Integrated Spatial and Single-Cell Transcriptomics Reveals Poor Prognostic Ligand–Receptor Pairs in Glioblastoma
Abstract
Highlights
- Poor prognostic ligand–receptor pairs are localized in specific regions within glioblastoma tissues.
- Our findings provide new insights into pathological cell–cell interactions and treatment for glioblastoma.
Abstract
1. Introduction
2. Materials and Methods
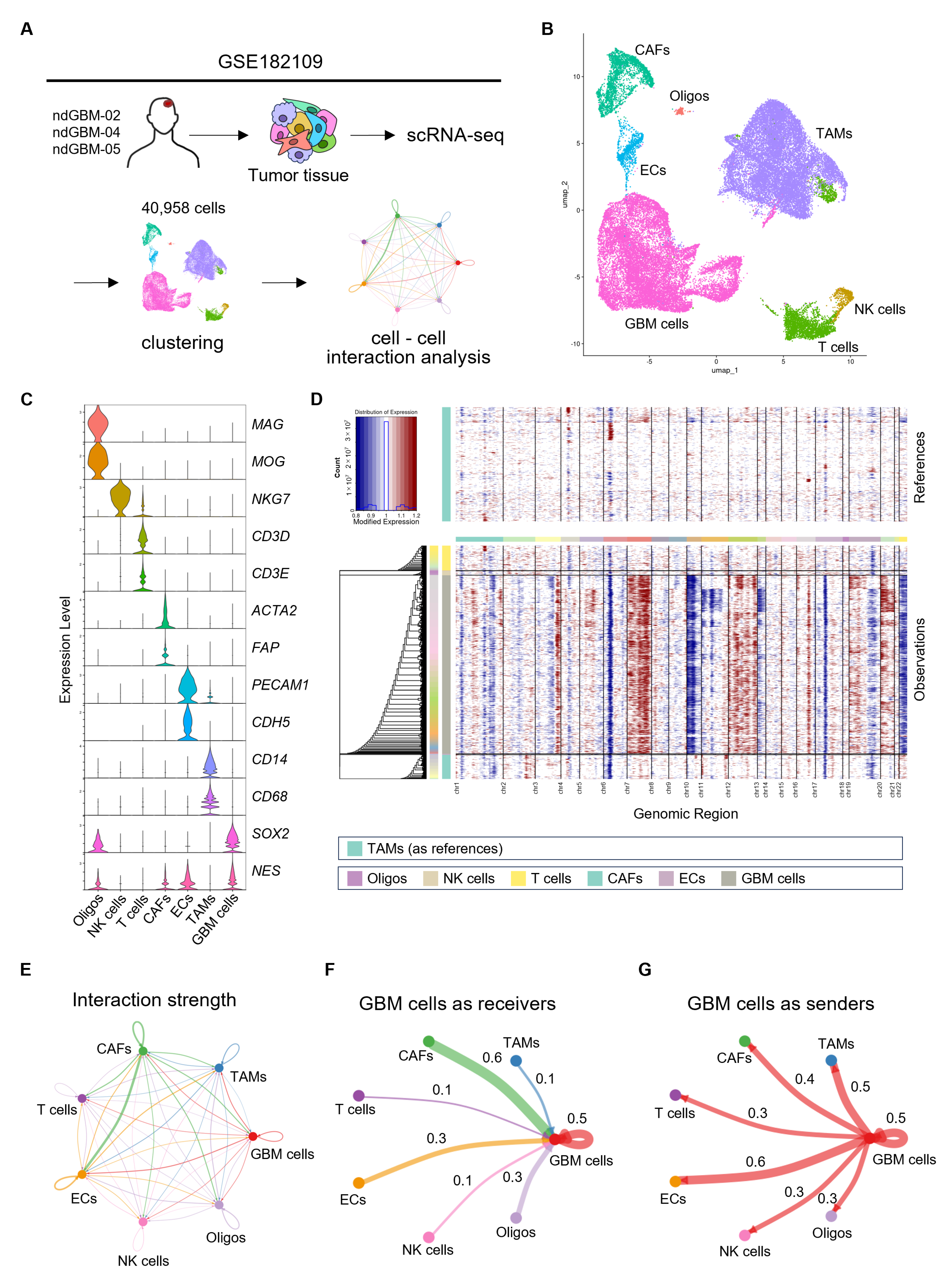
2.1. scRNA-seq Data Analysis
2.2. Cell–Cell Interaction Analysis
2.3. Bulk RNA-seq Data Analysis
2.4. Survival Analysis of the Patients with GBM and Selection of LR Pairs
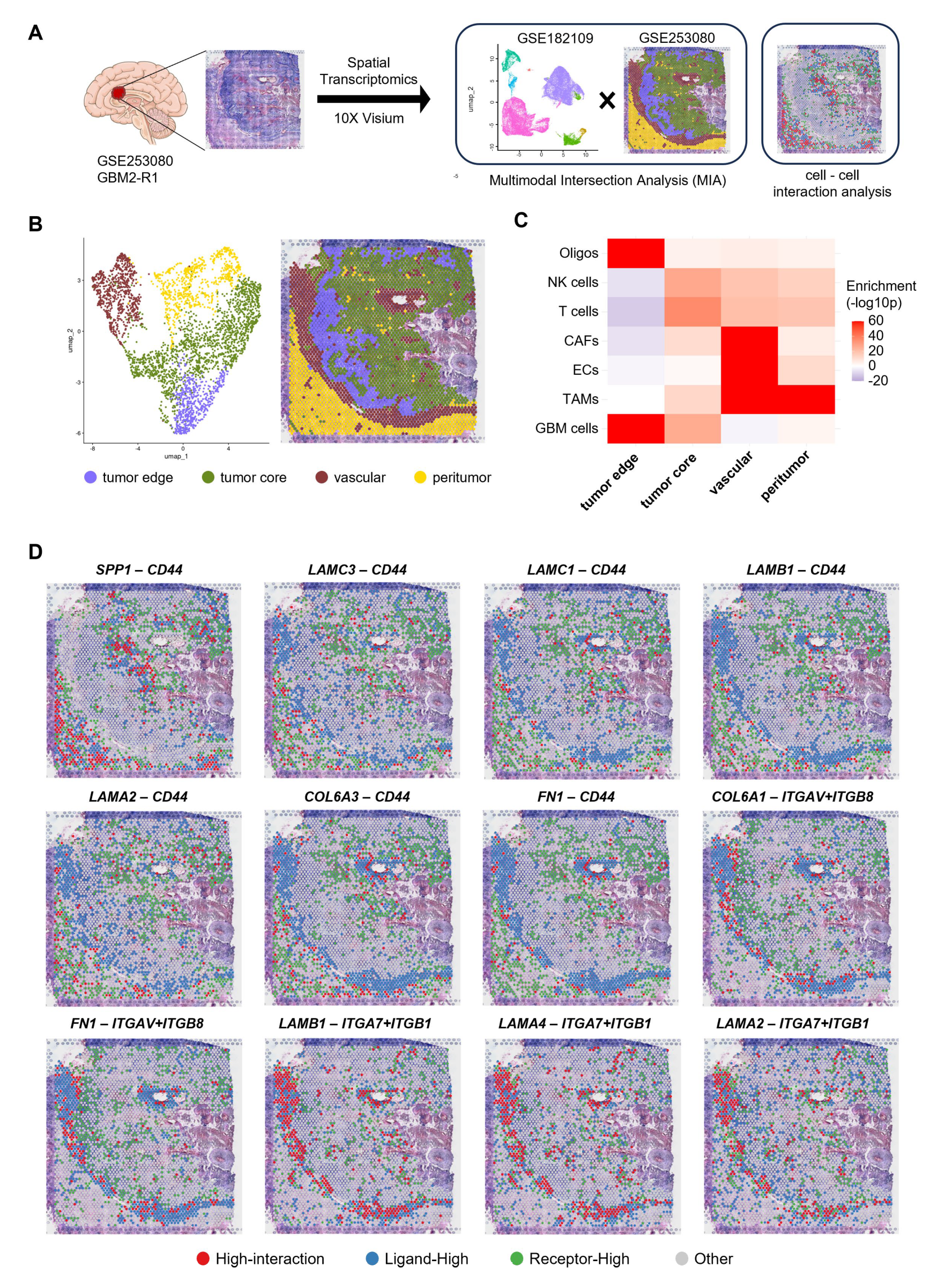
2.5. Spatial Transcriptomics Data Analysis
2.6. Multimodal Intersection Analysis
2.7. Ligand-Receptor Colocalization Analysis in GBM Tissue
2.8. Statistical Analysis
3. Results
3.1. Identification of Intercellular Networks in GBM with Bioinformatics Analysis
3.2. Pathway Enrichment Analysis of LR Pairs Activated in GBM
3.3. Identification of ECM-Related LR Pairs Associated with Poor Prognosis
3.4. Spatial Localization of ECM-Related LR Pairs with Poor Prognosis Within GBM Tissues
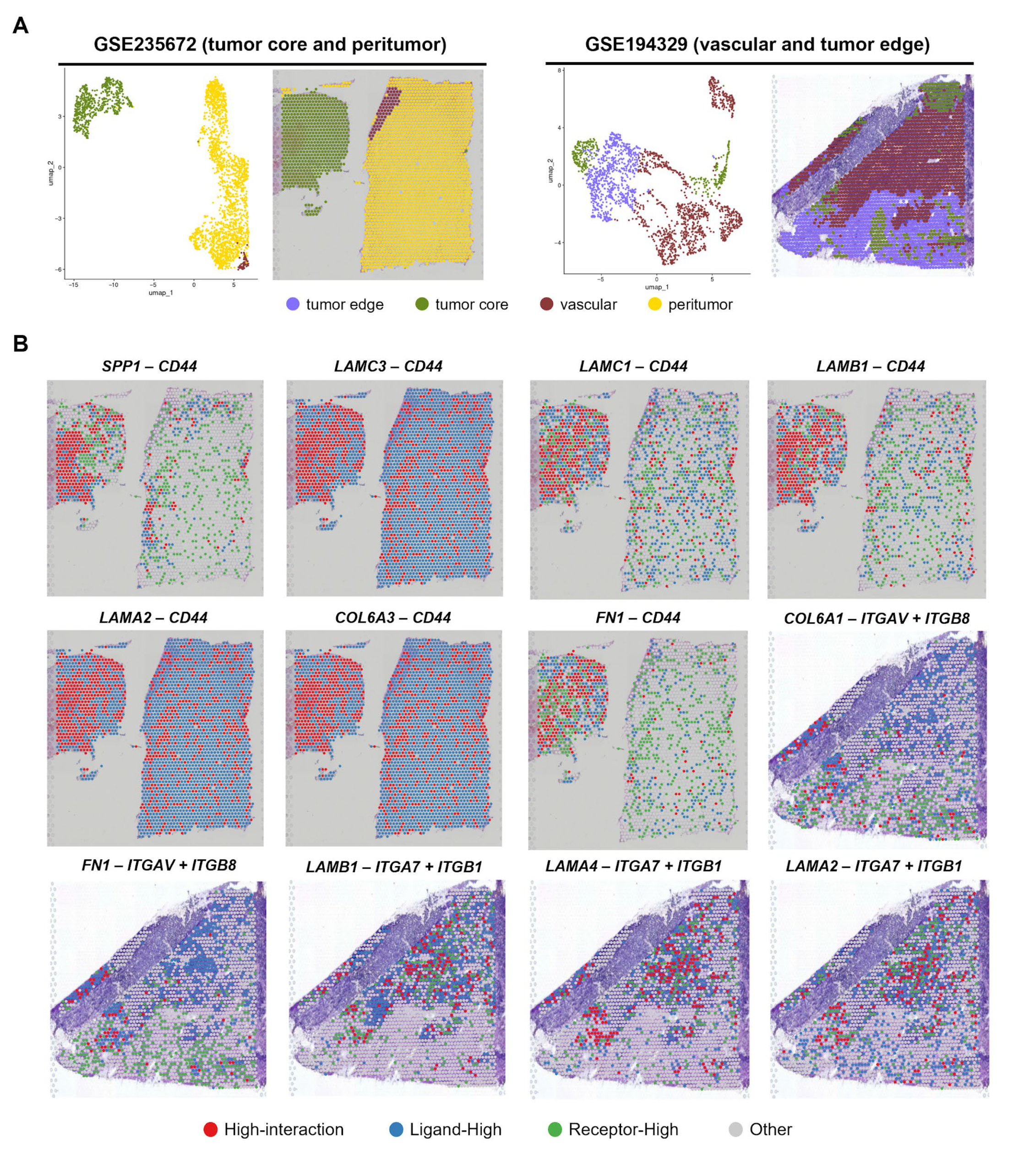
3.5. Validation of the Spatial Findings Using Independent Visium GBM Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAFs | Cancer-associated fibroblasts |
| CCIs | Cell–cell interactions |
| CGGA | Chinese glioma genome atlas |
| CNV | Copy number variation |
| ECM | Extra-cellular matrix |
| ECs | Endothelial cells |
| GBM | Glioblastoma |
| GSEA | Gene set enrichment analysis |
| LR | Ligand-receptor |
| MIA | Multimodal intersection analysis |
| MsigDB | Molecular signature database |
| NK cells | Natural killer cells |
| Oligos | Oligodendrocytes |
| PCA | Principal component analysis |
| ST | Spatial transcriptome |
| TAMs | Tumor-associated macrophages/microglia |
| TCGA | The cancer genome atlas |
| TME | Tumor microenvironment |
| UMAP | Uniform Manifold Approximation and Projection |
| scRNA-seq | Single cell RNA sequence |
| ssGSEA | Single sample gene set enrichment analysis |
References
