Natural Killer Cell-Secreted IFN-γ and TNF-α Mediated Differentiation in Lung Stem-like Tumors, Leading to the Susceptibility of the Tumors to Chemotherapeutic Drugs
Abstract
1. Introduction
2. Results
2.1. Enhanced Susceptibility of Chemotherapeutic Drugs Against Differentiated Tumors in Comparison to Their Stem-like Counterparts
2.2. Enhanced Susceptibility of Chemotherapeutic Drugs Against NK Cell-Differentiated Tumors Compared to Stem-like Tumors
2.3. Enhanced Susceptibility of rhIFN-γ- and rhTNF-α-Treated Tumors Against Chemotherapeutic Drugs
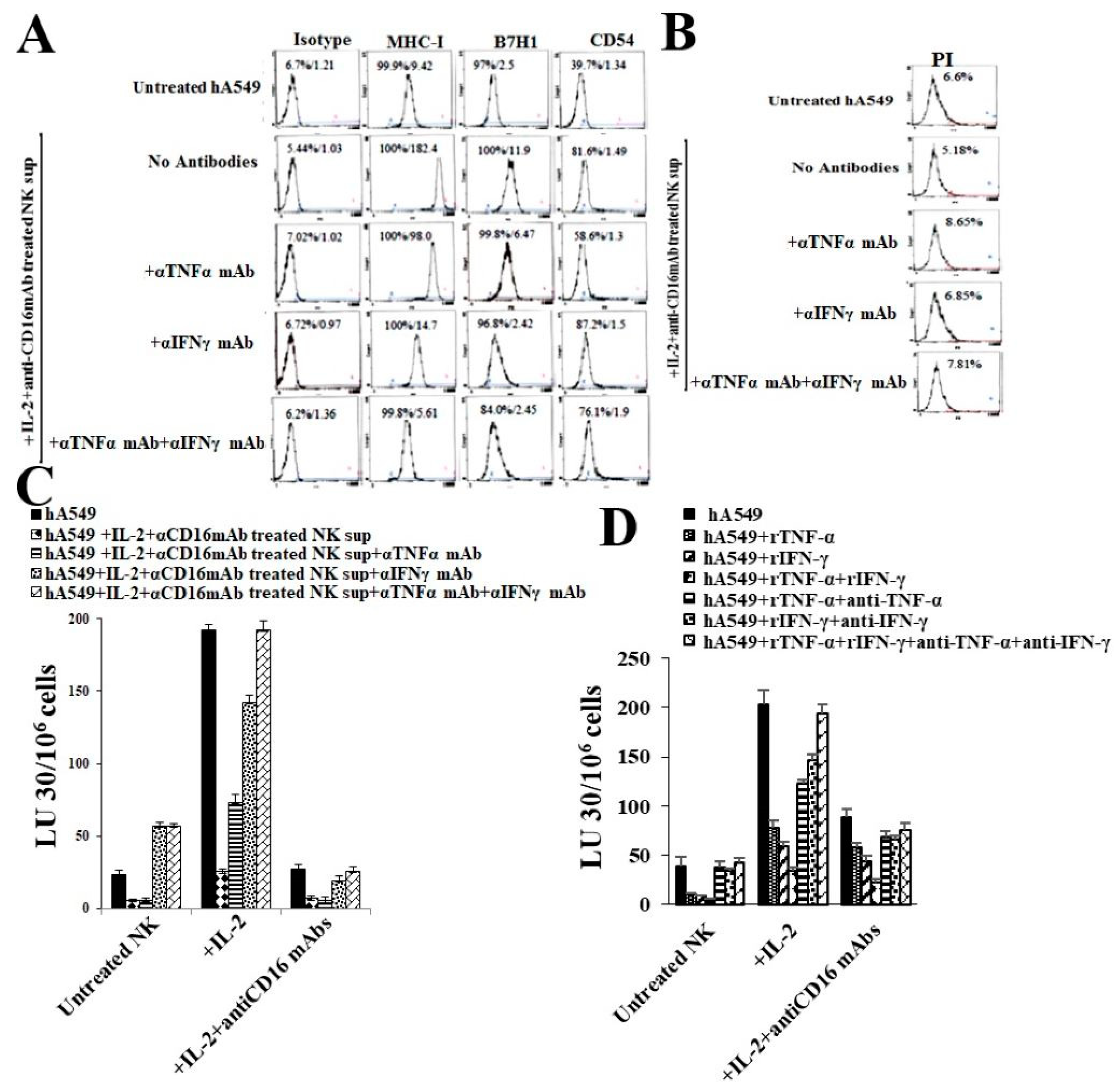
2.4. Anti-IFN-γ and Anti-TNF-α Inhibited Both NK Cells’ Supernatant Treated, or rhIFN-γ and rhTNF-α Mediated Differentiation of hA549 Cells
2.5. Anti-MHC-Class I Induces Different Effects in CSCs and Differentiated hA549 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies for Cell Cultures and Flow Cytometry
4.2. Isolation of NK Cells from PBMCs
4.3. Lung Cancer Stem Cell Differentiation Using NK Cells’ Supernatant
4.4. Surface Markers and Cell Death Analysis
4.5. Enzyme-Linked Immunosorbent Assays (ELISAs)
4.6. Four-Hour Chromium Release Cytotoxicity Assay
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NK Cells | Natural Killer Cells |
| MHC-Class I | major histocompatibility complex molecule class I |
| IFN-γ | interferon-gamma |
| TNF-α | tumor necrosis factor-α |
| CSCs | cancer stem cells |
| rhIL-2 | recombinant human IL-2 |
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo, G.; Imbimbo, M.; Garassino, M.C. Is the chemotherapy era in advanced non-small cell lung cancer really over? Maybe not yet. Tumori 2016, 2016, 223–225. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Damotte, D.; Alifano, M. New Therapeutic Strategies for Lung Cancer. Cancers 2021, 13, 1937. [Google Scholar] [CrossRef]
- Khajuria, O.; Sharma, N. Epigenetic targeting for lung cancer treatment via CRISPR/Cas9 technology. Adv. Cancer Biol. Metastasis 2021, 3, 100012. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Byrne, K. Tissue and Blood Biomarkers in Lung Cancer: A Review. Adv. Clin. Chem. 2018, 86, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.F.; Goggins, W.B.; Tse, S.L.A. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Denisenko, T.V.; Budkevich, I.N.; Zhivotovsky, B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018, 9, 117. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhan, Y.; Sun, L.; Zhu, W. Cancer Stem Cells and the Tumor Microenvironment in Tumor Drug Resistance. Stem Cell Rev. Rep. 2023, 19, 2141–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E.; et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 2012, 148, 259–272. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; de-Maya-Girones, J.D.; Calabuig-Fariñas, S.; Lucas, R.; Martínez, A.; Pardo-Sánchez, J.M.; Alonso, S.; Blasco, A.; Guijarro, R.; Martorell, M.; et al. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10, 660. [Google Scholar] [CrossRef]
