+T lymphocyte apoptosis in estrogen deficiency-induced osteoporosis. Mol. Med. Rep. 2018, 18, 920–930. [Google Scholar] [CrossRef]
- Shao, B.; Liao, L.; Yu, Y.; Shuai, Y.; Su, X.; Jing, H.; Yang, D.; Jin, Y. Estrogen preserves Fas ligand levels by inhibiting microRNA-181a in bone marrow-derived mesenchymal stem cells to maintain bone remodeling balance. FASEB J. 2015, 29, 3935–3944. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Wang, L.; Chen, H.; Fang, C.; Cui, J.; Hu, Y.; Cao, L.; Weng, W.; Zhou, Q.; Qin, L.; et al. l-tetrahydropalmatine suppresses osteoclastogenesis in vivo and in vitro via blocking RANK-TRAF6 interactions and inhibiting NF-κB and MAPK pathways. J. Cell Mol. Med. 2020, 24, 785–798. [Google Scholar] [CrossRef]
- Zainabadi, K.; Liu, C.J.; Caldwell, A.L.M.; Guarente, L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS ONE 2017, 12, e0185236. [Google Scholar] [CrossRef]
- Miao, Q.; Li, J.G.; Miao, S.; Hu, N.; Zhang, J.; Zhang, S.; Xie, Y.H.; Wang, J.B.; Wang, S.W. The bone-protective effect of genistein in the animal model of bilateral ovariectomy: Roles of phytoestrogens and PTH/PTHR1 against post-menopausal osteoporosis. Int. J. Mol. Sci. 2012, 13, 56–70. [Google Scholar] [CrossRef]
- Larrañaga-Vera, A.; Toti, K.S.; Flatow, J.S.; Haraczy, A.J.; Warnick, E.; Rao, H.; Gao, Z.G.; Sussman, S.M.; Mediero, A.; Leucht, P.; et al. Novel alendronate-CGS21680 conjugate reduces bone resorption and induces new bone formation in post-menopausal osteoporosis and inflammatory osteolysis mouse models. Arthritis Res. Ther. 2022, 24, 265. [Google Scholar] [CrossRef] [PubMed]
- Gera, S.; Sampathi, S.; Maddukuri, S.; Dodoala, S.; Junnuthula, V.; Dyawanapelly, S. Therapeutic Potential of Naringenin Nanosuspension: In Vitro and In Vivo Anti-Osteoporotic Studies. Pharmaceutics 2022, 14, 1449. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.S. The sheep as a model for osteoporosis in humans. Vet. J. 2002, 163, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.R.; Camassa, J.A.; Bordelo, J.A.; Babo, P.S.; Viegas, C.A.; Dourado, N.; Reis, R.L.; Gomes, M.E. Preclinical and Translational Studies in Small Ruminants (Sheep and Goat) as Models for Osteoporosis Research. Curr. Osteoporos. Rep. 2018, 16, 182–197. [Google Scholar] [CrossRef]
- Eschler, A.; Roepenack, P.; Roesner, J.; Herlyn, P.K.; Martin, H.; Reichel, M.; Rotter, R.; Vollmar, B.; Mittlmeier, T.; Gradl, G. Cementless Titanium Mesh Fixation of Osteoporotic Burst Fractures of the Lumbar Spine Leads to Bony Healing: Results of an Experimental Sheep Model. Biomed. Res. Int. 2016, 2016, 4094161. [Google Scholar] [CrossRef]
- Dittmer, K.E.; Chernyavtseva, A.; Marshall, J.C.; Cabrera, D.; Wolber, F.M.; Kruger, M. Expression of Renal Vitamin D and Phosphatonin-Related Genes in a Sheep Model of Osteoporosis. Animals 2021, 12, 67. [Google Scholar] [CrossRef]
- Rupp, M.; Biehl, C.; Malhan, D.; Hassan, F.; Attia, S.; Rosch, S.; Schäfer, A.B.; McMahon, E.; Kampschulte, M.; Heiss, C.; et al. Large Animal Model of Osteoporotic Defect Healing: An Alternative to Metaphyseal Defect Model. Life 2021, 11, 254. [Google Scholar] [CrossRef]