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Galbo, P.M.; Madsen, A.T.; Liu, Y.; Peng, M.; Wei, Y.; Ciesielski, M.J.; Fenstermaker, R.A.; Graff, S.; Montagna, C.; Segall, J.E.; et al. Functional Contribution and Clinical Implication of Cancer-Associated Fibroblasts in Glioblastoma. Clin. Cancer Res. 2024, 30, 865–876. [Google Scholar] [CrossRef]
- Hide, T.; Komohara, Y.; Miyasato, Y.; Nakamura, H.; Makino, K.; Takeya, M.; Kuratsu, J.; Mukasa, A.; Yano, S. Oligodendrocyte Progenitor Cells and Macrophages/Microglia Produce Glioma Stem Cell Niches at the Tumor Border. eBioMedicine 2018, 30, 94–104. [Google Scholar] [CrossRef]
- Huang, Y.; Hoffman, C.; Rajappa, P.; Kim, J.-H.; Hu, W.; Huse, J.; Tang, Z.; Li, X.; Weksler, B.; Bromberg, J.; et al. Oligodendrocyte Progenitor Cells Promote Neovascularization in Glioma by Disrupting the Blood–Brain Barrier. Cancer Res. 2014, 74, 1011–1021. [Google Scholar] [CrossRef]
- Jain, S.; Rick, J.W.; Joshi, R.S.; Beniwal, A.; Spatz, J.; Gill, S.; Chang, A.C.-C.; Choudhary, N.; Nguyen, A.T.; Sudhir, S.; et al. Single-cell RNA sequencing and spatial transcriptomics reveal cancer-associated fibroblasts in glioblastoma with protumoral effects. J. Clin. Investig. 2023, 133, e147087. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neuro-Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Hovis, G.; Chandra, N.; Kejriwal, N.; Hsieh, K.J.-Y.; Chu, A.; Yang, I.; Wadehra, M. Understanding the Role of Endothelial Cells in Glioblastoma: Mechanisms and Novel Treatments. Int. J. Mol. Sci. 2024, 25, 6118. [Google Scholar] [CrossRef]
- Wei, R.; Zhou, J.; Bui, B.; Liu, X. Glioma actively orchestrate a self-advantageous extracellular matrix to promote recurrence and progression. BMC Cancer 2024, 24, 974. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.; Vento-Tormo, M.; Teichmann, S.A.; Vento-Tormo, R. CellPhoneDB: Inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 2020, 15, 1484–1506. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Denisenko, E.; Ong, H.T.; Ramilowski, J.A.; Forrest, A.R.R. Predicting cell-to-cell communication networks using NATMI. Nat. Commun. 2020, 11, 5011. [Google Scholar] [CrossRef]
