Collagen Fiber Maturity and Architecture in MVP-Associated Fibrosis Quantified by Digital Pathology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Recruitment
2.2. Tissue Acquisition
2.3. Histologic Processing, Staining, and Digital Pathology
2.4. Quantitative Fibrosis Profiling
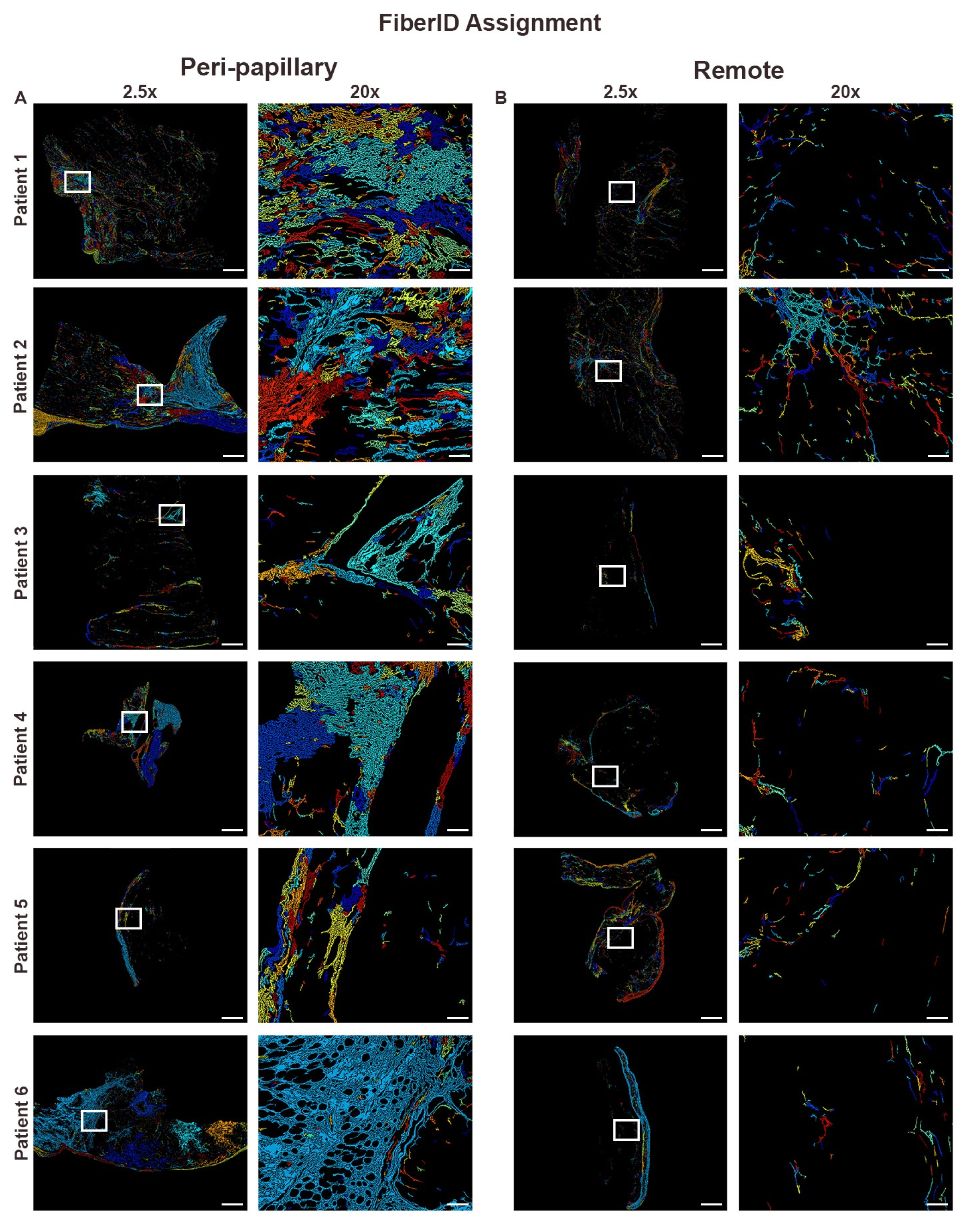
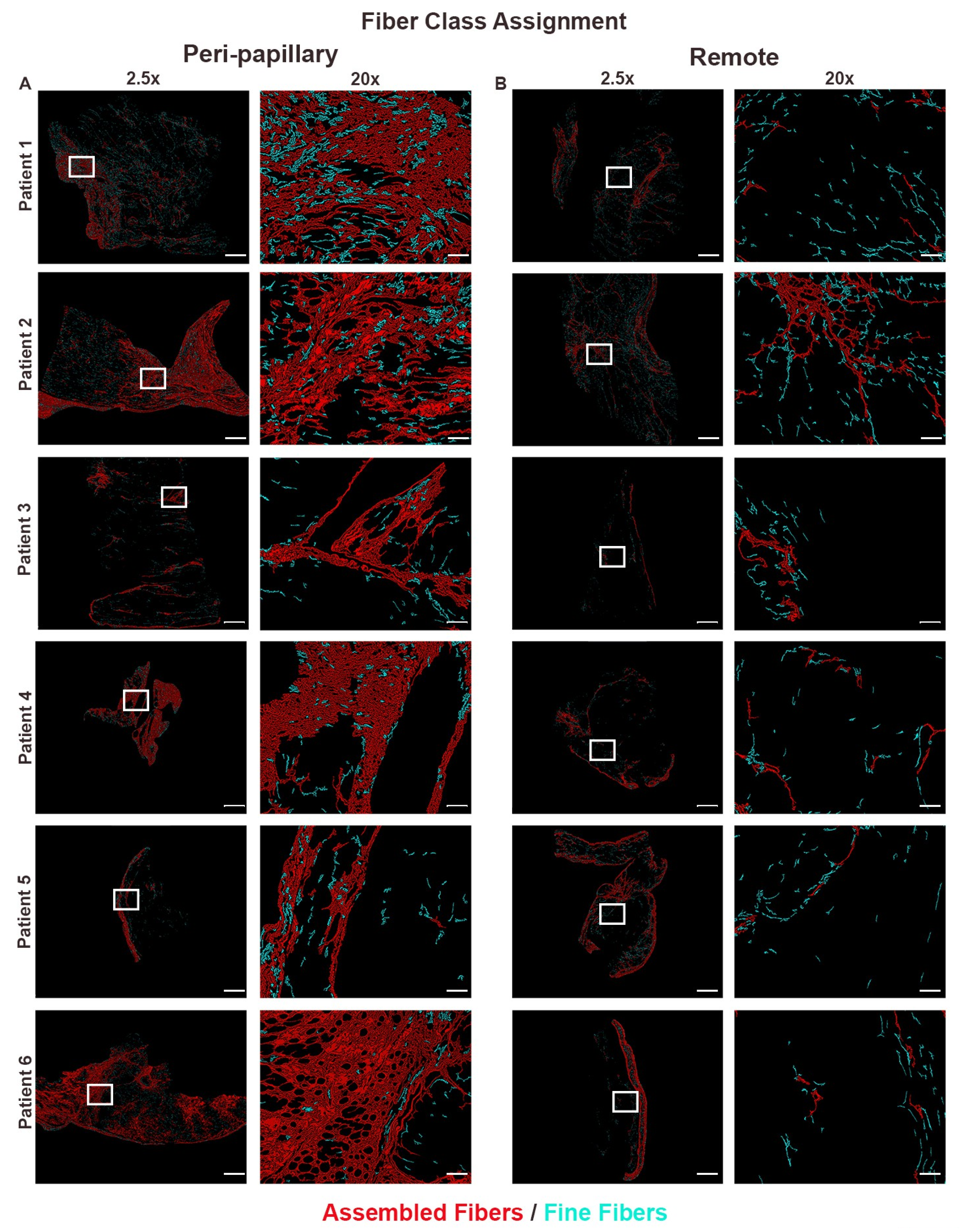
2.5. Fiber Segmentation and Classification
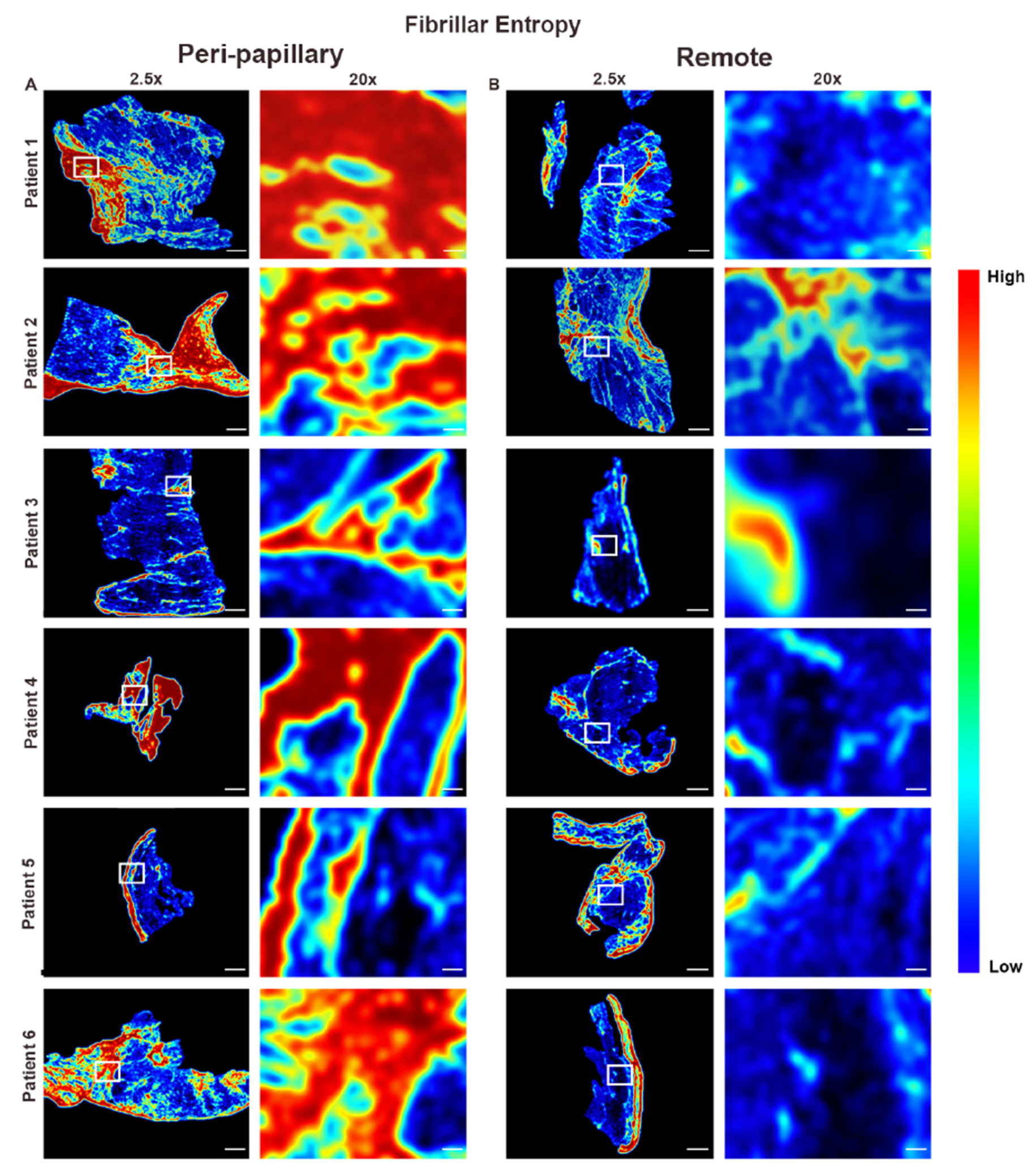
2.6. Fibrillar Entropy Mapping
2.7. Region of Interest (ROI) Annotations
2.8. Analytical Performance
2.9. Statistical Analysis
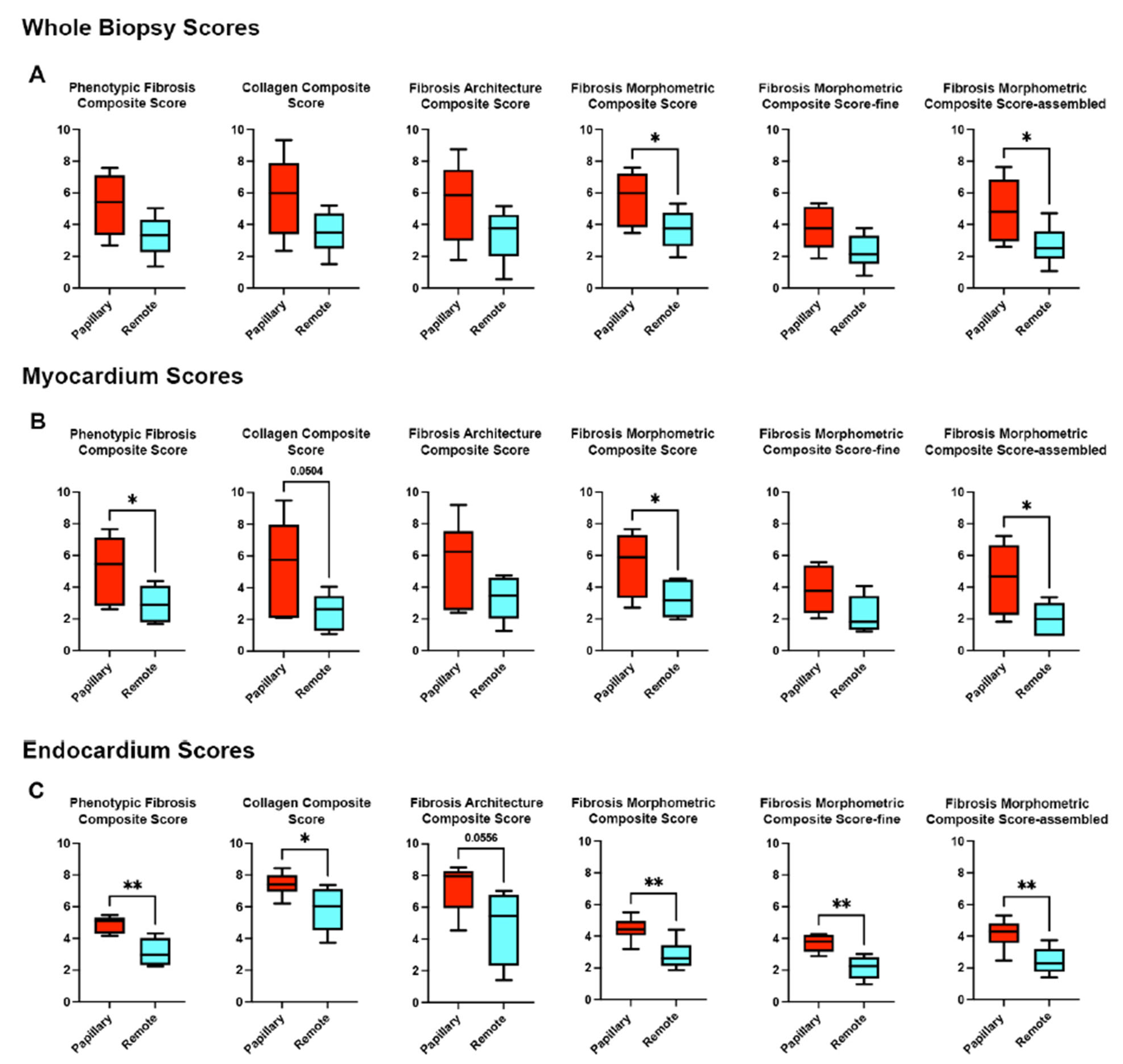
