Nicotinamide Counteracts the Detrimental Effect of Endothelin-1 on Uterine Decidualization During Early Pregnancy by Influencing EDNRB
Highlights
- ET-1 impairs endometrial decidualization and angiogenesis during early pregnancy.
- Nicotinamide (NAM) treatment mitigates the detrimental effect of ET-1.
- ET-1 causes insufficient decidualization, leading to impaired implantation and subsequent manifestation of preeclampsia.
- NAM supplementation has the potential to improve embryo implantation and pregnancy outcomes, especially pregnancy with implantation problems, including PE.
Abstract
1. Background
2. Materials and Methods
2.1. Mice
2.2. Matings
2.3. Three-Dimensional (3D) High-Frequency Ultrasonography (HFU)
2.4. Morphological Examination
2.5. Decidua Preparation
2.6. Biochemical Analyses
2.7. Western Blot
2.8. Immunohistochemistry (IHC)
2.9. Cell Culture
2.10. Assessment of Motility of HESCs with Wound Healing Assay
2.11. Trophoblast Spheroid Formation
2.12. Spheroid Attachment to Epithelial Cells
2.13. RNA Sequencing
2.14. Quantitative RT-PCR
2.15. Statistical Analysis
3. Results
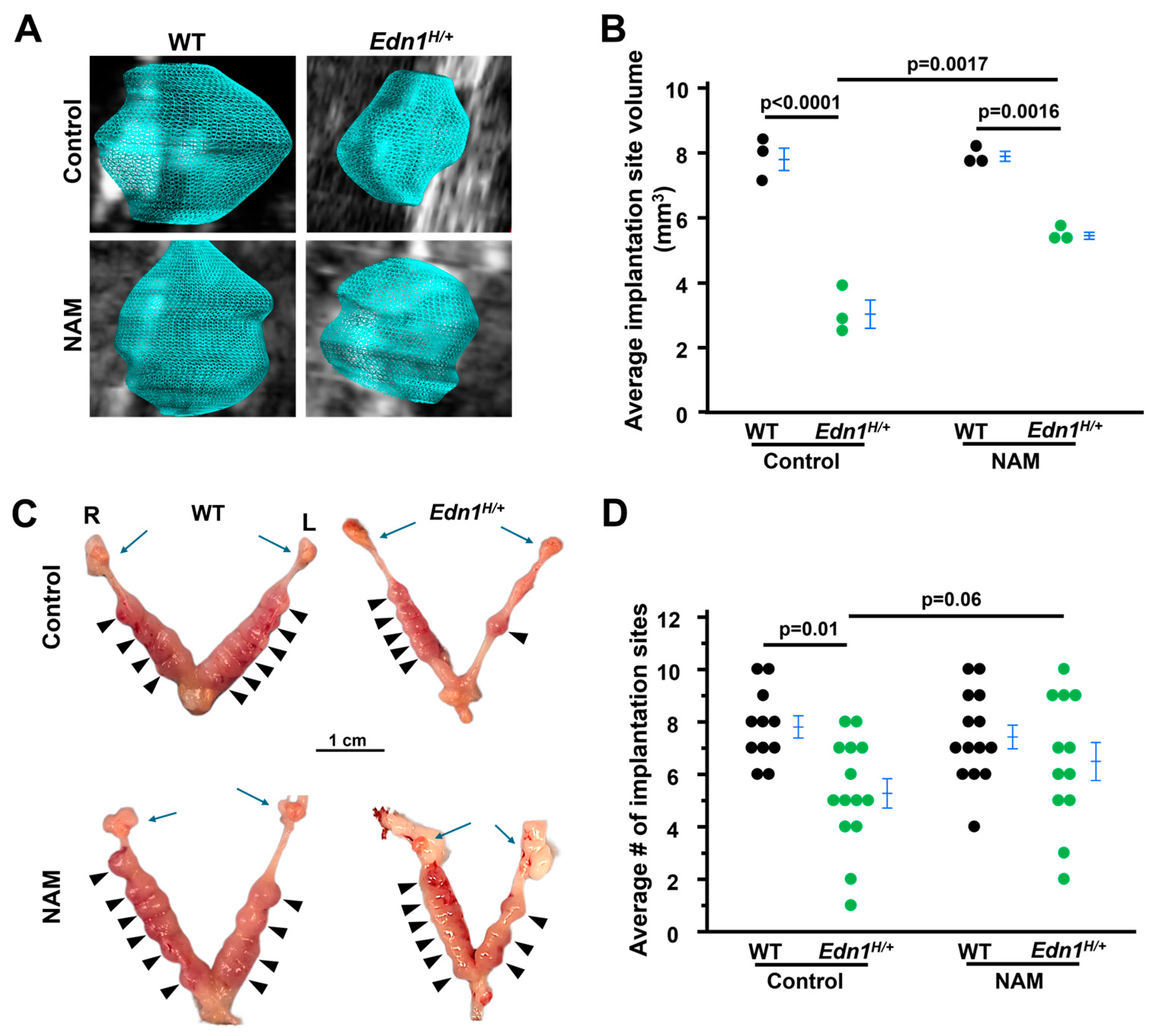
3.1. The Decrease in Implantation Site Volume in Edn1H/+ Dams Is Prevented by NAM Treatment
3.2. The Impaired Structure of Implantation Sites from Edn1H/+ Dams Is Improved by NAM
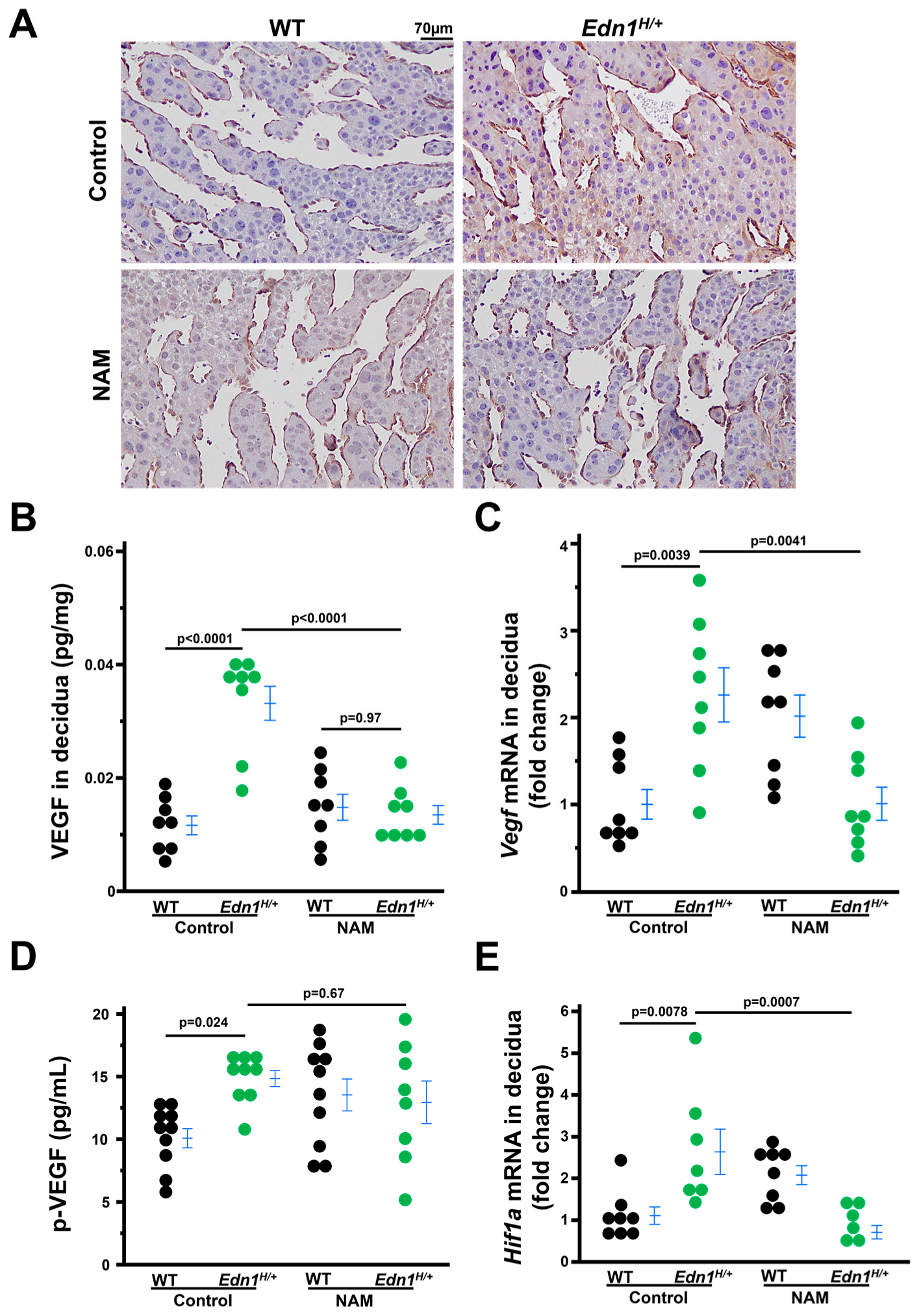
3.3. NAM Treatment Improves the Structure of Maternal–Embryo Interface During Early Pregnancy
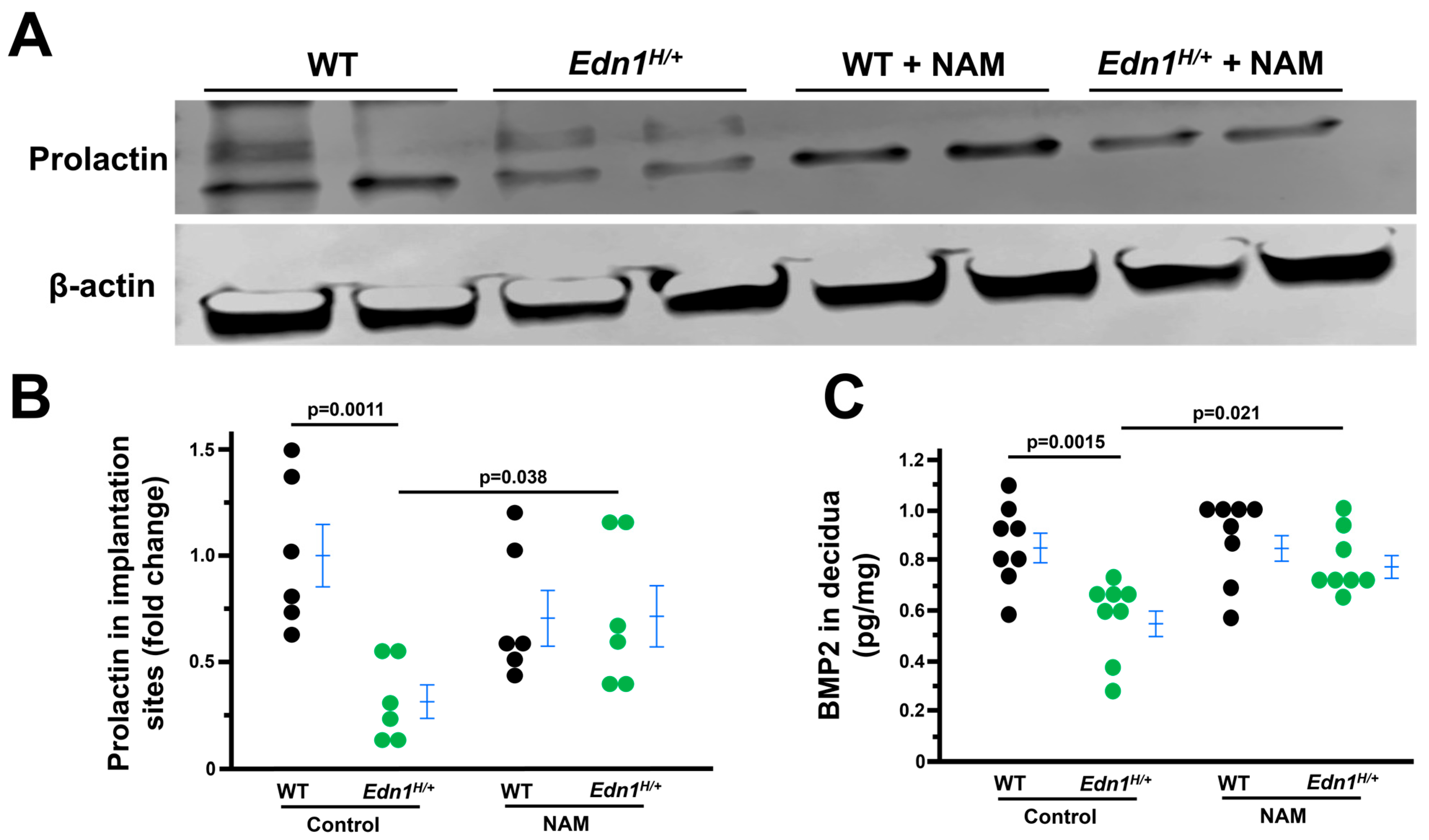
3.4. Decidualization Is Impaired in Edn1H/+ Dams, Which Is Mitigated by NAM
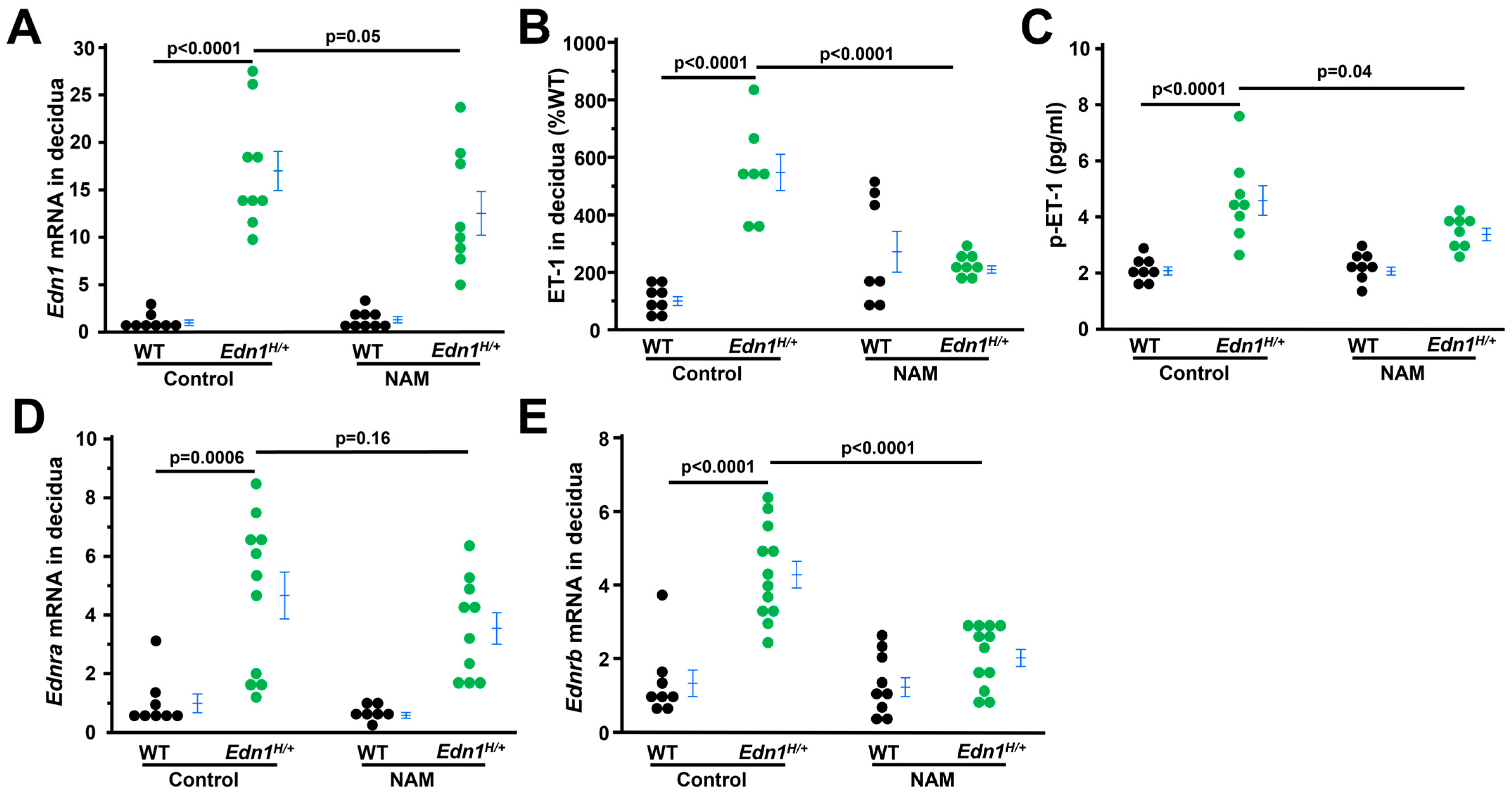
3.5. NAM Inhibits ET-1 Expression in the Deciduae of Edn1H/+ Dams
3.6. ET-1 Inhibits the Endometrial Stromal Cell Differentiation In Vitro, Which Was Corrected by NAM
3.7. The Beneficial Effects of NAM on Stromal Cell Decidualization Are Mediated Through EDNRB