- Cabello-Aguilar, S.; Alame, M.; Kon-Sun-Tack, F.; Fau, C.; Lacroix, M.; Colinge, J. SingleCellSignalR: Inference of intercellular networks from single-cell transcriptomics. Nucleic Acids Res. 2020, 48, e55. [Google Scholar] [CrossRef]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.-H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Suzuki, S.R.; Kuno, A.; Ozaki, H. Cell-to-cell interaction analysis of prognostic ligand-receptor pairs in human pancreatic ductal adenocarcinoma. Biochem. Biophys. Rep. 2021, 28, 101126. [Google Scholar] [CrossRef]
- Feng, B.; Zhao, D.; Zhang, Z.; Jia, R.; Schuler, P.J.; Hess, J. Ligand-receptor interactions combined with histopathology for improved prognostic modeling in HPV-negative head and neck squamous cell carcinoma. npj Precis. Oncol. 2025, 9, 57. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar] [CrossRef]
- Du, J.; Yang, Y.-C.; An, Z.-J.; Zhang, M.-H.; Fu, X.-H.; Huang, Z.-F.; Yuan, Y.; Hou, J. Advances in spatial transcriptomics and related data analysis strategies. J. Transl. Med. 2023, 21, 330. [Google Scholar] [CrossRef]
- Park, H.; Jo, S.H.; Lee, R.H.; Macks, C.P.; Ku, T.; Park, J.; Lee, C.W.; Hur, J.K.; Sohn, C.H. Spatial Transcriptomics: Technical Aspects of Recent Developments and Their Applications in Neuroscience and Cancer Research. Adv. Sci. 2023, 10, e2206939. [Google Scholar] [CrossRef]
- Wang, X.; Venet, D.; Lifrange, F.; Larsimont, D.; Rediti, M.; Stenbeck, L.; Dupont, F.; Rouas, G.; Garcia, A.J.; Craciun, L.; et al. Spatial transcriptomics reveals substantial heterogeneity in triple-negative breast cancer with potential clinical implications. Nat. Commun. 2024, 15, 10232. [Google Scholar] [CrossRef]
- Lv, X.; Wang, B.; Liu, K.; Li, M.J.; Yi, X.; Wu, X. Decoding heterogeneous and coordinated tissue architecture in glioblastoma using spatial transcriptomics. iScience 2024, 27, 110064. [Google Scholar] [CrossRef]
