Endothelial Cell Transition: Preliminary Data on Cross-Organ Shift from Brain to Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Data Analysis
2.3. Reagents and Animals
2.4. MBEC Culture
2.5. Brightfield Microscopy
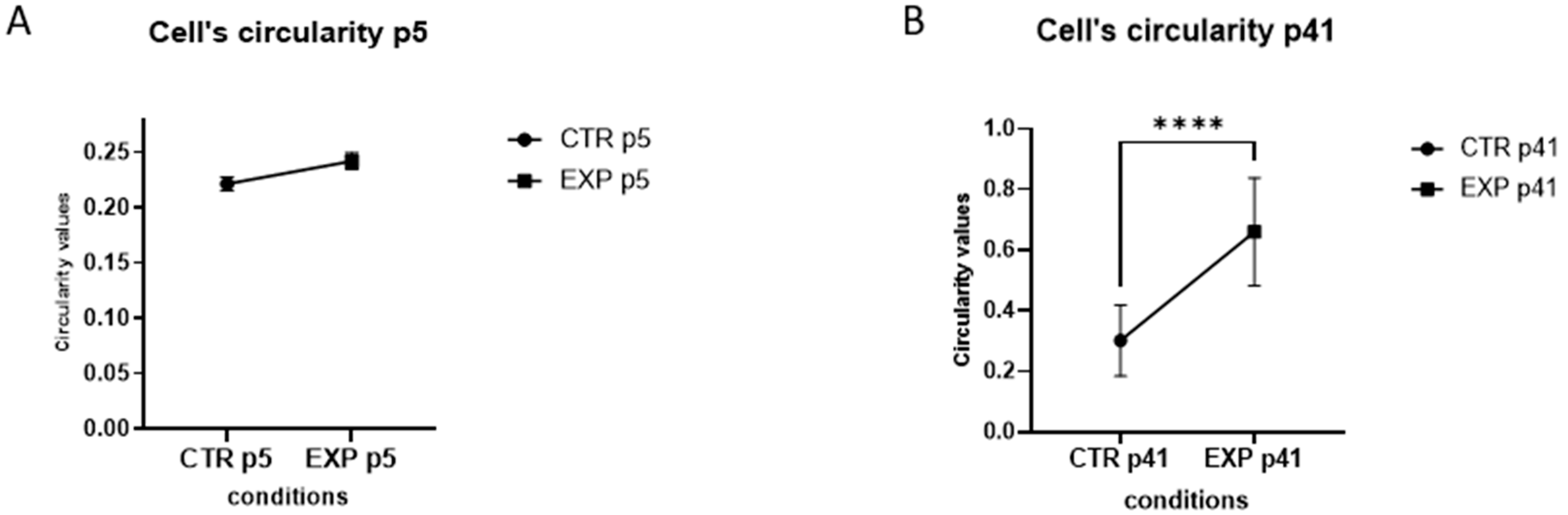
2.6. Circularity Assessment
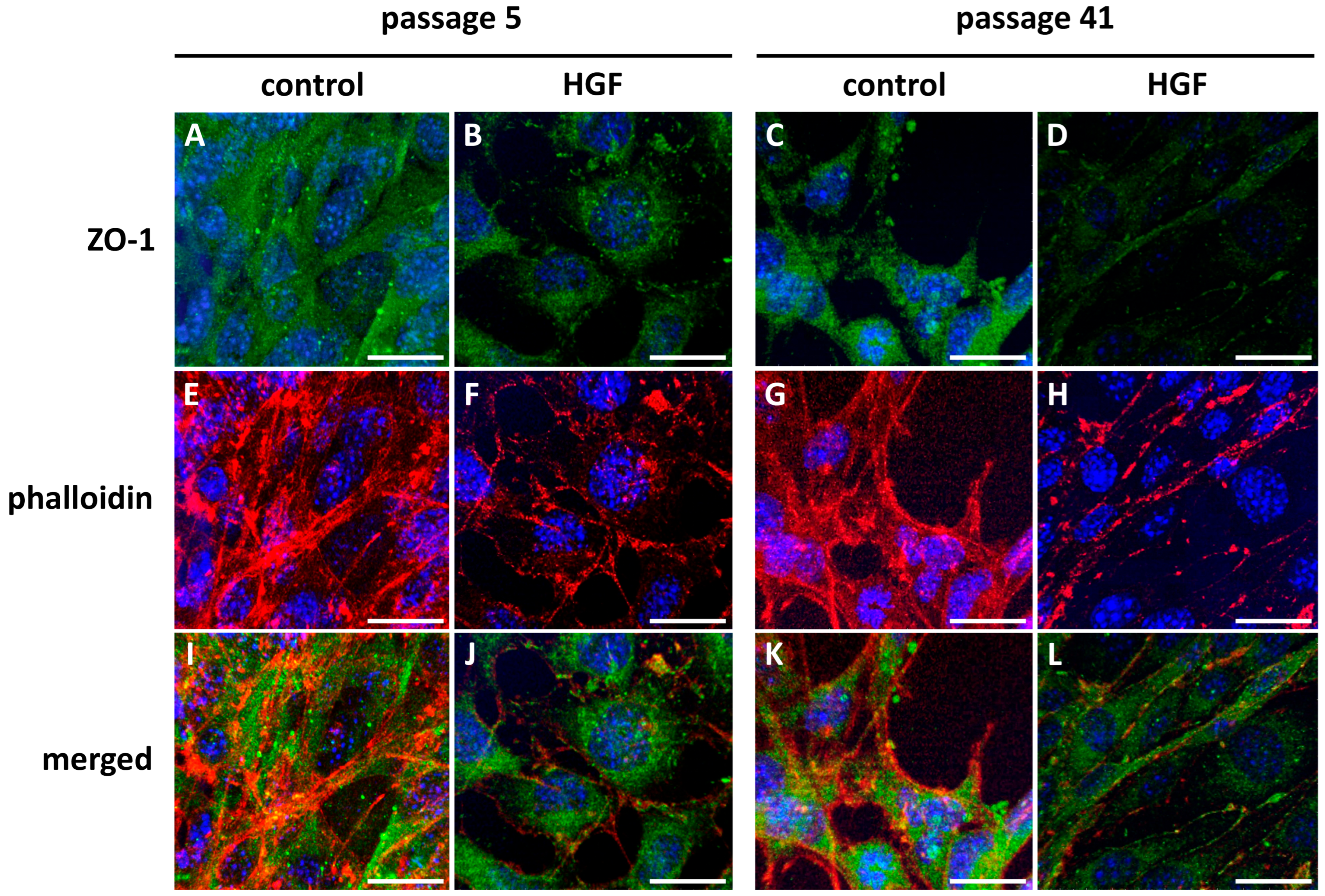
2.7. Endothelial Cell Immunostaining
2.8. Confocal Microscopy
2.9. Protein Quantification
2.10. Western Blot Analysis
2.11. Transendothelial Electric Resistance (TEER)
2.12. Transendothelial Dextran Permeability (TEDP)
2.13. Statistical Analysis
3. Results
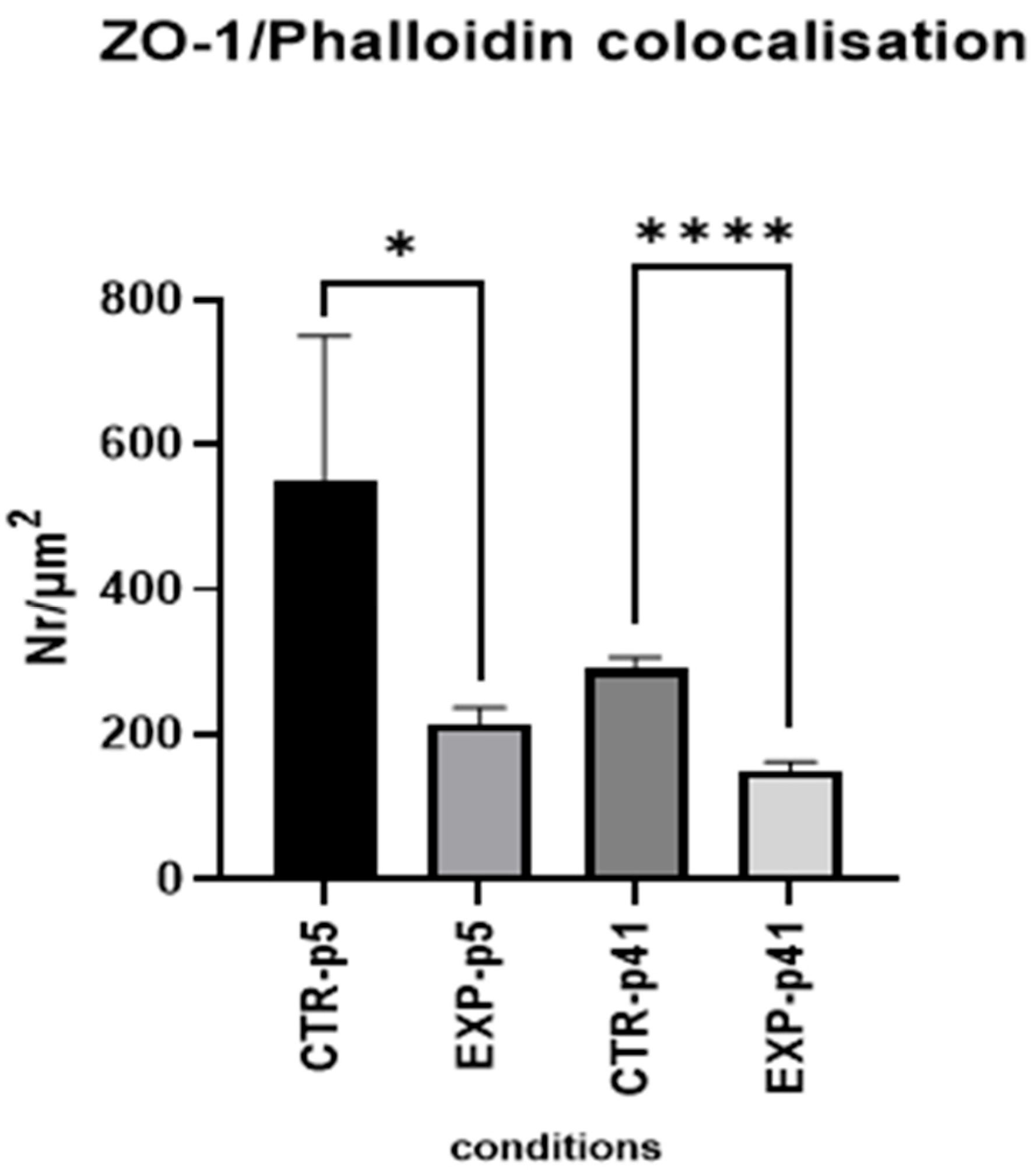
3.1. MBEC Morphology and Cytoarchitecture
3.1.1. Western Blot
3.1.2. Brightfield Microscopy
3.1.3. Endothelial Cell Immunostaining
3.1.4. Confocal Microscopy
3.2. MBEC Physiology and Barrier Function
3.2.1. TEER Measurements of MBECs Monolayers
3.2.2. Size-Selective Transendothelial Dextran Permeability (TEDP) in MBECs Monolayers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alpha-SMA | alpha 2 smooth muscle actin |
| BBB | Blood–Brain Barrier |
| Cadmec | Dermal microvascular cells |
| ECs | endothelial cells |
| EHT | endothelial to hematopoietic transition |
| EMT | endothelial to mesenchymal transition |
| FSP1 | fibroblast-specific protein-1 |
| GATA-2 | binding protein 2 |
| HGF | hepatocyte growth factor |
| HSCs | hematopoietic stem cells |
| MBEC(s) | mouse brain endothelial cell(s) |
| MLEC(s) | mouse liver endothelial cells |
| RUNX1 | runt-related transcription factor-1 |
| TAL1 | T-cell acute lymphocytic leukemia protein-1 |
| Tie2 | tyrosine kinase with immunoglobulin-like and EGF-like domains |
| TJs | tight junctions |
| VE | cadherin-vascular endothelial cadherin |
| vWf | von Willebrand factor, Anti-factor VIII |
References
- Zhou, J.; Li, Y.S.; Chien, S. Shear Stress-Initiated Signaling and Its Regulation of Endothelial Function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- Mierke, C.T. Mechanosensory Entities and Functionality of Endothelial Cells. Front. Cell Dev. Biol. 2024, 12, 1446452. [Google Scholar] [CrossRef]
- Charbonier, F.W.; Zamani, M.; Huang, N.F. Endothelial Cell Mechanotransduction in the Dynamic Vascular Environment. Adv. Biosyst. 2018, 3, 1800252. [Google Scholar] [CrossRef] [PubMed]
- Faller, D.V. Endothelial Cell Responses to Hypoxic Stress. Clin. Exp. Pharmacol. Physiol. 1999, 26, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, Q. Understanding the Oxygen-Sensing Pathway and Its Therapeutic Implications in Diseases. Am. J. Pathol. 2020, 190, 1584. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.; Brown, G.C.; Foxwell, N.; Moncada, S. On the Mechanism by Which Vascular Endothelial Cells Regulate Their Oxygen Consumption. Proc. Natl. Acad. Sci. USA 1999, 96, 1559–1562. [Google Scholar] [CrossRef]
- Zhang, C. The Role of Inflammatory Cytokines in Endothelial Dysfunction. Basic. Res. Cardiol. 2008, 103, 398. [Google Scholar] [CrossRef]
- Groten, S.A.; Smit, E.R.; Janssen, E.F.J.; van den Eshof, B.L.; van Alphen, F.P.J.; van der Zwaan, C.; Meijer, A.B.; Hoogendijk, A.J.; Biggelaar, M. van den Multi-Omics Delineation of Cytokine-Induced Endothelial Inflammatory States. Commun. Biol. 2023, 6, 525. [Google Scholar] [CrossRef]
- Matthews, B.D.; Overby, D.R.; Mannix, R.; Ingber, D.E. Cellular Adaptation to Mechanical Stress: Role of Integrins, Rho, Cytoskeletal Tension and Mechanosensitive Ion Channels. J. Cell Sci. 2006, 119, 508–518. [Google Scholar] [CrossRef]
