From Cytokines to Biomarkers: Mapping the Immunopathology of Inflammatory Bowel Disease
Abstract
1. Introduction
2. Normal and Disease Pathophysiology of the Gastrointestinal Immune System in IBD
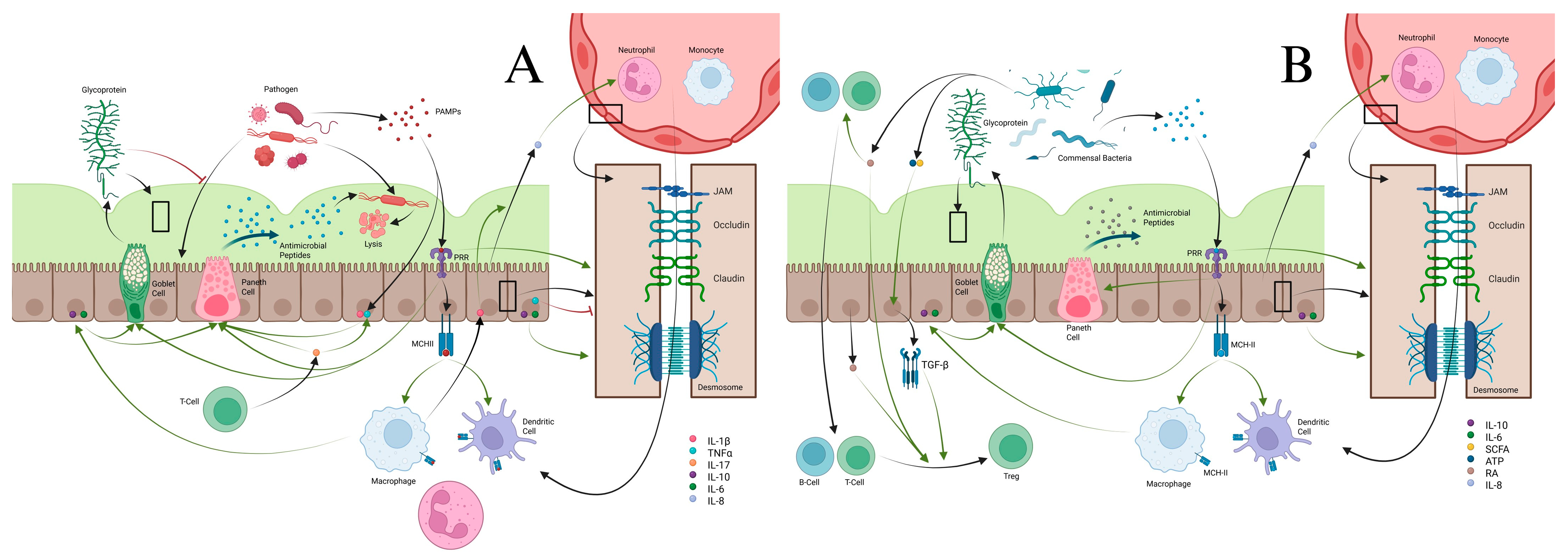
2.1. Mucous Layers and the Epithelial Barrier
2.2. Macrophages/Monocytes
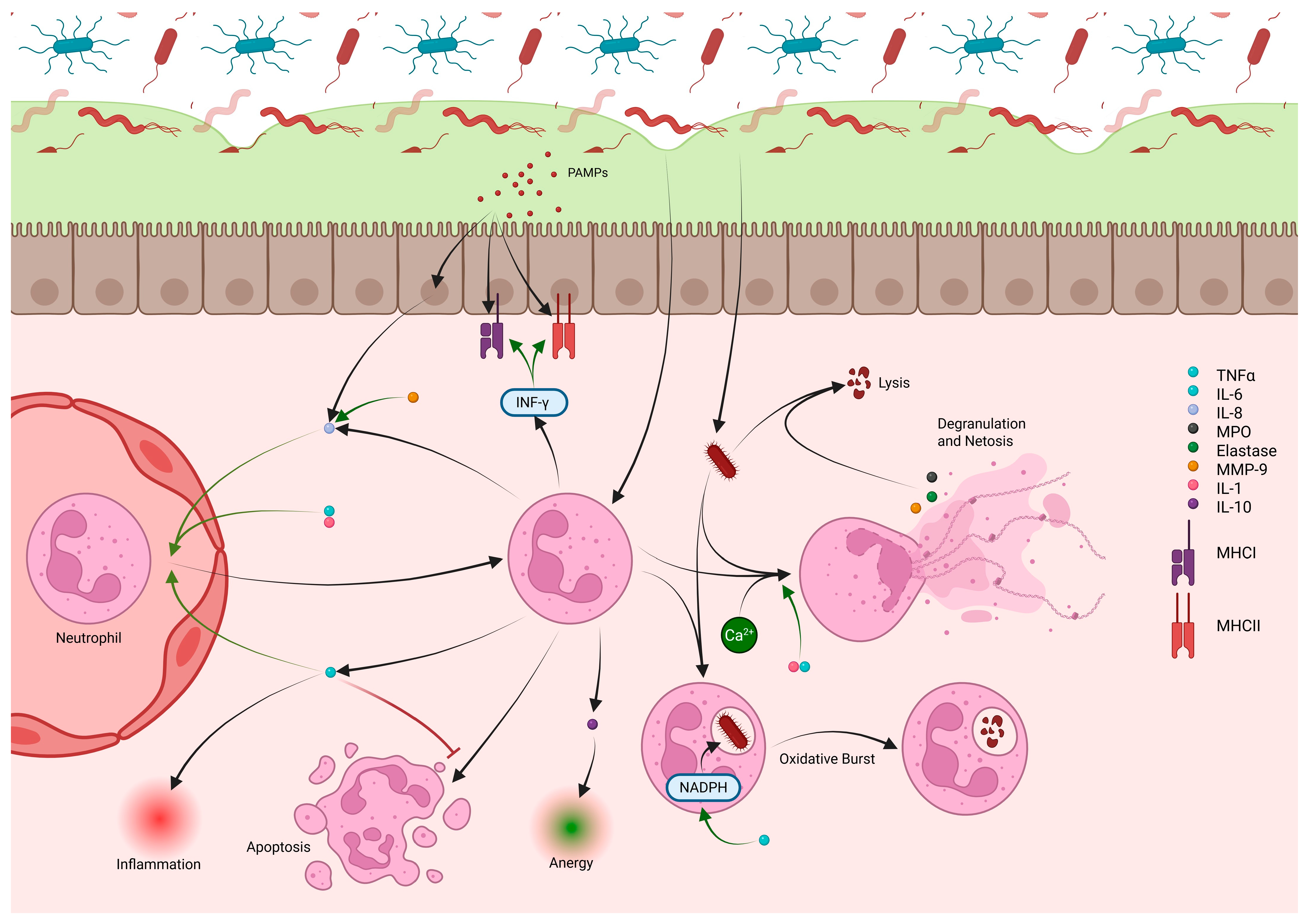
2.3. Neutrophils
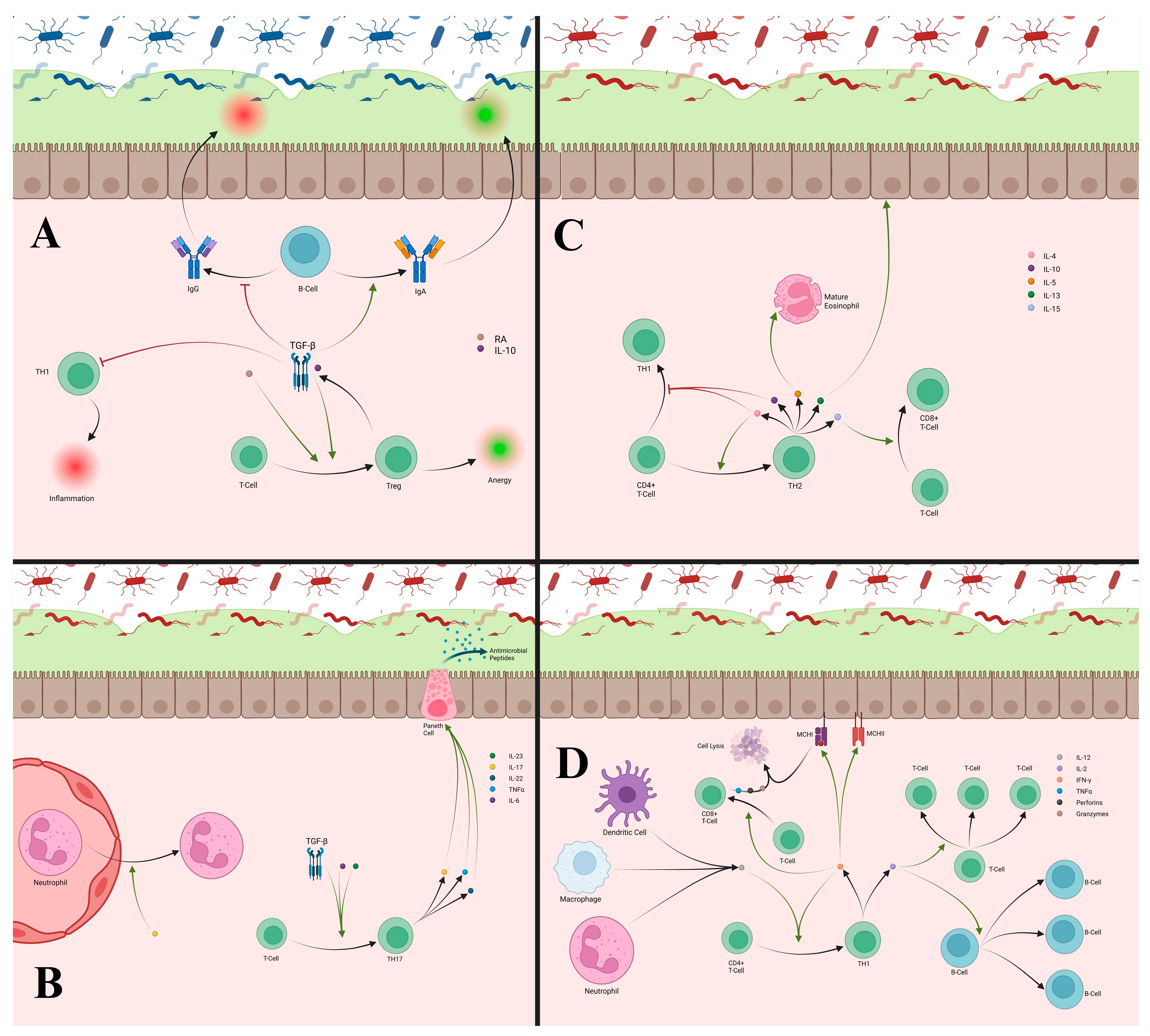
2.4. Innate Lymphoid Cells
2.5. Dendritic Cells
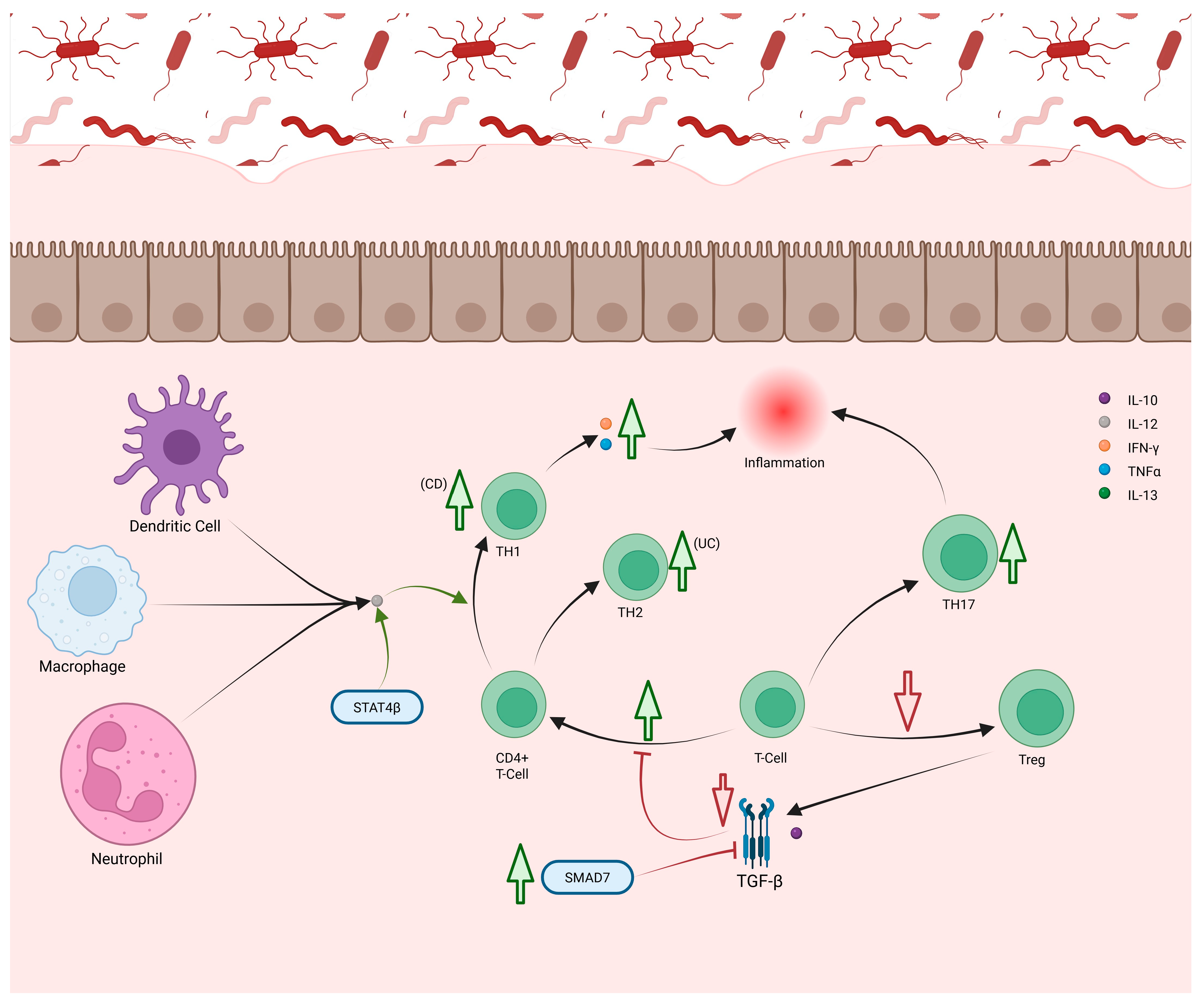
2.6. T-Cells
2.7. B-Cells
3. Biomarker Challenges and Future Directions
4. Treatment of IBD
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ASA | 5-Aminosalicylic Acid |
| AhR | Aryl Hydrocarbon Receptor |
| anti-TM5 | Anti-Tropomyosin 5 |
| AP-1 | Activator Protein 1 |
| APCs | Antigen-Presenting Cells |
| APRIL | A Proliferation-Inducing Ligand |
| ASCA | Anti-Saccharomyces Cerevisiae Antibodies |
| ATG | Autophagy-Related Gene |
| ATG16L1 | Autophagy-Related 16-Like 1 |
| ATOH1 | Atonal Homolog 1 |
| ATP | Adenosine Triphosphate |
| BAFF | B-Cell-Activating Factor |
| BCL-2 | B-Cell Lymphoma 2 |
| BLF-1 | BCL-2 Family Member A1 |
| Breg | Regulatory B-Cell |
| cAMP | Cyclic Adenosine Monophosphate |
| CAR-T | Chimeric Antigen Receptor T-Cell |
| CCL2/CCL7 | Chemokine (C-C Motif) Ligand 2 and 7 |
| CCR2 | C-C Motif Chemokine Receptor 2 |
| CD | Crohn’s Disease |
| cDCs | Conventional DCs |
| cGMP | Cyclic Guanosine Monophosphate |
| COSMC | Core 1 β3-Galactosyltransferase-Specific Molecular Chaperone |
| CRP | C-Reactive Protein |
| CXCL | C-X-C Motif Chemokine |
| CXCR2 | C-X-C Motif Chemokine Receptor 2 |
| DAMP | Damage-Associated Molecular Pattern Protein |
| M1 | Macrophage Type 1 |
| M2 | Macrophage Type 2 |
| MAdCAM | Mucosal Vascular Addressin Cell Adhesion Molecule |
| MAPK | Mitogen-Activated Protein Kinase |
| MCL-1 | Myeloid Leukemia 1 |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MHC | Major Histocompatibility Complexes |
| MiR | MicroRNA |
| MLCK | Myosin Light Chain Kinase |
| MMP-9 | Matrix Metalloproteinase 9 |
| MPO | Myeloperoxidase |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NETs | Neutrophil Extracellular Traps |
| NCR | Natural Cytotoxicity Receptor |
| NK1R | Neurokinin-1 Receptor |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B-Cells |
| NOD2 | Nucleotide-Binding Oligomerization Domain-Containing Protein 2 |
| NO | Nitric Oxide |
| NOX1 | NADPH Oxidase 1 |
| PAMPs | Pathogen-Associated Molecular Patterns |
| p-ANCA | Perinuclear Anti-Neutrophil Cytoplasmic Antibodies |
| PARS | Poly(ADP-ribose) Synthetase |
| PDE4 | Phosphodiesterase 4 |
| PD-1 | Programmed Death Receptor 1 |
| PD-L | Programmed Death Ligand |
| pDCs | Plasmacytoid DCs |
| PGP | ProlylGlycineProline |
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Wehkamp, J.; Götz, M.; Herrlinger, K.; Steurer, W.; Stange, E.F. Inflammatory Bowel Disease. Dtsch. Arztebl. Int. 2016, 113, 72–82. [Google Scholar]
- Ananthakrishnan, A.N. Environmental triggers for inflammatory bowel disease. Curr. Gastroenterol. Rep. 2013, 15, 302. [Google Scholar] [CrossRef]
- Ranasinghe, I.R.; Hsu, R. Crohn Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ma, S.; Zhang, J.; Liu, H.; Li, S.; Wang, Q. The Role of Tissue-Resident Macrophages in the Development and Treatment of Inflammatory Bowel Disease. Front. Cell Dev. Biol. 2022, 10, 896591. [Google Scholar] [CrossRef]
- Segal, A.W. The role of neutrophils in the pathogenesis of Crohn’s disease. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12983. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. BioMed Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.P.D.; Le, P.H. Histology, Goblet Cells. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Ferrand, A.; Al Nabhani, Z.; Tapias, N.S.; Mas, E.; Hugot, J.-P.; Barreau, F. NOD2 Expression in Intestinal Epithelial Cells Protects Toward the Development of Inflammation and Associated Carcinogenesis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 357–369. [Google Scholar] [CrossRef]
- Muniz, L.R.; Knosp, C.; Yeretssian, G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front. Immunol. 2012, 3, 310. [Google Scholar] [CrossRef]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V. Mucus Layers in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Bauché, D.; Marie, J.C. Transforming growth factor β: A master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef]
- Nemeth, Z.H.; Bogdanovski, D.A.; Barratt-Stopper, P.; Paglinco, S.R.; Antonioli, L.; Rolandelli, R.H. Crohn’s Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus 2017, 9, e1177. [Google Scholar] [CrossRef]
- Andoh, A.; Yagi, Y.; Shioya, M.; Nishida, A.; Tsujikawa, T.; Fujiyama, Y. Mucosal cytokine network in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 5154–5161. [Google Scholar] [CrossRef]
- Kaplanski, G.; Marin, V.; Montero-Julian, F.; Mantovani, A.; Farnarier, C. IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003, 24, 25–29. [Google Scholar] [CrossRef]
- Silva, A.C.O.; Faria, M.R.; Fontes, A.; Campos, M.S.; Cavalcanti, B.N. Interleukin-1 beta and interleukin-8 in healthy and inflamed dental pulps. J. Appl. Oral Sci. 2009, 17, 527–532. [Google Scholar] [CrossRef]
- Luo, P.; Yang, Z.; Chen, B.; Zhong, X. The multifaceted role of CARD9 in inflammatory bowel disease. J. Cell. Mol. Med. 2020, 24, 34–39. [Google Scholar] [CrossRef]
- Kang, Y.; Park, H.; Choe, B.-H.; Kang, B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front. Med. 2022, 9, 848344. [Google Scholar] [CrossRef]
- Liso, M.; De Santis, S.; Verna, G.; Dicarlo, M.; Calasso, M.; Santino, A.; Gigante, I.; Eri, R.; Raveenthiraraj, S.; Sobolewski, A.; et al. A Specific Mutation in Muc2 Determines Early Dysbiosis in Colitis-Prone Winnie Mice. Inflamm. Bowel Dis. 2020, 26, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Kyo, K.; Muto, T.; Nagawa, H.; Lathrop, G.M.; Nakamura, Y. Associations of distinct variants of the intestinal mucin gene MUC3A with ulcerative colitis and Crohn’s disease. J. Hum. Genet. 2001, 46, 5–20. [Google Scholar] [CrossRef]
- Lu, P.; Paassen, N.B.-V.; van der Sluis, M.; Witte-Bouma, J.; Kerckaert, J.-P.; van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. Colonic gene expression patterns of mucin Muc2 knockout mice reveal various phases in colitis development. Inflamm. Bowel Dis. 2011, 17, 2047–2057. [Google Scholar] [CrossRef]