3. Results
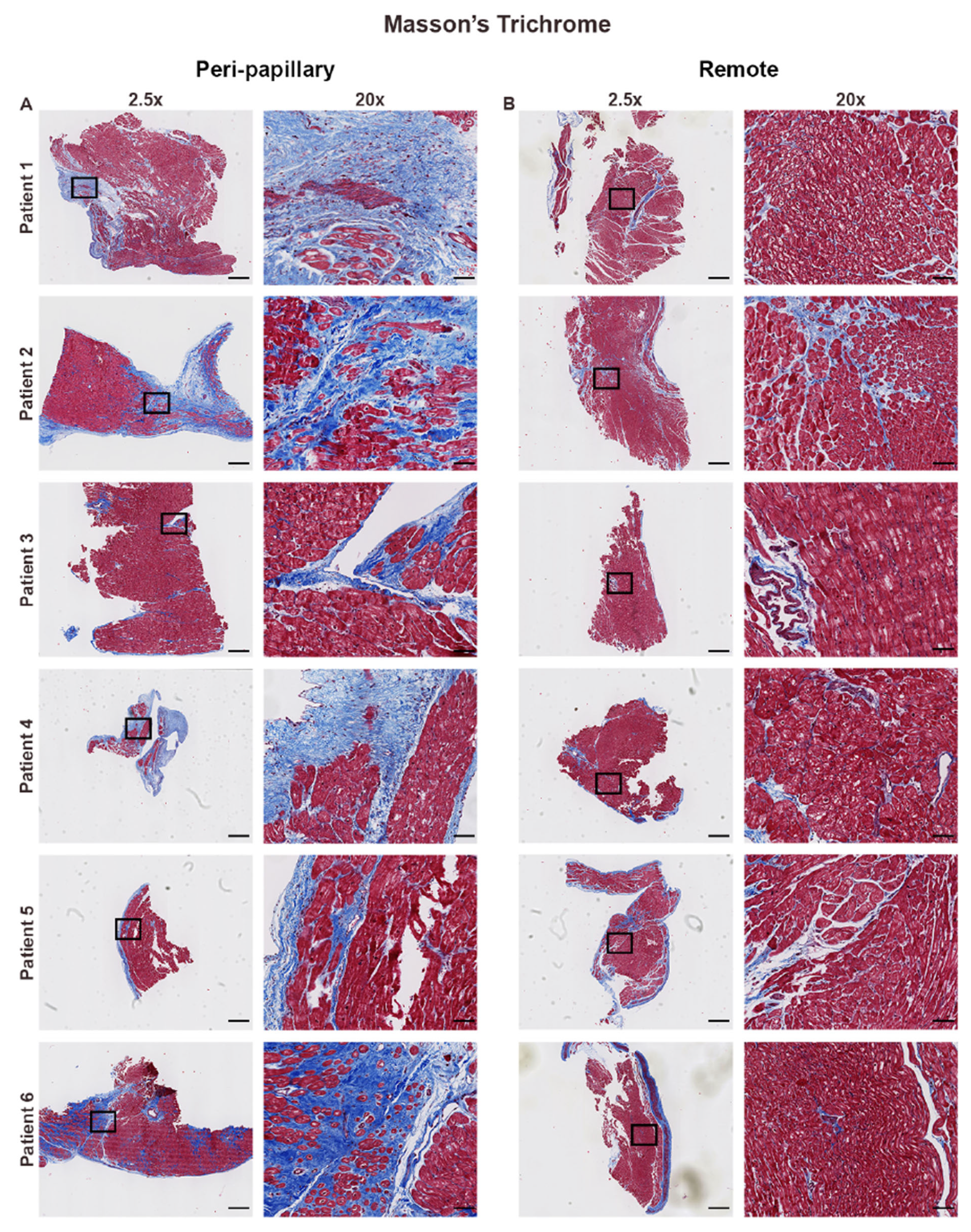
3.1. Histological Assessment Confirms Regional Fibrosis in MVP
3.2. Collagen Fiber Architecture Demonstrates Greater Maturity and Complexity in Papillary Region
3.3. Endocardial vs. Myocardial Fibrosis Differ in Morphology
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef]
- Delling, F.N.; Noseworthy, P.A.; Adams, D.H.; Basso, C.; Borger, M.; Bouatia-Naji, N.; Elmariah, S.; Evans, F.; Gerstenfeld, E.; Hung, J.; et al. Research Opportunities in the Treatment of Mitral Valve Prolapse: JACC Expert Panel. J. Am. Coll. Cardiol. 2022, 80, 2331–2347. [Google Scholar] [CrossRef]
