Pvalb8, a Type of Oncomodulin, Regulates Neuromast Development and Auditory Function in Zebrafish
Abstract
Highlights
- pvalb8 is highly and specifically expressed in supporting cells and hair cells.
- The pvalb8 mutation causes developmental defects in neuromasts and significant hearing loss.
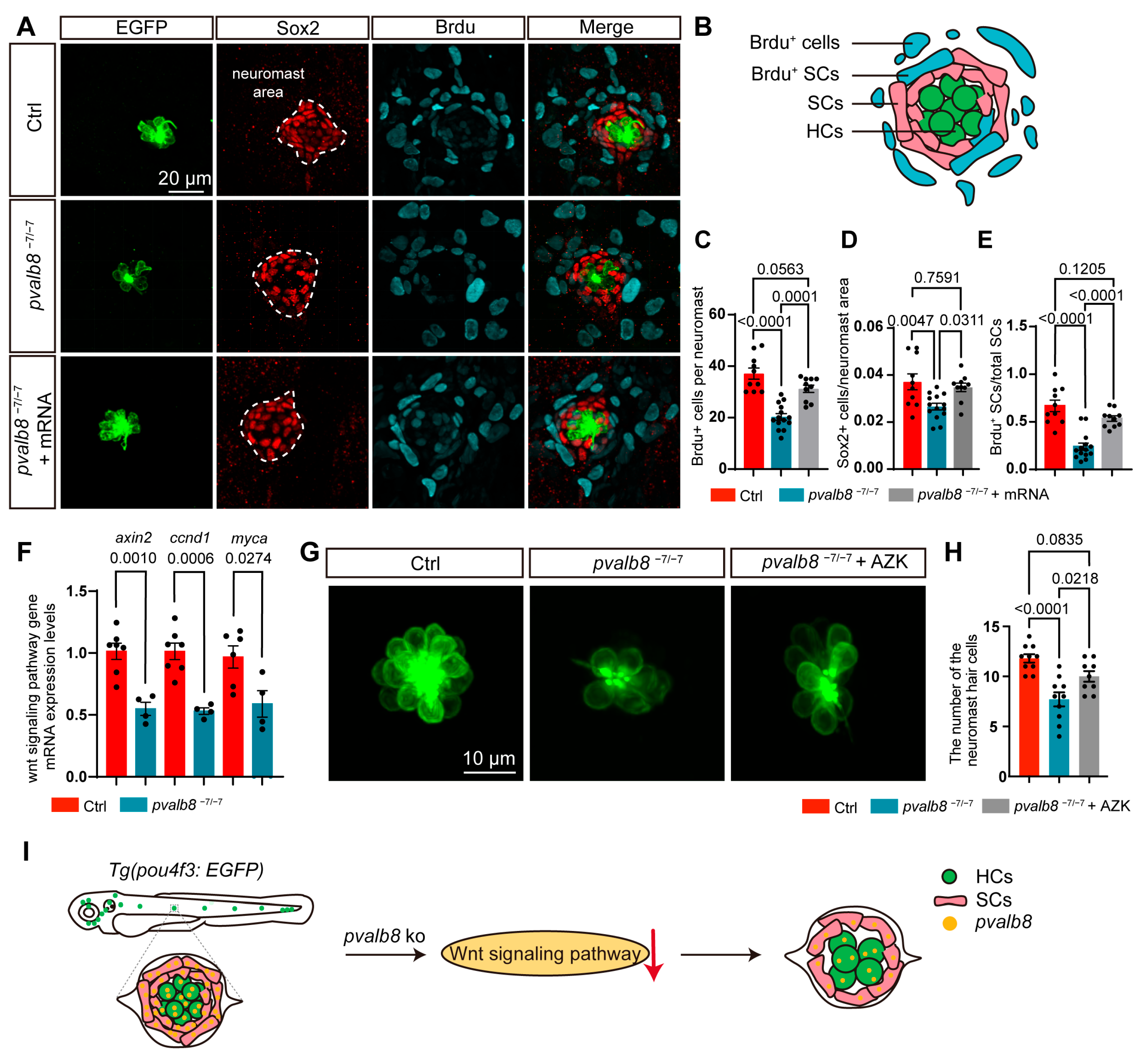
- Loss of pvalb8 suppresses supporting cell proliferation, limiting hair cell differentia-tion.
- Activation of the Wnt signaling pathway rescues the hair cell loss observed in pvalb8 mutants.
- pvalb8 regulates the development of neuromast and hair cells by mediating the Wnt signaling pathway.
