MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. miRNA Network Analysis
2.2. Patients and Tissue Samples
2.3. Cell Cultures
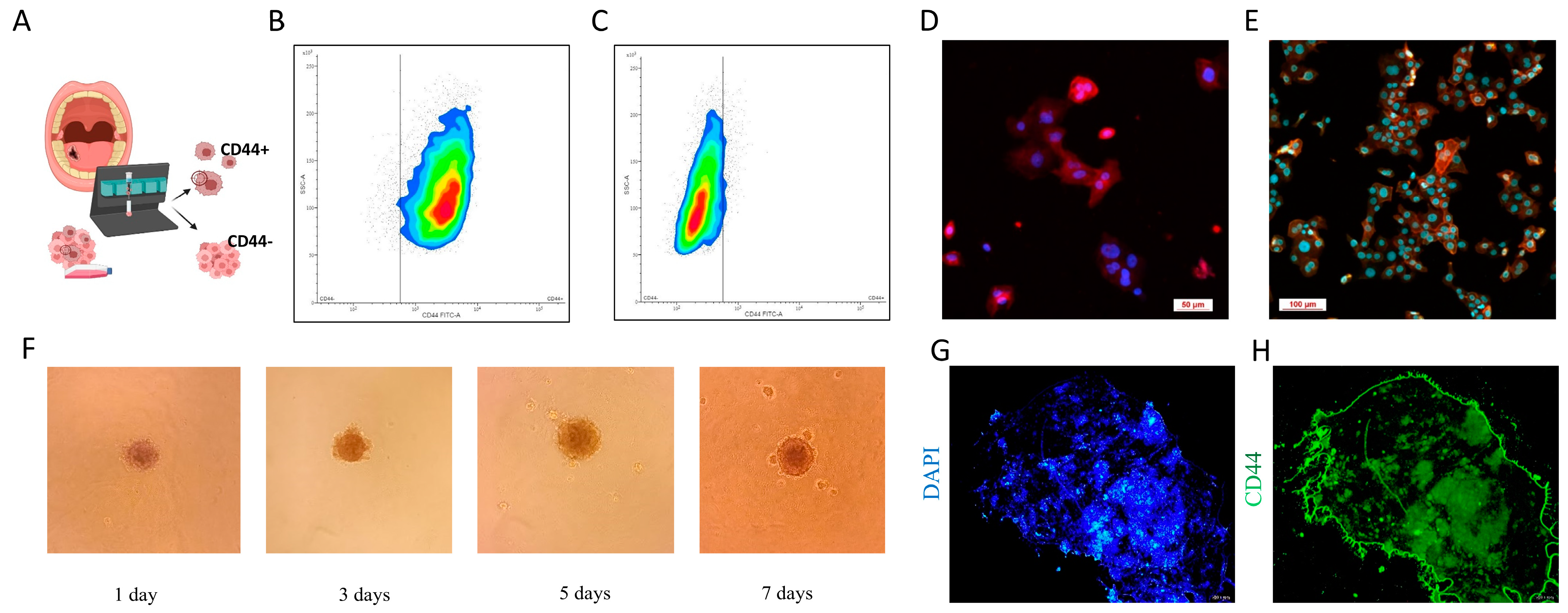
2.4. Magnetic Cell Sorting (MACS)
2.5. Spheroid Formation Assay
2.6. Immunocytochemistry
2.7. Transfection of miRNA-21 Inhibitor
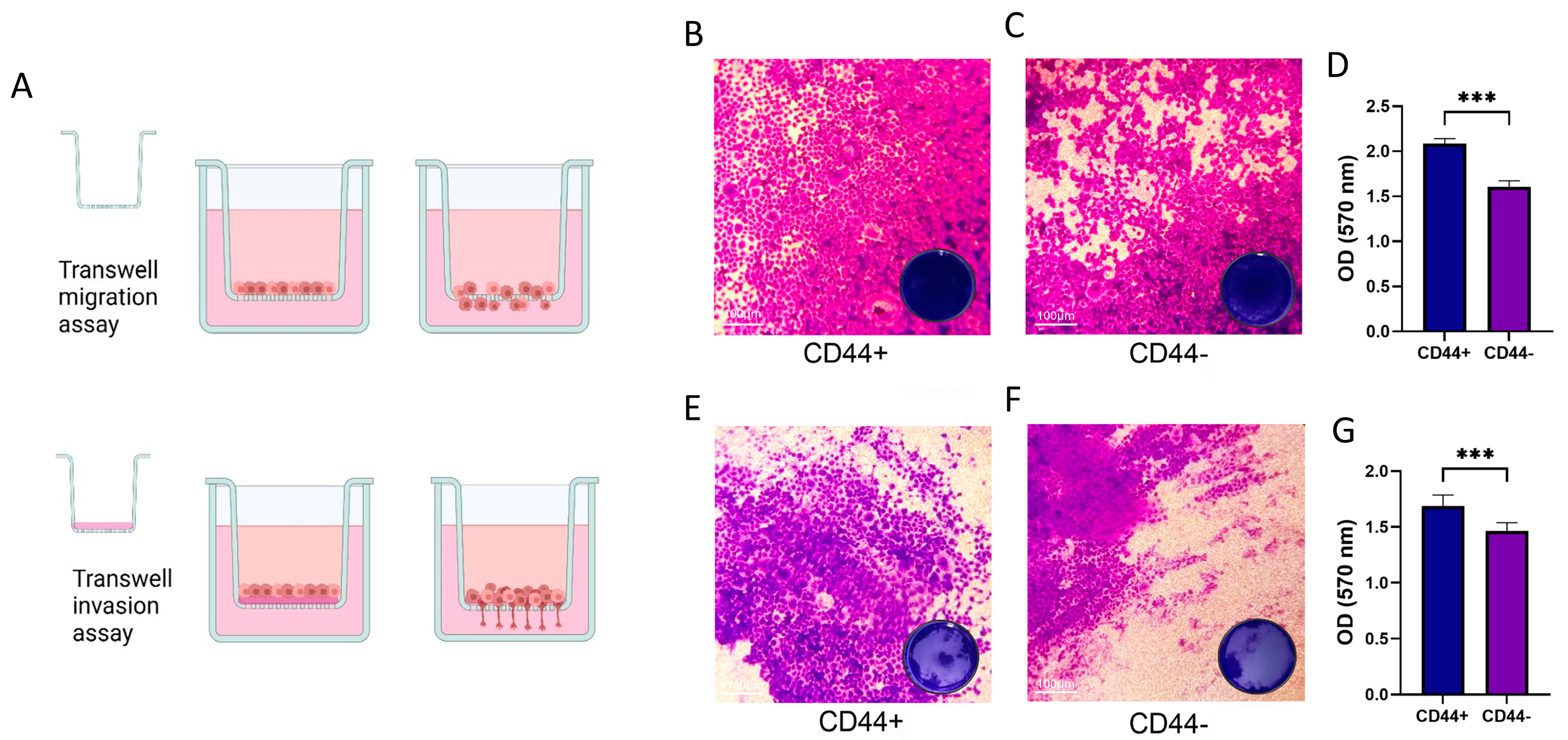
2.8. Transwell Cell Migration and Invasion Assay
2.9. Apoptosis Assay Annexin V
2.10. Cell Cycle Analysis
2.11. Isolation of RNA and Reverse-Transcription Polymerase Chain Reaction
2.12. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.13. TaqMan microRNA Assay
2.14. Statistical Analysis
3. Results
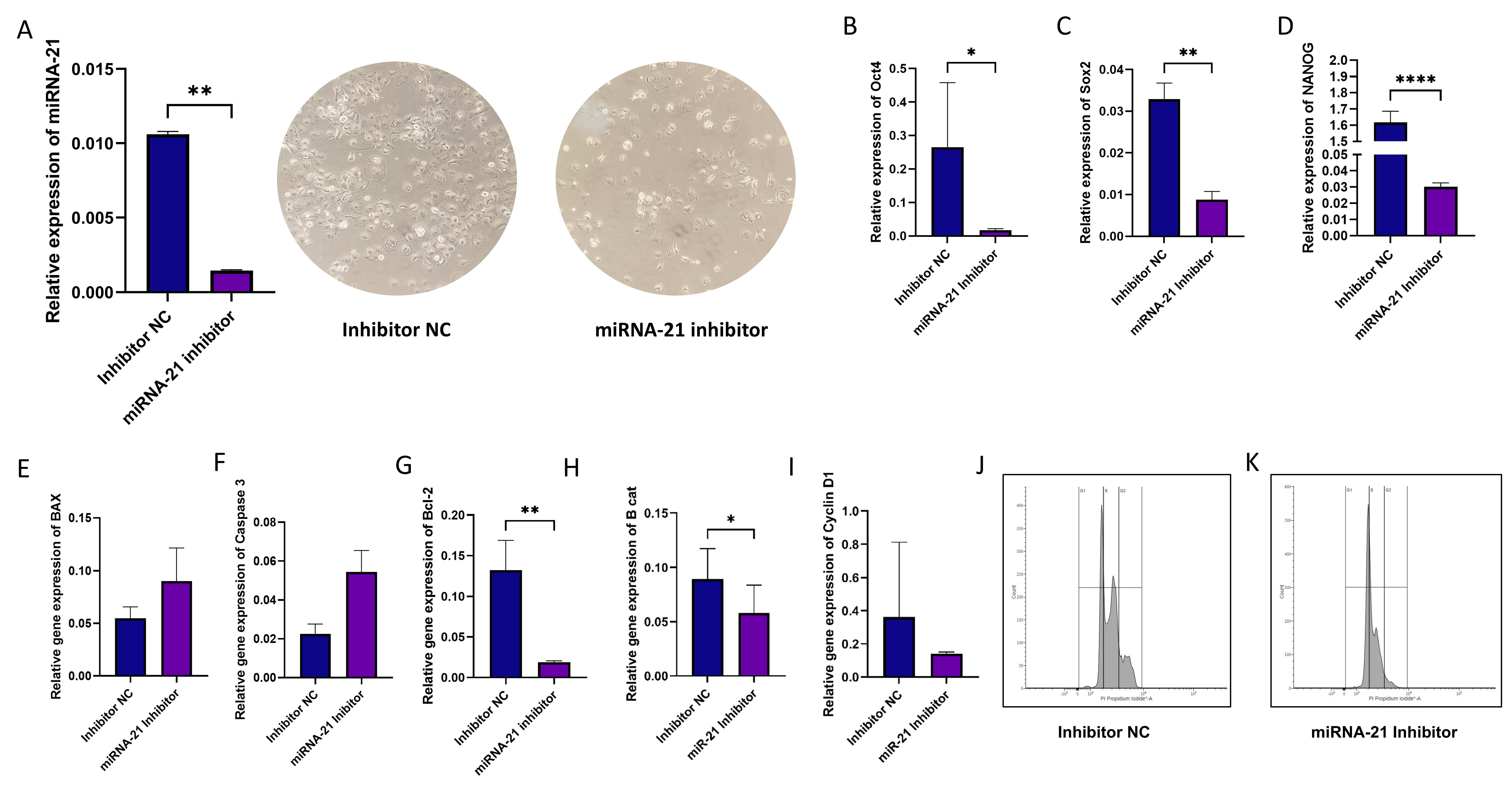
3.1. Interaction of miRNA-21 with Key Carcinogenesis-Related Genes in OSCC
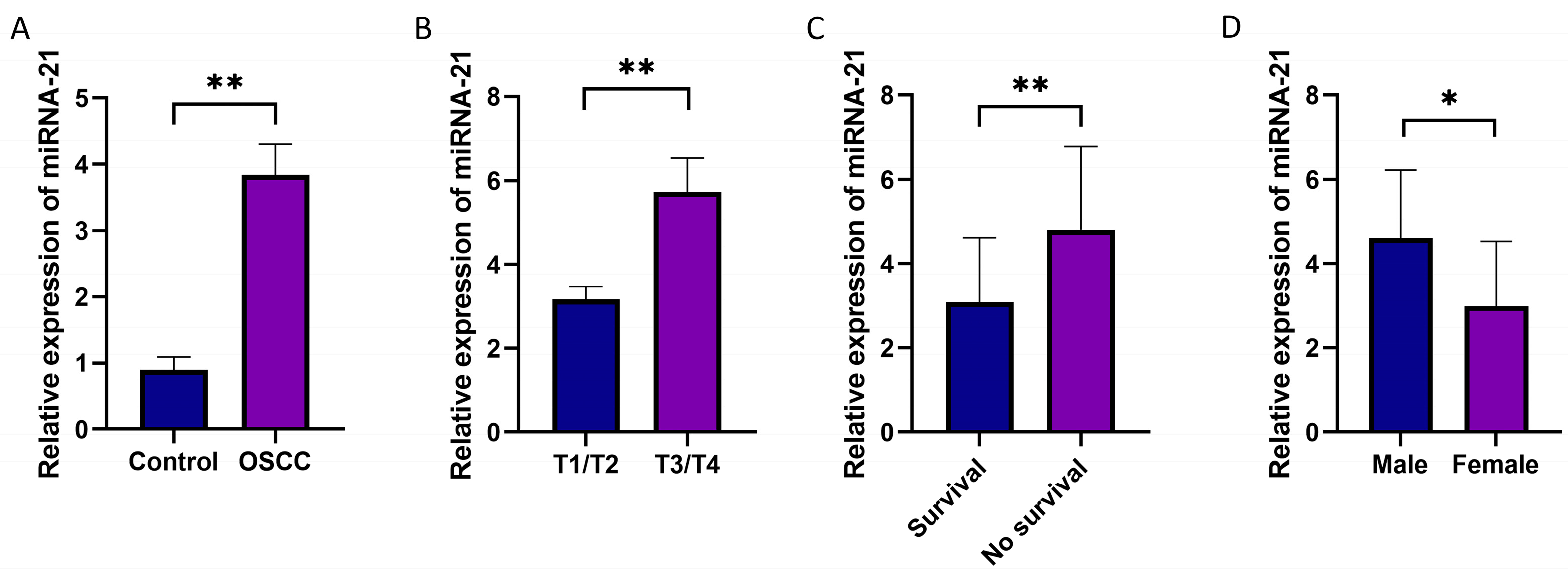
3.2. The Expression Levels of miRNA-21 in OSCC Tissues
3.3. CSC Characterization
3.4. CD44+ Cells Migration and Invasion
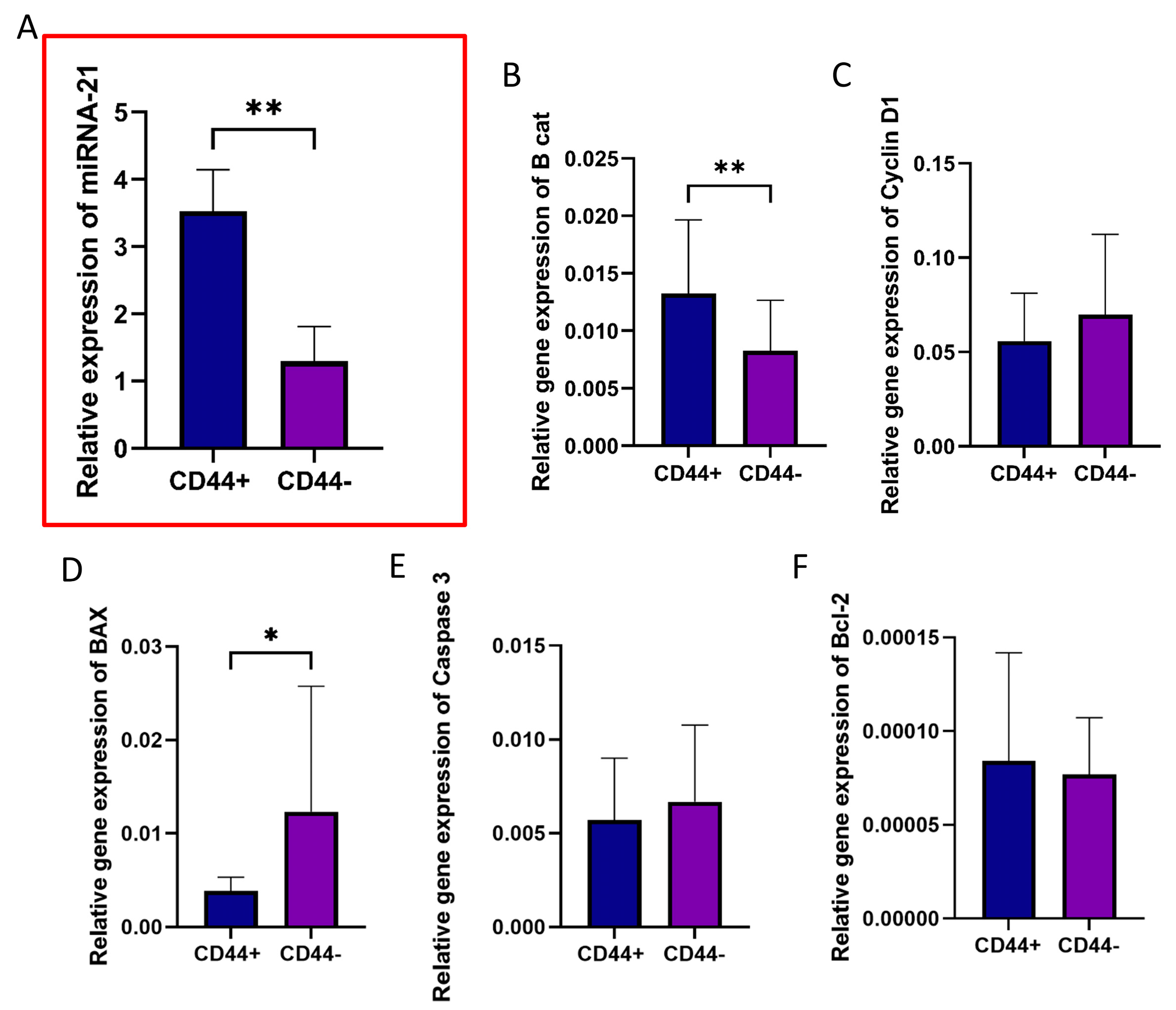
3.5. Gene Expression Profiles of CD44+ and CD44− Cells Isolated from OSCC Cultures
3.6. Effect of miR-21 Inhibition in CD44+ Cells
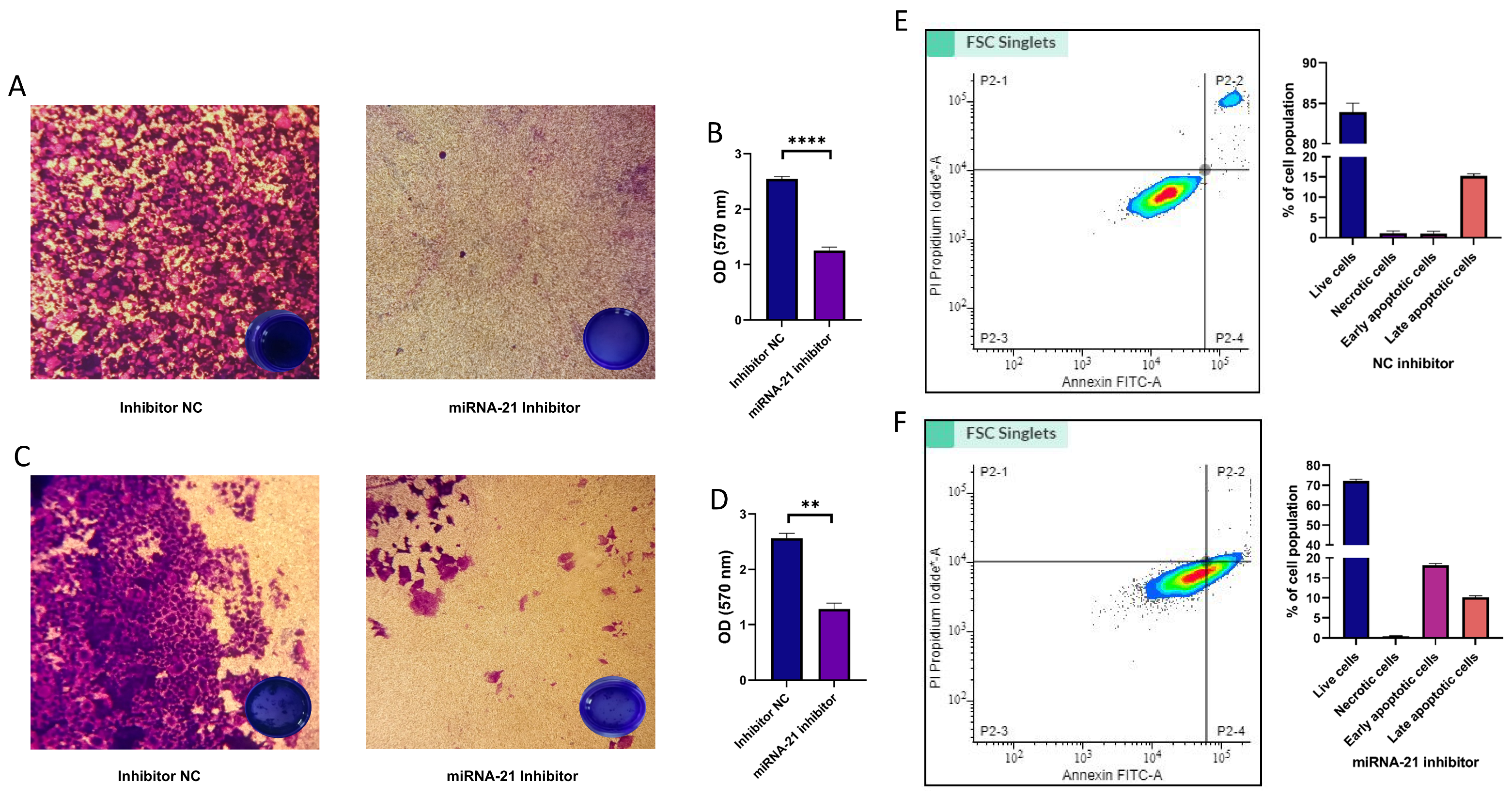
3.7. Migration, Invasion, and Cell Apoptosis in CSCs Following miR-21 Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jelovac, D.B.; Tepavčević, Z.; Nikolić, N.; Ilić, B.; Eljabo, N.; Popović, B.; Čarkić, J.; Konstantinović, V.; Vukadinović, M.; Miličić, B.; et al. The Amplification of C-Erb-B2 in Cancer-Free Surgical Margins Is a Predictor of Poor Outcome in Oral Squamous Cell Carcinoma. Int. J. Oral. Maxillofac. Surg. 2016, 45, 700–705. [Google Scholar] [CrossRef]