- Pellegata, A.F.; Tedeschi, A.M.; De Coppi, P. Whole Organ Tissue Vascularization: Engineering the Tree to Develop the Fruits. Front. Bioeng. Biotechnol. 2018, 6, 357762. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2016, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lu, H.; Liu, Y.; Zhao, Y.; Zhu, T.; Garcia-Barrio, M.T.; Chen, Y.E.; Zhang, J. Single-Cell Transcriptomics Reveals Endothelial Plasticity During Diabetic Atherogenesis. Front. Cell Dev. Biol. 2021, 9, 689469. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Watabe, T. Emerging Roles of Inflammation-Mediated Endothelial–Mesenchymal Transition in Health and Disease. Inflamm. Regen. 2022, 42, 9. [Google Scholar] [CrossRef]
- Krenning, G.; Barauna, V.G.; Krieger, J.E.; Harmsen, M.C.; Moonen, J.R.A.J. Endothelial Plasticity: Shifting Phenotypes through Force Feedback. Stem Cells Int. 2016, 2016, 9762959. [Google Scholar] [CrossRef]
- Greenspan, L.J.; Weinstein, B.M. To Be or Not to Be: Endothelial Cell Plasticity in Development, Repair, and Disease. Angiogenesis 2021, 24, 251. [Google Scholar] [CrossRef]
- Islam, S.; Boström, K.I.; Di Carlo, D.; Simmons, C.A.; Tintut, Y.; Yao, Y.; Hsu, J.J. The Mechanobiology of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease. Front. Physiol. 2021, 12, 734215. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Shan, D.; Cui, K.; Li, K.; Zhu, B.; Wu, H.; Wang, B.; Wong, S.; Norton, V.; Dong, Y.; et al. The Role of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease. Cells 2022, 11, 1834. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Ge, J.; Li, H. Endothelial-to-Mesenchymal Transition in Cardiovascular Diseases. Trends Mol. Med. 2025; S1471-4914(25)00113-3, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Camarero, P.; Toledo, B.; Diaz-Ruano, A.B.; González-Titos, A.; García-Ortega, M.B.; Perán, M. What Is the Impact of Endothelial-to-Mesenchymal Transition in Solid Tumours: A Qualitative Systematic Review and Quantitative Meta-Analysis. Int. J. Biol. Sci. 2025, 21, 2155–2178. [Google Scholar] [CrossRef]
- Jung, C.; Oh, J.E.; Lee, S.; Yoon, Y.S. Generation and Application of Directly Reprogrammed Endothelial Cells. Korean Circ. J. 2022, 52, 643–658. [Google Scholar] [CrossRef]
- Samarakkody, A.S.; Cantor, A.B. Opening the Window for Endothelial-to-Hematopoietic Transition. Genes. Dev. 2021, 35, 1398–1400. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.F.; Kishta, F.; Xu, Y.; Baker, A.H.; Kovacic, J.C. Endothelial to Mesenchymal Transition: At the Axis of Cardiovascular Health and Disease. Cardiovasc. Res. 2024, 120, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Hirschi, K.K.; Simons, M. The Molecular Basis of Endothelial Cell Plasticity. Nat. Commun. 2017, 8, 14361. [Google Scholar] [CrossRef]
- Choi, K.J.; Nam, J.K.; Kim, J.H.; Choi, S.H.; Lee, Y.J. Endothelial-to-Mesenchymal Transition in Anticancer Therapy and Normal Tissue Damage. Exp. Mol. Med. 2020, 52, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.-S.; Kim, J. Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Alvandi, Z.; Bischoff, J. Endothelial-Mesenchymal Transition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2357–2369. [Google Scholar] [CrossRef]