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Breeding
2.2. Single–Cell Data Analysis
2.3. Whole-Mount In Situ Hybridization (WISH)
2.4. MO-Mediated Gene Knockdown
2.5. Startle Response Experiment
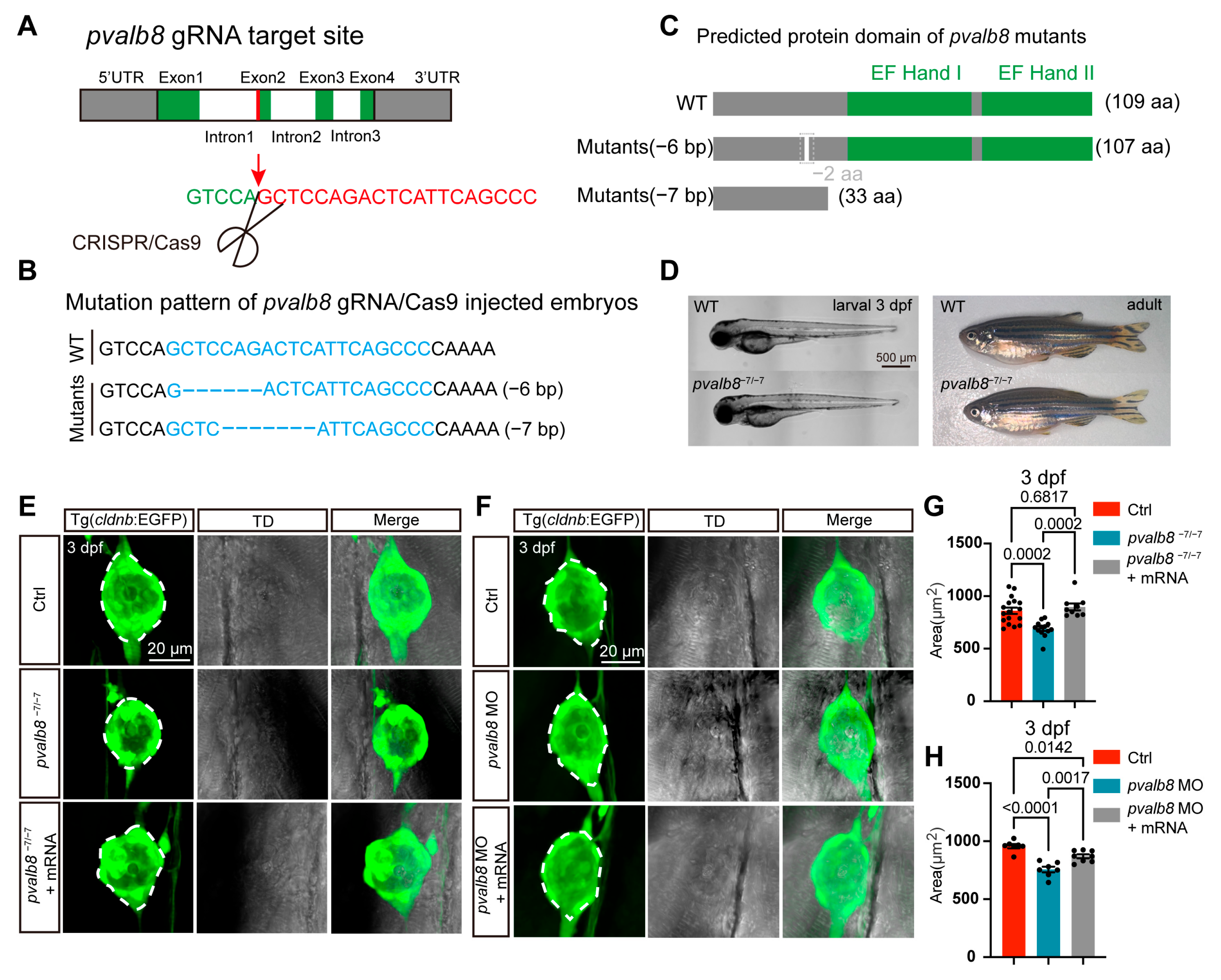
2.6. Generation of Knockout Zebrafish and Rescue Experiment
2.7. Quantitative RT-PCR
2.8. Cell Proliferation and Analysis
2.9. TUNEL Assay for Apoptosis Detection
2.10. FM4-64 and Wnt Activator Treatment
2.11. Imaging and Statistical Analysis
3. Results
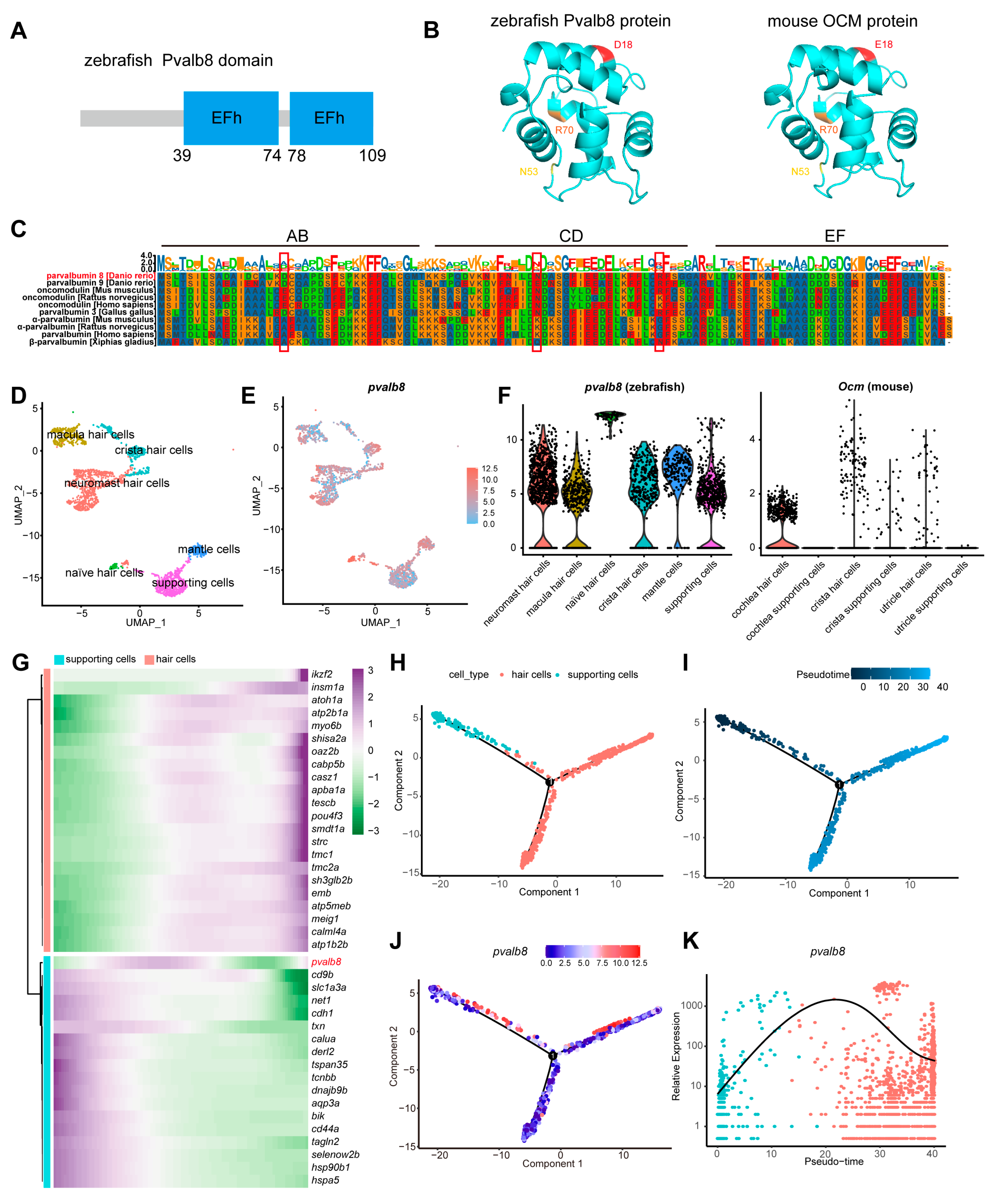
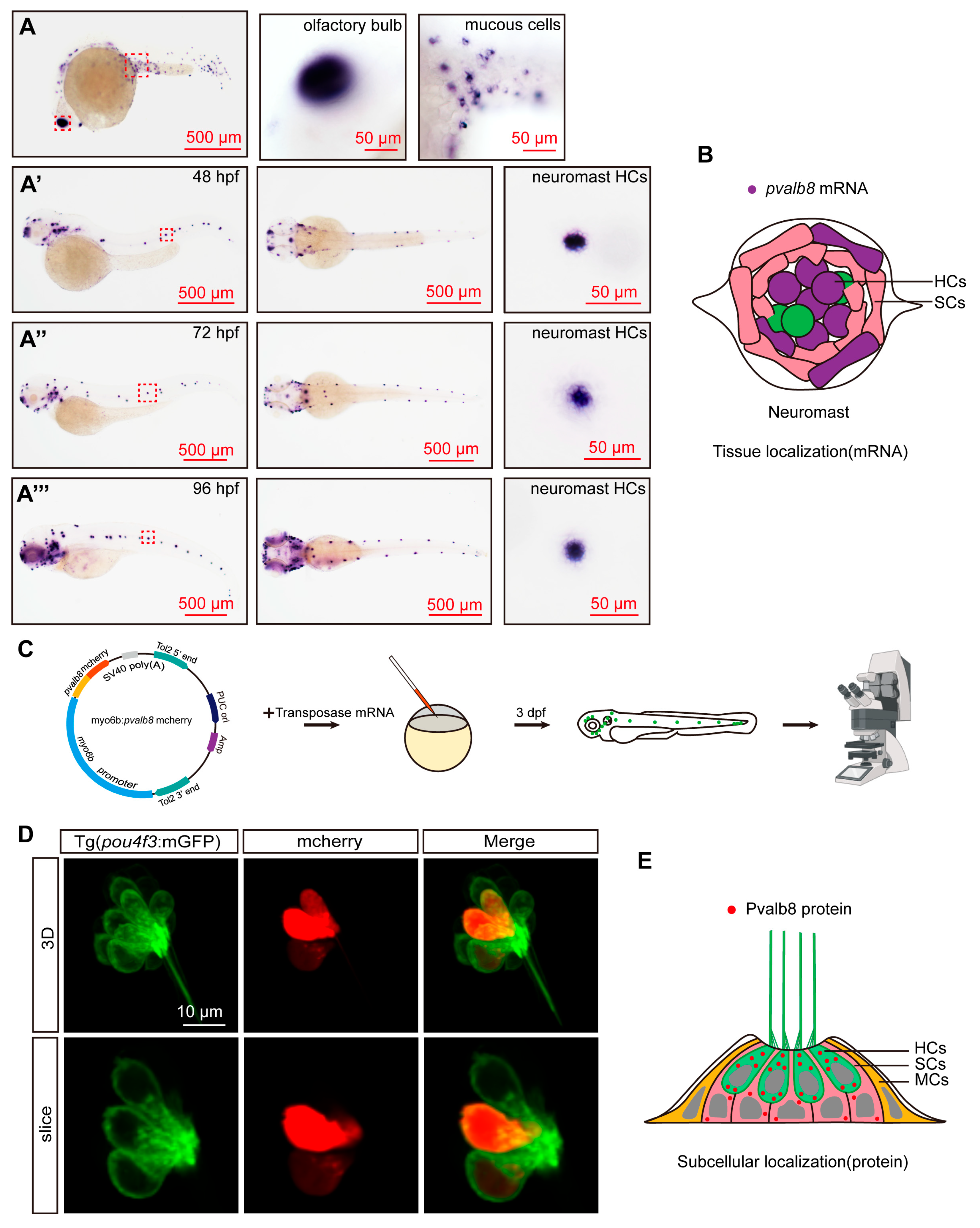
3.1. Pvalb8 Was Highly Expressed in the Neuromast Hair Cells
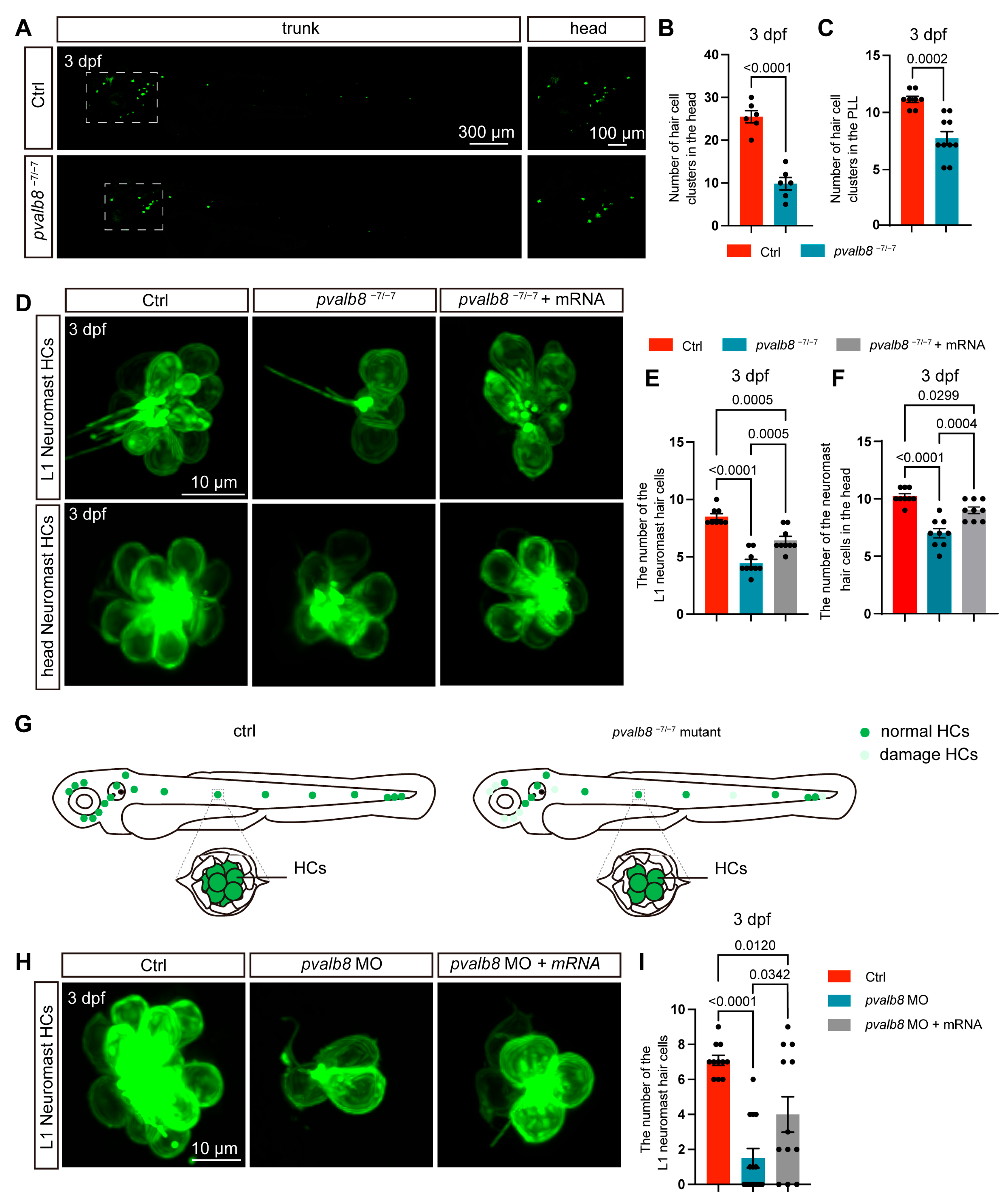
3.2. pvalb8 Is Required for Proper Neuromast Development
3.3. pvalb8 Regulates Hair Cell Development in Neuromasts
3.4. pvalb8 Deficiency Impairs Auditory Function
3.5. pvalb8 Deficiency Reduces Hair Cell Numbers by Impairing Proliferation Rather than Inducing Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maniaci, A.; La Via, L.; Lechien, J.R.; Sangiorgio, G.; Iannella, G.; Magliulo, G.; Pace, A.; Mat, Q.; Lavalle, S.; Lentini, M. Hearing Loss and Oxidative Stress: A Comprehensive Review. Antioxidants 2024, 13, 842. [Google Scholar] [CrossRef]
- Al-Ani, R.M. Various aspects of hearing loss in newborns: A narrative review. World J. Clin. Pediatr. 2023, 12, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, X.; Yang, L.; Chu, C.; Tan, F.; Yu, Z.; Ke, J.; Li, X.; Zheng, X.; Zhao, X.; et al. AAV-ie-K558R mediated cochlear gene therapy and hair cell regeneration. Signal Transduct. Target. Ther. 2022, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Huang, J.; Zhang, A.; Zhao, F.; Chen, T.; McDermott, Q.M.; Zheng, T.; Wang, H.; Zhang, R.; Zhang, X.; et al. Long-Lasting Auditory and Vestibular Recovery Following Gene Replacement Therapy in a Novel Usher Syndrome Type 1c Mouse Model. Adv. Sci. 2025, 12, e2410063. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Tan, F.; Zhang, L.; Lu, L.; Zhang, S.; Zhai, Y.; Lu, Y.; Qian, X.; Dong, W.; Zhou, Y.; et al. AAV-Mediated Gene Therapy Restores Hearing in Patients with DFNB9 Deafness. Adv. Sci. 2024, 11, e2306788. [Google Scholar] [CrossRef]
