Functional Characterization of Trypsin in the Induction of Biologically Live Bait Feeding in Mandarin Fish (Siniperca chuatsi) Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Farming and Sample Collection
2.2. The mRNA Expression of Trypsin in Mandarin Fish
2.3. Sequence and Phylogenetic Analysis
2.4. Whole-Mount in Situ Hybridization
2.5. Trypsin Functional Targeted Inhibition Assay
2.6. Determination of Digestive Enzyme Activity
2.7. The qRT-PCR and Statistical Analysis
3. Results
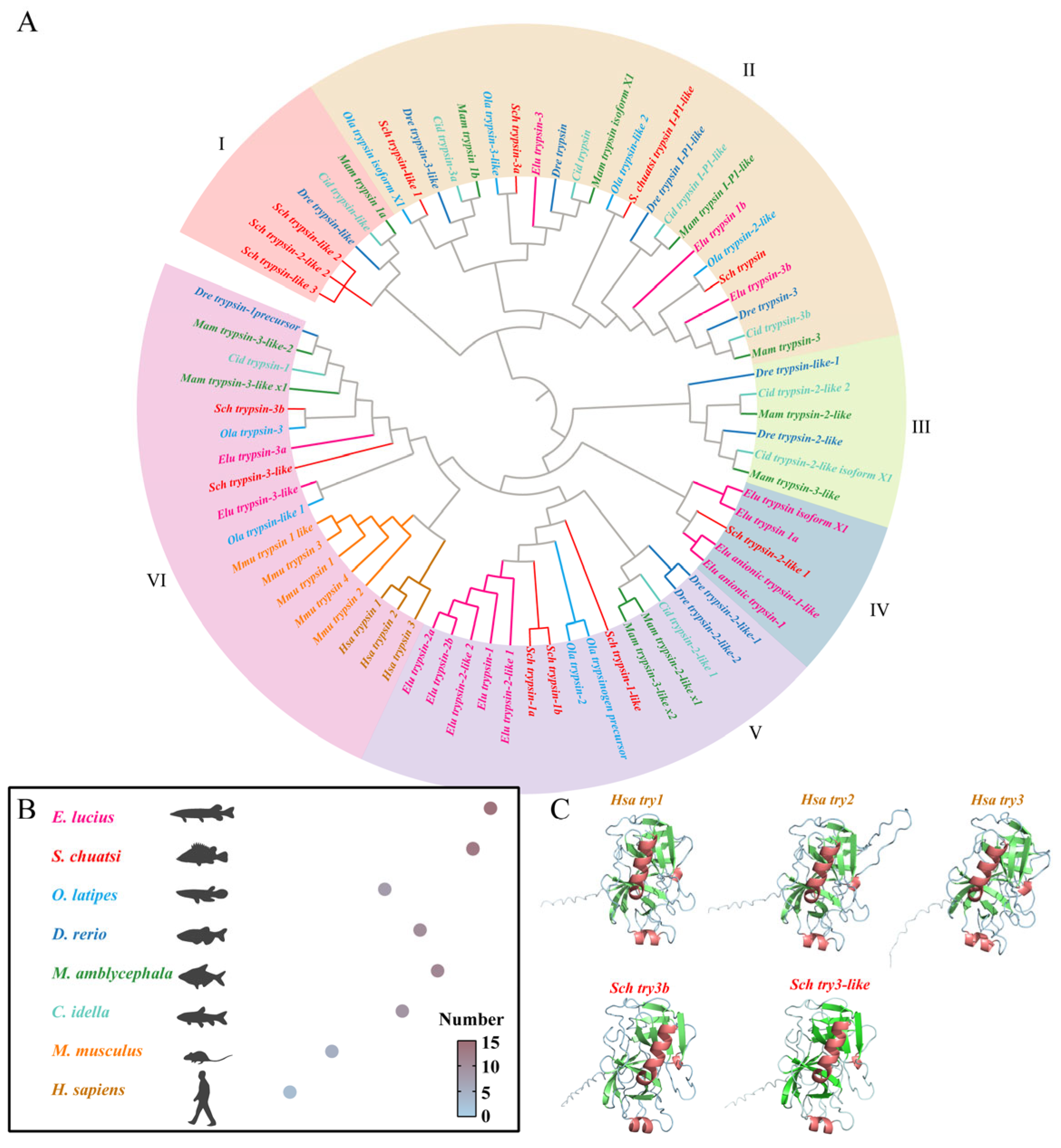
3.1. Phylogenetic Analysis of Trypsins
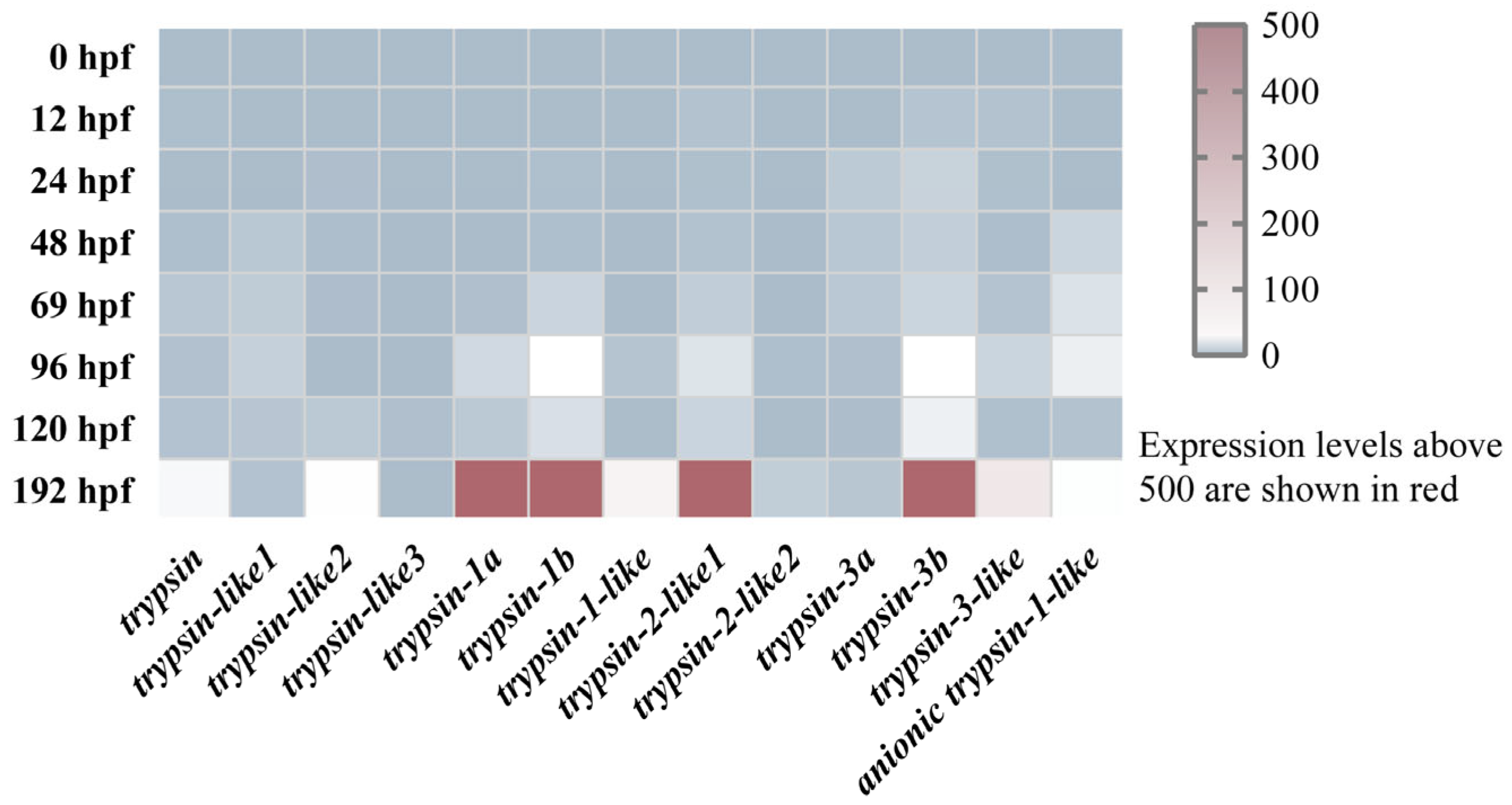
3.2. Expression Patterns of Trypsins During Different Developmental Stages in Mandarin Fish
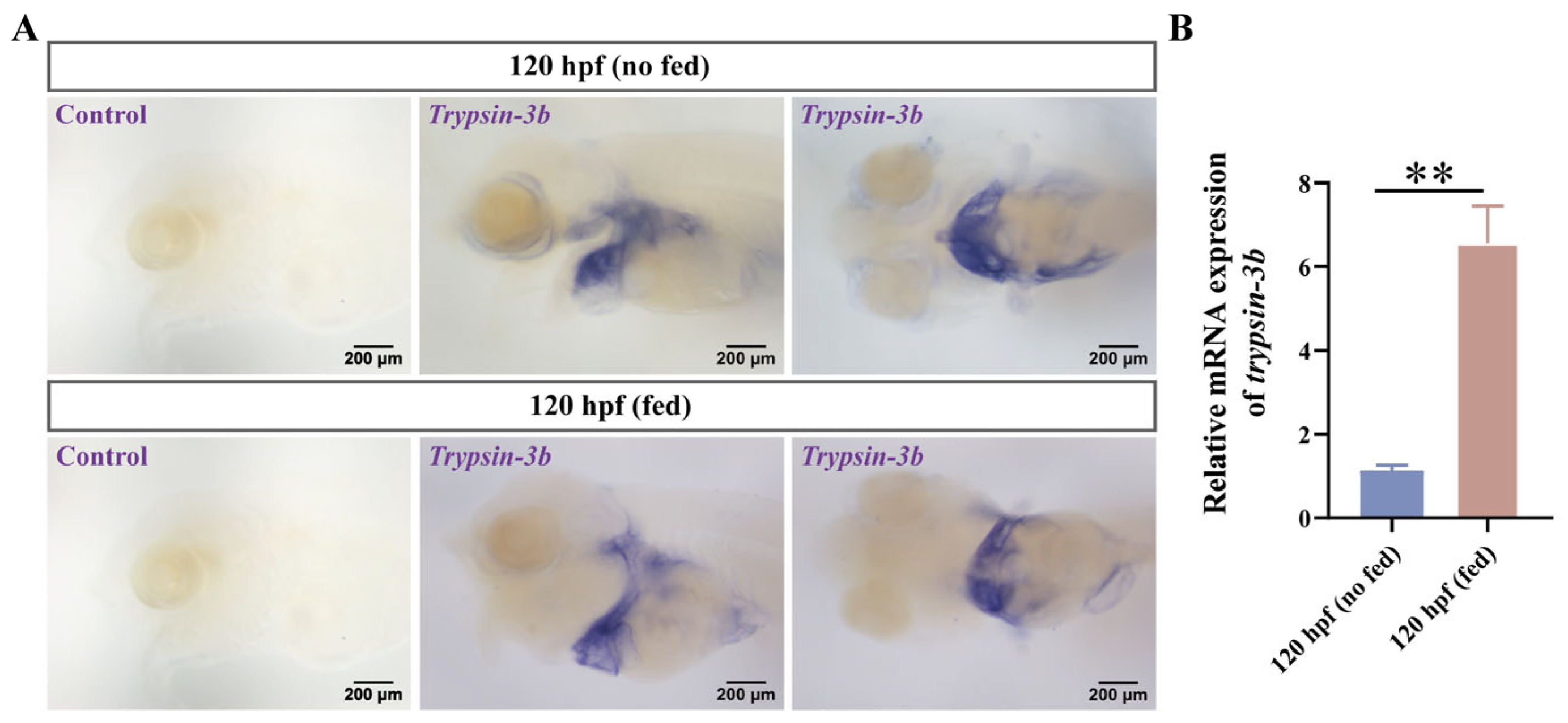
3.3. Expression Localization of Trypsin-3b During the Development of Mandarin Fish Larvae
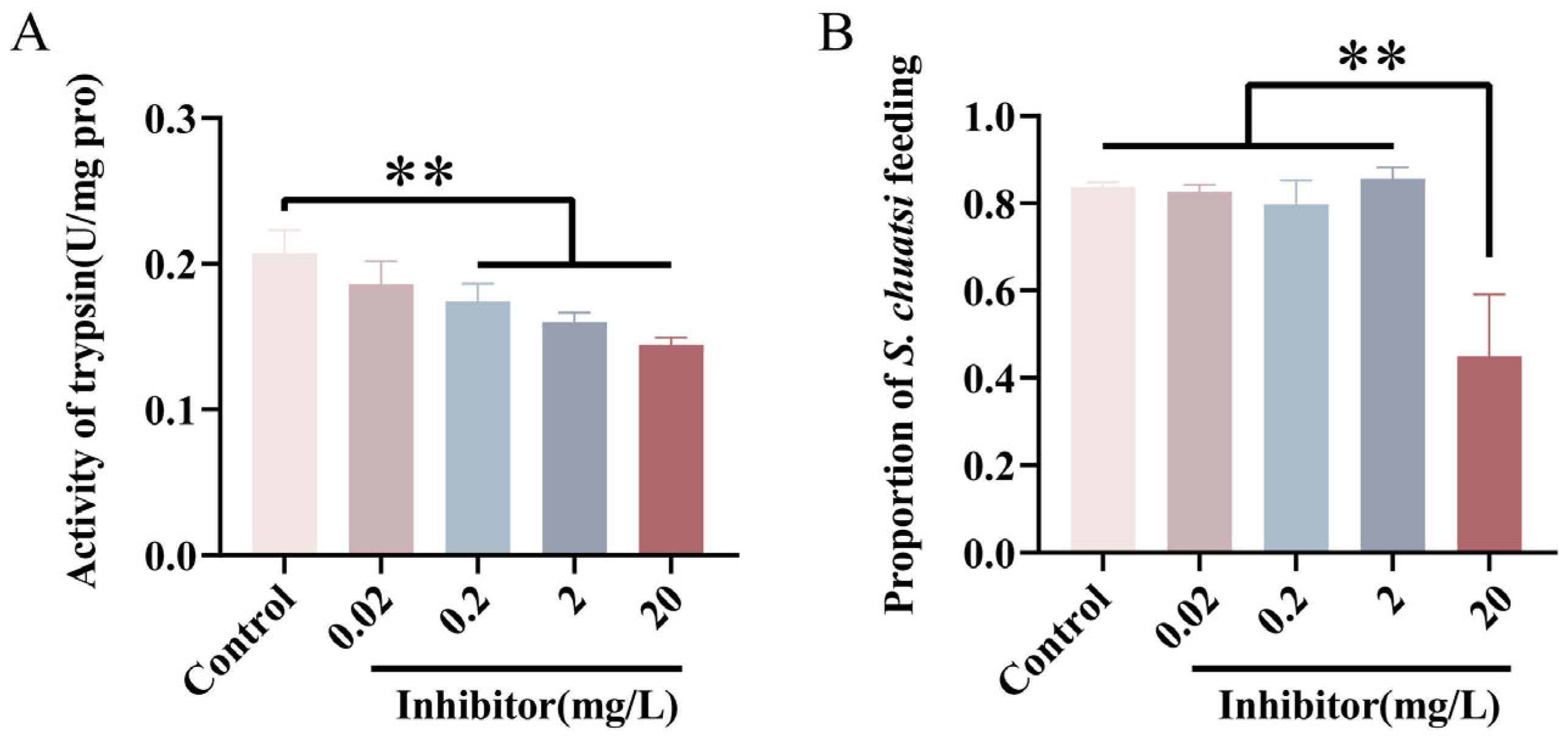
3.4. Effects of Trypsin Inhibitor on Feeding and Digestion in Mandarin Fish Larvae
3.5. Trypsin Exerts Its Appetite-Suppressing Effects Through Anorexigenic Factors CCK and POMC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Poonsin, T.; Simpson, B.K.; Benjakul, S.; Visessanguan, W.; Yoshida, A.; Osatomi, K.; Klomklao, S. Anionic trypsin from the spleen of albacore tuna (Thunnus alalunga): Purification, biochemical properties and its application for proteolytic degradation of fish muscle. Int. J. Biol. Macromol. 2019, 15, 971–979. [Google Scholar] [CrossRef]
- Miura, Y.; Kageyama, T.; Moriyama, A. Pepsinogens and pepsins from largemouth bass, purification and characterization with special reference to high proteolytic activities of bass enzymes. Comp. Biochem. Phys. B 2015, 183, 42–48. [Google Scholar] [CrossRef]