- Raniszewska, A.; Polubiec-Kownacka, M.; Rutkowska, E.; Domagala-Kulawik, J. PD-L1 Expression on Lung Cancer Stem Cells in Metastatic Lymph Nodes Aspirates. Stem Cell Rev. Rep. 2019, 15, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Raniszewska, A.; Vroman, H.; Dumoulin, D.; Cornelissen, R.; Aerts, J.; Domagała-Kulawik, J. PD-L1(+) lung cancer stem cells modify the metastatic lymph-node immunomicroenvironment in nsclc patients. Cancer Immunol. Immunother. 2021, 70, 453–461. [Google Scholar] [CrossRef]
- Raniszewska, A.; Kwiecień, I.; Sokołowski, R.; Rutkowska, E.; Domagała-Kulawik, J. Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates. Cancers 2020, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Bui, V.T.; Tseng, H.C.; Kozlowska, A.; Maung, P.O.; Kaur, K.; Topchyan, P.; Jewett, A. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Front. Immunol. 2015, 6, 576. [Google Scholar] [CrossRef]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef]
- Kozlowska, A.K.; Topchyan, P.; Kaur, K.; Tseng, H.C.; Teruel, A.; Hiraga, T.; Jewett, A. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J. Cancer 2017, 8, 537–554. [Google Scholar] [CrossRef]
- Hersey, P.; Edwards, A.; Honeyman, M.; McCarthy, W.H. Low natural-killer-cell activity in familial melanoma patients and their relatives. Br. J. Cancer 1979, 40, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kuss, I.; Saito, T.; Johnson, J.T.; Whiteside, T.L. Clinical significance of decreased zeta chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin. Cancer Res. 1999, 5, 329–334. [Google Scholar] [PubMed]
- Guillerey, C. NK Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Lee, K.M.; Kim, D.W.; Heo, D.S. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J. Immunol. 2004, 172, 7335–7340. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Pietra, G.; Manzini, C.; Rivara, S.; Vitale, M.; Cantoni, C.; Petretto, A.; Balsamo, M.; Conte, R.; Benelli, R.; Minghelli, S.; et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012, 72, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Hershey, P.; Edwards, A.; Milton, G.W.; McCarthy, W.H. Relationship of cell-mediated cytotoxicity against melanoma cells to prognosis in melanoma patients. Br. J. Cancer 1978, 37, 505–513. [Google Scholar] [CrossRef]