- Lv, J.; Wang, H.; Cheng, X.; Chen, Y.; Wang, D.; Zhang, L.; Cao, Q.; Tang, H.; Hu, S.; Gao, K.; et al. AAV1-hOTOF gene therapy for autosomal recessive deafness 9: A single-arm trial. Lancet 2024, 403, 2317–2325. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Lv, J.; Cheng, X.; Cao, Q.; Wang, D.; Zhang, L.; Zhu, B.; Shen, M.; Xu, C.; et al. Bilateral gene therapy in children with autosomal recessive deafness 9: Single-arm trial results. Nat. Med. 2024, 30, 1898–1904. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, L.; Lu, L.; Tan, F.; Cheng, C.; Lu, Y.; Dong, W.; Zhou, Y.; Fu, X.; Jiang, L.; et al. AAV gene therapy for autosomal recessive deafness 9: A single-arm trial. Nat. Med. 2025, 31, 2917–2926. [Google Scholar] [CrossRef]
- Qi, J.; Huang, W.; Lu, Y.; Yang, X.; Zhou, Y.; Chen, T.; Wang, X.; Yu, Y.; Sun, J.Q.; Chai, R. Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear. Neurosci. Bull. 2024, 40, 113–126. [Google Scholar] [CrossRef]
- Zheng, W.; Holt, J.R. The Mechanosensory Transduction Machinery in Inner Ear Hair Cells. Annu. Rev. Biophys. 2021, 50, 31–51. [Google Scholar] [CrossRef]
- Zhang, S.; Qiang, R.; Dong, Y.; Zhang, Y.; Chen, Y.; Zhou, H.; Gao, X.; Chai, R. Hair cell regeneration from inner ear progenitors in the mammalian cochlea. Am. J. Stem Cells 2020, 9, 25–35. [Google Scholar] [PubMed]
- Sato, M.P.; Benkafadar, N.; Heller, S. Hair cell regeneration, reinnervation, and restoration of hearing thresholds in the avian hearing organ. Cell Rep. 2024, 43, 113822. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Gil-Longo, J.; Campos-Toimil, M. Calcium binding proteins. Adv. Exp. Med. Biol. 2012, 740, 461–482. [Google Scholar] [CrossRef]