- Visschedijk, M.C.; Alberts, R.; Mucha, S.; Deelen, P.; de Jong, D.J.; Pierik, M.; Spekhorst, L.M.; Imhann, F.; Jong, A.E.v.d.M.-D.; van der Woude, C.J.; et al. Pooled Resequencing of 122 Ulcerative Colitis Genes in a Large Dutch Cohort Suggests Population-Specific Associations of Rare Variants in MUC2. PLoS ONE 2016, 11, e0159609. [Google Scholar] [CrossRef]
- Sun, J.; Shen, X.; Li, Y.; Guo, Z.; Zhu, W.; Zuo, L.; Zhao, J.; Gu, L.; Gong, J.; Li, J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Park, E.T.; Oh, H.K.; Gum Jr, J.R.; Crawley, S.C.; Kakar, S.; Engel, J.; Leow, C.C.; Gao, W.Q.; Kim, Y.S. HATH1 Expression in Mucinous Cancers of the Colorectum and Related Lesions. Clin. Cancer Res. 2006, 12, 5403–5410. [Google Scholar] [CrossRef]
- Chang, D.; Gao, F.; Slavney, A.; Ma, L.; Waldman, Y.Y.; Sams, A.J.; Billing-Ross, P.; Madar, A.; Spritz, R.; Keinan, A. Accounting for eXentricities: Analysis of the X chromosome in GWAS reveals X-linked genes implicated in autoimmune diseases. PLoS ONE 2014, 9, e113684. [Google Scholar] [CrossRef]
- Dorofeyev, A.E.; Vasilenko, I.V.; Rassokhina, O.A.; Kondratiuk, R.B. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol. Res. Pract. 2013, 2013, 431231. [Google Scholar] [CrossRef]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef]
- Cadwell, K.; Liu, J.Y.; Brown, S.L.; Miyoshi, H.; Loh, J.; Lennerz, J.K.; Kishi, C.; Kc, W.; Carrero, J.A.; Hunt, S.; et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008, 456, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gulati, A.S.; Cantillana, V.; Henry, S.C.; Schmidt, E.A.; Daniell, X.; Grossniklaus, E.; Schoenborn, A.A.; Sartor, R.B.; Taylor, G.A. Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, G573–G584. [Google Scholar]
- Wehkamp, J.; Harder, J.; Weichenthal, M.; Schwab, M.; Schäffeler, E.; Schlee, M.; Herrlinger, K.R.; Stallmach, A.; Noack, F.; Fritz, P.; et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 2004, 53, 1658–1664. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Kollar, P.; Vítězová, M.; Drago, L. Hydrogen Sulfide as a Toxic Product in the Small-Large Intestine Axis and its Role in IBD Development. J. Clin. Med. 2019, 8, 1054. [Google Scholar] [CrossRef]
- Levine, J.; Ellis, C.J.; Furne, J.K.; Springfield, J.; Levitt, M.D. Fecal hydrogen sulfide production in ulcerative colitis. Am. J. Gastroenterol. 1998, 93, 83–87. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Kammermeier, J.; Elawad, M.; Glocker, E.-O. Interleukin-10 and interleukin-10-receptor defects in inflammatory bowel disease. Curr. Allergy Asthma Rep. 2012, 12, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Parvinroo, S.; Kopper, J.; Wannemuehler, M.J. Rules of Engagement: Epithelial-Microbe Interactions and Inflammatory Bowel Disease. Front. Med. 2021, 8, 669913. [Google Scholar] [CrossRef]
- Tanaka, H.; Takechi, M.; Kiyonari, H.; Shioi, G.; Tamura, A.; Tsukita, S. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut 2015, 64, 1529–1538. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions at a glance. J. Cell Sci. 2008, 121, 3677–3682. [Google Scholar] [CrossRef]
- Gitter, A.H.; Wullstein, F.; Fromm, M.; Schulzke, J.D. Epithelial barrier defects in ulcerative colitis: Characterization and quantification by electrophysiological imaging. Gastroenterology 2001, 121, 1320–1328. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576. [Google Scholar] [CrossRef]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’toole, P.W.; Motherway, M.O.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. 2013, 71, 183–203. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.X.; Liu, Y.; Xi, E.Z.; An, J.J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef]
- Wang, H.; Chao, K.; Ng, S.C.; Bai, A.H.; Yu, Q.; Yu, J.; Li, M.; Cui, Y.; Chen, M.; Hu, J.-F.; et al. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.; Neumann, P.-A.; Kamaly, N.; Quiros, M.; Nishio, H.; Jones, H.R.; Sumagin, R.; Hilgarth, R.S.; Alam, A.; Fredman, G.; et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Investig. 2015, 125, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.; Alam, A.; Neumann, P.-A.; Lambeth, J.D.; Cheng, G.; McCoy, J.; Hilgarth, R.S.; Kundu, K.; Murthy, N.; Kusters, D.; et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Investig. 2013, 123, 443–454. [Google Scholar] [CrossRef]
- Bakirtzi, K.; Law, I.K.M.; Fang, K.; Iliopoulos, D.; Pothoulakis, C. MiR-21 in Substance P-induced exosomes promotes cell proliferation and migration in human colonic epithelial cells. Am. J. Physiol. Liver Physiol. 2019, 317, G802–G810. [Google Scholar] [CrossRef] [PubMed]
- Avdagić, N.; Zaćiragić, A.; Babić, N.; Hukić, M.; Šeremet, M.; Lepara, O.; Nakaš-Ićindić, E. Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn. J. Basic Med Sci. 2013, 13, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, S.; Rajamanickam, S.; Motamarry, A.; Ramakrishna, B.S.; Amirtharaj, J.G.; Ramachandran, A.; Pulimood, A.; Venkatraman, A. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 2158–2168. [Google Scholar] [CrossRef]
- Kamalian, A.; Asl, M.S.; Dolatshahi, M.; Afshari, K.; Shamshiri, S.; Roudsari, N.M.; Momtaz, S.; Rahimi, R.; Abdollahi, M.; Abdolghaffari, A.H. Interventions of natural and synthetic agents in inflammatory bowel disease, modulation of nitric oxide pathways. World J. Gastroenterol. 2020, 26, 3365–3400. [Google Scholar] [CrossRef] [PubMed]
- Ljung, T.; Lundberg, S.; Varsanyi, M.; Johansson, C.; Schmidt, P.T.; Herulf, M.; Lundberg, J.O.; Hellström, P.M. Rectal nitric oxide as biomarker in the treatment of inflammatory bowel disease: Responders versus nonresponders. World J. Gastroenterol. 2006, 12, 3386–3392. [Google Scholar]
- Kimura, H.; Miura, S.; Shigematsu, T.; Ohkubo, N.; Tsuzuki, Y.; Kurose, I.; Higuchi, H.; Akiba, Y.; Hokari, R.; Hirokawa, M.; et al. Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn’s disease. Dig. Dis. Sci. 1997, 42, 1047–1054. [Google Scholar] [CrossRef]
- Boughton-Smith, N.; Evans, S.; Whittle, B.; Moncada, S.; Hawkey, C.; Cole, A.; Balsitis, M. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet 1993, 342, 338-e2. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Stamler, J.S.; Bachwich, D.; Karmeli, F.; Ackerman, Z.; Podolsky, D.K. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut 1995, 36, 718–723. [Google Scholar] [CrossRef]