3.8. Excess ET-1 Does Not Alter the Receptivity of Uterine Luminal Epithelial Cells (Ishikawa Cells)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thaete, L.G.; Neerhof, M.G.; Caplan, M.S. Endothelin receptor A antagonism prevents hypoxia-induced intrauterine growth restriction in the rat. Am. J. Obstet. Gynecol. 1997, 176 Pt 1, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.P.; Spradley, F.T.; Bakrania, B.A. The Endothelin System: A Critical Player in the Pathophysiology of Preeclampsia. Curr. Hypertens. Rep. 2018, 20, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saleh, L.; Verdonk, K.; Visser, W.; van den Meiracker, A.H.; Danser, A.H. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther. Adv. Cardiovasc. Dis. 2016, 10, 282–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aggarwal, P.K.; Jain, V.; Srinivasan, R.; Jha, V. Maternal EDN1 G5665T polymorphism influences circulating endothelin-1 levels and plays a role in determination of preeclampsia phenotype. J. Hypertens. 2009, 27, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, W.; Liu, M.S.; Mao, L.J.; Wang, X.H. Potential correlation between EDN1 gene polymorphisms with preeclampsia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.G.; Baker, R.S.; Yang, D.; Clark, K.E. Effects of continuous infusion of endothelin-1 in pregnant sheep. Hypertension 1997, 30, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kakoki, M.; Smid, M.; Boggess, K.; Wilder, J.; Hiller, S.; Bounajim, C.; Parnell, S.E.; Sulik, K.K.; Smithies, O.; et al. Causative Effects of Genetically Determined High Maternal/Fetal Endothelin-1 on Preeclampsia-Like Conditions in Mice. Hypertension 2018, 71, 894–903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thai, T.L.; Arendshorst, W.J. ADP-ribosyl cyclase and ryanodine receptors mediate endothelin ETA and ETB receptor-induced renal vasoconstriction in vivo. Am. J. Physiol. Renal Physiol. 2008, 295, F360–F368. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.L.; Fellner, S.K.; Arendshorst, W.J. ADP-ribosyl cyclase and ryanodine receptor activity contribute to basal renal vasomotor tone and agonist-induced renal vasoconstriction in vivo. Am. J. Physiol. Renal Physiol. 2007, 293, F1107–F1114. [Google Scholar] [CrossRef] [PubMed]