- Elies, J.; Yanez, M.; Pereira, T.M.C.; Gil-Longo, J.; MacDougall, D.A.; Campos-Toimil, M. An Update to Calcium Binding Proteins. Adv. Exp. Med. Biol. 2020, 1131, 183–213. [Google Scholar] [CrossRef] [PubMed]
- Nouvian, R. Letting the calcium flow. Elife 2024, 13, e96139. [Google Scholar] [CrossRef]
- Liu, W.; Chen, H.; Zhu, X.; Yu, H. Expression of Calbindin-D28K in the Developing and Adult Mouse Cochlea. J. Histochem. Cytochem. 2022, 70, 583–596. [Google Scholar] [CrossRef]
- Bottoms, C.A.; Schuermann, J.P.; Agah, S.; Henzl, M.T.; Tanner, J.J. Crystal structure of rat alpha-parvalbumin at 1.05 Angstrom resolution. Protein Sci. 2004, 13, 1724–1734. [Google Scholar] [CrossRef]
- Jia, Y.; Perez, J.C. Recombinant expression and affinity purification of snake venom gland parvalbumin in Escherichia coli. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 303–308. [Google Scholar] [CrossRef]
- Schwaller, B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010, 2, a004051. [Google Scholar] [CrossRef]
- Dijkstra, J.M.; Kondo, Y. Comprehensive Sequence Analysis of Parvalbumins in Fish and Their Comparison with Parvalbumins in Tetrapod Species. Biology 2022, 11, 1713. [Google Scholar] [CrossRef]
- Butera, G.; Vecellio Reane, D.; Canato, M.; Pietrangelo, L.; Boncompagni, S.; Protasi, F.; Rizzuto, R.; Reggiani, C.; Raffaello, A. Parvalbumin affects skeletal muscle trophism through modulation of mitochondrial calcium uptake. Cell Rep. 2021, 35, 109087. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bartoš, O.; Zdeňková, K.; Hanák, P.; Horká, P.; Musilova, Z. Evolution of the Parvalbumin Genes in Teleost Fishes after the Whole-Genome Duplication. Fishes 2021, 6, 70. [Google Scholar] [CrossRef]
- Permyakov, E.A.; Uversky, V.N. What Is Parvalbumin for? Biomolecules 2022, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Druga, R.; Salaj, M.; Al-Redouan, A. Parvalbumin—Positive Neurons in the Neocortex: A Review. Physiol. Res. 2023, 72, S173–S191. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, C.; Huo, L.; Cao, J.; Mao, X.; He, Z.; Hu, C.; Sun, H.; Deng, W.; He, W.; et al. Complexin-1 enhances ultrasound neurotransmission in the mammalian auditory pathway. Nat. Genet. 2024, 56, 1503–1515. [Google Scholar] [CrossRef]