- Tanji, M.; Yakabe, E.; Kubota, K.; Kageyama, T.; Ichinose, M.; Miki, K.; Ito, H.; Takahashi, K. Structural and phylogenetic comparison of three pepsinogens from Pacific bluefin tuna: Molecular evolution of fish pepsinogens. Comp. Biochem. Phys. B 2009, 152, 9–19. [Google Scholar] [CrossRef]
- Chen, B.N.; Qin, J.G.; Kumar, M.S.; Hutchinson, W.G.; Clarke, S.M. Ontogenetic development of digestive enzymes in yellowtail kingfish Seriola lalandi larvae. Aquaculture 2006, 260, 264–271. [Google Scholar] [CrossRef]
- Feng, S.Z.; Li, W.S.; Lin, H.R. Characterization and expression of the pepsinogen C gene and determination of pepsin-like enzyme activity from orange-spotted grouper (Epinephelus coioides). Comp. Biochem. Phys. B 2008, 149, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Guo, H.Y.; Zheng, P.L.; Wang, L.; Jiang, S.G.; Qin, J.G.; Zhang, D.C. Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol. Biochem. 2014, 40, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.L.Z.; Cahu, C.L. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp. Biochem. Phys. C 2001, 130, 477–487. [Google Scholar] [CrossRef]
- Ronnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Sæle, O.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquac. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- Suzer, C.; Çoban, D.; Yildirim, S.; Hekimoglu, M.; Kamaci, H.O.; Firat, K.; Saka, S. Stage-Specific Ontogeny of Digestive Enzymes in the Cultured Common Dentex (Dentex dentex) Larvae. Turk. J. Fish. Aquat. Sci. 2014, 14, 759–768. [Google Scholar] [CrossRef]
- Youson, J.H.; Al-Mahrouki, A.A.; Amemiya, Y.; Graham, L.C.; Montpetit, C.; Irwin, D.M. The fish endocrine pancreas: Review, new data, and future research directions in ontogeny and phylogeny. Gen. Comp. Endocr. 2006, 148, 105–115. [Google Scholar] [CrossRef]