- Gaikwad, A.V.; Lu, W.; Dey, S.; Bhattarai, P.; Haug, G.; Larby, J.; Chia, C.; Jaffar, J.; Westall, G.; Singhera, G.K.; et al. Endothelial-to-Mesenchymal Transition: A Precursor to Pulmonary Arterial Remodelling in Patients with Idiopathic Pulmonary Fibrosis. ERJ Open Res. 2023, 9, 00487–02022. [Google Scholar] [CrossRef]
- Dahal, S.; Huang, P.; Murray, B.T.; Mahler, G.J. Endothelial to Mesenchymal Transformation Is Induced by Altered Extracellular Matrix in Aortic Valve Endothelial Cells. J. Biomed. Mater. Res. A 2017, 105, 2729–2741. [Google Scholar] [CrossRef]
- Wilkus-Adamczyk, K.; Brodaczewska, K.; Majewska, A.; Kieda, C. Microenvironment Commits Breast Tumor ECs to Dedifferentiation by Micro-RNA-200-b-3p Regulation and Extracellular Matrix Remodeling. Front. Cell Dev. Biol. 2023, 11, 1125077. [Google Scholar] [CrossRef]
- Yoneda, Y.; Kato, H.; Maezawa, Y.; Yokote, K.; Nakanishi, M. Real-Time Imaging of Human Endothelial-to-Hematopoietic Transition in Vitro Using Pluripotent Stem Cell Derived Hemogenic Endothelium. Biophys. Physicobiol 2024, 21, e211015. [Google Scholar] [CrossRef]
- Gao, X.; Xu, C.; Asada, N.; Frenette, P.S. The Hematopoietic Stem Cell Niche: From Embryo to Adult. Development 2018, 145, dev139691. [Google Scholar] [CrossRef]
- Wu, Y.; Hirschi, K.K. Regulation of Hemogenic Endothelial Cell Development and Function. Annu. Rev. Physiol. 2021, 83, 17. [Google Scholar] [CrossRef]
- Canu, G.; Ruhrberg, C. First Blood: The Endothelial Origins of Hematopoietic Progenitors. Angiogenesis 2021, 24, 199. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.K.; Ingram, D.A.; Yoder, M.C. Assessing Identity, Phenotype, and Fate of Endothelial Progenitor Cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1584–1595. [Google Scholar] [CrossRef]
- Ottersbach, K. Endothelial-to-Haematopoietic Transition: An Update on the Process of Making Blood. Biochem. Soc. Trans. 2019, 47, 591–601. [Google Scholar] [CrossRef]
- Neo, W.H.; Lie-A-Ling, M.; Fadlullah, M.Z.H.; Lacaud, G. Contributions of Embryonic HSC-Independent Hematopoiesis to Organogenesis and the Adult Hematopoietic System. Front. Cell Dev. Biol. 2021, 9, 631699. [Google Scholar] [CrossRef]
- Oliver, G.; Srinivasan, R.S. Endothelial Cell Plasticity: How to Become and Remain a Lymphatic Endothelial Cell. Development 2010, 137, 363–372. [Google Scholar] [CrossRef]
- Lv, J.; Meng, S.; Gu, Q.; Zheng, R.; Gao, X.; Kim, J.; Chen, M.; Xia, B.; Zuo, Y.; Zhu, S.; et al. Epigenetic Landscape Reveals MECOM as an Endothelial Lineage Regulator. Nat. Commun. 2023, 14, 2390. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Restrepo, N.K.; Chestnut, B.; Klimkaite, L.; Sumanas, S. Single-Cell Transcriptomic Analysis of Vascular Endothelial Cells in Zebrafish Embryos. Sci. Rep. 2022, 12, 13065. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Le Bras, A.; Margariti, A.; Xu, Q. Reprogramming towards Endothelial Cells for Vascular Regeneration. Genes. Dis. 2016, 3, 186–197. [Google Scholar] [CrossRef]
- Cho, S.; Aakash, P.; Lee, S.; Yoon, Y. sup Endothelial Cell Direct Reprogramming: Past, Present, and Future. J. Mol. Cell Cardiol. 2023, 180, 22. [Google Scholar] [CrossRef]
- Choi, Y.G.; Ma, X.; Das, S.; Sierra-Pagan, J.E.; Larson, T.; Gong, W.; Sadek, H.A.; Zhang, J.; Garry, M.G.; Garry, D.J. ETV2 Transcriptionally Activates Rig1 Gene Expression and Promotes Reprogramming of the Endothelial Lineage. Sci. Rep. 2024, 14, 28688. [Google Scholar] [CrossRef]