- Le Campion, A.C.O.V.; Ribeiro, C.M.B.; Luiz, R.R.; da Silva Júnior, F.F.; Barros, H.C.S.; dos Santos, K.d.C.B.; Ferreira, S.J.; Gonçalves, L.S.; Ferreira, S.M.S. Low Survival Rates of Oral and Oropharyngeal Squamous Cell Carcinoma. Int. J. Dent. 2017, 2017, 5815493. [Google Scholar] [CrossRef]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular Mechanisms and Pathways as Targets for Cancer Prevention and Progression with Dietary Compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef] [PubMed]
- Baillie, R.; Tan, S.T.; Itinteang, T. Cancer Stem Cells in Oral Cavity Squamous Cell Carcinoma: A Review. Front. Oncol. 2017, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as Oncogenes and Tumor Suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamamoto, Y.; Ochiya, T. miRNA Signaling Networks in Cancer Stem Cells. Regen. Ther. 2021, 17, 1–7. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hsieh, P.-L.; Liao, Y.-W.; Peng, C.-Y.; Lu, M.-Y.; Yang, C.-H.; Yu, C.-C.; Liu, C.-M. Berberine-Targeted miR-21 Chemosensitizes Oral Carcinomas Stem Cells. Oncotarget 2017, 8, 80900–80908. [Google Scholar] [CrossRef]