- Lai, P.; Rabinowich, H.; Crowley-Nowick, P.A.; Bell, M.C.; Mantovani, G.; Whiteside, T.L. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin. Cancer Res. 1996, 2, 161–173. [Google Scholar] [PubMed]
- Burke, S.; Lakshmikanth, T.; Colucci, F.; Carbone, E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010, 31, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Brittenden, J.; Heys, S.D.; Ross, J.; Eremin, O. Natural killer cells and cancer. Cancer 1996, 77, 1226–1243. [Google Scholar] [CrossRef]
- Chary, A. Culturing Human Lung Adenocarcinoma Cells in a Serum-Free Environment. Methods Mol. Biol. 2023, 2645, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Koul, A.M.; Wani, U.M.; Farooq, F.; Amin, B.; Wani, Z.; Lone, A.; Qadri, A.; Qadri, R.A. Dissection of paracrine/autocrine interplay in lung tumor microenvironment mimicking cancer cell-monocyte co-culture models reveals proteins that promote inflammation and metastasis. BMC Cancer 2023, 23, 926. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Magister, S.; Obermajer, N.; Mirkovic, B.; Svajger, U.; Renko, M.; Softic, A.; Romih, R.; Colbert, J.D.; Watts, C.; Kos, J. Regulation of cathepsins S and L by cystatin F during maturation of dendritic cells. Eur. J. Cell Biol. 2012, 91, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kozlowska, A.K.; Topchyan, P.; Ko, M.W.; Ohanian, N.; Chiang, J.; Cook, J.; Maung, P.O.; Park, S.H.; Cacalano, N.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers 2019, 12, 63. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Arasteh, A.; Paranjpe, A.; Teruel, A.; Yang, W.; Behel, A.; Alva, J.A.; Walter, G.; Head, C.; Ishikawa, T.-o.; et al. Increased Lysis of Stem Cells but Not Their Differentiated Cells by Natural Killer Cells; De-Differentiation or Reprogramming Activates NK Cells. PLoS ONE 2010, 5, e11590. [Google Scholar] [CrossRef]
- Kaur, K.; Cook, J.; Park, S.-H.; Topchyan, P.; Kozlowska, A.; Ohanian, N.; Fang, C.; Nishimura, I.; Jewett, A. Novel Strategy to Expand Super-Charged NK Cells with Significant Potential to Lyse and Differentiate Cancer Stem Cells: Differences in NK Expansion and Function between Healthy and Cancer Patients. Front. Immunol. 2017, 8, 297. [Google Scholar] [CrossRef]
- Kaur, K.; Chen, P.-C.; Ko, M.-W.; Mei, A.; Senjor, E.; Malarkannan, S.; Kos, J.; Jewett, A. Sequential therapy with supercharged NK cells with either chemotherapy drug cisplatin or anti-PD-1 antibody decreases the tumor size and significantly enhances the NK function in Hu-BLT mice. Front. Immunol. 2023, 14, 1132807. [Google Scholar] [CrossRef] [PubMed]