- Morningstar, J.E.; Nieman, A.; Wang, C.; Beck, T.; Harvey, A.; Norris, R.A. Mitral Valve Prolapse and Its Motley Crew-Syndromic Prevalence, Pathophysiology, and Progression of a Common Heart Condition. J. Am. Heart Assoc. 2021, 10, e020919. [Google Scholar] [CrossRef]
- Spampinato, R.A.; Marin-Cuartas, M.; van Kampen, A.; Fahr, F.; Sieg, F.; Strotdrees, E.; Jahnke, C.; Klaeske, K.; Wiesner, K.; Morningstar, J.E.; et al. Left ventricular fibrosis and CMR tissue characterization of papillary muscles in mitral valve prolapse patients. Int. J. Cardiovasc. Imaging 2023, 40, 213–224. [Google Scholar] [CrossRef]
- Nagata, Y.; Bertrand, P.B.; Baliyan, V.; Kochav, J.; Kagan, R.D.; Ujka, K.; Alfraidi, H.; van Kampen, A.; Morningstar, J.E.; Dal-Bianco, J.P.; et al. Abnormal Mechanics Relate to Myocardial Fibrosis and Ventricular Arrhythmias in Patients with Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2023, 16, e014963. [Google Scholar] [CrossRef]
- Dieterlen, M.T.; Klaeske, K.; Spampinato, R.; Marin-Cuartas, M.; Wiesner, K.; Morningstar, J.; Norris, R.A.; Melnitchouk, S.; Levine, R.A.; van Kampen, A.; et al. Histopathological