- Cui Zhou, D.; Jayasinghe, R.G.; Chen, S.; Herndon, J.M.; Iglesia, M.D.; Navale, P.; Wendl, M.C.; Caravan, W.; Sato, K.; Storrs, E.; et al. Spatially restricted drivers and transitional cell populations cooperate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat. Genet. 2022, 54, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.; Kumar, P.; Wang, C.; Leu, J.-S.; Flynn, W.F.; Gao, R.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Wood, S.L.; et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 2022, 13, 767. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Sadamori, K.; Tokumura, K.; Tanaka, Y.; Fukasawa, K.; Hinoi, E. Bioinformatic analysis reveals potential relationship between chondrocyte senescence and protein glycosylation in osteoarthritis pathogenesis. Front. Endocrinol. 2023, 14, 1153689. [Google Scholar] [CrossRef]
- Fukasawa, K.; Lyu, J.; Kubo, T.; Tanaka, Y.; Suzuki, A.; Horie, T.; Tomizawa, A.; Osumi, R.; Iwahashi, S.; Tokumura, K.; et al. MEK5-ERK5 Axis Promotes Self-renewal and Tumorigenicity of Glioma Stem Cells. Cancer Res. Commun. 2023, 3, 148–159. [Google Scholar] [CrossRef]
- Tokumura, K.; Sadamori, K.; Yoshimoto, M.; Tomizawa, A.; Tanaka, Y.; Fukasawa, K.; Hinoi, E. The Bioinformatics Identification of Potential Protein Glycosylation Genes Associated with a Glioma Stem Cell Signature. BioMedInformatics 2024, 4, 75–88. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, X.; Zhang, J.; Liu, D.; He, J.; Huang, C.; Liao, J.; Wang, Y.; Feng, Y.; Li, H.; et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat. Cancer 2023, 4, 1273–1291. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, Z.; Zhou, L.; Xiao, P.; Song, J.; He, P.; Xie, C.; Zhou, R.; Li, M.; Dong, X.; et al. Spatial transcriptomics reveals niche-specific enrichment and vulnerabilities of radial glial stem-like cells in malignant gliomas. Nat. Commun. 2023, 14, 1028. [Google Scholar] [CrossRef]