- Colman, R.J. Non-human primates as a model for aging. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864 Pt A, 2733–2741. [Google Scholar] [CrossRef]
- Colman, R.J.; Kemnitz, J.W.; Lane, M.A.; Abbott, D.H.; Binkley, N. Skeletal Effects of Aging and Menopausal Status in Female Rhesus Macaques. J. Clin. Endocrinol. Metab. 1999, 84, 4144–4148. [Google Scholar] [CrossRef]
- Grynpas, M.D.; Chachra, D.; Lundon, K. Bone quality in animal models of osteoporosis. Drug Dev. Res. 2000, 49, 146–158. [Google Scholar] [CrossRef]
- Saltzman, W.; Abbott, D.H.; Binkley, N.; Colman, R.J. Maintenance of bone mass despite estrogen depletion in female common marmoset monkeys (Callithrix jacchus). Am. J. Primatol. 2019, 81, e22905. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Mutsuzaki, H.; Hara, Y.; Nagashima, K.; Okano, E.; Yanagisawa, Y.; Noguchi, H.; Sankai, T.; Yamazaki, M. Safety and Osteointegration of Titanium Screws Coated with a Fibroblast Growth Factor-2-Calcium Phosphate Composite Layer in Non-Human Primates: A Pilot Study. J. Funct. Biomater. 2023, 14, 261. [Google Scholar] [CrossRef]
- Wen, C.; Wang, D.; Zhang, Z.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; Xu, X. Intervention Effects of Deer-Tendon Collagen Hydrolysates on Osteoporosis In Vitro and In Vivo. Molecules 2023, 28, 6275. [Google Scholar] [CrossRef]
- Xue, C.; Pan, W.; Lu, X.; Guo, J.; Xu, G.; Sheng, Y.; Yuan, G.; Zhao, N.; Sun, J.; Guo, X.; et al. Effects of compound deer bone extract on osteoporosis model mice and intestinal microflora. J. Food Biochem. 2021, 45, e13740. [Google Scholar] [CrossRef]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. A Systematic Review of Animal Models of Disuse-Induced Bone Loss. Calcif. Tissue Int. 2021, 108, 561–575. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Silva, M.J. HIF-1α regulates bone formation after osteogenic mechanical loading. Bone 2015, 73, 98–104. [Google Scholar] [CrossRef]
- Xue, Y.; Li, Z.; Wang, Y.; Zhu, X.; Hu, R.; Xu, W. Role of the HIF-1α/SDF-1/CXCR4 signaling axis in accelerated fracture healing after craniocerebral injury. Mol. Med. Rep. 2020, 22, 2767–2774. [Google Scholar] [CrossRef]
- Bie, M.; Tang, Y.; Xia, Y.; Zhang, Q.; Tian, Y.; Cheng, C.; Li, X.; Qi, X.; Kang, F. HIF-1α mediates osteoclast-induced disuse osteoporosis via cytoophidia in the femur of mice. Bone 2023, 168, 116648. [Google Scholar] [CrossRef]
- Lyu, F.; Wang, L.; Jia, Y.; Wang, Y.; Qi, H.; Dai, Z.; Zhou, X.; Zhu, H.; Li, B.; Xu, Y.; et al. Analysis of Zinc and Stromal Immunity in Disuse Osteoporosis: Mendelian Randomization and Transcriptomic Analysis. Orthop. Surg. 2023, 15, 2947–2959. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Muraca, M.; Teti, A.; Rucci, N. Circulating Extracellular Vesicles Express Receptor Activator of Nuclear Factor κB Ligand and Other Molecules Informative of the Bone Metabolic Status of Mouse Models of Experimentally Induced Osteoporosis. Calcif. Tissue Int. 2023, 112, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zuo, B.; Tao, B.; Wang, C.; Li, Y.; Peng, J.; Shen, C.; Cui, Y.; Zhu, J.; Chen, X. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann. Transl. Med. 2021, 9, 798. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, F.; Liang, C.; Hu, L.; Li, D.; Zhang, Y.; Yin, C.; Chen, L.; Wang, L.; Lin, X.; et al. Silencing of miR-138-5p sensitizes bone anabolic action to mechanical stimuli. Theranostics 2020, 10, 12263–12278. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Wang, H.; Wang, Y.; Tan, Y.; Dang, L.; Wang, K.; Sun, Z.; Li, G.; Cao, X.; et al. Targeted silencing of miRNA-132-3p expression rescues disuse osteopenia by promoting mesenchymal stem cell osteogenic differentiation and osteogenesis in mice. Stem. Cell Res. Ther. 2020, 11, 58. [Google Scholar] [CrossRef]
- Ge, X.; Zhou, G. Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway. Am. J. Transl. Res. 2021, 13, 6330–6341. [Google Scholar]
- Li, M.; Yang, N.; Hao, L.; Zhou, W.; Li, L.; Liu, L.; Yang, F.; Xu, L.; Yao, G.; Zhu, C.; et al. Melatonin Inhibits the Ferroptosis Pathway in Rat Bone Marrow Mesenchymal Stem Cells by Activating the PI3K/AKT/mTOR Signaling Axis to Attenuate Steroid-Induced Osteoporosis. Oxid. Med. Cell Longev. 2022, 2022, 8223737. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Mohd Ramli, E.S.; Abdullah Sani, N.; Abd Ghafar, N.; Soelaiman, I.N.; Chin, K.Y. Tocotrienol as a Protecting Agent against Glucocorticoid-Induced Osteoporosis: A Mini Review of Potential Mechanisms. Molecules 2022, 27, 5862. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.S. Animal models of osteoporosis--necessity and limitations. Eur. Cell Mater. 2001, 1, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Reinwald, S.; Burr, D. Review of Nonprimate, Large Animal Models for Osteoporosis Research. J. Bone Miner. Res. 2008, 23, 1353–1368. [Google Scholar] [CrossRef]
- Feng, P.; Shu, S.; Zhao, F. Anti-osteoporosis Effect of Fisetin against Ovariectomy Induced Osteoporosis in Rats: In silico, in vitro and in vivo Activity. J. Oleo Sci. 2022, 71, 105–118. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.C.; González-Burgos, E.; Lalatsa, A.; Serrano, D.R. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar] [CrossRef]
- Tao, L.Y.; Łagosz-Ćwik, K.B.; Hogervorst, J.M.A.; Schoenmaker, T.; Grabiec, A.M.; Forouzanfar, T.; van der Weijden, F.A.; de Vries, T.J. Diabetes Medication Metformin Inhibits Osteoclast Formation and Activity in In Vitro Models for Periodontitis. Front. Cell Dev. Biol. 2022, 9, 777450. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, H.; Qi, G.; Jiang, C.; Chen, K.; Yan, Z. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: An in vitro and in vivo study. IUBMB Life 2022, 74, 1052–1069. [Google Scholar] [CrossRef]
- Zhu, S.; Häussling, V.; Aspera-Werz, R.H.; Chen, T.; Braun, B.; Weng, W.; Histing, T.; Nussler, A.K. Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model. Int. J. Mol. Sci. 2021, 22, 53. [Google Scholar] [CrossRef]