- Anezaki, K.; Asakura, H.; Honma, T.; Ishizuka, K.; Funakoshi, K.; Tsukada, Y.; Narisawa, R. Correlations between Interleukin-8, and Myeloperoxidase or Luminol-Dependent Chemiluminescence in Inflamed Mucosa of Ulcerative Colitis. Intern. Med. 1998, 37, 253–258. [Google Scholar] [CrossRef]
- Chami, B.; Martin, N.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef]
- O’BRien, D.; O’COnnor, T.; Shanahan, F.; O’COnnell, J. Activation of the p38 MAPK and ERK1/2 pathways is required for Fas-induced IL-8 production in colonic epithelial cells. Ann. N. Y. Acad. Sci. 2002, 973, 161–165. [Google Scholar] [CrossRef]
- Leung, T.H.; Zhang, L.F.; Wang, J.; Ning, S.; Knox, S.J.; Kim, S.K. Topical hypochlorite ameliorates NF-κB-mediated skin diseases in mice. J. Clin. Investig. 2013, 123, 5361–5370. [Google Scholar] [CrossRef]
- Saiki, T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med J. 1998, 45, 69–73. [Google Scholar] [CrossRef]
- Peterson, C.G.B.; Sangfelt, P.; Wagner, M.; Hansson, T.; Lettesjö, H.; Carlson, M. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand. J. Clin. Lab. Investig. 2007, 67, 810–820. [Google Scholar] [CrossRef]
- Swaminathan, A.; Borichevsky, G.M.; Edwards, T.S.; Hirschfeld, E.; Mules, T.C.; A Frampton, C.M.; Day, A.S.; Hampton, M.B.; Kettle, A.J.; Gearry, R.B. Faecal Myeloperoxidase as a Biomarker of Endoscopic Activity in Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 16, 1862–1873. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Chan, L.; Zhou, S.-W. Omentin: Linking metabolic syndrome and cardiovascular disease. Curr. Vasc. Pharmacol. 2014, 12, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, I.; Kochhar, R.; Dutta, U.; Vaishnavi, C.; Prasad, K.K.; Vaiphei, K.; Hussain, S.; Singh, K. Evaluation of fecal myeloperoxidase as a biomarker of disease activity and severity in ulcerative colitis. Dig. Dis. Sci. 2012, 57, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Li, L.; Tao, Y.; Yu, J.; Wei, S.; Liu, M.; Li, J. The Inhibitory Effect of Artesunate on Excessive Endoplasmic Reticulum Stress Alleviates Experimental Colitis in Mice. Front. Pharmacol. 2021, 12, 629798. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.; Zhang, C.; Zhou, Y.; Zhang, H. Omentin-1 attenuates inflammation and barrier damage in DSS-induced ulcerative colitis in mice by inhibiting endoplasmic reticulum stress. Gen. Physiol. Biophys. 2022, 41, 221–230. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, L.; Liu, L.; Feng, Y.; Lu, L.; Ren, X.; Dong, X.; Sang, W. Serum omentin-1 as a disease activity marker for Crohn’s disease. Dis. Markers 2014, 2014, 162517. [Google Scholar] [CrossRef]
- Komiya, T.; Tanigawa, Y.; Hirohashi, S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem. Biophys. Res. Commun. 1998, 251, 759–762. [Google Scholar] [CrossRef]
- Yin, J.; Hou, P.; Wu, Z.; Nie, Y. Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med Sci. Monit. 2015, 21, 118–122. [Google Scholar] [CrossRef]
- Cunningham, K.E.; Turner, J.R. Myosin light chain kinase: Pulling the strings of epithelial tight junction function. Ann. N. Y. Acad. Sci. 2012, 1258, 34–42. [Google Scholar]
- Al-Sadi, R.; Youssef, M.; Rawat, M.; Guo, S.; Dokladny, K.; Haque, M.; Watterson, M.D.; Ma, T.Y. MMP-9-induced increase in intestinal epithelial tight permeability is mediated by p38 kinase signaling pathway activation of MLCK gene. Am. J. Physiol. Gastrointest Liver Physiol. 2019, 316, G278–G290. [Google Scholar]
- Annahazi, A.; Molnar, T.; Farkas, K.; Rosztoczy, A.; Izbeki, F.; Gecse, K.; Inczefi, O.; Nagy, F.; Foldesi, I.; Szucs, M.; et al. Fecal MMP-9: A new noninvasive differential diagnostic and activity marker in ulcerative colitis. J. Crohn’s Colitis 2013, 19, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.A.A.; Jagannathan, S.; Malek, E.; Sayed, D.M.; Elgammal, S.A.; El-Azeem, H.G.A.; Thabet, N.M.; Driscoll, J.J. Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma. Oncotarget 2015, 6, 3098–3110. [Google Scholar] [CrossRef] [PubMed]
- Gren, S.T.; Grip, O. Role of Monocytes and Intestinal Macrophages in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1992–1998. [Google Scholar] [PubMed]
- Smythies, L.E.; Shen, R.; Bimczok, D.; Novak, L.; Clements, R.H.; Eckhoff, D.E.; Bouchard, P.; George, M.D.; Hu, W.K.; Dandekar, S.; et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J. Biol. Chem. 2010, 285, 19593–19604. [Google Scholar] [CrossRef]
- Chen, H.; Wu, X.; Xu, C.; Lin, J.; Liu, Z. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis. Clin. Med. 2021, 4, 246–257. [Google Scholar] [CrossRef]
- Farkas, K.; SaróDi, Z.; BáLint, A.; FöLdesi, I.; Tiszlavicz, L.; Cs, M.S.; NyáRi, T.; Tajti, J.; Nagy, F.; Szepes, Z.; et al. The diagnostic value of a new fecal marker, matrix metalloprotease-9, in different types of inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 231–237. [Google Scholar] [CrossRef]
- Buisson, A.; Vazeille, E.; Minet-Quinard, R.; Goutte, M.; Bouvier, D.; Goutorbe, F.; Pereira, B.; Barnich, N.; Bommelaer, G. Fecal Matrix Metalloprotease-9 and Lipocalin-2 as Biomarkers in Detecting Endoscopic Activity in Patients with Inflammatory Bowel Diseases. J. Clin. Gastroenterol. 2018, 52, e53–e62. [Google Scholar] [CrossRef] [PubMed]
- Yablecovitch, D.; Kopylov, U.; Lahat, A.; Amitai, M.M.; Klang, E.; Shor, D.B.-A.; Neuman, S.; Levhar, N.; Fudim, E.; Avidan, B.; et al. Serum MMP-9: A novel biomarker for prediction of clinical relapse in patients with quiescent Crohn’s disease, a post hoc analysis. Ther. Adv. Gastroenterol. 2019, 12, 1756284819881590. [Google Scholar] [CrossRef]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef]
- Monteleone, G.; Kumberova, A.; Croft, N.M.; McKenzie, C.; Steer, H.W.; MacDonald, T.T. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J. Clin. Investig. 2001, 108, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Yang, C.; Liu, L.; Mai, G.; Li, H.; Wu, L.; Jin, M.; Chen, Y. Commensal bacteria-derived extracellular vesicles suppress ulcerative colitis through regulating the macrophages polarization and remodeling the gut microbiota. Microb. Cell Factories 2022, 21, 88. [Google Scholar] [CrossRef]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; E Collins, J.; Cummings, J.R.F.; Sanchez-Elsner, T. Human Intestinal Macrophages Are Involved in the Pathology of Both Ulcerative Colitis and Crohn Disease. Inflamm. Bowel Dis. 2021, 27, 1641–1652. [Google Scholar] [CrossRef]