- Muhlia-Almazán, A.; Sánchez-Paz, A.; García-Carreño, F.L. Invertebrate trypsins: A review. J. Comp. Physiol. B 2008, 178, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Kukor, Z.; Le Maréchal, U.; Tóth, M.; Tsakiris, L.; Raguénes, O.; Férec, C.; Sahin-Tóth, M. Evolution of trypsinogen activation peptides. Mol. Biol. Evol. 2003, 20, 1767–1777. [Google Scholar] [CrossRef]
- Gieseler, F.; Ungefroren, H.; Settmacher, U.; Hollenberg, M.D.; Kaufmann, R. Proteinase-activated receptors (PARs)—Focus on receptor-receptor-interactions and their physiological and pathophysiological impact. Cell Commun. Signal 2013, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.D.; Lu, K.; Liang, X.F. Neuropeptide Y receptor Y8b (npy8br) regulates feeding and digestion in Japanese medaka (Oryzias latipes) larvae: Evidence from gene knockout. J. Zhejiang Univ.-Sc. B 2024, 25, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Koji, M.; Fukada, H.; Ronnestad, I.; Kurokawa, T.; Masumoto, T. Nutrient control of release of pancreatic enzymes in yellowtail (Seriola quinqueradiata): Involvement of CCK and PY in the regulatory loop. Comp. Biochem. Phys. A 2008, 150, 438–443. [Google Scholar] [CrossRef]
- Tillner, R.; Ronnestad, I.; Harboe, T.; Ueberschär, B. Hormonal control of tryptic enzyme activity in Atlantic cod larvae (Gadus morhua): Involvement of cholecystokinin during ontogeny and diurnal rhythm. Aquaculture 2013, 402, 133–140. [Google Scholar] [CrossRef]
- Volkoff, H.; Hoskins, L.J.; Tuziak, S.M. Influence of intrinsic signals and environmental cues on the endocrine control of feeding in fish: Potential application in aquaculture. Gen. Comp. Endocr. 2010, 167, 352–359. [Google Scholar] [CrossRef]