- Jiao, X.; Qian, X.; Wu, L.; Li, B.; Wang, Y.; Kong, X.; Xiong, L. microRNA: The Impact on Cancer Stemness and Therapeutic Resistance. Cells 2020, 9, 8. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, H.; Shi, Y.; Yin, L.; Zhu, Y.; Liu, R. Identification of Cancer Stem Cell Molecular Markers and Effects of Hsa-miR-21-3p on Stemness in Esophageal Squamous Cell Carcinoma. Cancers 2019, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Medina, P.P.; Nolde, M.; Slack, F.J. OncomiR Addiction in an in Vivo Model of microRNA-21-Induced Pre-B-Cell Lymphoma. Nature 2010, 467, 86–90. [Google Scholar] [CrossRef]
- Volinia, S.; Calin, G.A.; Liu, C.-G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA Expression Signature of Human Solid Tumors Defines Cancer Gene Targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Earle, C.; Wong, G.; Spevak, C.C.; Krueger, K. Stem Cell Marker (Nanog) and Stat-3 Signaling Promote MicroRNA-21 Expression and Chemoresistance in Hyaluronan/CD44-Activated Head and Neck Squamous Cell Carcinoma Cells. Oncogene 2012, 31, 149–160. [Google Scholar] [CrossRef]
- Mortoglou, M.; Miralles, F.; Arisan, E.D.; Dart, A.; Jurcevic, S.; Lange, S.; Uysal Onganer, P. microRNA-21 Regulates Stemness in Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 1275. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, N.; Hu, J.; Wang, L. Inhibitory Effect of RNA-Mediated Knockdown of Zinc Finger Protein 91 Pseudogene on Pancreatic Cancer Cell Growth and Invasion. Oncol. Lett. 2016, 12, 1343–1348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rhim, J.; Baek, W.; Seo, Y.; Kim, J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells 2022, 11, 2791. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Yang, J.; Pan, T.; Zhang, S.; Wang, Z. MiR-21 Protected Human Glioblastoma U87MG Cells from Chemotherapeutic Drug Temozolomide Induced Apoptosis by Decreasing Bax/Bcl-2 Ratio and Caspase-3 Activity. Brain Res. 2010, 1352, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Takahashi, M.; Hur, K.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum miR-21 as a Diagnostic and Prognostic Biomarker in Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 849–859. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, Z.; Li, B.; Li, H.; Yang, B.; Li, T.; Mei, Z. miRNA-21 May Serve as a Promising Noninvasive Marker of Glioma with a High Diagnostic Performance: A Pooled Analysis of 997 Patients. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987650. [Google Scholar] [CrossRef]
- Pilotto Heming, C.; Niemeyer Filho, P.; Moura-Neto, V.; Aran, V. Recent Advances in the Use of Liquid Biopsy to Fight Central Nervous System Tumors. Cancer Treat. Res. Commun. 2023, 35, 100709. [Google Scholar] [CrossRef]
- Rajan, C.; Roshan, V.G.D.; Khan, I.; Manasa, V.G.; Himal, I.; Kattoor, J.; Thomas, S.; Kondaiah, P.; Kannan, S. MiRNA Expression Profiling and Emergence of New Prognostic Signature for Oral Squamous Cell Carcinoma. Sci. Rep. 2021, 11, 7298. [Google Scholar] [CrossRef]