- Cha, M.; Lee, K.M.; Lee, J.H. Positive Effects of Bisphosphonates on Osteogenic Differentiation in Patient-Derived Mesenchymal Stem Cells for the Treatment of Osteoporosis. Tissue Eng. Regen. Med. 2018, 15, 467–475. [Google Scholar] [CrossRef]
- Hu, W.-X.; Li, H.; Jiang, J.-Z. MiR-491-3p is down-regulated in postmenopausal osteoporosis and affects growth, differentiation and apoptosis of hFOB1.19 cells through targeting CTSS. Folia Histochem. Cytobiol. 2020, 58, 9–16. [Google Scholar] [CrossRef]
- Lv, H.; Yang, T.; He, A.; Wang, M.; Jia, H.; Ma, M.; Li, S. miR-27b attenuates dexamethasone-inhibited proliferation and osteoblastic differentiation in MC3T3-E1 cells by targeting PPARγ2. Exp. Ther. Med. 2022, 23, 127. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Brom, V.C.; Strauss, A.C.; Sieberath, A.; Salber, J.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Agonistic and antagonistic targeting of immune checkpoint molecules differentially regulate osteoclastogenesis. Front. Immunol. 2023, 14, 988365. [Google Scholar] [CrossRef] [PubMed]
- Mandatori, D.; Penolazzi, L.; Pelusi, L.; Lambertini, E.; Michelucci, F.; Porreca, A.; Cerritelli, P.; Pipino, C.; Di Iorio, A.; Bruni, D.; et al. Three-Dimensional Co-Culture System of Human Osteoblasts and Osteoclast Precursors from Osteoporotic Patients as an Innovative Model to Study the Role of Nutrients: Focus on Vitamin K2. Nutrients 2021, 13, 2823. [Google Scholar] [CrossRef] [PubMed]
- Pelusi, L.; Mandatori, D.; Di Pietrantonio, N.; Del Pizzo, F.; Di Tomo, P.; Di Pietro, N.; Buda, R.; Genovese, S.; Epifano, F.; Pandolfi, A.; et al. Estrogen Receptor 1 (ESR1) and the Wnt/beta-Catenin Pathway Mediate the Effect of the Coumarin Derivative Umbelliferon on Bone Mineralization. Nutrients 2022, 14, 3209. [Google Scholar] [CrossRef]
- Hulley, P.A.; Papadimitriou-Olivgeri, I.; Knowles, H.J. Osteoblast–Osteoclast Coculture Amplifies Inhibitory Effects of FG-4592 on Human Osteoclastogenesis and Reduces Bone Resorption. JBMR Plus. 2020, 4, e10370. [Google Scholar] [CrossRef]
- Cramer, E.E.A.; Ito, K.; Hofmann, S. Ex vivo Bone Models and Their Potential in Preclinical Evaluation. Curr. Osteoporos. Rep. 2021, 19, 75–87. [Google Scholar] [CrossRef]
- Salamanna, F.; Borsari, V.; Brogini, S.; Torricelli, P.; Cepollaro, S.; Cadossi, M.; Fini, M. A Human 3D In Vitro Model to Assess the Relationship Between Osteoporosis and Dissemination to Bone of Breast Cancer Tumor Cells. J. Cell. Physiol. 2017, 232, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Mansoorifar, A.; Gordon, R.; Bergan, R.; Bertassoni, L.E. Bone-on-a-chip: Microfluidic technologies and microphysiologic models of bone tissue. Adv. Funct. Mater. 2021, 31, 2006796. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Liu, X. Osteon: Structure, Turnover, and Regeneration. Tissue Eng. Part B Rev. 2022, 28, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.; Kim, S.; Tak, S.; Kim, M.K.; Park, J.; Chung, S.; Park, T.H.; Kim, J.A. A high-throughput biomimetic bone-on-a-chip platform with artificial intelligence-assisted image analysis for osteoporosis drug testing. Bioeng. Transl. Med. 2023, 8, e10313. [Google Scholar] [CrossRef]