- Qin, X. Why is damage limited to the mucosa in ulcerative colitis but transmural in Crohn’s disease? World J. Gastrointest. Pathophysiol. 2013, 4, 63–64. [Google Scholar] [CrossRef]
- Kredel, L.I.; Batra, A.; Stroh, T.; A Kühl, A.; Zeitz, M.; Erben, U.; Siegmund, B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut 2013, 62, 852–862. [Google Scholar] [CrossRef]
- Noguchi, E.; Homma, Y.; Kang, X.; Netea, M.G.; Ma, X. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat. Immunol. 2009, 10, 471–479. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J.; Lin, P.L. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011, 4, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ballou, S.P.; Lozanski, G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine 1992, 4, 361–368. [Google Scholar] [CrossRef]
- Thiriot, J.D.; Martinez-Martinez, Y.B.; Endsley, J.J.; Torres, A.G. Hacking the host: Exploitation of macrophage polarization by intracellular bacterial pathogens. Pathog. Dis. 2020, 78, ftaa009. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426–431. [Google Scholar] [CrossRef]
- Mavropoulou, E.; Mechie, N.-C.; Knoop, R.; Petzold, G.; Ellenrieder, V.; Kunsch, S.; Pilavakis, Y.; Amanzada, A. Association of serum interleukin-6 and soluble interleukin-2-receptor levels with disease activity status in patients with inflammatory bowel disease: A prospective observational study. PLoS ONE 2020, 15, e0233811. [Google Scholar] [CrossRef]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Stratis, A.; Wixler, V.; Völler, T.; Thurainayagam, S.; Jorch, S.K.; Zenker, S.; Dreiling, A.; Chakraborty, D.; Fröhling, M.; et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J. Clin. Investig. 2018, 128, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Gonzalez, F.; Dubuquoy, L.; Rousseaux, C.; Dubuquoy, C.; Decourcelle, C.; Saudemont, A.; Tachon, M.; Béclin, E.; Odou, M.-F.; et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 2012, 61, 78–85. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; di Patti, M.C.B.; Musci, G.; Valenti, P. Lactoferrin Efficiently Counteracts the Inflammation-Induced Changes of the Iron Homeostasis System in Macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin activates macrophages via TLR4-dependent and-independent signaling pathways. Cell. Immunol. 2006, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Elass-Rochard, E.; Legrand, D.; Salmon, V.; Roseanu, A.; Trif, M.; Tobias, P.S.; Mazurier, J.; Spik, G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 1998, 66, 486–491. [Google Scholar] [CrossRef]
- Lutaty, A.; Soboh, S.; Schif-Zuck, S.; Ariel, A. Resolution-Associated Lactoferrin Peptides Limit LPS Signaling and Cytokine Secretion from Human Macrophages. Int. J. Mol. Sci. 2020, 21, 5166. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Miedzybrodzki, R.; Szymaniec, S. Oral treatment of rats with bovine lactoferrin inhibits carrageenan-induced inflammation; correlation with decreased cytokine production. Arch. Immunol. Et Ther. Exp. 1998, 46, 361–365. [Google Scholar]
- Machnicki, M.; Zimecki, M.; Zagulski, T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Pathol. 1993, 74, 433–439. [Google Scholar]
- Lutaty, A.; Soboh, S.; Schif-Zuck, S.; Zeituni-Timor, O.; Rostoker, R.; Podolska, M.J.; Schauer, C.; Herrmann, M.; Muñoz, L.E.; Ariel, A. A 17-kDa Fragment of Lactoferrin Associates with the Termination of Inflammation and Peptides within Promote Resolution. Front. Immunol. 2018, 9, 644. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Trummler, M.; Seeholzer, P.; Criblez, D.H.; Seibold, F. Accuracy of four fecal assays in the diagnosis of colitis. Dis. Colon Rectum 2007, 50, 1697–1706. [Google Scholar] [CrossRef]
- Stephens, W.Z.; Kubinak, J.L.; Ghazaryan, A.; Bauer, K.M.; Bell, R.; Buhrke, K.; Chiaro, T.R.; Weis, A.M.; Tang, W.W.; Monts, J.K.; et al. Epithelial-myeloid exchange of MHC class II constrains immunity and microbiota composition. Cell Rep. 2021, 37, 109916. [Google Scholar] [CrossRef]
- Daig, R.; Rogler, G.; Aschenbrenner, E.; Vogl, D.; Falk, W.; Gross, V.; Schölmerich, J.; Andus, T. Human intestinal epithelial cells secrete interleukin-1 receptor antagonist and interleukin-8 but not interleukin-1 or interleukin-6. Gut 2000, 46, 350–358. [Google Scholar] [CrossRef]
- Johnson, B.L., 3rd; Goetzman, H.S.; Prakash, P.S.; Caldwell, C.C. Mechanisms underlying mouse TNF-α stimulated neutrophil derived microparticle generation. Biochem. Biophys. Res. Commun. 2013, 437, 591–596. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Johswich, K.; Martin, M.; Bleich, A.; Kracht, M.; Dittrich-Breiholz, O.; Gessner, E.J.; Suerbaum, S.; Wende, E.; Rheinheimer, C.; Klos, A. Role of the C5a Receptor (C5aR) in Acute and Chronic Dextran Sulfate-Induced Models of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 1812–1823. [Google Scholar] [CrossRef]
- Khameneh, H.J.; Ho, A.W.S.; Laudisi, F.; Derks, H.; Kandasamy, M.; Sivasankar, B.; Teng, G.G.; Mortellaro, A. C5a Regulates IL-1β Production and Leukocyte Recruitment in a Murine Model of Monosodium Urate Crystal-Induced Peritonitis. Front. Pharmacol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Graham, D.B.; Robertson, C.M.; Bautista, J.; Mascarenhas, F.; Diacovo, M.J.; Montgrain, V.; Lam, S.K.; Cremasco, V.; Dunne, W.M.; Faccio, R.; et al. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J. Clin. Investig. 2007, 117, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Reumaux, D.; Hordijk, P.L.; Duthilleul, P.; Roos, D. Priming by tumor necrosis factor-alpha of human neutrophil NADPH-oxidase activity induced by anti-proteinase-3 or anti-myeloperoxidase antibodies. J. Leukoc. Biol. 2006, 80, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Wéra, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef]
- Cross, A.; Moots, R.J.; Edwards, S.W. The dual effects of TNFalpha on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood 2008, 111, 878–884. [Google Scholar] [CrossRef]
- A Lokuta, M.; Huttenlocher, A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J. Leukoc. Biol. 2005, 78, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Pyrillou, K.; Burzynski, L.C.; Clarke, M.C.H. Alternative Pathways of IL-1 Activation, and Its Role in Health and Disease. Front. Immunol. 2020, 11, 613170. [Google Scholar] [CrossRef]