- Moncada, R.; Barkley, D.; Wagner, F.; Chiodin, M.; Devlin, J.C.; Baron, M.; Hajdu, C.H.; Simeone, D.M.; Yanai, I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020, 38, 333–342. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Liu, T.; Lu, H.; Lin, X.; Wang, W.; Liu, Y.; Huang, Y.; Huang, G.; Sun, H.; et al. Single-cell multi-omics sequencing uncovers region-specific plasticity of glioblastoma for complementary therapeutic targeting. Sci. Adv. 2024, 10, eadn4306. [Google Scholar] [CrossRef]
- Wang, D.; Anderson, J.C.; Gladson, C.L. The Role of the Extracellular Matrix in Angiogenesis in Malignant Glioma Tumors. Brain Pathol. 2005, 15, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Menna, G.; Di Bonaventura, R.; Lisi, L.; Mattogno, P.; Figà, F.; Bilgin, L.; D’Alessandris, Q.G.; Olivi, A.; Della Pepa, G.M. The Extracellular Matrix in Glioblastomas: A Glance at Its Structural Modifications in Shaping the Tumoral Microenvironment—A Systematic Review. Cancers 2023, 15, 1879. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, E.; Yoo, K.; Zhao, Y.; Kang, J.; Lim, E.; Shin, I.; Kang, S.; Lim, H.W.; Lee, S. Glioblastoma-educated mesenchymal stem-like cells promote glioblastoma infiltration via extracellular matrix remodelling in the tumour microenvironment. Clin. Transl. Med. 2022, 12, e997. [Google Scholar] [CrossRef]
- Horta, C.A.; Doan, K.; Yang, J. Mechanotransduction pathways in regulating epithelial-mesenchymal plasticity. Curr. Opin. Cell Biol. 2023, 85, 102245. [Google Scholar] [CrossRef]
- Kolliopoulos, C.; Ali, M.M.; Castillejo-Lopez, C.; Heldin, C.-H.; Heldin, P. CD44 Depletion in Glioblastoma Cells Suppresses Growth and Stemness and Induces Senescence. Cancers 2022, 14, 3747. [Google Scholar] [CrossRef]
- Radotra, B.; McCormick, D.; Crockard, A. CD44 plays a role in adhesive interactions between glioma cells and extracellular matrix components. Neuropathol. Appl. Neurobiol. 1994, 20, 399–405. [Google Scholar] [CrossRef]
- Johansson, E.; Grassi, E.S.; Pantazopoulou, V.; Tong, B.; Lindgren, D.; Berg, T.J.; Pietras, E.J.; Axelson, H.; Pietras, A. CD44 Interacts with HIF-2α to Modulate the Hypoxic Phenotype of Perinecrotic and Perivascular Glioma Cells. Cell Rep. 2017, 20, 1641–1653. [Google Scholar] [CrossRef]