- Glaser, D.E.; Curtis, M.B.; Sariano, P.A.; Rollins, Z.A.; Shergill, B.S.; Anand, A.; Deely, A.M.; Shirure, V.S.; Anderson, L.; Lowen, J.M.; et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials 2022, 280, 121245. [Google Scholar] [CrossRef]
- Vis, M.A.M.; Zhao, F.; Bodelier, E.S.R.; Bood, C.M.; Bulsink, J.; van Doeselaar, M.; Amirabadi, H.E.; Ito, K.; Hofmann, S. Osteogenesis and osteoclastogenesis on a chip: Engineering a self-assembling 3D coculture. Bone 2023, 173, 116812. [Google Scholar] [CrossRef]
- Defranoux, N.A.; Stokes, C.L.; Young, D.L.; Kahn, A.J. In Silico Modeling and Simulation of Bone Biology: A Proposal. J. Bone Miner. Res. 2005, 20, 1079–1084. [Google Scholar] [CrossRef]
- Imanpour, A.; Kolahi Azar, H.; Makarem, D.; Nematollahi, Z.; Nahavandi, R.; Rostami, M.; Beheshtizadeh, N. In silico engineering and simulation of RNA interferences nanoplatforms for osteoporosis treating and bone healing promoting. Sci. Rep. 2023, 13, 18185, Erratum in Sci. Rep. 2024, 14, 2963. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Gregson, C.L.; Tang, H.; van der Kamp, M.; Leo, P.; McInerney-Leo, A.M.; Zheng, J.; Brandi, M.L.; Tang, J.C.Y.; Fraser, W.; et al. Rare and Common Variants in GALNT3 May Affect Bone Mass Independently of Phosphate Metabolism. J. Bone Miner. Res. 2023, 38, 678–691. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, C.; Li, R.; Chen, B.; Zhang, Y.; Sun, Z.; Wei, J.; Zhou, H.; Gu, Q.; Xu, J. Identification of a binding site on soluble RANKL that can be targeted to inhibit soluble RANK-RANKL interactions and treat osteoporosis. Nat. Commun. 2022, 13, 5338. [Google Scholar] [CrossRef]
- Kato, H.; Ansh, A.J.; Lester, E.R.; Kinoshita, Y.; Hidaka, N.; Hoshino, Y.; Koga, M.; Taniguchi, Y.; Uchida, T.; Yamaguchi, H.; et al. Identification of ENPP1 Haploinsufficiency in Patients With Diffuse Idiopathic Skeletal Hyperostosis and Early-Onset Osteoporosis. J. Bone Miner. Res. 2022, 37, 1125–1135. [Google Scholar] [CrossRef]
- Govindan, R.; El-Sherbiny, M.; Ibraheem, K.M.M.; Narasimhan, S.; Salama, M.E.-D.M.; Ahmad, F.; Jayaraman, S.; Veeraraghavan, V.P.; Vengadassalapathy, S.; Mohan, S.K.; et al. Thyroid-Stimulating Hormone Favors Runx2-Mediated Matrix Mineralization in HOS and SaOS2 Cells: An In Vitro and In Silico Approach. Molecules 2022, 27, 613. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Shehab, D.; Jarallah, K.A.; Gupta, R.; Raghupathy, R. Circulatory Levels of RANKL, OPG, and Oxidative Stress Markers in Postmenopausal Women With Normal or Low Bone Mineral Density. Biomark. Insights 2019, 14, 1177271919843825. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.N. Use of Biochemical Markers of Bone Turnover in Osteoporosis: Up to Date; updated 17 November 2023. 2023. Available online: https://www.uptodate.com/contents/use-of-biochemical-markers-of-bone-turnover-in-osteoporosis (accessed on 14 August 2025).