- Townley-Tilson, W.H.; Wu, Y.; Ferguson, J.E., 3rd; Patterson, C. The ubiquitin ligase ASB4 promotes trophoblast differentiation through the degradation of ID2. PLoS ONE 2014, 9, e89451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, F.; Fushima, T.; Oyanagi, G.; Townley-Tilson, H.W.; Sato, E.; Nakada, H.; Oe, Y.; Hagaman, J.R.; Wilder, J.; Li, M.; et al. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc. Natl. Acad. Sci. USA 2016, 113, 13450–13455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fushima, T.; Sekimoto, A.; Oe, Y.; Sato, E.; Ito, S.; Sato, H.; Takahashi, N. Nicotinamide ameliorates a preeclampsia-like condition in mice with reduced uterine perfusion pressure. Am. J. Physiol. Renal Physiol. 2017, 312, F366–F372. [Google Scholar] [CrossRef] [PubMed]
- Peavey, M.C.; Reynolds, C.L.; Szwarc, M.M.; Gibbons, W.E.; Valdes, C.T.; DeMayo, F.J.; Lydon, J.P. Three-Dimensional High-Frequency Ultrasonography for Early Detection and Characterization of Embryo Implantation Site Development in the Mouse. PLoS ONE 2017, 12, e0169312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Peavey, M.C.; Reynolds, C.L.; Szwarc, M.M.; Gibbons, W.E.; Valdes, C.T.; DeMayo, F.J.; Lydon, J.P. A Novel Use of Three-dimensional High-frequency Ultrasonography for Early Pregnancy Characterization in the Mouse. J. Vis. Exp. 2017, 128, 56207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heyward, C.Y.; Sones, J.L.; Lob, H.E.; Yuen, L.C.; Abbott, K.E.; Huang, W.; Begun, Z.R.; Butler, S.D.; August, A.; Leifer, C.A.; et al. The decidua of preeclamptic-like BPH/5 mice exhibits an exaggerated inflammatory response during early pregnancy. J. Reprod. Immunol. 2017, 120, 27–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, F.; Bahnson, E.M.; Wilder, J.; Siletzky, R.; Hagaman, J.; Nickekeit, V.; Hiller, S.; Ayesha, A.; Feng, L.; Levine, J.S.; et al. Oral high dose vitamin B12 decreases renal superoxide and post-ischemia/reperfusion injury in mice. Redox. Biol. 2020, 32, 101504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestre Citrinovitz, A.C.; Langer, L.; Strowitzki, T.; Germeyer, A. Resveratrol enhances decidualization of human endometrial stromal cells. Reproduction 2020, 159, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Rytkonen, K.T.; Erkenbrack, E.M.; Poutanen, M.; Elo, L.L.; Pavlicev, M.; Wagner, G.P. Decidualization of Human Endometrial Stromal Fibroblasts is a Multiphasic Process Involving Distinct Transcriptional Programs. Reprod. Sci. 2019, 26, 323–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ssengonzi, R.; Wang, Y.; Zhou, J.; Kayashima, Y.; Townley-Tilson, W.H.D.; Rao, B.; Ma, Q.; Maeda-Smithies, N.; Li, F. Inhibitor of DNA Binding Protein 2 (ID2) Mediates the Anti-Proliferative and Pro-Differentiation Effects of Insulin-like Growth Factor-1 (IGF-1). Life 2024, 14, 1663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gellersen, B.; Wolf, A.; Kruse, M.; Schwenke, M.; Bamberger, A.M. Human endometrial stromal cell-trophoblast interactions: Mutual stimulation of chemotactic migration and promigratory roles of cell surface molecules CD82 and CEACAM1. Biol. Reprod. 2013, 88, 80. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Takamura, M.; Tochigi, H.; Mizuno, Y.; Mizuno, Y.; Sato, T.; Tamaru, S.; Kusama, K.; Tamura, K.; Kamei, Y.; et al. Loss of miR-424 and miR-503 promotes decidualization of human endometrial stromal cells by increasing SCARA5 expression. Med. Mol. Morphol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Nandi, P.; Lim, H.; Torres-Garcia, E.J.; Lala, P.K. Human trophoblast stem cell self-renewal and differentiation: Role of decorin. Sci. Rep. 2018, 8, 8977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bowser, J.L.; Blackburn, M.R.; Shipley, G.L.; Molina, J.G.; Dunner, K., Jr.; Broaddus, R.R. Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J. Clin. Investig. 2016, 126, 220–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buck, V.U.; Gellersen, B.; Leube, R.E.; Classen-Linke, I. Interaction of human trophoblast cells with gland-like endometrial spheroids: A model system for trophoblast invasion. Hum. Reprod. 