Expression of Genes Encoding Receptors for Classical Neurotransmitters, Neuropeptides and Hormones in the Substantia Nigra, Especially in Dopaminergic Neurons, in Intact Mice and Mouse Models of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
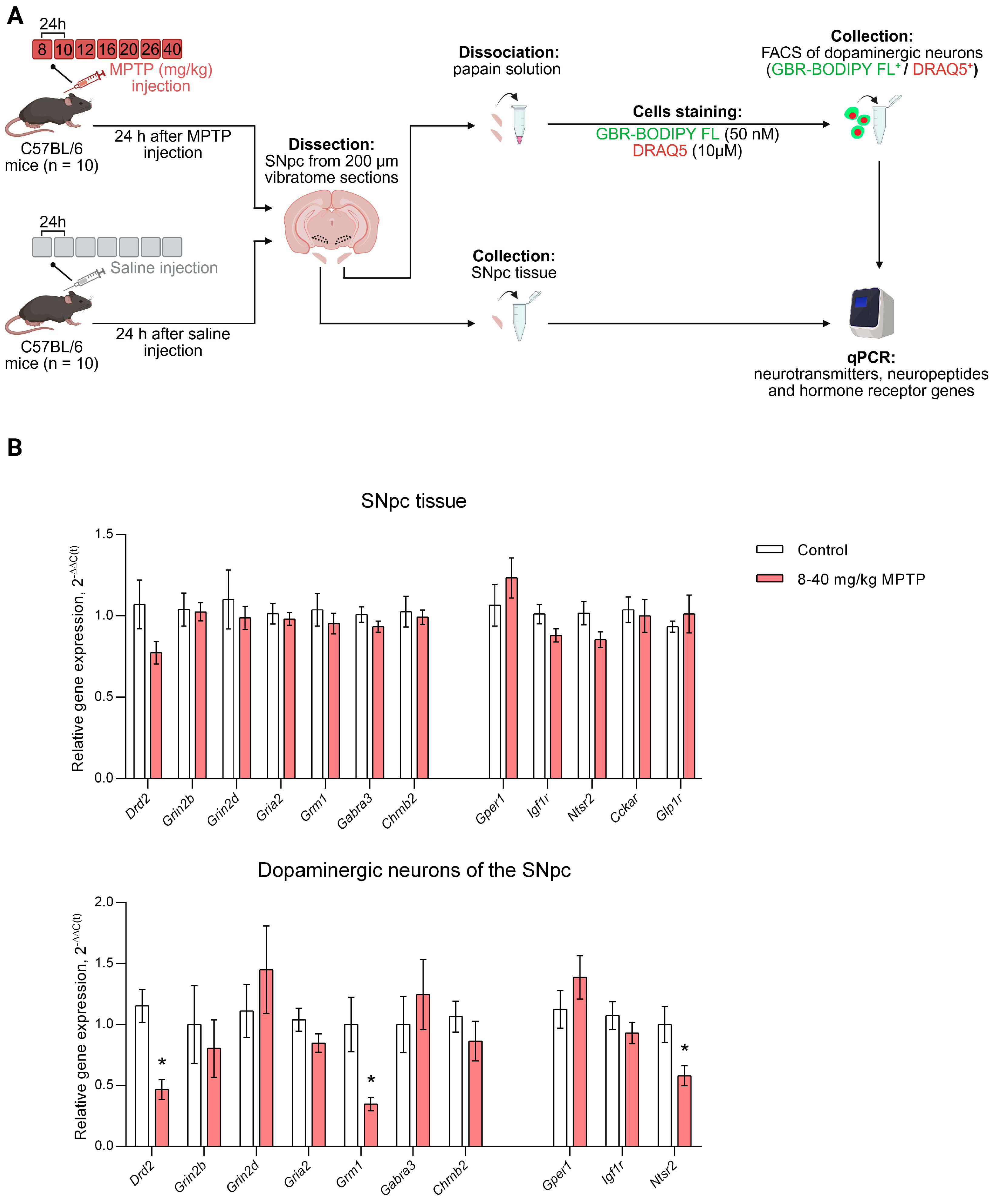
2.1. Animals and Experimental Procedures
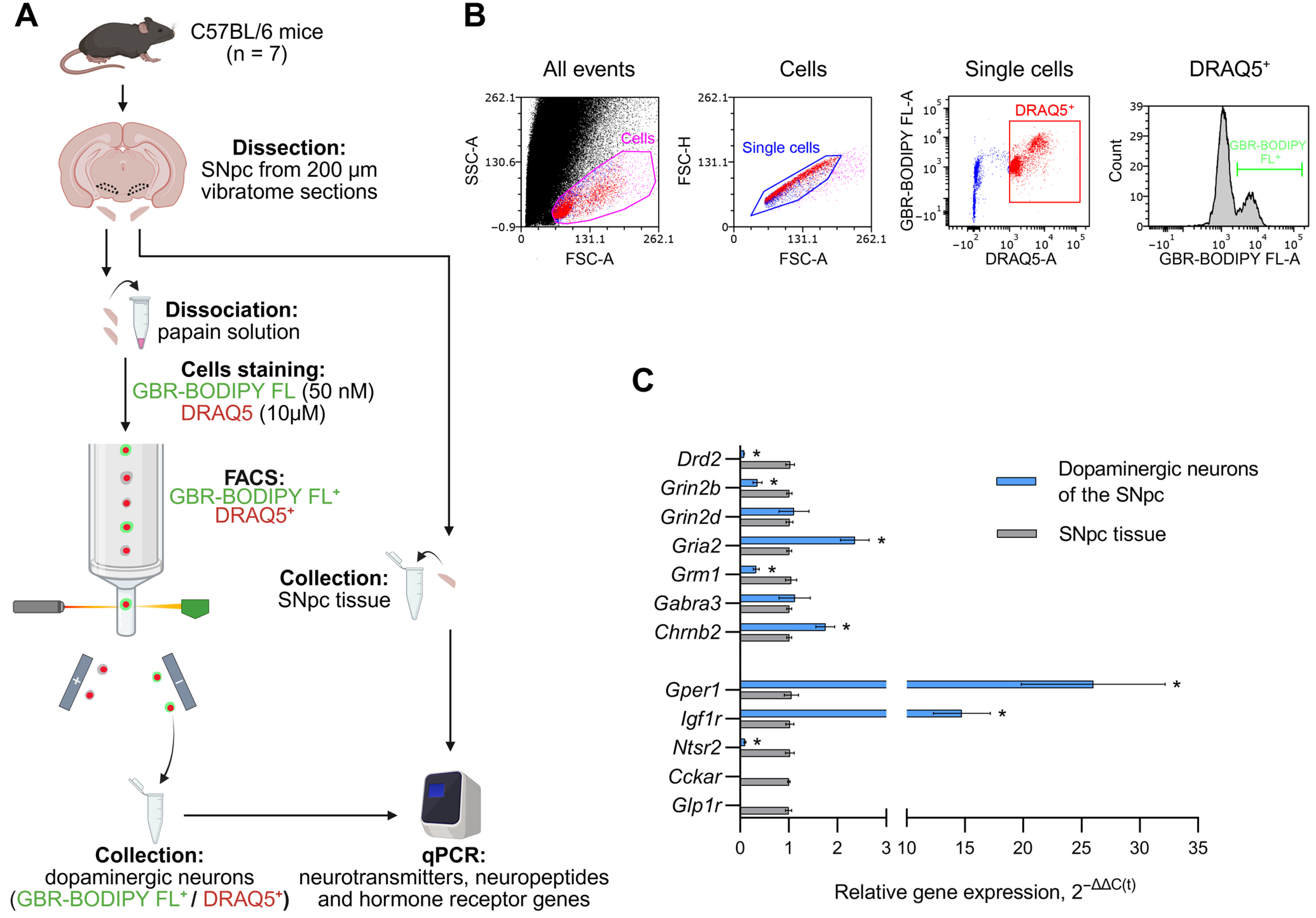
2.2. Dissection of the Substantia Nigra Pars Compacta, Preparing a Cell Suspension, and Staining Dopaminergic Neurons
2.3. Fluorescence-Activated Cell Sorting
2.4. Real-Time Polymerase Chain Reaction
2.5. Statistical Analysis
3. Results
3.1. Expression of Genes Encoding Neurotransmitter, Neuropeptide and Hormone Receptors in the Substantia Nigra Pars Compacta Tissue and in Sorted Dopaminergic Neurons of the Substantia Nigra Pars Compacta in Intact Mice
3.2. Expression of Genes Encoding Neurotransmitter, Neuropeptide and Hormone Receptors in the Substantia Nigra Pars Compacta Tissue and in Sorted Dopaminergic Neurons of the Substantia Nigra Pars Compacta in Mice When Modeling the Preclinical Stage of Parkinson’s Disease
3.3. Gene Expression of Neurotransmitter, Neuropeptide and Hormone Receptors in the Substantia Nigra Pars Compacta Tissue and in Sorted Dopaminergic Neurons of the Substantia Nigra Pars Compacta in Mice When Modeling the Clinical Stage of Parkinson’s Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| DNs | Dopaminergic neurons |
| GABA | Gamma-aminobutyric acid |
| GPER1 | G protein-coupled estrogen receptor 1 |
| IGF1 | Insulin-like growth factor 1 |
| mGluRs | Glutamate metabotropic receptors |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| nAChRs | Nicotinic acetylcholine receptors |
| NMDA | N-methyl-D-aspartate |
| NT | Neurotensin |
| PD | Parkinson’s disease |
| qPCR | Quantitative polymerase chain reaction |
| SNpc | Substantia nigra pars compacta |
References
- Agid, Y. Parkinson’s Disease: Pathophysiology. Lancet 1991, 337, 1321–1324. [Google Scholar] [CrossRef]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci. 2016, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M. Development of Early Diagnosis of Parkinson’s Disease: Illusion or Reality? CNS Neurosci. Ther. 2020, 26, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.; Jenner, P. Animal Models of Parkinson’s Disease: A Source of Novel Treatments and Clues to the Cause of the Disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Yue, Z.; Kirik, D.; Spillantini, M.G. Animal Models of Parkinson’s Disease: Limits and Relevance to Neuroprotection Studies. Mov. Disord. 2013, 28, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Liu, C.; Xue, Y.; Chen, L. Changed Firing Activity of Nigra Dopaminergic Neurons in Parkinson’s Disease. Neurochem. Int. 2023, 162, 105465. [Google Scholar] [CrossRef]