- Cheung, K.C.P.; Fanti, S.; Mauro, C.; Wang, G.; Nair, A.S.; Fu, H.; Angeletti, S.; Spoto, S.; Fogolari, M.; Romano, F.; et al. Preservation of Microvascular Barrier Function Requires CD31 Receptor-Induced Metabolic Reprogramming. Nat. Commun. 2020, 11, 3595. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, N.V.; Verin, A.D. The Role of Cytoskeleton in the Regulation of Vascular Endothelial Barrier Function. Microvasc. Res. 2008, 76, 202. [Google Scholar] [CrossRef]
- Lee, S.; Park, C.; Han, J.W.; Kim, J.Y.; Cho, K.; Kim, E.J.; Kim, S.; Lee, S.J.; Oh, S.Y.; Tanaka, Y.; et al. Direct Reprogramming of Human Dermal Fibroblasts Into Endothelial Cells Using ER71/ETV2. Circ. Res. 2016, 120, 848. [Google Scholar] [CrossRef]
- Chavkin, N.W.; Genet, G.; Poulet, M.; Jeffery, E.D.; Marziano, C.; Genet, N.; Vasavada, H.; Nelson, E.A.; Acharya, B.R.; Kour, A.; et al. Endothelial Cell Cycle State Determines Propensity for Arterial-Venous Fate. Nat. Commun. 2022, 13, 5891. [Google Scholar] [CrossRef]
- De Val, S.; Black, B.L. Transcriptional Control of Endothelial Cell Development. Dev. Cell 2009, 16, 180. [Google Scholar] [CrossRef]
- Garratt, H.; Ashburn, R.; Sopić, M.; Nogara, A.; Caporali, A.; Mitić, T. Long Non-Coding RNA Regulation of Epigenetics in Vascular Cells. Noncoding RNA 2021, 7, 62. [Google Scholar] [CrossRef]
- Hudson, J.; Farkas, L. Epigenetic Regulation of Endothelial Dysfunction and Inflammation in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 12098. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Dong, G.; Yang, C.; Zheng, W.; Zhong, C.; Shen, Q.; Lu, Y.; Zhao, Y. Broadening Horizons: Molecular Mechanisms and Disease Implications of Endothelial-to-Mesenchymal Transition. Cell Commun. Signal 2025, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Desole, C.; Gallo, S.; Vitacolonna, A.; Montarolo, F.; Bertolotto, A.; Vivien, D.; Comoglio, P.; Crepaldi, T. HGF and MET: From Brain Development to Neurological Disorders. Front. Cell Dev. Biol. 2021, 9, 1321. [Google Scholar] [CrossRef]
- Xia, J.L.; Dai, C.; Michalopoulos, G.K.; Liu, Y. Hepatocyte Growth Factor Attenuates Liver Fibrosis Induced by Bile Duct Ligation. Am. J. Pathol. 2006, 168, 1500. [Google Scholar] [CrossRef]
- Yepes Barreto, I.D.J.; Múnera Contreras, M.N.; Suárez Causado, A. Relación Entre El Factor de Crecimiento Hepático y El Estadio de La Cirrosis. Rev. Colomb. Gastroenterol. 2017, 32, 24. [Google Scholar] [CrossRef]
- Hervieu, A.; Kermorgant, S. The Role of PI3K in Met Driven Cancer: A Recap. Front. Mol. Biosci. 2018, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Bu, R.; Uddin, S.; Bavi, P.; Hussain, A.R.; Al-Dayel, F.; Ghourab, S.; Ahmed, M.; Al-Kuraya, K.S. HGF/c-Met Pathway Has a Prominent Role in Mediating Antiapoptotic Signals through AKT in Epithelial Ovarian Carcinoma. Lab. Investig. 2010, 91, 124–137. [Google Scholar] [CrossRef]
- Organ, S.L.; Tsao, M.-S. An Overview of the C-MET Signaling Pathway. Ther. Adv. Med. Oncol. 2011, 3, S7–S19. [Google Scholar] [CrossRef]
- Meng, W.; Chen, T. Association between the HGF/c-MET Signaling Pathway and Tumorigenesis, Progression and Prognosis of Hepatocellular Carcinoma (Review). Oncol. Rep. 2021, 46, 191. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, W.; Wang, Y.D.; Chen, W.D. HGF/c-Met: A Key Promoter in Liver Regeneration. Front. Pharmacol. 2022, 13, 808855. [Google Scholar] [CrossRef]