- Gisbert, J.P.; McNicholl, A.G.; Gomollon, F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1746–1754. [Google Scholar] [CrossRef]
- Zhou, H.; Xiao, J.; Wu, N.; Liu, C.; Xu, J.; Liu, F.; Wu, L. MicroRNA-223 Regulates the Differentiation and Function of Intestinal Dendritic Cells and Macrophages by Targeting C/EBPβ. Cell Rep. 2015, 13, 1149–1160. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Zhang, Y.; Song, Z.; Bian, J.; Yi, H.; Ma, Z. Identifying neutrophil-associated subtypes in ulcerative colitis and confirming neutrophils promote colitis-associated colorectal cancer. Front. Immunol. 2023, 14, 1095098. [Google Scholar] [CrossRef]
- Bjarnason, I. The Use of Fecal Calprotectin in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2017, 13, 53–56. [Google Scholar]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Ricciuto, A.; Griffiths, A.M. Clinical value of fecal calprotectin. Crit. Rev. Clin. Lab. Sci. 2019, 56, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Manolakis, A.C.; Kapsoritakis, A.N.; Tiaka, E.K.; Potamianos, S.P. Calprotectin, calgranulin C, and other members of the s100 protein family in inflammatory bowel disease. Dig. Dis. Sci. 2011, 56, 1601–1611. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Sunderkötter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; He, J.; Shen, Y.; Zhang, C.; Wang, J.; Chen, Y. New Frontiers in Genetics, Gut Microbiota, and Immunity: A Rosetta Stone for the Pathogenesis of Inflammatory Bowel Disease. BioMed Res. Int. 2017, 2017, 8201672. [Google Scholar] [CrossRef]
- Hwang, S.J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M., Jr.; Boerwinkle, E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef]
- Moein, S.; Qujeq, D.; Tabari, M.V.; Kashifard, M.; Hajian-Tilaki, K. Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic. Casp. J. Intern. Med. 2017, 8, 178–182. [Google Scholar]
- Kennedy, N.A.; Jones, G.-R.; Plevris, N.; Patenden, R.; Arnott, I.D.; Lees, C.W. Association Between Level of Fecal Calprotectin and Progression of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 2269–2276.e4. [Google Scholar]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Zhou, F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int. Rev. Immunol. 2009, 28, 239–260. [Google Scholar]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, J.; Ji, M.; Liang, H.-E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Mold, C.; Baca, R.; Du Clos, T.W. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J. Autoimmun. 2002, 19, 147–154. [Google Scholar] [CrossRef]
- Tillinger, W.; Jilch, R.; Jilma, B.; Brunner, H.; Koeller, U.; Lichtenberger, C.; Waldhör, T.; Reinisch, W. Expression of the high-affinity IgG receptor FcRI (CD64) in patients with inflammatory bowel disease: A new biomarker for gastroenterologic diagnostics. Am. J. Gastroenterol. 2009, 104, 102–109. [Google Scholar] [CrossRef]
- Imam, T.; Park, S.; Kaplan, M.H.; Olson, M.R. Effector T Helper Cell Subsets in Inflammatory Bowel Diseases. Front. Immunol. 2018, 9, 1212. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Kuhnt, A.; Neurath, M.F.; Wirtz, S.; Atreya, I. Innate Lymphoid Cells as Regulators of Epithelial Integrity: Therapeutic Implications for Inflammatory Bowel Diseases. Front. Med. 2021, 8, 656745. [Google Scholar] [CrossRef] [PubMed]
- Frisbee, A.L.; Saleh, M.M.; Young, M.K.; Leslie, J.L.; Simpson, M.E.; Abhyankar, M.M.; Cowardin, C.A.; Ma, J.Z.; Pramoonjago, P.; Turner, S.D.; et al. IL-33 drives group 2 innate lymphoid cell-mediated protection during Clostridium difficile infection. Nat. Commun. 2019, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Shi, S.; Ashworth, G.; Dong, C.; Liu, J.; Xing, F. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 2019, 10, 315. [Google Scholar] [CrossRef]
- Stagg, A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef]
- Ye, Y.; Gaugler, B.; Mohty, M.; Malard, F. Plasmacytoid dendritic cell biology and its role in immune-mediated diseases. Clin. Transl. Immunol. 2020, 9, e1139. [Google Scholar] [CrossRef]
- Blanco, P.; Palucka, A.; Pascual, V.; Banchereau, J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008, 19, 41–52. [Google Scholar] [CrossRef]
- Castro-Dopico, T.; Dennison, T.W.; Ferdinand, J.R.; Mathews, R.J.; Fleming, A.; Clift, D.; Stewart, B.J.; Jing, C.; Strongili, K.; Labzin, L.I.; et al. Anti-commensal IgG Drives Intestinal Inflammation and Type 17 Immunity in Ulcerative Colitis. Immunity 2019, 50, 1099–1114.e10. [Google Scholar] [CrossRef]
- Sewell, G.W.; Kaser, A. Interleukin-23 in the Pathogenesis of Inflammatory Bowel Disease and Implications for Therapeutic Intervention. J. Crohn’s Colitis 2022, 16 (Suppl. 2), ii3–ii19. [Google Scholar] [CrossRef]
- Geremia, A.; Arancibia-Cárcamo, C.V. Innate Lymphoid Cells in Intestinal Inflammation. Front. Immunol. 2017, 8, 1296. [Google Scholar] [CrossRef]
- Bates, J.; Diehl, L. Dendritic cells in IBD pathogenesis: An area of therapeutic opportunity? J. Pathol. 2014, 232, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Stagg, A.J.; Hart, A.L.; Knight, S.C.; A Kamm, M. The dendritic cell: Its role in intestinal inflammation and relationship with gut bacteria. Gut 2003, 52, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Di Sabatino, A. Dendritic cells in intestinal homeostasis and disease. J. Clin. Investig. 2009, 119, 2441–2450. [Google Scholar] [CrossRef]
- Na, H.; Cho, M.; Chung, Y. Regulation of Th2 Cell Immunity by Dendritic Cells. Immune Netw. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Möbs, C.; Salheiser, M.; Bleise, F.; Witt, M.; Mayer, J.U. Basophils control T cell priming through soluble mediators rather than antigen presentation. Front. Immunol. 2022, 13, 1032379. [Google Scholar] [CrossRef]
- van Wijk, F.; Cheroutre, H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev. Clin. Immunol. 2010, 6, 559–566. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Liu, F. Immunological Impact of Intestinal T Cells on Metabolic Diseases. Front. Immunol. 2021, 12, 639902. [Google Scholar] [PubMed]
- Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef]
- Takeuchi, T.; Ohno, H. IgA in human health and diseases: Potential regulator of commensal microbiota. Front. Immunol. 2022, 13, 1024330. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Jacobson, N.G.; Szabo, S.J.; Weber-Nordt, R.M.; Zhong, Z.; Schreiber, R.D.; Darnell, J.E., Jr.; Murphy, K.M. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J. Exp. Med. 1995, 181, 1755–1762. [Google Scholar] [CrossRef]