2015, 30, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Ayesha, A.; Bahnson, E.M.; Kayashima, Y.; Wilder, J.; Huynh, P.K.; Hiller, S.; Maeda-Smithies, N.; Li, F. Vitamin B12 does not increase cell viability after hydrogen peroxide induced damage in mouse kidney proximal tubular cells and brain endothelial cells. Adv. Redox Res. 2022, 4, 100029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winterhager, E.; Gellhaus, A.; Blois, S.M.; Hill, L.A.; Barr, K.J.; Kidder, G.M. Decidual angiogenesis and placental orientation are altered in mice heterozygous for a dominant loss-of-function Gja1 (connexin43) mutation. Biol. Reprod. 2013, 89, 111. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Liu, L.X.; Date, T.; Belanger, A.J.; Vincent, K.A.; Akita, G.Y.; Kuriyama, T.; Cheng, S.H.; Gregory, R.J.; Jiang, C. Hypoxia-Inducible Factor-1 Mediates Activation of Cultured Vascular Endothelial Cells by Inducing Multiple Angiogenic Factors. Circ. Res. 2003, 93, 664–673. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sato, E. Uterine angiogenesis during implantation and decidualization in mice. Reprod. Med. Biol. 2006, 5, 81–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zambuto, S.G.; Theriault, H.; Jain, I.; Crosby, C.O.; Pintescu, I.; Chiou, N.; Oyen, M.L.; Zoldan, J.; Underhill, G.H.; Harley, B.A.C.; et al. Endometrial decidualization status modulates endometrial microvascular complexity and trophoblast outgrowth in gelatin methacryloyl hydrogels. NPJ Womens Health 2024, 2, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Apa, R.; Miceli, F.; de Feo, D.; Mastrandrea, M.L.; Mancuso, S.; Napolitano, M.; Lanzone, A. Endothelin-1 inhibits basal and human chorionic gonadotrophin-stimulated progesterone production. Hum. Reprod. 1998, 13, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Morey, A.K.; Pedram, A.; Razandi, M.; Prins, B.A.; Hu, R.M.; Biesiada, E.; Levin, E.R. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology 1997, 138, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Knutsen, G.R.; Brown, M.D.; Ruest, L.B. Control of endothelin-a receptor expression by progesterone is enhanced by synergy with Gata2. Mol. Endocrinol. 