- Rademacher, K.; Nakamura, K. Role of Dopamine Neuron Activity in Parkinson’s Disease Pathophysiology. Exp. Neurol. 2024, 373, 114645. [Google Scholar] [CrossRef]
- Limousin, P.; Pollak, P.; Benazzouz, A.; Hoffmann, D.; Bas, J.-F.L.; Perret, J.E.; Benabid, A.-L.; Broussolle, E. Effect on Parkinsonian Signs and Symptoms of Bilateral Subthalamic Nucleus Stimulation. Lancet 1995, 345, 91–95. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Brontë-Stewart, H. High Frequency Deep Brain Stimulation and Neural Rhythms in Parkinson’s Disease. Neuropsychol. Rev. 2015, 25, 384–397. [Google Scholar] [CrossRef]
- Björklund, A.; Parmar, M. Neuronal Replacement as a Tool for Basal Ganglia Circuitry Repair: 40 Years in Perspective. Front. Cell. Neurosci. 2020, 14, 146. [Google Scholar] [CrossRef]
- Guerra-Crespo, M.; la Herrán-Arita, A.K.D.; Hernández-Cruz, A.; Bargas, J.; Drucker-Colín, R.; Guerra-Crespo, M.; la Herrán-Arita, A.K.D.; Hernández-Cruz, A.; Bargas, J.; Drucker-Colín, R. Cell Therapy for Parkinson’s Disease: Failure or Success? In Stem Cells in Clinic and Research; IntechOpen: London, UK, 2011; ISBN 978-953-307-797-0. [Google Scholar][Green Version]
- Fisher, A. Cholinergic Modulation of Amyloid Precursor Protein Processing with Emphasis on M1 Muscarinic Receptor: Perspectives and Challenges in Treatment of Alzheimer’s Disease. J. Neurochem. 2012, 120, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, P.D.; Wonnacott, S. Nicotinic Acetylcholine Receptors and the Ascending Dopamine Pathways. Biochem. Pharmacol. 2009, 78, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Tschumi, C.W.; Beckstead, M.J. Neurotensin Speeds Inhibition of Dopamine Neurons through Temporal Modulation of GABAA and GABAB Receptor-Mediated Synaptic Input. Neuropharmacology 2018, 131, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Pristerà, A.; Blomeley, C.; Lopes, E.; Threlfell, S.; Merlini, E.; Burdakov, D.; Cragg, S.; Guillemot, F.; Ang, S.-L. Dopamine Neuron-Derived IGF-1 Controls Dopamine Neuron Firing, Skill Learning, and Exploration. Proc. Natl. Acad. Sci. USA 2019, 116, 3817–3826. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.; Xie, J.; Shi, L. The Emerging Role of Neuropeptides in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 646726. [Google Scholar] [CrossRef] [PubMed]
- Kolacheva, A.; Bannikova, A.; Pavlova, E.; Bogdanov, V.; Ugrumov, M. Modeling of the Progressive Degradation of the Nigrostriatal Dopaminergic System in Mice to Study the Mechanisms of Neurodegeneration and Neuroplasticity in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 24, 683. [Google Scholar] [CrossRef]
- Troshev, D.; Blokhin, V.; Ukrainskaya, V.; Kolacheva, A.; Ugrumov, M. Isolation of Living Dopaminergic Neurons Labeled with a Fluorescent Ligand of the Dopamine Transporter from Mouse Substantia Nigra as a New Tool for Basic and Applied Research. Front. Mol. Neurosci. 2022, 15, 1020070. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-12-391057-8. [Google Scholar]