- Zhou, F.; Jing Zhou, J.; Shao, Q.; Wang, Y.; Li, H.; Gao, S.; Hua, Y. Effects of arginine-deficient and replete diets on growth performance, digestive enzyme activities and genes expression of black sea bream, Acanthopagrus schlegelii, Juveniles. J. World Aquac. Soc. 2012, 43, 828–839. [Google Scholar] [CrossRef]
- Albarazanji, K.; Hinke, S.A.; Cavanaugh, C.; Liu, J.Y.; Beck, S.; Shukla, N.; Meng, R.; Ho, G.; Raul, C.; Camacho, R.C.; et al. Role of CCK1 receptor in metabolic benefits of intestinal enteropeptidase inhibition in mice. PLoS ONE 2025, 20, e0312927. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y. Siniperca chuatsi aquaculture status and aquaculture trend in 2024. Sci. Fish Farming 2024, 07, 3–5. [Google Scholar] [CrossRef]
- Wu, X.F.; Zhao, J.L.; Qian, Y.Z.; Wu, C. Histological study of the digestive system organogenesis for the mandarin fish, Siniperca chuatsi. Zool. Res. 2007, 28, 511–518. [Google Scholar]
- Chen, J.Y.; Zeng, C.S.; Jerry, D.R.; Cobcroft, J.M. Recent advances of marine ornamental fish larviculture: Broodstock reproduction, live prey and feeding regimes, and comparison between demersal and pelagic spawners. Rev. Aquac. 2020, 12, 1518–1541. [Google Scholar] [CrossRef]
- Liang, X.F.; Lin, X.T.; Li, S.Q.; Liu, J.K. Impact of environmental and innate factors on the food habit of Chinese perch Siniperca chuatsi (Basilewsky) (Percichthyidae). Aquac. Res. 2008, 39, 150–157. [Google Scholar] [CrossRef]
- Shen, Y.; Song, L.; Chen, T.; Jiang, H.; Yang, G.; Zhang, Y.; Zhang, X.; Lim, K.K.; Meng, X.; Zhao, J.; et al. Identification of hub genes in digestive system of mandarin fish (Siniperca chuatsi) fed with artificial diet by weighted gene co-expression network analysis. Comp. Biochem. Phys. D 2023, 47, 101112. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Chitramuthu, B.P.; Bennett, H.P.J. High resolution whole mount in situ hybridization within zebrafish embryos to study gene expression and function. Jove-J. Vis. Exp. 2013, 19, e50644. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-resolution hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Khoa, T.N.D.; Waqalevu, V.; Honda, A.; Shiozaki, K.; Kotani, T. Comparative study on early digestive enzyme activity and expression in red sea bream (Pagrus major) fed on live feed and micro-diet. Aquaculture 2020, 519, 734721. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hughes, L.C.; Ortí, G.; Huang, Y.; Sun, Y.; Baldwin, C.C.; Thompson, A.W.; Arcila, D.; Betancur-R, R.; Li, C.H.; Becker, L.; et al. Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc. Natl. Acad. Sci. USA 2018, 115, 6249–6254. [Google Scholar] [CrossRef]
- Srichanun, M.; Tantikitti, C.; Utarabhand, P.; Kortner, T.M. Gene expression and activity of digestive enzymes during the larval development of Asian seabass (Lates calcarifer). Comp. Biochem. Phys. B 2013, 165, 1–9. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, L.; Limbu, S.M.; Yin, H.; Xie, Y.Q.; Yang, Z.H.; Shang, Z.M.; Kong, L.F.; Rong, H. A comparison of digestive strategies for fishes with different feeding habits: Digestive enzyme activities, intestinal morphology, and gut microbiota. Ecol. Evol. 2023, 13, e10499. [Google Scholar] [CrossRef]