- Konjar, Š.; Ferreira, C.; Blankenhaus, B.; Veldhoen, M. Intestinal Barrier Interactions with Specialized CD8 T Cells. Front. Immunol. 2017, 8, 1281. [Google Scholar] [CrossRef]
- Junqueira, C.; Barbosa, C.R.R.; Costa, P.A.C.; Teixeira-Carvalho, A.; Castro, G.; Santara, S.S.; Barbosa, R.P.; Dotiwala, F.; Pereira, D.B.; Antonelli, L.R.; et al. Cytotoxic CD8(+) T cells recognize and kill Plasmodium vivax-infected reticulocytes. Nat. Med. 2018, 24, 1330–1336. [Google Scholar] [CrossRef]
- van Damme, N.; De Keyser, F.; Demetter, P.; Baeten, D.; Mielants, H.; Verbruggen, G.; Cuvelier, C.; Veys, E.M.; De Vos, M. The proportion of Th1 cells, which prevail in gut mucosa, is decreased in inflammatory bowel syndrome. Clin. Exp. Immunol. 2001, 125, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Hural, J. Functions of IL-4 and control of its expression. Crit. Rev. Immunol. 1997, 17, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Bamias, G.; Cominelli, F. Role of type 2 immunity in intestinal inflammation. Curr. Opin. Gastroenterol. 2015, 31, 471–476. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.C. IL-15 converts human intestinal intraepithelial lymphocytes to CD94 producers of IFN-gamma and IL-10, the latter promoting Fas ligand-mediated cytotoxicity. Immunology 2005, 115, 118–126. [Google Scholar] [CrossRef]
- Mahajan, S.; Cervera, A.; MacLeod, M.; Fillatreau, S.; Perona-Wright, G.; Meek, S.; Smith, A.; MacDonald, A.; Gray, D. The role of ICOS in the development of CD4 T cell help and the reactivation of memory T cells. Eur. J. Immunol. 2007, 37, 1796–1808. [Google Scholar] [CrossRef]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, R.; Liu, J.; Karunarathne, D.; Edmundson, A.; Winterford, C.; Nguyen, T.H.; A Simms, L.; Radford-Smith, G.; Wykes, M. Crohn’s disease is facilitated by a disturbance of programmed death-1 ligand 2 on blood dendritic cells. Clin. Transl. Immunol. 2019, 8, e01071. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Kayama, H.; Nishimura, J.; Sekido, Y.; Osawa, H.; Barman, S.; Ogino, T.; Takahashi, H.; Haraguchi, N.; Hata, T.; et al. CD103+ Dendritic Cell Function Is Altered in the Colons of Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1524–1534. [Google Scholar] [CrossRef]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Voskens, C.; Stoica, D.; Rosenberg, M.; Vitali, F.; Zundler, S.; Ganslmayer, M.; Knott, H.; Wiesinger, M.; Wunder, J.; Kummer, M.; et al. Autologous regulatory T-cell transfer in refractory ulcerative colitis with concomitant primary sclerosing cholangitis. Gut 2023, 72, 49–53. [Google Scholar] [CrossRef]
- Smids, C.; Horje, C.S.H.T.; Drylewicz, J.; Roosenboom, B.; Groenen, M.J.M.; van Koolwijk, E.; van Lochem, E.G.; Wahab, P.J. Intestinal T Cell Profiling in Inflammatory Bowel Disease: Linking T Cell Subsets to Disease Activity and Disease Course. J. Crohn’s Colitis 2018, 12, 465–475. [Google Scholar] [CrossRef]
- Garduño, R.C.; Däbritz, J. New Insights on CD8(+) T Cells in Inflammatory Bowel Disease and Therapeutic Approaches. Front. Immunol. 2021, 12, 738762. [Google Scholar]
- Clough, J.N.; Omer, O.S.; Tasker, S.; Lord, G.M.; Irving, P.M. Regulatory T-cell therapy in Crohn’s disease: Challenges and advances. Gut 2020, 69, 942–952. [Google Scholar] [CrossRef]
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014, 2014, 928461. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 2195–2205. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Lu, G.; Shen, Y.; Peng, L.; Zhu, C.; Cui, M.; Wang, W.; Arnaboldi, P.; Tang, M.; et al. T cell–derived inducible nitric oxide synthase switches off Th17 cell differentiation. J. Exp. Med. 2013, 210, 1447–1462. [Google Scholar] [CrossRef]
- Pène, J.; Gauchat, J.-F.; Lécart, S.; Drouet, E.; Guglielmi, P.; Boulay, V.; Delwail, A.; Foster, D.; Lecron, J.-C.; Yssel, H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 2004, 172, 5154–5157. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Castro-Dopico, T.; Clatworthy, M.R. B cell class switching in intestinal immunity in health and disease. Scand. J. Immunol. 2022, 95, e13139. [Google Scholar] [CrossRef]
- Gommerman, J.L.; Rojas, O.L.; Fritz, J.H. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes 2014, 5, 652–662. [Google Scholar] [CrossRef]
- Rahman, Z.S. Impaired clearance of apoptotic cells in germinal centers: Implications for loss of B cell tolerance and induction of autoimmunity. Immunol. Res. 2011, 51, 125–133. [Google Scholar] [CrossRef]
- Niedbala, W.; Cai, B.; Liew, F.Y. Role of nitric oxide in the regulation of T cell functions. Ann. Rheum. Dis. 2006, 65 (Suppl. 3), iii37–iii40. [Google Scholar] [CrossRef] [PubMed]
- Wilk, K.M.; Hwang, S.-A.; Actor, J.K. Lactoferrin modulation of antigen-presenting-cell response to BCG infection. Postep. Hig. Med. Dosw. 2007, 61, 277–282. [Google Scholar]
- Yu, D. Omentin Activates AMP-Activated Protein Kinase and Plays a Role in Energy Metabolism and Immune Response. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2011. [Google Scholar]
- Timmermans, W.M.C.; van Laar, J.A.M.; van der Houwen, T.B.; Kamphuis, L.S.J.; Bartol, S.J.W.; Lam, K.H.; Ouwendijk, R.J.; Sparrow, M.P.; Gibson, P.R.; van Hagen, P.M.; et al. B-Cell Dysregulation in Crohn’s Disease Is Partially Restored with Infliximab Therapy. PLoS ONE 2016, 11, e0160103. [Google Scholar] [CrossRef]
- Rabe, H.; Malmquist, M.; Barkman, C.; Östman, S.; Gjertsson, I.; Saalman, R.; E Wold, A. Distinct patterns of naive, activated and memory T and B cells in blood of patients with ulcerative colitis or Crohn’s disease. Clin. Exp. Immunol. 2019, 197, 111–129. [Google Scholar] [CrossRef]
- Castro-Dopico, T.; Colombel, J.-F.; Mehandru, S. Targeting B cells for inflammatory bowel disease treatment: Back to the future. Curr. Opin. Pharmacol. 2020, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’aMico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Saikam, V.; Skrada, K.A.; Merlin, D.; Iyer, S.S. Inflammatory bowel disease biomarkers. Med. Res. Rev. 2022, 42, 1856–1887. [Google Scholar] [CrossRef]
- Poullis, A.P.; Zar, S.; Sundaram, K.K.; Moodie, S.J.; Risley, P.; Theodossi, A.; Mendall, M.A. A new, highly sensitive assay for C-reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur. J. Gastroenterol. Hepatol. 2002, 14, 409–412. [Google Scholar] [CrossRef]