- Henzl, M.T.; Agah, S. Divalent ion-binding properties of the two avian β-parvalbumins. Proteins 2006, 62, 270–278. [Google Scholar] [CrossRef]
- Heller, S.; Bell, A.M.; Denis, C.S.; Choe, Y.; Hudspeth, A.J. Parvalbumin 3 is an abundant Ca2+ buffer in hair cells. J. Assoc. Res. Otolaryngol. 2002, 3, 488–498. [Google Scholar] [CrossRef]
- Climer, L.K.; Cox, A.M.; Reynolds, T.J.; Simmons, D.D. Oncomodulin: The Enigmatic Parvalbumin Protein. Front. Mol. Neurosci. 2019, 12, 235. [Google Scholar] [CrossRef]
- Henzl, M.T.; Shibasaki, O.; Comegys, T.H.; Thalmann, I.; Thalmann, R. Oncomodulin is abundant in the organ of Corti. Hear. Res. 1997, 106, 105–111. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Henzl, M.T.; Thalmann, I.; Thalmann, R.; Schulte, B.A. Oncomodulin is expressed exclusively by outer hair cells in the organ of Corti. J. Histochem. Cytochem. 1998, 46, 29–40. [Google Scholar] [CrossRef]
- Simmons, D.D.; Tong, B.; Schrader, A.D.; Hornak, A.J. Oncomodulin identifies different hair cell types in the mammalian inner ear. J. Comp. Neurol. 2010, 518, 3785–3802. [Google Scholar] [CrossRef]
- Tong, B.; Hornak, A.J.; Maison, S.F.; Ohlemiller, K.K.; Liberman, M.C.; Simmons, D.D. Oncomodulin, an EF-Hand Ca2+ Buffer, Is Critical for Maintaining Cochlear Function in Mice. J. Neurosci. 2016, 36, 1631–1635. [Google Scholar] [CrossRef]
- Climer, L.K.; Hornak, A.J.; Murtha, K.; Yang, Y.; Cox, A.M.; Simpson, P.L.; Le, A.; Simmons, D.D. Deletion of Oncomodulin Gives Rise to Early Progressive Cochlear Dysfunction in C57 and CBA Mice. Front. Aging Neurosci. 2021, 13, 749729. [Google Scholar] [CrossRef] [PubMed]
- Giffen, K.P.; Liu, H.; Yamane, K.L.; Li, Y.; Chen, L.; Kramer, K.L.; Zallocchi, M.; He, D.Z. Molecular specializations underlying phenotypic differences in inner ear hair cells of zebrafish and mice. Front. Neurol. 2024, 15, 1437558. [Google Scholar] [CrossRef] [PubMed]