- Ganjibakhsh, M.; Aminishakib, P.; Farzaneh, P.; Karimi, A.; Fazeli, S.A.S.; Rajabi, M.; Nasimian, A.; Naini, F.B.; Rahmati, H.; Gohari, N.S.; et al. Establishment and Characterization of Primary Cultures from Iranian Oral Squamous Cell Carcinoma Patients by Enzymatic Method and Explant Culture. J. Dent. 2017, 14, 191–202. [Google Scholar]
- Jaksic Karisik, M.; Lazarevic, M.; Mitic, D.; Nikolic, N.; Milosevic Markovic, M.; Jelovac, D.; Milasin, J. Osteogenic and Adipogenic Differentiation Potential of Oral Cancer Stem Cells May Offer New Treatment Modalities. Int. J. Mol. Sci. 2023, 24, 4704. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, M.; Milošević, M.; Trišić, D.; Toljić, B.; Simonović, J.; Nikolić, N.; Miković, N.; Jelovac, D.; Petrović, M.; Vukadinović, M.; et al. Putative Cancer Stem Cells Are Present in Surgical Margins of Oral Squamous Cell Carcinoma. J. BUON 2018, 23, 1686–1692. [Google Scholar] [PubMed]
- Zheng, Y.; Xie, J.; Jiang, F.; Li, Y.; Chang, G.; Ma, H. Inhibition of miR-21 Promotes Cell Apoptosis in Oral Squamous Cell Carcinoma by Upregulating PTEN. Oncol. Rep. 2018, 40, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kawakita, D.; Matsuo, K. Alcohol and Head and Neck Cancer. Cancer Metastasis Rev. 2017, 36, 425–434. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, Y.; Cui, Z. MicroRNA-21 Depletion by CRISPR/Cas9 in CNE2 Nasopharyngeal Cells Hinders Proliferation and Induces Apoptosis by Targeting the PI3K/AKT/MOTOR Signaling Pathway. Int. J. Clin. Exp. Pathol. 2020, 13, 738–745. [Google Scholar] [PubMed]
- Chan, J.A.; Krichevsky, A.M.; Kosik, K.S. MicroRNA-21 Is an Antiapoptotic Factor in Human Glioblastoma Cells. Cancer Res. 2005, 65, 6029–6033. [Google Scholar] [CrossRef]
- Zhou, C.; Ding, J.; Wu, Y. Resveratrol Induces Apoptosis of Bladder Cancer Cells via miR-21 Regulation of the Akt/Bcl-2 Signaling Pathway. Mol. Med. Rep. 2014, 9, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Y.; Sun, X.C. LincRNA-ROR functions as a ceRNA to regulate Oct4, Sox2, and Nanog expression by sponging miR-145 and its effect on biologic characteristics of colonic cancer stem cells. Zhonghua Bing Li Xue Za Zhi 2018, 47, 284–290. [Google Scholar] [CrossRef]
- Kanwal, N.; Al Samarrai, O.R.; Al-Zaidi, H.M.H.; Mirzaei, A.R.; Heidari, M.J. Comprehensive Analysis of microRNA (miRNA) in Cancer Cells. Cell. Mol. Biomed. Rep. 2023, 3, 89–97. [Google Scholar] [CrossRef]
- Hashemi, M.; Mirdamadi, M.S.A.; Talebi, Y.; Khaniabad, N.; Banaei, G.; Daneii, P.; Gholami, S.; Ghorbani, A.; Tavakolpournegari, A.; Farsani, Z.M.; et al. Pre-Clinical and Clinical Importance of miR-21 in Human Cancers: Tumorigenesis, Therapy Response, Delivery Approaches and Targeting Agents. Pharmacol. Res. 2023, 187, 106568. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, A.N.; Sharma, R.; Mateen, S.; Shukla, B.; Singh, A.; Chandel, S. Circulating MicroRNA-21 Expression as a Novel Serum Biomarker for Oral Sub-Mucous Fibrosis and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Chin, K.-Y.; Das, S. miRNA-Regulated Cancer Stem Cells: Understanding the Property and the Role of miRNA in Carcinogenesis. Tumor Biol. 2016, 37, 13039–13048. [Google Scholar] [CrossRef] [PubMed]
- Yeh, D.-W.; Chen, Y.-S.; Lai, C.-Y.; Liu, Y.-L.; Lu, C.-H.; Lo, J.-F.; Chen, L.; Hsu, L.-C.; Luo, Y.; Xiang, R.; et al. Downregulation of COMMD1 by miR-205 Promotes a Positive Feedback Loop for Amplifying Inflammatory- and Stemness-Associated Properties of Cancer Cells. Cell Death Differ. 2016, 23, 841–852. [Google Scholar] [CrossRef]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Rajendra, S.G.; Levi, E.; Majumdar, A.P. miR-21 and miR-145 Cooperation in Regulation of Colon Cancer Stem Cells. Mol. Cancer 2015, 14, 98. [Google Scholar] [CrossRef]
- Sekar, D.; Krishnan, R.; Panagal, M.; Sivakumar, P.; Gopinath, V.; Basam, V. Deciphering the Role of microRNA 21 in Cancer Stem Cells (CSCs). Genes Dis. 2016, 3, 277–281. [Google Scholar] [CrossRef]

| Variables | Values (n = 20) |
|---|---|
| Gender (%) | |
| Male | 11 (55) |
| Female | 9 (45) |
| Age (years) | |