- Troshev, D.; Kolacheva, A.; Pavlova, E.; Blokhin, V.; Ugrumov, M. Application of OpenArray Technology to Assess Changes in the Expression of Functionally Significant Genes in the Substantia Nigra of Mice in a Model of Parkinson’s Disease. Genes 2023, 14, 2202. [Google Scholar] [CrossRef]
- Rydbirk, R.; Folke, J.; Winge, K.; Aznar, S.; Pakkenberg, B.; Brudek, T. Assessment of Brain Reference Genes for RT-qPCR Studies in Neurodegenerative Diseases. Sci. Rep. 2016, 6, 37116, Erratum in Sci. Rep. 2020, 10, 12559. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ståhlberg, A.; Rorsman, P.; Kubista, M. Gene Expression Profiling in Single Cells from the Pancreatic Islets of Langerhans Reveals Lognormal Distribution of mRNA Levels. Genome Res. 2005, 15, 1388–1392. [Google Scholar] [CrossRef]
- Ford, C.P. The Role of D2-Autoreceptors in Regulating Dopamine Neuron Activity and Transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Schuebel, K.E.; Jair, K.; Cimbro, R.; De Biase, L.M.; Goldman, D.; Bonci, A. Ventral Midbrain Astrocytes Display Unique Physiological Features and Sensitivity to Dopamine D2 Receptor Signaling. Neuropsychopharmacology 2019, 44, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiantehrani, S.; Ron, D. Dopamine D2 Receptor Activation Leads to an Up-Regulation of Glial Cell Line–Derived Neurotrophic Factor via Gβγ-Erk1/2-Dependent Induction of Zif268. J. Neurochem. 2013, 125, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Meijide, A.; Rodriguez-Perez, A.I.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopamine Modulates Astroglial and Microglial Activity via Glial Renin-Angiotensin System in Cultures. Brain Behav. Immun. 2017, 62, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Mai, D.; Qu, S. Molecular Mechanisms of Glutamate Toxicity in Parkinson’s Disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef]
- Jones, S.; Gibb, A.J. Functional NR2B- and NR2D-Containing NMDA Receptor Channels in Rat Substantia Nigra Dopaminergic Neurones. J. Physiol. 2005, 569, 209–221. [Google Scholar] [CrossRef]
- Morris, P.G. Functions of GluN2D-Containing NMDA Receptors in Dopamine Neurons of the Substantia Nigra Pars Compacta. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Albers, D.S.; Weiss, S.W.; Iadarola, M.J.; Standaert, D.G. Immunohistochemical Localization of N-Methyl-d-Aspartate and α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionate Receptor Subunits in the Substantia Nigra Pars Compacta of the Rat. Neuroscience 1999, 89, 209–220. [Google Scholar] [CrossRef]