- Pietras, A.; Katz, A.M.; Ekström, E.J.; Wee, B.; Halliday, J.J.; Pitter, K.L.; Werbeck, J.L.; Amankulor, N.M.; Huse, J.T.; Holland, E.C. Osteopontin-CD44 Signaling in the Glioma Perivascular Niche Enhances Cancer Stem Cell Phenotypes and Promotes Aggressive Tumor Growth. Cell Stem Cell 2014, 14, 357–369. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, Z.; Xu, N.; Liu, B.; Fu, Z.; Lian, C.; Guo, H. Connective tissue growth factor promotes temozolomide resistance in glioblastoma through TGF-β1-dependent activation of Smad/ERK signaling. Cell Death Dis. 2017, 8, e2885. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Ha, C.P.; Hua, T.N.M.; Vo, V.T.A.; Om, J.; Han, S.; Cha, S.-K.; Park, K.-S.; Jeong, Y. Humanin activates integrin αV–TGFβ axis and leads to glioblastoma progression. Cell Death Dis. 2024, 15, 464. [Google Scholar] [CrossRef]
- Guerrero, P.A.; Tchaicha, J.H.; Chen, Z.; Morales, J.E.; McCarty, N.; Wang, Q.; Sulman, E.P.; Fuller, G.; Lang, F.F.; Rao, G.; et al. Glioblastoma stem cells exploit the αvβ8 integrin-TGFβ1 signaling axis to drive tumor initiation and progression. Oncogene 2017, 36, 6568–6580. [Google Scholar] [CrossRef]
- Reyes, S.B.; Narayanan, A.S.; Lee, H.S.; Tchaicha, J.H.; Aldape, K.D.; Lang, F.F.; Tolias, K.F.; McCarty, J.H. αvβ8 integrin interacts with RhoGDI1 to regulate Rac1 and Cdc42 activation and drive glioblastoma cell invasion. Mol. Biol. Cell 2013, 24, 474–482. [Google Scholar] [CrossRef]
- Haas, T.L.; Sciuto, M.R.; Brunetto, L.; Valvo, C.; Signore, M.; Fiori, M.E.; Di Martino, S.; Giannetti, S.; Morgante, L.; Boe, A.; et al. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma. Cell Stem Cell 2017, 21, 35–50.e9. [Google Scholar] [CrossRef]
- Renner, G.; Janouskova, H.; Noulet, F.; Koenig, V.; Guerin, E.; Bär, S.; Nuesch, J.; Rechenmacher, F.; Neubauer, S.; Kessler, H.; et al. Integrin α5β1 and p53 convergent pathways in the control of anti-apoptotic proteins PEA-15 and survivin in high-grade glioma. Cell Death Differ. 2016, 23, 640–653. [Google Scholar] [CrossRef]