- Lush, M.E.; Piotrowski, T. Sensory hair cell regeneration in the zebrafish lateral line. Dev. Dyn. 2014, 243, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Bandmann, O.; Burton, E.A. Genetic zebrafish models of neurodegenerative diseases. Neurobiol. Dis. 2010, 40, 58–65. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Q.; Deng, J.; Wu, S.; Zhang, G.; Hu, Y.; Shen, Y.; Liu, D.; Wu, H.; Gong, J. Rhpn2 regulates the development and function of vestibular sensory hair cells through the RhoA signaling in zebrafish. J. Genet. Genom. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Xu, J.; Li, B.; Liu, X.; Xie, G.; Duan, X.; Liu, D. Noncaloric monosaccharides induce excessive sprouting angiogenesis in zebrafish via foxo1a-marcksl1a signal. eLife 2024, 13, RP95427. [Google Scholar] [CrossRef]
- Qian, F.; Wei, G.; Gao, Y.; Wang, X.; Gong, J.; Guo, C.; Wang, X.; Zhang, X.; Zhao, J.; Wang, C.; et al. Single-cell RNA-sequencing of zebrafish hair cells reveals novel genes potentially involved in hearing loss. Cell Mol. Life Sci. 2022, 79, 385. [Google Scholar] [CrossRef]
- Lu, Y.F.; Liu, D.W.; Li, I.C.; Lin, J.; Wang, C.M.; Chu, K.C.; Kuo, H.H.; Lin, C.Y.; Yih, L.H.; Jiang, Y.J.; et al. Delta/Jagged-mediated Notch signaling induces the differentiation of agr2-positive epidermal mucous cells in zebrafish embryos. PLoS Genet. 2021, 17, e1009969. [Google Scholar] [CrossRef]
- Janicke, M.; Renisch, B.; Hammerschmidt, M. Zebrafish grainyhead-like1 is a common marker of different non-keratinocyte epidermal cell lineages, which segregate from each other in a Foxi3-dependent manner. Int. J. Dev. Biol. 2010, 54, 837–850. [Google Scholar] [CrossRef]
- Xiao, T.; Roeser, T.; Staub, W.; Baier, H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development 2005, 132, 2955–2967. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Muller, U. Molecular Structure of the Hair Cell Mechanoelectrical Transduction Complex. Cold Spring Harb. Perspect. Med. 2019, 9, a033167. [Google Scholar] [CrossRef] [PubMed]