- Yuan, X.C.; Liang, X.F.; Cai, W.J.; He, S.; Guo, W.J.; Mai, K.S. Expansion of sweet taste receptor genes in grass carp (Ctenopharyngodon idellus) coincided with vegetarian adaptation. BMC Evol. Biol. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Pajic, P.; Pavlidis, P.; Dean, K.; Neznanova, L.; Romano, R.A.; Garneau, D.; Daugherity, E.; Globig, A.; Ruhl, S.; Gokcumen, O. Independent amylase gene copy number bursts correlate with dietary preferences in mammals. eLife 2019, 14, e44628. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.M.; Perez-Casanova, J.C.; Gallant, J.W.; Johnson, S.C.; Douglas, S.E. Trypsinogen expression during the development of the exocrine pancreas in winter flounder (Pleuronectes americanus). Comp. Biochem. Phys. A 2004, 138, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liang, X.F.; He, S.; Zhang, Y.; Peng, D.; Feng, H. Development of digestive enzymatic activity and gene expression during the early ontogeny of Chinese perch (Siniperca chuatsi). Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Gawlicka, A.K.; Horn, M.H. Trypsin gene expression by quantitative in situ hybridization in carnivorous and herbivorous prickleback fishes (Teleostei: Stichaeidae): Ontogenetic, dietary, and phylogenetic effects. Physiol. Biochem. Zool. 2006, 79, 120–132. [Google Scholar] [CrossRef]
- Drewe, K.E.; Horn, M.H.; Dickson, K.A.; Gawlicka, A. Insectivore to frugivore: Ontogenetic changes in gut morphology and digestive enzyme activity in the characid fish Brycon guatemalensis from Costa Rican rain forest streams. J. Fish. Biol. 2004, 64, 890–902. [Google Scholar] [CrossRef]
- Axelsson, E.; Ratnakumar, A.; Arendt, M.L.; Maqbool, K.; Webster, M.T.; Perloski, M.; Liberg, O.; Arnemo, J.M.; Hedhammar, Å.; Lindblad-Toh, K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 2013, 495, 360–364. [Google Scholar] [CrossRef]
- Chi, L.; Liu, Q.; Xu, S.; Xiao, Z.; Ma, D.; Li, J. Maternally derived trypsin may have multiple functions in the early development of turbot (Scopthalmus maximus). Comp. Biochem. Phys. A 2015, 188, 148–155. [Google Scholar] [CrossRef]