| Mean ± SD | 58.6 ± 11.7 |
| Min–Max | 43–83 |
| 5-year survival rate | |
| Survival | 11 (55) |
| No survival | 9 (45) |
| Alcohol consumption (%) | |
| Yes | 6 (30) |
| No | 14 (70) |
| Smoking (%) | |
| Yes | 16 (80) |
| No | 4 (20) |
| T status * (%) | |
| T1/2 | 13 (65) |
| T3/4 | 7 (35) |
| N status ** (%) | |
| N0 | 16 (80) |
| N1 | 3 (15) |
| N2 | 1 (5) |
| M status *** (%) | |
| M0 | 20 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaksic Karisik, M.; Lazarevic, M.; Mitic, D.; Milosevic Markovic, M.; Riberti, N.; Jelovac, D.; Milasin, J. MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer. Cells 2025, 14, 91. https://doi.org/10.3390/cells14020091
Jaksic Karisik M, Lazarevic M, Mitic D, Milosevic Markovic M, Riberti N, Jelovac D, Milasin J. MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer. Cells. 2025; 14(2):91. https://doi.org/10.3390/cells14020091
Chicago/Turabian StyleJaksic Karisik, Milica, Milos Lazarevic, Dijana Mitic, Maja Milosevic Markovic, Nicole Riberti, Drago Jelovac, and Jelena Milasin. 2025. "MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer" Cells 14, no. 2: 91. https://doi.org/10.3390/cells14020091
APA StyleJaksic Karisik, M., Lazarevic, M., Mitic, D., Milosevic Markovic, M., Riberti, N., Jelovac, D., & Milasin, J. (2025). MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer. Cells, 14(2), 91. https://doi.org/10.3390/cells14020091