- You, W.K.; McDonald, D.M. The Hepatocyte Growth Factor/c-Met Signaling Pathway as a Therapeutic Target to Inhibit Angiogenesis. BMB Rep. 2008, 41, 833. [Google Scholar] [CrossRef]
- Vimalraj, S. A Concise Review of VEGF, PDGF, FGF, Notch, Angiopoietin, and HGF Signalling in Tumor Angiogenesis with a Focus on Alternative Approaches and Future Directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Dejana, E.; Mcdonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Czupalla, C.; Yousef, H.; Wyss-Coray, T.; Butcher, E. Collagenase-Based Single Cell Isolation of Primary Murine Brain Endothelial Cells Using Flow Cytometry. Bio Protoc. 2018, 8, e3092. [Google Scholar] [CrossRef]
- Bolden, C.T.; Skibber, M.A.; Olson, S.D.; Zamorano Rojas, M.; Milewicz, S.; Gill, B.S.; Cox, C.S. Validation and Characterization of a Novel Blood–Brain Barrier Platform for Investigating Traumatic Brain Injury. Sci. Rep. 2023, 13, 16150. [Google Scholar] [CrossRef]
- De Jong, E.; Williams, D.S.; Abdelmohsen, L.K.E.A.; Van Hest, J.C.M.; Zuhorn, I.S. A Filter-Free Blood-Brain Barrier Model to Quantitatively Study Transendothelial Delivery of Nanoparticles by Fluorescence Spectroscopy. J. Control. Release 2018, 289, 14–22. [Google Scholar] [CrossRef]
- Okunishi, K.; Dohi, M.; Nakagome, K.; Tanaka, R.; Mizuno, S.; Matsumoto, K.; Miyazaki, J.; Nakamura, T.; Yamamoto, K. A Novel Role of Hepatocyte Growth Factor as an Immune Regulator through Suppressing Dendritic Cell Function. J. Immunol. 2005, 175, 4745–4753. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The Discovery of Hepatocyte Growth Factor (HGF) and Its Significance for Cell Biology, Life Sciences and Clinical Medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. Biological Roles of Hepatocyte Growth Factor-Met Signaling from Genetically Modified Animals (Review). Biomed. Rep. 2017, 7, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Wang, T.; Wassertheil-Smoller, S.; Strickler, H.D.; Kaplan, R.C.; McGinn, A.P.; Wildman, R.P.; Rosenbaum, D.; Rohan, T.E.; Scherer, P.E.; et al. Hepatocyte Growth Factor and the Risk of Ischemic Stroke Developing Among Postmenopausal Women. Stroke 2010, 41, 857–862. [Google Scholar] [CrossRef]
- Meng, S.; Xia, F.; Xu, J.; Zhang, X.; Xue, M.; Gu, M.; Guo, F.; Huang, Y.; Qiu, H.; Yang, Y.; et al. Hepatocyte Growth Factor Protects Pulmonary Endothelial Barrier against Oxidative Stress and Mitochondria-Dependent Apoptosis. Chin. Med. J. (Engl.) 2022, 135, 837–848. [Google Scholar] [CrossRef]
- Jiang, W.G.; Martin, T.A.; Matsumoto, K.; Nakamura, T.; Mansel, R.E. Hepatocyte Growth Factor/Scatter Factor Decreases the Expression of Occludin and Transendothelial Resistance (TER) and Increases Paracellular Permeability in Human Vascular Endothelial Cells. J. Cell. Physiol. 1999, 181, 319–329. [Google Scholar] [CrossRef]
- Abounader, R.; Laterra, J. Scatter Factor/Hepatocyte Growth Factor in Brain Tumor Growth and Angiogenesis. Neuro Oncol. 2005, 7, 436. [Google Scholar] [CrossRef]
- Clermont, A.C.; Cahill, M.; Salti, H.; Rook, S.L.; Rask-Madsen, C.; Goddard, L.; Wong, J.S.; Bursell, D.; Bursell, S.E.; Aiello, L.P. Hepatocyte Growth Factor Induces Retinal Vascular Permeability via MAP-Kinase and PI-3 Kinase without Altering Retinal Hemodynamics. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2701–2708. [Google Scholar] [CrossRef]