- Shacka, J.J.; Garner, M.A.; Gonzalez, J.D.; Ye, Y.Z.; D’Alessandro, T.L.; Estévez, A.G. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 2006, 13, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, K.S.; Hassan, H.A.; Abdel-Aziz, S.A.; Marzouk, A.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. JNK signaling as a target for anticancer therapy. Pharmacol. Rep. 2021, 73, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Thielens, A.; Vivier, E.; Romagné, F. NK cell MHC class I specific receptors (KIR): From biology to clinical intervention. Curr. Opin. Immunol. 2012, 24, 239–245. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Prompsy, P.; Kimeswenger, S.; Tsai, Y.-C.; Ignatova, D.; Pavlova, O.; Iselin, C.; French, L.E.; Levesque, M.P.; Kuonen, F.; et al. MHC-I upregulation safeguards neoplastic T cells in the skin against NK cell-mediated eradication in mycosis fungoides. Nat. Commun. 2024, 15, 752. [Google Scholar] [CrossRef] [PubMed]
- Joetham, A.T.K.; Miyahara, N.; Matsubara, S.; Ohnishi, H.; Koya, T.; Dakhama, A.; Gelfand, E.W. Activation of naturally occurring lung CD54+CD25+ regulatory T cells requires CD8 and MHC 1 interaction. Proc. Natl. Acad. Sci. USA 2007, 104, 1507–1516. [Google Scholar] [CrossRef]
- Jewett, A.; Bonavida, B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J. Immunol. 1996, 156, 907–915. [Google Scholar] [CrossRef]
- Jewett, A.; Wang, M.Y.; Teruel, A.; Poupak, Z.; Bostanian, Z.; Park, N.H. Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex class I antigens by nuclear factor kappaB in HEp2 tumor cell line: Effect on the function of natural killer cells. Hum. Immunol. 2003, 64, 505–520. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, K.; Celis, A.P.; Jewett, A. Natural Killer Cell-Secreted IFN-γ and TNF-α Mediated Differentiation in Lung Stem-like Tumors, Leading to the Susceptibility of the Tumors to Chemotherapeutic Drugs. Cells 2025, 14, 90. https://doi.org/10.3390/cells14020090
Kaur K, Celis AP, Jewett A. Natural Killer Cell-Secreted IFN-γ and TNF-α Mediated Differentiation in Lung Stem-like Tumors, Leading to the Susceptibility of the Tumors to Chemotherapeutic Drugs. Cells. 2025; 14(2):90. https://doi.org/10.3390/cells14020090
Chicago/Turabian StyleKaur, Kawaljit, Angie Perez Celis, and Anahid Jewett. 2025. "Natural Killer Cell-Secreted IFN-γ and TNF-α Mediated Differentiation in Lung Stem-like Tumors, Leading to the Susceptibility of the Tumors to Chemotherapeutic Drugs" Cells 14, no. 2: 90. https://doi.org/10.3390/cells14020090
APA StyleKaur, K., Celis, A. P., & Jewett, A. (2025). Natural Killer Cell-Secreted IFN-γ and TNF-α Mediated Differentiation in Lung Stem-like Tumors, Leading to the Susceptibility of the Tumors to Chemotherapeutic Drugs. Cells, 14(2), 90. https://doi.org/10.3390/cells14020090