- Yuan, H.; Wen, B.; Liu, X.; Gao, C.; Yang, R.; Wang, L.; Chen, S.; Chen, Z.; de The, H.; Zhou, J.; et al. CCAAT/enhancer-binding protein α is required for hepatic outgrowth via the p53 pathway in zebrafish. Sci. Rep. 2015, 29, 15838. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tang, S.; Lu, K.; Peng, D.; Wu, J.; Zhang, Q.; Wang, Q.; Xu, D.; Xie, R.; Liang, X.F. The essential role of mTOR in promoting feeding behavior through regulating NPY and AgRP expression in mandarin fish (Siniperca chuatsi) larvae. Water Biol. Secur. 2025; in press. [Google Scholar] [CrossRef]
- Heiser, P.W.; Lau, J.; Taketo, M.M.; Herrera, P.L.; Hebrok, M. Stabilization of beta-catenin impacts pancreas growth. Development 2006, 133, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, W.; Lu, M.; Li, Z.; Qiao, X.; Sun, B.; Zhang, W.; Xue, D. Role of the c-Jun N-terminal kinase signaling pathway in the activation of trypsinogen in rat pancreatic acinar cells. Int. J. Mol. Med. 2018, 41, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Iinuma, N.; Unuma, T.; Tanaka, H.; Kagawa, H.; Ohta, H.; Suzuki, T. Development of endocrine system regulating exocrine pancreas and estimation of feeding and digestive ability in Japanese eel larvae. Aquaculture 2004, 234, 513–525. [Google Scholar] [CrossRef]
- Ping, H.C.; Feng, K.; Zhang, G.R.; Wei, K.J.; Zou, G.W.; Wang, W.M. Ontogeny expression of ghrelin, neuropeptide Y and cholecystokinin in blunt snout bream, Megalobrama amblycephala. J. Anim. Physiol. N. 2014, 98, 338–346. [Google Scholar] [CrossRef]