- Schaffenrath, J.; Wyss, T.; He, L.; Rushing, E.J.; Delorenzi, M.; Vasella, F.; Regli, L.; Neidert, M.C.; Keller, A. Blood-brain barrier alterations in human brain tumors revealed by genome-wide transcriptomic profiling. Neuro-Oncology 2021, 23, 2095–2106. [Google Scholar] [CrossRef]
- Lathia, J.D.; Li, M.; Hall, P.E.; Gallagher, J.; Hale, J.S.; Wu, Q.; Venere, M.; Levy, E.; Rani, M.R.S.; Huang, P.; et al. Laminin alpha 2 enables glioblastoma stem cell growth. Ann. Neurol. 2012, 72, 766–778. [Google Scholar] [CrossRef]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue mechanics promote IDH1-dependent HIF1α–tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Neyns, B.; Goldbrunner, R.; Schlegel, U.; Clement, P.M.J.; Grabenbauer, G.G.; Ochsenbein, A.F.; Simon, M.; Dietrich, P.-Y.; et al. Phase I/IIa Study of Cilengitide and Temozolomide with Concomitant Radiotherapy Followed by Cilengitide and Temozolomide Maintenance Therapy in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2010, 28, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Mikkelsen, T.; Hegi, M.E.; Ye, X.; Batchelor, T.; Lesser, G.; Peereboom, D.; Rosenfeld, M.R.; Olsen, J.; Brem, S.; et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306). Cancer 2012, 118, 5601–5607. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Wang, Y.; Buzdin, A.; Li, X. A practical guide for choosing an optimal spatial transcriptomics technology from seven major commercially available options. BMC Genom. 2025, 26, 47. [Google Scholar] [CrossRef]
- Janesick, A.; Shelansky, R.; Gottscho, A.D.; Wagner, F.; Williams, S.R.; Rouault, M.; Beliakoff, G.; Morrison, C.A.; Oliveira, M.F.; Sicherman, J.T.; et al. High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat. Commun. 2023, 14, 8353. [Google Scholar] [CrossRef]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth andangiogenesis. Neuro-Oncology 2005, 7, 134–153. [Google Scholar] [CrossRef]
- Carrera-Aguado, I.; Marcos-Zazo, L.; Carrancio-Salán, P.; Guerra-Paes, E.; Sánchez-Juanes, F.; Muñoz-Félix, J.M. The Inhibition of Vessel Co-Option as an Emerging Strategy for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 921. [Google Scholar] [CrossRef]