- Paquet, M.; Tremblay, M.; Soghomonian, J.-J.; Smith, Y. AMPA and NMDA Glutamate Receptor Subunits in Midbrain Dopaminergic Neurons in the Squirrel Monkey: An Immunohistochemical and In Situ Hybridization Study. J. Neurosci. 1997, 17, 1377–1396. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Albers, D.S.; Zeevalk, G.D. Role of Glutamate in Neurodegeneration of Dopamine Neurons in Several Animal Models of Parkinsonism. Amino Acids 1998, 14, 69–74. [Google Scholar] [CrossRef]
- Doble, A. The Role of Excitotoxicity in Neurodegenerative Disease: Implications for Therapy. Pharmacol. Ther. 1999, 81, 163–221. [Google Scholar] [CrossRef]
- Blandini, F. An Update on the Potential Role of Excitotoxicity in the Pathogenesis of Parkinson’s Disease. Funct. Neurol. 2010, 25, 65–71. [Google Scholar] [PubMed]
- Chen, L.-W.; Wei, L.-C.; Lang, B.; Ju, G.; Chan, Y.S. Differential Expression of AMPA Receptor Subunits in Dopamine Neurons of the Rat Brain: A Double Immunocytochemical Study. Neuroscience 2001, 106, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, M.; Hartley, M.; Heinemann, S. Ca2+ Permeability of KA-AMPA—Gated Glutamate Receptor Channels Depends on Subunit Composition. Science 1991, 252, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, C.M.; Standaert, D.G.; Testa, C.M.; Penney, J.B., Jr.; Young, A.B. Expression of Metabotropic Glutamate Receptor 1 Isoforms in the Substantia Nigra Pars Compacta of the Rat. Neuroscience 1998, 86, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Testa, C.M.; Standaert, D.G.; Young, A.B.; Penney, J.B. Metabotropic Glutamate Receptor mRNA Expression in the Basal Ganglia of the Rat. J. Neurosci. 1994, 14, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Ledonne, A.; Nobili, A.; Latagliata, E.C.; Cavallucci, V.; Guatteo, E.; Puglisi-Allegra, S.; D’Amelio, M.; Mercuri, N.B. Neuregulin 1 Signalling Modulates mGluR1 Function in Mesencephalic Dopaminergic Neurons. Mol. Psychiatry 2015, 20, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Bolam, J.P.; Smith, Y. The GABA and Substance P Input to Dopaminergic Neurones in the Substantia Nigra of the Rat. Brain Res. 1990, 529, 57–78. [Google Scholar] [CrossRef]
- Tepper, J.M.; Lee, C.R. GABAergic Control of Substantia Nigra Dopaminergic Neurons. In Progress in Brain Research; Tepper, J.M., Abercrombie, E.D., Bolam, J.P., Eds.; Gaba and the Basal Ganglia; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 189–208. [Google Scholar]
- Okada, H.; Matsushita, N.; Kobayashi, K.; Kobayashi, K. Identification of GABAA Receptor Subunit Variants in Midbrain Dopaminergic Neurons. J. Neurochem. 2004, 89, 7–14. [Google Scholar] [CrossRef]
- Waldvogel, H.J.; Baer, K.; Gai, W.-P.; Gilbert, R.T.; Rees, M.I.; Mohler, H.; Faull, R.L.M. Differential Localization of GABAA Receptor Subunits within the Substantia Nigra of the Human Brain: An Immunohistochemical Study. J. Comp. Neurol. 2008, 506, 912–929. [Google Scholar] [CrossRef]