- Ido, A.; Moriuchi, A.; Numata, M.; Murayama, T.; Teramukai, S.; Marusawa, H.; Yamaji, N.; Setoyama, H.; Kim, I.D.; Chiba, T.; et al. Safety and Pharmacokinetics of Recombinant Human Hepatocyte Growth Factor (Rh-HGF) in Patients with Fulminant Hepatitis: A Phase I/II Clinical Trial, Following Preclinical Studies to Ensure Safety. J. Transl. Med. 2011, 9, 55. [Google Scholar] [CrossRef]
- Solozafy Bemena, B.M.; Totohasina, A.; Feno, D.R.; Rakotoarivelo, R.A. New Branch and Definitions against Emerging Infectious Diseases. Acad. Med. 2025, 2, 1–14. [Google Scholar] [CrossRef]
- Tsubouchi, H.; Niitani, Y.; Hirono, S.; Nakayama, H.; Gohda, E.; Arakaki, N.; Sakiyama, O.; Takahashi, K.; Kimoto, M.; Kawakami, S. Levels of the Human Hepatocyte Growth Factor in Serum of Patients with Various Liver Diseases Determined by an Enzyme-Linked Immunosorbent Assay. Hepatology 1991, 13, 1–5. [Google Scholar] [CrossRef]
- Verghese, G.M.; McCormick-Shannon, K.; Mason, R.J.; Matthay, M.A. Hepatocyte Growth Factor and Keratinocyte Growth Factor in the Pulmonary Edema Fluid of Patients with Acute Lung Injury. Biologic and Clinical Significance. Am. J. Respir. Crit. Care Med. 1998, 158, 386–394. [Google Scholar] [CrossRef]
- Seidel, C.; Børset, M.; Turesson, I.; Abildgaard, N.; Sundan, A.; Waage, A. Elevated Serum Concentrations of Hepatocyte Growth Factor in Patients with Multiple Myeloma. Blood 1998, 91, 806–812. [Google Scholar] [CrossRef]
- Larionov, A.; Hammer, C.M.; Fiedler, K.; Filgueira, L. Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease. Cells 2024, 13, 1276. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In Vitro Models of the Blood–Brain Barrier: An Overview of Commonly Used Brain Endothelial Cell Culture Models and Guidelines for Their Use. J. Cereb. Blood Flow Metab. 2016, 36, 862. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for in Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107. [Google Scholar] [CrossRef]
- Zhang, X.; Olsavszky, V.; Yin, Y.; Wang, B.; Engleitner, T.; Öllinger, R.; Schledzewski, K.; Koch, P.S.; Rad, R.; Schmid, R.M.; et al. Angiocrine Hepatocyte Growth Factor Signaling Controls Physiological Organ and Body Size and Dynamic Hepatocyte Proliferation to Prevent Liver Damage during Regeneration. Am. J. Pathol. 2020, 190, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Catizone, A. Pleiotropic Activities of HGF/c-Met System in Testicular Physiology: Paracrine and Endocrine Implications. Front. Endocrinol. 2014, 5, 76610. [Google Scholar] [CrossRef]
- Ricci, G. Editorial of Special Issue “New Aspects of the Hepatocyte Growth Factor/c-Met System”. Biomedicines 2015, 3, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, T.; Chen, X.; Li, Q.; Liao, M.; Fu, L.; Huang, J.; Yuan, K.; Wang, Z.; Zeng, Y. Molecular Mechanisms in Liver Repair and Regeneration: From Physiology to Therapeutics. Signal Transduct. Target. Ther. 2025, 10, 63. [Google Scholar] [CrossRef]
- Clavien, P.A. Liver Regeneration: A Spotlight on the Novel Role of Platelets and Serotonin. Swiss Med. Wkly. 2008, 138, 361–370. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver Regeneration: Biological and Pathological Mechanisms and Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef]
- McAinch, A.J.; Naderpoor, N.; Loomes, K.; Oliveira, A.G.; A Saad, M.J.; Mja, S.; Araújo, T.G.; de Melo Carvalho, B.; Rocha, G.Z.; Santos, A. The Role of Hepatocyte Growth Factor (HGF) in Insulin Resistance and Diabetes. Diabetes. Front. Endocrinol. 2018, 9, 503. [Google Scholar] [CrossRef]