- Inflammatory Bowel Disease: Laboratory Support for Diagnosis and Management; Quest Diagnositics. Available online: https://testdirectory.questdiagnostics.com/test/test-guides/CF_IBD/inflammatory-bowel-disease-laboratory-support-for-diagnosis-and-management (accessed on 29 September 2024).
- Chang, S.; Malter, L.; Hudesman, D. Disease monitoring in inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 11246–11259. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Shi, K.; Peng, J. Serologic testing of a panel of five antibodies in inflammatory bowel diseases: Diagnostic value and correlation with disease phenotype. Biomed. Rep. 2017, 6, 401–410. [Google Scholar] [CrossRef]
- Sendid, B.; Cornu, M.; Cordier, C.; Bouckaert, J.; Colombel, J.F.; Poulain, D. From ASCA breakthrough in Crohn’s disease and Candida albicans research to thirty years of investigations about their meaning in human health. Autoimmun. Rev. 2024, 23, 103486. [Google Scholar] [CrossRef]
- Zonna, X.; Banta, C.; Hossein-Javaheri, N. The Association Between Crohn’s Disease and Patient Response to Yeast: A Review of the Literature. Gastroenterol. Insights 2024, 15, 1064–1074. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-P.; Niedbala, W.; Wei, X.-Q.; Xu, D.; Feng, G.-J.; Robinson, J.H.; Lam, C.; Liew, F.Y. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 1998, 28, 4062–4070. [Google Scholar] [CrossRef]
- Reinders, C.I.; Herulf, M.; Ljung, T.; Hollenberg, J.; Weitzberg, E.; Lundberg, J.O.; Hellström, P.M. Rectal mucosal nitric oxide in differentiation of inflammatory bowel disease and irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2005, 3, 777–783. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Omar, N.M. Serum omentin-1 as a predictor of activity in Crohn’s disease. Egypt. J. Intern. Med. 2020, 31, 514–521. [Google Scholar] [CrossRef]
- Pan, Y.; Li, A.; Huang, X.; Zhou, Z.; Zhang, Y.; Yang, X.; Gao, C.; He, C. Association between serum omentin-1 and mucosal disease activity in patients with ulcerative colitis. Postgrad. Med J. 2024, 100, 327–333. [Google Scholar] [CrossRef]
- Abdul-Aali, T.S. Evaluation of Mirna-155 and Mirna-223 in Blood in Patients with Ulcerative Colitis. Int. J. Multidiscip. Res. Anal. 2023, 6, 10. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Yu, Q.; Yang, G.; Guo, J.; Li, M.; Zeng, Z.; He, Y.; Chen, B.; Chen, M. Circulating MicroRNA223 is a New Biomarker for Inflammatory Bowel Disease. Medicine 2016, 95, e2703. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Hossain, B.; Siddiqua, T.; Kabir, M.; Noor, Z.; Ahmed, M.; Haque, R. Fecal MicroRNAs as Potential Biomarkers for Screening and Diagnosis of Intestinal Diseases. Front. Mol. Biosci. 2020, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Nakarai, A.; Kato, J.; Hiraoka, S.; Inokuchi, T.; Takei, D.; Morito, Y.; Akita, M.; Takahashi, S.; Hori, K.; Harada, K.; et al. Slight increases in the disease activity index and platelet count imply the presence of active intestinal lesions in C-reactive protein-negative Crohn’s disease patients. Intern. Med. 2014, 53, 1905–1911. [Google Scholar] [CrossRef]
- Whitehead, S.J.; Ford, C.; Gama, R.M.; Ali, A.; McKaig, B.; Waldron, J.L.; Steed, H.; Brookes, M.J. Effect of faecal calprotectin assay variability on the management of inflammatory bowel disease and potential role of faecal S100A12. J. Clin. Pathol. 2017, 70, 1049–1056. [Google Scholar] [CrossRef]
- Kaiser, T.; Langhorst, J.; Wittkowski, H.; Becker, K.; Friedrich, A.W.; Rueffer, A.; Dobos, G.J.; Roth, J.; Foell, D. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut 2007, 56, 1706–1713. [Google Scholar] [CrossRef]
- Shi, J.-T.; Zhang, Y.; She, Y.; Goyal, H.; Wu, Z.-Q.; Xu, H.-G. Diagnostic Utility of Non-invasive Tests for Inflammatory Bowel Disease: An Umbrella Review. Front. Med. 2022, 9, 920732. [Google Scholar] [CrossRef]
- Ekoff, H.; Rydell, N.; Hellström, P.M.; Movérare, R. Fecal and Serum Granulocyte Protein Levels in Inflammatory Bowel Disease and Irritable Bowel Syndrome and Their Relation to Disease Activity. Clin. Transl. Gastroenterol. 2024, 15, e1. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Gilmer, J.F.; Medina, C. Matrix metalloproteinases in inflammatory bowel disease: An update. Mediat. Inflamm. 2015, 2015, 964131. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F. Biomarkers for Personalizing IBD Therapy: The Quest Continues. Clin. Gastroenterol. Hepatol. 2024, 22, 1353–1364. [Google Scholar] [CrossRef]
- Chandrakumar, A.; Georgy, M.; Agarwal, P.; Jong, G.W.; El-Matary, W. Anti-Saccharomyces cerevisiae Antibodies as a Prognostic Biomarker in Children With Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 82–87. [Google Scholar] [CrossRef]
- Imakiire, S.; Takedatsu, H.; Mitsuyama, K.; Sakisaka, H.; Tsuruta, K.; Morita, M.; Kuno, N.; Abe, K.; Funakoshi, S.; Ishibashi, H.; et al. Role of Serum Proteinase 3 Antineutrophil Cytoplasmic Antibodies in the Diagnosis, Evaluation of Disease Severity, and Clinical Course of Ulcerative Colitis. Gut Liver 2022, 16, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Z.; Wang, Z.; Zhu, W.; Li, Q. Fecal miR-223 is a noninvasive biomarker for estimating Crohn’s disease activity. Immun. Inflamm. Dis. 2023, 11, e1131. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro-Iglesias, R.; Barreiro-de Acosta, M.; Lorenzo-Gonzalez, A.; Dominguez-Muñoz, J.E. Accuracy of Consecutive Fecal Calprotectin Measurements to Predict Relapse in Inflammatory Bowel Disease Patients Under Maintenance with Anti-TNF Therapy: A Prospective Longitudinal Cohort Study. J. Clin. Gastroenterol. 2018, 52, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Carlsen, H.S.; Halstensen, T.S. The B-cell system in inflammatory bowel disease. Adv. Exp. Med. Biol. 2006, 579, 149–167. [Google Scholar]
- Wang, X.; Zhu, Y.; Zhang, M.; Wang, H.; Jiang, Y.; Gao, P. Ulcerative Colitis Is Characterized by a Decrease in Regulatory B Cells. J. Crohn’s Colitis 2016, 10, 1212–1223. [Google Scholar] [CrossRef]
- Uzzan, M.; Martin, J.C.; Mesin, L.; Livanos, A.E.; Castro-Dopico, T.; Huang, R.; Petralia, F.; Magri, G.; Kumar, S.; Zhao, Q.; et al. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat. Med. 2022, 28, 766–779. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, M. A review of the biological and pharmacological activities of mesalazine or 5-aminosalicylic acid (5-ASA): An anti-ulcer and anti-oxidant drug. Inflammopharmacology 2021, 29, 1279–1290. [Google Scholar] [CrossRef]