- Romero-Carvajal, A.; Navajas Acedo, J.; Jiang, L.; Kozlovskaja-Gumbriene, A.; Alexander, R.; Li, H.; Piotrowski, T. Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways. Dev. Cell 2015, 34, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Chai, R.; Li, H. Hair Cell Regeneration. Adv. Exp. Med. Biol. 2019, 1130, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Khvotchev, M.; Soloviev, M. Copines, a Family of Calcium Sensor Proteins and Their Role in Brain Function. Biomolecules 2024, 14, 255. [Google Scholar] [CrossRef]
- Melo, C.S.; Arantes Faria, J.A.; Correa, N.C.; de Andrade, C.; Carvalho, J.L.; Goes, A.M.; Rodrigues, M.A.; Gomes, D.A. Cytoplasmic-targeted parvalbumin blocks the proliferation of multipotent mesenchymal stromal cells in prophase. Stem Cell Res. Ther. 2013, 4, 92. [Google Scholar] [CrossRef]
- Arslan-Ergul, A.; Adams, M.M. Gene expression changes in aging zebrafish (Danio rerio) brains are sexually dimorphic. BMC Neurosci. 2014, 15, 29. [Google Scholar] [CrossRef]
- Baek, S.; Tran, N.T.T.; Diaz, D.C.; Tsai, Y.Y.; Navajas Acedo, J.; Lush, M.E.; Piotrowski, T. Single-cell transcriptome analysis reveals three sequential phases of gene expression during zebrafish sensory hair cell regeneration. Dev. Cell 2022, 57, 799–819 e796. [Google Scholar] [CrossRef]
- Haas, P.; Gilmour, D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev. Cell 2006, 10, 673–680. [Google Scholar] [CrossRef]
- Kniss, J.S.; Jiang, L.; Piotrowski, T. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 2016, 40, 32–40. [Google Scholar] [CrossRef]
- Sendin, G.; Bourien, J.; Rassendren, F.; Puel, J.L.; Nouvian, R. Spatiotemporal pattern of action potential firing in developing inner hair cells of the mouse cochlea. Proc. Natl. Acad. Sci. USA 2014, 111, 1999–2004. [Google Scholar] [CrossRef]