- Ohmichi, H.; Matsumoto, K.; Nakamura, T. In Vivo Mitogenic Action of HGF on Lung Epithelial Cells: Pulmotrophic Role in Lung Regeneration. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1996, 270, L1031–L1039. [Google Scholar] [CrossRef] [PubMed]
- Coudriet, G.M.; He, J.; Trucco, M.; Mars, W.M.; Piganelli, J.D. Hepatocyte Growth Factor Modulates Interleukin-6 Production in Bone Marrow Derived Macrophages: Implications for Inflammatory Mediated Diseases. PLoS ONE 2010, 5, e15384. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Shi, M.; Zhang, T.; Lu, W.; Yang, S.; Cui, Q.; Li, Z. Potential Mechanisms of the Impact of Hepatocyte Growth Factor Gene-Modified Tendon Stem Cells on Tendon Healing. Front. Cell Dev. Biol. 2021, 9, 659389. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakamura, T. Hepatocyte Growth Factor: Molecular Structure and Implications for a Central Role in Liver Regeneration. J. Gastroenterol. Hepatol. 1991, 6, 509–519. [Google Scholar] [CrossRef]
- Imamura, R.; Matsumoto, K. Hepatocyte Growth Factor in Physiology and Infectious Diseases. Cytokine 2017, 98, 97–106. [Google Scholar] [CrossRef]
- Antoljak, N.; Topić, E.; Duvnjak, M.; Vrkić, N.; Zuntar, I. Hepatocyte Growth Factor Levels in Croatian Healthy and Alcoholic Liver Cirrhosis Patients. Coll. Antropol. 2001, 25, 341–348. [Google Scholar]
- Yamagamim, H.; Moriyama, M.; Matsumura, H.; Aoki, H.; Shimizu, T.; Saito, T.; Kaneko, M.; Shioda, A.; Tanaka, N.; Arakawa, Y. Serum Concentrations of Human Hepatocyte Growth Factor Is a Useful Indicator for Predicting the Occurrence of Hepatocellular Carcinomas in C-Viral Chronic Liver Diseases. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2002, 95, 824–834. [Google Scholar] [CrossRef]
- Shiota, G.; Umeki, K.; Okano, J.I.; Kawasaki, H. Hepatocyte Growth Factor and Acute Phase Proteins in Patients with Chronic Liver Diseases. J. Med. 1995, 26, 295–308. [Google Scholar]
- Sato, T.; Yoshinouchi, T.; Sakamoto, T.; Fujieda, H.; Murao, S.; Sato, H.; Kobayashi, H.; Ohe, T. Hepatocyte Growth Factor (HGF): A New Biochemical Marker for Acute Myocardial Infarction. Heart Vessel. 1997, 12, 241–246. [Google Scholar] [CrossRef]
- Funakoshi, H.; Nakamura, T. Hepatocyte Growth Factor (HGF) Levels and Diseases. Nihon Rinsho Jpn. J. Clin. Med. 1999, 57, 821–826. [Google Scholar]
- Vejchapipat, P.; Tangkijvanich, P.; Theamboonlers, A.; Chongsrisawat, V.; Chittmittrapap, S.; Poovorawan, Y. Association between Serum Hepatocyte Growth Factor and Survival in Untreated Hepatocellular Carcinoma. J. Gastroenterol. 2004, 39, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, J.C.; Tallis, S.; Delfante, A.; Souto, P.A.; Lemberg, A.; Eizayaga, F.X.; Romay, S. Hepatic Encephalopathy: An Approach to Its Multiple Pathophysiological Features. World J. Hepatol. 2012, 4, 50–65. [Google Scholar] [CrossRef]
- López-Ornelas, A.; Jiménez, A.; Pérez-Sánchez, G.; Rodríguez-Pérez, C.E.; Corzo-Cruz, A.; Velasco, I.; Estudillo, E. The Impairment of Blood-Brain Barrier in Alzheimer’s Disease: Challenges and Opportunities with Stem Cells. Int. J. Mol. Sci. 2022, 23, 10136. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, Y.; Kakimoto, K.; Nakajima, M.; Akatsu, H.; Yamamoto, T.; Ogawa, K.; Ohnishi, T.; Daikuhara, Y.; Yamada, T. Increased Hepatocyte Growth Factor Level in Cerebrospinal Fluid in Alzheimer’s Disease. Acta Neurol. Scand. 2003, 107, 81–86. [Google Scholar] [CrossRef]