- Navarro-Guillén, C.; Ronnestad, I.; Jordal, A.E.O.; Moyano, F.J.; Yúfera, M. Involvement of cholecystokinin (CCK) in the daily pattern of gastrointestinal regulation of Senegalese sole (Solea senegalensis) larvae reared under different feeding regimes. Comp. Biochem. Phys. A 2017, 203, 126–132. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef]
- Volkoff, H. The neuroendocrine regulation of food intake in fish: A review of current knowledge. Front. Neurosci. 2016, 29, 540. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, X.F.; Yuan, X.C.; Li, J.; He, Y.; Fang, L.; Guo, X.Z.; Liu, L.W.; Li, B.; Shen, D. Neuropeptide Y stimulates food intake and regulates metabolism in grass carp, Ctenopharyngodon idellus. Aquaculture 2013, 380, 52–61. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Lu, K.; Wu, J.; Wang, Q.; Liang, X.-f. Functional Characterization of Trypsin in the Induction of Biologically Live Bait Feeding in Mandarin Fish (Siniperca chuatsi) Larvae. Cells 2025, 14, 1537. https://doi.org/10.3390/cells14191537
Dong X, Lu K, Wu J, Wang Q, Liang X-f. Functional Characterization of Trypsin in the Induction of Biologically Live Bait Feeding in Mandarin Fish (Siniperca chuatsi) Larvae. Cells. 2025; 14(19):1537. https://doi.org/10.3390/cells14191537
Chicago/Turabian StyleDong, Xiaoru, Ke Lu, Jiaqi Wu, Qiuling Wang, and Xu-fang Liang. 2025. "Functional Characterization of Trypsin in the Induction of Biologically Live Bait Feeding in Mandarin Fish (Siniperca chuatsi) Larvae" Cells 14, no. 19: 1537. https://doi.org/10.3390/cells14191537
APA StyleDong, X., Lu, K., Wu, J., Wang, Q., & Liang, X.-f. (2025). Functional Characterization of Trypsin in the Induction of Biologically Live Bait Feeding in Mandarin Fish (Siniperca chuatsi) Larvae. Cells, 14(19), 1537. https://doi.org/10.3390/cells14191537