- Zloh, M.; Kirton, S.B. The benefits of in silico modeling to identify possible small-molecule drugs and their off-target interactions. Future Med. Chem. 2018, 10, 423–432. [Google Scholar] [CrossRef] [PubMed]
| Trigger | Specification |
|---|---|
| Drugs | Alcohol, anticoagulants, anticonvulsants, antidepressants, aromatase inhibitors, medroxyprogesterone acetate, GnRH agonists, cyclosporines, calcineurin inhibitors, GCs, loop diuretics, proton pump inhibitors, thiazolidinediones |
| Gastrointestinal disorders | Celiac disease, inflammatory disease, gastric bypass, anorexia nervosa, hemochromatosis, chronic liver disease |
| Hematological disorders | Monoclonal gammopathy of uncertain significance, multiple myeloma, systemic mastocytosis, beta thalassemia major |
| Rheumatic diseases | Rheumatoid arthritis, sarcoidosis, systemic lupus erythematosus |
| Endocrine disorders | Acromegaly, cushing syndrome, diabetes mellitus, hypogonadism, thyroid dysregulation, growth hormone deficiency |
| Nutritional deficiencies | Calcium, magnesium, phosphate, vitamin D |
| Renal disorders | Idiopathic hypercalciuris, renal tubular acidosis, chronic kidney disease |
| Class of Drug | Examples |
|---|---|
| Estrogens | |
| Selective estrogen receptor modulators | Bazedoxifene, raloxifene |
| Bisphosphonates | Alendronic acid, risendronic acid, zolendronic acid, ibandronic acid |
| Antibodies | Denosumab, Romosozumab |
| PTH derivates | Teriparatide, abaloparatide |
| Species | Purpose—Basic Research | Purpose—Translational Research | Similarity to Human Bone |
|---|---|---|---|
| Mouse |
|
|
|
|
| ||
| Rat |
|
|
|
| |||
| Dog |
|
|
|
| Sheep/Goat |
|
|
|
| Non-human primates |
|
| |
|
| Ovariectomy | Hormonal Manipulation | Naturally Aging | |||
|---|---|---|---|---|---|
| Advantages | Drawbacks | Advantages | Drawbacks | Advantages | Drawbacks |
| - Well established - Diversified data | - Stress and strain - Educated surgeon | - Not invasive - Well controllable | - Chemically induced - Massive side effects | - Not invasive - No stress for animals | - Long timeframe - Low productivity |
| Tail Suspension | Immobilization | Transgenic Models | |||
|---|---|---|---|---|---|
| Advantages | Drawbacks | Advantages | Drawbacks | Advantages | Drawbacks |
| - Well established - Diversified data | - Stress and pain - Effects on other physiological systems | - targeted examination of specific limbs - easy-to-use | - Stress and pain | - Specific tasks - No stress for animals | - non-specific effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plank, J.; Damerau, A.; Chacon, M.S.; Hoff, P.; Buttgereit, F.; Pfeiffenberger, M. Modelling Osteoporosis in Pre-Clinical Research—Challenges, Trends and New Approaches. Cells 2025, 14, 1649. https://doi.org/10.3390/cells14211649
Plank J, Damerau A, Chacon MS, Hoff P, Buttgereit F, Pfeiffenberger M. Modelling Osteoporosis in Pre-Clinical Research—Challenges, Trends and New Approaches. Cells. 2025; 14(21):1649. https://doi.org/10.3390/cells14211649
Chicago/Turabian StylePlank, Johannes, Alexandra Damerau, Madison Skye Chacon, Paula Hoff, Frank Buttgereit, and Moritz Pfeiffenberger. 2025. "Modelling Osteoporosis in Pre-Clinical Research—Challenges, Trends and New Approaches" Cells 14, no. 21: 1649. https://doi.org/10.3390/cells14211649
APA StylePlank, J., Damerau, A., Chacon, M. S., Hoff, P., Buttgereit, F., & Pfeiffenberger, M. (2025). Modelling Osteoporosis in Pre-Clinical Research—Challenges, Trends and New Approaches. Cells, 14(21), 1649. https://doi.org/10.3390/cells14211649