2013, 27, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramathal, C.Y.; Bagchi, I.C.; Taylor, R.N.; Bagchi, M.K. Endometrial decidualization: Of mice and men. Semin. Reprod. Med. 2010, 28, 17–26. [Google Scholar] [CrossRef]
- Kaya Okur, H.S.; Das, A.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Roles of Estrogen Receptor-alpha and the Coactivator MED1 During Human Endometrial Decidualization. Mol. Endocrinol. 2016, 30, 302–313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, J.J.; Youssef, A.H.; Yandle, T.G.; Lewis, L.K.; Nicholls, M.G. Endothelin-1 directly modulates its own secretion: Studies utilising the cell immunoblot technique. Regul. Pept. 2003, 113, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Stow, L.R.; Jacobs, M.E.; Wingo, C.S.; Cain, B.D. Endothelin-1 gene regulation. FASEB J. 2011, 25, 16–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kannan, A.; Wang, W.; Demayo, F.J.; Taylor, R.N.; Bagchi, M.K.; Bagchi, I.C. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J. Biol. Chem. 2007, 282, 31725–31732. [Google Scholar] [CrossRef]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for study of human embryo implantation: Choice of cell lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Kubota, T.; Masuda, M.; Aso, T. Role of nitric oxide synthase in release of endothelin from cultured human endometrial cells. Eur. J. Endocrinol. 1998, 138, 467–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohnen, G.; Campbell, S.; Irvine, G.A.; Church, H.J.; MacLachlan, F.; Titterington, M.; Cameron, I.T. Endothelin receptor expression in human decidua. Mol. Hum. Reprod. 1998, 4, 185–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, J.P.; Tian, Q.; Zhu, Q.Y.; Liu, J.L. Single-cell analysis of mouse uterus at the invasion phase of embryo implantation. Cell Biosci. 2022, 12, 13. [Google Scholar] [CrossRef]