- Salehi, Z.; Rajaei, F. Expression of Hepatocyte Growth Factor in the Serum and Cerebrospinal Fluid of Patients with Parkinson’s Disease. J. Clin. Neurosci. 2010, 17, 1553–1556. [Google Scholar] [CrossRef]
- Li, F.; Liu, P.; Huang, Y.; Li, L.; Zhang, S.; Yang, Z.; Wang, R.; Tao, Z.; Han, Z.; Fan, J.; et al. The Incremental Prognostic Value of Hepatocyte Growth Factor in First-Ever Acute Ischemic Stroke: An Early Link Between Growth Factor and Interleukins. Front. Neurol. 2021, 12, 691886. [Google Scholar] [CrossRef]
- Decker, P.A.; Larson, N.B.; Bell, E.J.; Pankow, J.S.; Hanson, N.Q.; Wassel, C.L.; Tsai, M.Y.; Bielinski, S.J. Increased Hepatocyte Growth Factor Levels over 2 Years Are Associated with Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. Heart J. 2019, 213, 30. [Google Scholar] [CrossRef]
- Schröder, K.; Schütz, S.; Schlöffel, I.; Bätz, S.; Takac, I.; Weissmann, N.; Michaelis, U.R.; Koyanagi, M.; Brandes, R.P. Hepatocyte Growth Factor Induces a Proangiogenic Phenotype and Mobilizes Endothelial Progenitor Cells by Activating Nox2. Antioxid. Redox Signal 2011, 15, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Usatyuk, P.V.; Fu, P.; Mohan, V.; Epshtein, Y.; Jacobson, J.R.; Gomez-Cambronero, J.; Wary, K.K.; Bindokas, V.; Dudek, S.M.; Salgia, R.; et al. Role of C-Met/Phosphatidylinositol 3-Kinase (PI3k)/Akt Signaling in Hepatocyte Growth Factor (HGF)-Mediated Lamellipodia Formation, Reactive Oxygen Species (ROS) Generation, and Motility of Lung Endothelial Cells. J. Biol. Chem. 2014, 289, 13476–13491. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Alekseeva, E.; Mikaelyan, A.; Birukov, K.G. HGF Attenuates Thrombin-Induced Endothelial Permeability by Tiam1-Mediated Activation of the Rac Pathway and by Tiam1/Rac-Dependent Inhibition of the Rho Pathway. FASEB J. 2007, 21, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Pandey, E.; Nour, A.S.; Harris, E.N. Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease. Front. Physiol. 2020, 11, 547714. [Google Scholar] [CrossRef]
- Guo, Q.; Furuta, K.; Aly, A.; Ibrahim, S.H. Isolation and Characterization of Mouse Primary Liver Sinusoidal Endothelial Cells. J. Vis. Exp. 2021, 201, 10-3791. [Google Scholar] [CrossRef]
- Bhandari, S.; Kyrrestad, I.; Simón-Santamaría, J.; Li, R.; Szafranska, K.J.; Dumitriu, G.; Sánchez Romano, J.; Smedsrød, B.; Sørensen, K.K. Mouse Liver Sinusoidal Endothelial Cell Responses to the Glucocorticoid Receptor Agonist Dexamethasone. Front. Pharmacol. 2024, 15, 1377136. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larionov, A.; Filgueira, L.; Hammer, C.M. Endothelial Cell Transition: Preliminary Data on Cross-Organ Shift from Brain to Liver. Cells 2025, 14, 1538. https://doi.org/10.3390/cells14191538
Larionov A, Filgueira L, Hammer CM. Endothelial Cell Transition: Preliminary Data on Cross-Organ Shift from Brain to Liver. Cells. 2025; 14(19):1538. https://doi.org/10.3390/cells14191538
Chicago/Turabian StyleLarionov, Alexey, Luis Filgueira, and Christian M. Hammer. 2025. "Endothelial Cell Transition: Preliminary Data on Cross-Organ Shift from Brain to Liver" Cells 14, no. 19: 1538. https://doi.org/10.3390/cells14191538
APA StyleLarionov, A., Filgueira, L., & Hammer, C. M. (2025). Endothelial Cell Transition: Preliminary Data on Cross-Organ Shift from Brain to Liver. Cells, 14(19), 1538. https://doi.org/10.3390/cells14191538