- Smith, S.J.; Fenwick, P.S.; Nicholson, A.G.; Kirschenbaum, F.; Finney-Hayward, T.K.; Higgins, L.S.; A Giembycz, M.; Barnes, P.J.; E Donnelly, L. Inhibitory effect of p38 mitogen-activated protein kinase inhibitors on cytokine release from human macrophages. Br. J. Pharmacol. 2006, 149, 393–404. [Google Scholar] [CrossRef]
- Morse, D.; Pischke, S.E.; Zhou, Z.; Davis, R.J.; Flavell, R.A.; Loop, T.; Otterbein, S.L.; Otterbein, L.E.; Choi, A.M.K. Suppression of Inflammatory Cytokine Production by Carbon Monoxide Involves the JNK Pathway and AP-1. J. Biol. Chem. 2003, 278, 36993–36998. [Google Scholar] [CrossRef]
- Oh-Oka, K.; Kojima, Y.; Uchida, K.; Yoda, K.; Ishimaru, K.; Nakajima, S.; Hemmi, J.; Kano, H.; Fujii-Kuriyama, Y.; Katoh, R.; et al. Induction of Colonic Regulatory T Cells by Mesalamine by Activating the Aryl Hydrocarbon Receptor. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 135–151. [Google Scholar] [CrossRef]
- Carter, M.J.; Lobo, A.J.; Travis, S.P.L. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004, 53 (Suppl. 5), v1–v16. [Google Scholar] [CrossRef]
- Necela, B.M.; Cidlowski, J.A. Mechanisms of Glucocorticoid Receptor Action in Noninflammatory and Inflammatory Cells. Proc. Am. Thorac. Soc. 2004, 1, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Auwardt, R.B.; Mudge, S.J.; Chen, C.G.; A Power, D. Regulation of nuclear factor kappaB by corticosteroids in rat mesangial cells. J. Am. Soc. Nephrol. 1998, 9, 1620–1628. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, Y.; Zhao, Y.; Hu, Y.; Li, Z.; Guo, Q.; Zhao, K.; Lu, N. LL202 protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting MAPK/AP-1 signaling. Oncotarget 2016, 7, 63981–63994. [Google Scholar] [CrossRef] [PubMed]
- Constantin, A.; Loubet-Lescoulié, P.; Lambert, N.; Yassine-Diab, B.; Abbal, M.; Mazières, B.; de Préval, C.; Cantagrel, A. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: Evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum. 1998, 41, 48–57. [Google Scholar]
- Jefremow, A.; Neurath, M.F. Novel Small Molecules in IBD: Current State and Future Perspectives. Cells 2023, 12, 1730. [Google Scholar] [CrossRef]
- Ben Ghezala, I.; Charkaoui, M.; Michiels, C.; Bardou, M.; Luu, M. Small Molecule Drugs in Inflammatory Bowel Diseases. Pharmaceuticals 2021, 14, 637. [Google Scholar] [CrossRef]
- van Dieren, J.M.; Kuipers, E.J.; Samsom, J.N.; Nieuwenhuis, E.E.; van der Woude, J.C. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: Their mechanisms of action and role in the treatment of IBD. Inflamm. Bowel Dis. 2006, 12, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Nakase, H.; Honzawa, Y.; Matsumura, K.; Yamamoto, S.; Takeda, Y.; Ueno, S.; Uza, N.; Masuda, S.; Inui, K.; et al. Immunosuppressive effects of tacrolimus on macrophages ameliorate experimental colitis. Inflamm. Bowel Dis. 2010, 16, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Schwartz, D.A. The Expanding Role of Anti-IL-12 and/or Anti-IL-23 Antibodies in the Treatment of Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2019, 15, 255–265. [Google Scholar]


| Crohn’s Disease | Ulcerative Colitis | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | Use | Sensitivity | Specificity | Cytokine Association | Use | Sensitivity | Specificity | Cytokine Association |
| Serologic Biomarkers | ||||||||
| CRP * | Endoscopic disease activity | 49% | 92% | IL-6, IL-1β, TNF-α | - | - | - | - |
| Diagnosis | 63% | 88% | ||||||
| ASCA/ p-ANCA | Diagnosis (CD vs. UC) (ASCA+/p-ANCA-) | 50.7% | 80.5% | TNF-α, IL-12 | Diagnosis (UC vs. CD) (ASCA-/p-ANCA+) | 31.7% | 94.4% | - |
| High Sensitivity CRP | Endoscopic disease activity | 70% | - | IL-6, IL-1β, TNF-α | Endoscopic disease activity | 50% | - | IL-6, IL-1β, TNF-α |
| ESR * | Diagnosis | 66% | 84% | - | - | - | - | - |
| MiR-223 | - | - | - | IL-23, IL-17, TNF-α, IL-1β, IL-6 | - | - | - | IL-23, IL-17, TNF-α, IL-1β, IL-6 |
| Omentin-1 | Disease activity | 94.9% | 88.7% | TNF-α, IL-6 | Disease activity | 72.4% | 82% | TNF-α, IL-6 |
| Fecal Biomarkers | ||||||||
| Calprotectin | Diagnosis * | 88% | 80% | IL-1α, IL-1β, IL-6, IL-8, TNF-α | Diagnosis | - | - | IL-1α, IL-1β, IL-6, IL-8, TNF-α |
| Endoscopic disease activity | 82% | 72% | Endoscopic disease activity | 87% | 77% | |||
| Treatment response | 83% | 74% | ||||||
| Endoscopic healing | 79% | 57% | ||||||
| Lactoferrin | Diagnosis | 75% | 100% | IL-6, TNF-α, IL-12, IL-10 | Diagnosis | 82% | 100% | IL-6, TNF-α, IL-12, IL-10 |
| Endoscopic disease activity | 82% | 71% | Endoscopic disease activity | 81% | 82% | |||
| MPO * | Diagnosis | 74.7% | 84.6% | IL-8, TNF-α | - | - | - | - |
| MMP-9 | Endoscopic disease Activity | 90% | 63.6% | IL-8, TNF-α | Endoscopic disease activity | 96% | 75% | IL-8, TNF-α |
| MiR-223 | - | - | - | IL-23, IL-17, TNF-α, IL-1β, IL-6 | Diagnosis | 86.7% | 90% | IL-23, IL-17, TNF-α, IL-1β, IL-6 |
| Prediction of Treatment success | 25% | 92.5% | ||||||
| NO | Endoscopic disease activity | 88% | 69% | IL-12, IL-8, TNF-α, IL-1α, IFNγ, IL-4, IL-13 | Endoscopic disease activity | 100% | 100% | IL-12, IL-8, TNF-α, IL-1α, IFNγ, IL-4, IL-13 |
| S100A12 | Diagnosis | 96% | 92% | - | Diagnosis | 91% | 96% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baum, S.; Hamedi, K.; Loftus, C.; Loftus, G.; Zhou, E.-R.; Arce, S. From Cytokines to Biomarkers: Mapping the Immunopathology of Inflammatory Bowel Disease. Cells 2025, 14, 1589. https://doi.org/10.3390/cells14201589
Baum S, Hamedi K, Loftus C, Loftus G, Zhou E-R, Arce S. From Cytokines to Biomarkers: Mapping the Immunopathology of Inflammatory Bowel Disease. Cells. 2025; 14(20):1589. https://doi.org/10.3390/cells14201589
Chicago/Turabian StyleBaum, Sarah, Kamron Hamedi, Caroline Loftus, Gannett Loftus, Emily-Rose Zhou, and Sergio Arce. 2025. "From Cytokines to Biomarkers: Mapping the Immunopathology of Inflammatory Bowel Disease" Cells 14, no. 20: 1589. https://doi.org/10.3390/cells14201589
APA StyleBaum, S., Hamedi, K., Loftus, C., Loftus, G., Zhou, E.-R., & Arce, S. (2025). From Cytokines to Biomarkers: Mapping the Immunopathology of Inflammatory Bowel Disease. Cells, 14(20), 1589. https://doi.org/10.3390/cells14201589