- Charron, F.; Tsimiklis, G.; Arcand, M.; Robitaille, L.; Liang, Q.; Molkentin, J.D.; Meloche, S.; Nemer, M. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes. Dev. 2001, 15, 2702–2719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilson, D.B.; Dorfman, D.M.; Orkin, S.H. A nonerythroid GATA-binding protein is required for function of the human preproendothelin-1 promoter in endothelial cells. Mol. Cell Biol. 1990, 10, 4854–4862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schorlemmer, A.; Matter, M.L.; Shohet, R.V. Cardioprotective signaling by endothelin. Trends Cardiovasc. Med. 2008, 18, 233–239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farhat, N.; Matouk, C.C.; Mamarbachi, A.M.; Marsden, P.A.; Allen, B.G.; Thorin, E. Activation of ETB receptors regulates the abundance of ET-1 mRNA in vascular endothelial cells. Br. J. Pharmacol. 2008, 153, 1420–1431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinella, F.; Rosano, L.; Di Castro, V.; Natali, P.G.; Bagnato, A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J. Biol. Chem. 2002, 277, 27850–27855. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Rai, A.; Kambham, N.; Sung, J.F.; Singh, N.; Petitt, M.; Dhal, S.; Agrawal, R.; Sutton, R.E.; Druzin, M.L.; et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J. Clin. Investig. 2014, 124, 4941–4952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, Z.; Karakone, M.; Nagarajan, S.; Nagy, N.; Mildenberger, W.; Petrova, E.; Hinte, L.C.; Bijnen, M.; Hane, P.; Nelius, E.; et al. Macrophages preserve endothelial cell specialization in the adrenal gland to modulate aldosterone secretion and blood pressure. Cell Rep. 2024, 43, 114395. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Kodithuwakku, S.P.; Chan, R.W.S.; Yeung, W.S.B.; Yao, Y.; Ng, E.H.Y.; Chiu, P.C.N.; Lee, C.L. Three-dimensional culture models of human endometrium for studying trophoblast-endometrium interaction during implantation. Reprod. Biol. Endocrinol. 