- Mameli-Engvall, M.; Evrard, A.; Pons, S.; Maskos, U.; Svensson, T.H.; Changeux, J.-P.; Faure, P. Hierarchical Control of Dopamine Neuron-Firing Patterns by Nicotinic Receptors. Neuron 2006, 50, 911–921. [Google Scholar] [CrossRef]
- Klink, R.; d’Exaerde, A.d.K.; Zoli, M.; Changeux, J.-P. Molecular and Physiological Diversity of Nicotinic Acetylcholine Receptors in the Midbrain Dopaminergic Nuclei. J. Neurosci. 2001, 21, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Azam, L.; Winzer-Serhan, U.H.; Chen, Y.; Leslie, F.M. Expression of Neuronal Nicotinic Acetylcholine Receptor Subunit mRNAs within Midbrain Dopamine Neurons. J. Comp. Neurol. 2002, 444, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-P.; Mazella, J.; Kitabgi, P. Neurotensin and Neurotensin Receptors. Trends Pharmacol. Sci. 1999, 20, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Sarret, P.; Krzywkowski, P.; Segal, L.; Nielsen, M.S.; Petersen, C.M.; Mazella, J.; Stroh, T.; Beaudet, A. Distribution of NTS3 Receptor/Sortilin mRNA and Protein in the Rat Central Nervous System. J. Comp. Neurol. 2003, 461, 483–505. [Google Scholar] [CrossRef]
- Piccart, E.; Courtney, N.A.; Branch, S.Y.; Ford, C.P.; Beckstead, M.J. Neurotensin Induces Presynaptic Depression of D2 Dopamine Autoreceptor-Mediated Neurotransmission in Midbrain Dopaminergic Neurons. J. Neurosci. 2015, 35, 11144–11152. [Google Scholar] [CrossRef]
- Sarret, P.; Beaudet, A.; Vincent, J.-P.; Mazella, J. Regional and Cellular Distribution of Low Affinity Neurotensin Receptor mRNA in Adult and Developing Mouse Brain. J. Comp. Neurol. 1998, 394, 344–356. [Google Scholar] [CrossRef]
- Walker, N.; Lepee-Lorgeoux, I.; Fournier, J.; Betancur, C.; Rostene, W.; Ferrara, P.; Caput, D. Tissue Distribution and Cellular Localization of the Levocabastine-Sensitive Neurotensin Receptor mRNA in Adult Rat Brain. Mol. Brain Res. 1998, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sarret, P.; Perron, A.; Stroh, T.; Beaudet, A. Immunohistochemical Distribution of NTS2 Neurotensin Receptors in the Rat Central Nervous System. J. Comp. Neurol. 2003, 461, 520–538. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, H.L.; Perez-Bonilla, P.A.; Beekly, B.G.; Lewis, T.J.; Leinninger, G.M. Identification of Neurotensin Receptor Expressing Cells in the Ventral Tegmental Area across the Lifespan. eNeuro 2018, 5, 1–17. [Google Scholar] [CrossRef]
- Quesada, A.; Romeo, H.E.; Micevych, P. Distribution and Localization Patterns of Estrogen Receptor-β and Insulin-like Growth Factor-1 Receptors in Neurons and Glial Cells of the Female Rat Substantia Nigra: Localization of ERβ and IGF-1R in Substantia Nigra. J. Comp. Neurol. 2007, 503, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Micevych, P.E. Estrogen Interacts with the IGF-1 System to Protect Nigrostriatal Dopamine and Maintain Motoric Behavior after 6-Hydroxdopamine Lesions. J. Neurosci. Res. 2004, 75, 107–116. [Google Scholar] [CrossRef]