- Han, L. Modulation of the Blood–Brain Barrier for Drug Delivery to Brain. Pharmaceutics 2021, 13, 2024. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef]


| Ligand | Receptor | HR (TCGA) | 95% CI (TCGA) | HR (CGGA) | 95% CI (CGGA) |
|---|---|---|---|---|---|
| COL4A5 | CD44 | 1.32 | 1.05–1.65 | 1.38 | 1.03–1.82 |
| COL6A1 | ITGAV/ITGB8 | 1.29 | 1.02–1.61 | 1.35 | 1.02–1.79 |
| COL6A3 | CD44 | 1.46 | 1.16–1.82 | 1.36 | 1.03–1.80 |
| FN1 | CD44 | 1.28 | 1.02–1.60 | 1.47 | 1.11–1.95 |
| FN1 | ITGAV/ITGB8 | 1.25 | 1.00–1.56 | 1.44 | 1.08–1.90 |
| LAMA2 | CD44 | 1.39 | 1.11–1.74 | 1.39 | 1.04–1.84 |
| LAMA2 | ITGA7/ITGB1 | 1.28 | 1.02–1.60 | 1.38 | 1.04–1.82 |
| LAMA4 | ITGA7/ITGB1 | 1.26 | 1.00–1.57 | 1.36 | 1.02–1.80 |
| LAMB1 | CD44 | 1.43 | 1.14–1.79 | 1.33 | 1.00–1.75 |
| LAMB1 | ITGA7/ITGB1 | 1.26 | 1.00–1.57 | 1.45 | 1.09–1.92 |
| LAMC1 | CD44 | 1.30 | 1.03–1.62 | 1.38 | 1.04–1.82 |
| LAMC3 | CD44 | 1.34 | 1.07–1.67 | 1.36 | 1.02–1.80 |
| SPP1 | CD44 | 1.29 | 1.03–1.61 | 1.52 | 1.14–2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimoto, M.; Sugihara, K.; Tokumura, K.; Tsuji, S.; Hinoi, E. Integrated Spatial and Single-Cell Transcriptomics Reveals Poor Prognostic Ligand–Receptor Pairs in Glioblastoma. Cells 2025, 14, 1540. https://doi.org/10.3390/cells14191540
Yoshimoto M, Sugihara K, Tokumura K, Tsuji S, Hinoi E. Integrated Spatial and Single-Cell Transcriptomics Reveals Poor Prognostic Ligand–Receptor Pairs in Glioblastoma. Cells. 2025; 14(19):1540. https://doi.org/10.3390/cells14191540
Chicago/Turabian StyleYoshimoto, Makoto, Kengo Sugihara, Kazuya Tokumura, Shohei Tsuji, and Eiichi Hinoi. 2025. "Integrated Spatial and Single-Cell Transcriptomics Reveals Poor Prognostic Ligand–Receptor Pairs in Glioblastoma" Cells 14, no. 19: 1540. https://doi.org/10.3390/cells14191540
APA StyleYoshimoto, M., Sugihara, K., Tokumura, K., Tsuji, S., & Hinoi, E. (2025). Integrated Spatial and Single-Cell Transcriptomics Reveals Poor Prognostic Ligand–Receptor Pairs in Glioblastoma. Cells, 14(19), 1540. https://doi.org/10.3390/cells14191540