2022, 20, 120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujimura, T.; Tamura, I.; Yoshimura, A.; Yoneda, T.; Takasaki, H.; Shiroshita, A.; Shirafuta, Y.; Sato, S.; Sugino, N. Establishment of an in vitro implantation model using a newly developed mouse endometrial organoid. Development 2025, 152, dev204461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huynh, P.K.; Wilder, J.; Hiller, S.; Hagaman, J.; Takahashi, N.; Maeda-Smithies, N.; Li, F. Beneficial effects of nicotinamide on hypertensive mice with impaired endothelial nitric oxide function. J. Exp. Nephrol. 2020, 1, 1–8. [Google Scholar] [PubMed] [PubMed Central]
- Kumakura, S.; Sato, E.; Sekimoto, A.; Hashizume, Y.; Yamakage, S.; Miyazaki, M.; Ito, S.; Harigae, H.; Takahashi, N. Nicotinamide Attenuates the Progression of Renal Failure in a Mouse Model of Adenine-Induced Chronic Kidney Disease. Toxins 2021, 13, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, Q.; Chen, M.; Kayashima, Y.; Zhou, J.; Rao, B.; Bowser, J.L.; Yi, X.; Maeda-Smithies, N.; Li, F. Nicotinamide Counteracts the Detrimental Effect of Endothelin-1 on Uterine Decidualization During Early Pregnancy by Influencing EDNRB. Cells 2025, 14, 1645. https://doi.org/10.3390/cells14211645
Wang Y, Ma Q, Chen M, Kayashima Y, Zhou J, Rao B, Bowser JL, Yi X, Maeda-Smithies N, Li F. Nicotinamide Counteracts the Detrimental Effect of Endothelin-1 on Uterine Decidualization During Early Pregnancy by Influencing EDNRB. Cells. 2025; 14(21):1645. https://doi.org/10.3390/cells14211645
Chicago/Turabian StyleWang, Yuye, Qing Ma, Meitong Chen, Yukako Kayashima, Jiayi Zhou, Balaji Rao, Jessica L. Bowser, Xianwen Yi, Nobuyo Maeda-Smithies, and Feng Li. 2025. "Nicotinamide Counteracts the Detrimental Effect of Endothelin-1 on Uterine Decidualization During Early Pregnancy by Influencing EDNRB" Cells 14, no. 21: 1645. https://doi.org/10.3390/cells14211645
APA StyleWang, Y., Ma, Q., Chen, M., Kayashima, Y., Zhou, J., Rao, B., Bowser, J. L., Yi, X., Maeda-Smithies, N., & Li, F. (2025). Nicotinamide Counteracts the Detrimental Effect of Endothelin-1 on Uterine Decidualization During Early Pregnancy by Influencing EDNRB. Cells, 14(21), 1645. https://doi.org/10.3390/cells14211645