- Yuan, L.-J.; Wang, X.-W.; Wang, H.-T.; Zhang, M.; Sun, J.-W.; Chen, W.-F. G Protein-Coupled Estrogen Receptor Is Involved in the Neuroprotective Effect of IGF-1 against MPTP/MPP+-Induced Dopaminergic Neuronal Injury. J. Steroid Biochem. Mol. Biol. 2019, 192, 105384. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Dun, S.L.; Brailoiu, G.C.; Mizuo, K.; Sklar, L.A.; Oprea, T.I.; Prossnitz, E.R.; Dun, N.J. Distribution and Characterization of Estrogen Receptor G Protein-Coupled Receptor 30 in the Rat Central Nervous System. J. Endocrinol. 2007, 193, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Reich, N.; Hölscher, C. The Neuroprotective Effects of Glucagon-like Peptide 1 in Alzheimer’s and Parkinson’s Disease: An in-Depth Review. Front. Neurosci. 2022, 16, 970925. [Google Scholar] [CrossRef]
- Reich, N.; Hölscher, C. Cholecystokinin (CCK): A Neuromodulator with Therapeutic Potential in Alzheimer’s and Parkinson’s Disease. Front. Neuroendocrinol. 2024, 73, 101122. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Obeso, J.A.; Olanow, C.W. Subthalamic Nucleus-Mediated Excitotoxicity in Parkinson’s Disease: A Target for Neuroprotection. Ann. Neurol. 1998, 44, S175–S188. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, J.; Schlaudraff, F.; Klose, V.; Bingmer, M.; Seino, S.; Magill, P.J.; Zaghloul, K.A.; Schneider, G.; Liss, B.; Roeper, J. K-ATP Channels in Dopamine Substantia Nigra Neurons Control Bursting and Novelty-Induced Exploration. Nat. Neurosci. 2012, 15, 1272–1280. [Google Scholar] [CrossRef]
- Lin, M.; Mackie, P.M.; Shaerzadeh, F.; Gamble-George, J.; Miller, D.R.; Martyniuk, C.J.; Khoshbouei, H. In Parkinson’s Patient-Derived Dopamine Neurons, the Triplication of α-Synuclein Locus Induces Distinctive Firing Pattern by Impeding D2 Receptor Autoinhibition. Acta Neuropathol. Commun. 2021, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Nam, M.-H.; Yoon, H.H.; Kim, J.; Hwang, Y.J.; Won, W.; Woo, D.H.; Lee, J.A.; Park, H.-J.; Jo, S.; et al. Aberrant Tonic Inhibition of Dopaminergic Neuronal Activity Causes Motor Symptoms in Animal Models of Parkinson’s Disease. Curr. Biol. 2020, 30, 276–291.e9. [Google Scholar] [CrossRef]
- He, Y.; Janssen, W.G.M.; Morrison, J.H. Synaptic Coexistence of AMPA and NMDA Receptors in the Rat Hippocampus: A Postembedding Immunogold Study. J. Neurosci. Res. 1998, 54, 444–449. [Google Scholar] [CrossRef]
- Christoffersen, C.L.; Meltzer, L.T. Evidence for N-Methyl-d-Aspartate and AMPA Subtypes of the Glutamate Receptor on Substantia Nigra Dopamine Neurons: Possible Preferential Role for N-Methyl-d-Aspartate Receptors. Neuroscience 1995, 67, 373–381. [Google Scholar] [CrossRef]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional Changes of the Basal Ganglia Circuitry in Parkinson’s Disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef]