- Whitfield, T.T.; Riley, B.B.; Chiang, M.Y.; Phillips, B. Development of the zebrafish inner ear. Dev. Dyn. 2002, 223, 427–458. [Google Scholar] [CrossRef]
- Zatulovskiy, E.; Skotheim, J.M. On the Molecular Mechanisms Regulating Animal Cell Size Homeostasis. Trends Genet. 2020, 36, 360–372. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Navajas Acedo, J.; Voas, M.G.; Alexander, R.; Woolley, T.; Unruh, J.R.; Li, H.; Moens, C.; Piotrowski, T. PCP and Wnt pathway components act in parallel during zebrafish mechanosensory hair cell orientation. Nat. Commun. 2019, 10, 3993. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Murtha, K.; Climer, L.K.; Ceriani, F.; Thompson, P.; Hornak, A.J.; Marcotti, W.; Simmons, D.D. Oncomodulin regulates spontaneous calcium signalling and maturation of afferent innervation in cochlear outer hair cells. J. Physiol. 2023, 601, 4291–4308. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, T. The genetics of hair-cell function in zebrafish. J. Neurogenet. 2017, 31, 102–112. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Li, Q.; Xu, Y.; Zhao, H.; Yang, C.; Liu, D.; Gong, J. Pvalb8, a Type of Oncomodulin, Regulates Neuromast Development and Auditory Function in Zebrafish. Cells 2025, 14, 1572. https://doi.org/10.3390/cells14191572
Zhang G, Li Q, Xu Y, Zhao H, Yang C, Liu D, Gong J. Pvalb8, a Type of Oncomodulin, Regulates Neuromast Development and Auditory Function in Zebrafish. Cells. 2025; 14(19):1572. https://doi.org/10.3390/cells14191572
Chicago/Turabian StyleZhang, Guiyi, Qianqian Li, Ying Xu, Hanmeng Zhao, Chao Yang, Dong Liu, and Jie Gong. 2025. "Pvalb8, a Type of Oncomodulin, Regulates Neuromast Development and Auditory Function in Zebrafish" Cells 14, no. 19: 1572. https://doi.org/10.3390/cells14191572
APA StyleZhang, G., Li, Q., Xu, Y., Zhao, H., Yang, C., Liu, D., & Gong, J. (2025). Pvalb8, a Type of Oncomodulin, Regulates Neuromast Development and Auditory Function in Zebrafish. Cells, 14(19), 1572. https://doi.org/10.3390/cells14191572