- Kim, K.-S.; Jeon, M.T.; Kim, E.S.; Lee, C.H.; Kim, D.-G. Activation of NMDA Receptors in Brain Endothelial Cells Increases Transcellular Permeability. Fluids Barriers CNS 2022, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Correction to “Glutamate Receptor Ion Channels: Structure, Regulation, and Function”. Pharmacol. Rev. 2014, 66, 1141, Erratum in Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Good, C.H.; Hoffman, A.F.; Hoffer, B.J.; Chefer, V.I.; Shippenberg, T.S.; Bäckman, C.M.; Larsson, N.-G.; Olson, L.; Gellhaar, S.; Galter, D.; et al. Impaired Nigrostriatal Function Precedes Behavioral Deficits in a Genetic Mitochondrial Model of Parkinson’s Disease. FASEB J. 2011, 25, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Mingazov, E.R.; Khakimova, G.R.; Kozina, E.A.; Medvedev, A.E.; Buneeva, O.A.; Bazyan, A.S.; Ugrumov, M.V. MPTP Mouse Model of Preclinical and Clinical Parkinson’s Disease as an Instrument for Translational Medicine. Mol. Neurobiol. 2018, 55, 2991–3006. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, E.; Poetschke, C.; Duda, J.; Schlaudraff, F.; Lammel, S.; Schiemann, J.; Fauler, M.; Hetzel, A.; Watanabe, M.; Lujan, R.; et al. Cav1.3 Channels Control D2-Autoreceptor Responses via NCS-1 in Substantia Nigra Dopamine Neurons. Brain 2014, 137, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.; Tantucci, M.; Marchi, S.; Mazzocchetti, P.; Morari, M.; Pinton, P.; Mancini, A.; Calabresi, P. Dopamine D2 Receptor-Mediated Neuroprotection in a G2019S Lrrk2 Genetic Model of Parkinson’s Disease. Cell Death Dis. 2018, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, K.; Tachibana, Y.; Imanishi, M.; Kita, H.; Shigemoto, R.; Nambu, A.; Takada, M. Down-Regulation of Metabotropic Glutamate Receptor 1α in Globus Pallidus and Substantia Nigra of Parkinsonian Monkeys. Eur. J. Neurosci. 2005, 22, 3241–3254. [Google Scholar] [CrossRef]
- Yamasaki, T.; Fujinaga, M.; Kawamura, K.; Furutsuka, K.; Nengaki, N.; Shimoda, Y.; Shiomi, S.; Takei, M.; Hashimoto, H.; Yui, J.; et al. Dynamic Changes in Striatal mGluR1 But Not mGluR5 During Pathological Progression of Parkinson’s Disease in Human Alpha-Synuclein A53T Transgenic Rats: A Multi-PET Imaging Study. J. Neurosci. 2016, 36, 375–384. [Google Scholar] [CrossRef]
- Uhl, G.R.; Whitehouse, P.J.; Price, D.L.; Tourtelotte, W.W.; Kuhar, M.J. Parkinson’s Disease: Depletion of Substantia Nigra Neurotensin Receptors. Brain Res. 1984, 308, 186–190. [Google Scholar] [CrossRef]
- Fernandez, A.; Jenner, P.; Marsden, C.D.; de Ceballos, M.L. Characterization of Neurotensin-like Immunoreactivity in Human Basal Ganglia: Increased Neurotensin Levels in Substantia Nigra in Parkinson’s Disease. Peptides 1995, 16, 339–346. [Google Scholar] [CrossRef][Green Version]
- Tschumi, C.W.; Blankenship, H.E.; Sharma, R.; Lynch, W.B.; Beckstead, M.J. Neurotensin Release from Dopamine Neurons Drives Long-Term Depression of Substantia Nigra Dopamine Signaling. J. Neurosci. 2022, 42, 6186–6194. [Google Scholar] [CrossRef]


| Gene * | Protein | Forward Primer | Reverse Primer |
|---|---|---|---|
| Cyc1 | Cytochrome C1 | GCGGCCAGGGAAGTTGT | GCCAGTGAGCAGGGAAAATAC |
| Drd2 | Dopamine receptor 2 | GAACAGGCGGAGAATGGA | GGATGGATCGGGGAGAGT |
| Grin2b | Glutamate ionotropic receptor NMDA, subunit 2B | CGGCAGCACTCCTACGAC | CCAGCTGGCATCTCAAACATA |
| Grin2d | Glutamate ionotropic receptor NMDA, subunit 2D | TCCGCCTCAAGTACCCTCTAT | AAACCCTTGCAGCATCTCTTC |
| Gria2 | Glutamate ionotropic receptor AMPA, subunit 2 | GGGCGCTGATCAAGAATACA | TAAAACCCAAAAATCGCATAGAC |
| Grm1 | Glutamate metabotropic receptor 1 | ATGAACAAAAGCGGAATGGTA | CTCGAGGTAACGGATAGTAATGG |
| Gabra3 | Gamma-aminobutyric acid type A receptor subunit alpha3 | GCCGTCTGTTATGCCTTTGTAT | AGCAGCAGACTTGGAGATGGT |
| Chrnb2 | Cholinergic receptor nicotinic beta 2 subunit | CTACACCATCAACCTCATCATCC | GCCAGCAGCACAGAAATACAA |
| Cckar | Cholecystokinin A receptor | AATAACCAGACGGCGAACAT | GTAAGCCACCACCATCACAAC |
| Gper1 | G protein-coupled estrogen receptor 1 | TCCGGGAGAAGATGACCA | GAGGAAGAAGACGCTGCTGTA |
| Glp1r | Glucagon-like peptide 1 receptor | CGGCGTCAACTTTCTTATCTTC | GGTTCCTCGGGCGTGTT |
| Igf1r | Insulin-like growth factor 1 receptor | CCGGACAACTGCCCTGATA | GGCTTGTTCTCCTCGCTGTAG |
| Ntsr2 | Neurotensin receptor 2 | GCGGTTATCATGGGACAGA | AAGGGGAGAACGAAGGAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troshev, D.; Pavlova, E.; Bogdanov, V.; Ugrumov, M. Expression of Genes Encoding Receptors for Classical Neurotransmitters, Neuropeptides and Hormones in the Substantia Nigra, Especially in Dopaminergic Neurons, in Intact Mice and Mouse Models of Parkinson’s Disease. Cells 2025, 14, 1570. https://doi.org/10.3390/cells14191570
Troshev D, Pavlova E, Bogdanov V, Ugrumov M. Expression of Genes Encoding Receptors for Classical Neurotransmitters, Neuropeptides and Hormones in the Substantia Nigra, Especially in Dopaminergic Neurons, in Intact Mice and Mouse Models of Parkinson’s Disease. Cells. 2025; 14(19):1570. https://doi.org/10.3390/cells14191570
Chicago/Turabian StyleTroshev, Dmitry, Ekaterina Pavlova, Vsevolod Bogdanov, and Michael Ugrumov. 2025. "Expression of Genes Encoding Receptors for Classical Neurotransmitters, Neuropeptides and Hormones in the Substantia Nigra, Especially in Dopaminergic Neurons, in Intact Mice and Mouse Models of Parkinson’s Disease" Cells 14, no. 19: 1570. https://doi.org/10.3390/cells14191570
APA StyleTroshev, D., Pavlova, E., Bogdanov, V., & Ugrumov, M. (2025). Expression of Genes Encoding Receptors for Classical Neurotransmitters, Neuropeptides and Hormones in the Substantia Nigra, Especially in Dopaminergic Neurons, in Intact Mice and Mouse Models of Parkinson’s Disease. Cells, 14(19), 1570. https://doi.org/10.3390/cells14191570