insights into mitral valve prolapse-induced fibrosis. Front. Cardiovasc. Med. 2023, 10, 1057986. [Google Scholar] [CrossRef]
- Morningstar, J.E.; Gensemer, C.; Moore, R.; Fulmer, D.; Beck, T.C.; Wang, C.; Moore, K.; Guo, L.; Sieg, F.; Nagata, Y.; et al. Mitral Valve Prolapse Induces Regionalized Myocardial Fibrosis. J. Am. Heart Assoc. 2021, 10, e022332. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Bertrand, P.; Dal-Bianco, J.P.; Melnitchouk, S.; Baliyan, V.; Holmvang, F.; Norris, R.A.; Guerrero, J.L.; Borger, M.A.; Moore, R.; et al. Myocardial Fibrosis Relates to Abnormal Myocardial Mechanics in Patients with Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 75, 1547. [Google Scholar] [CrossRef]
- Borger, M.A.; Mansour, M.C.; Levine, R.A. Atrial Fibrillation and Mitral Valve Prolapse: Time to Intervene? J. Am. Coll. Cardiol. 2019, 73, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Jerosch-Herold, M.; Hajjar, R.J. Mitral Valve Prolapse: A Disease of Valve and Ventricle. J. Am. Coll. Cardiol. 2018, 72, 835–837. [Google Scholar] [CrossRef]
- Fukuda, S.; Song, J.K.; Mahara, K.; Kuwaki, H.; Jang, J.Y.; Takeuchi, M.; Sun, B.J.; Kim, Y.J.; Miyamoto, T.; Oginosawa, Y.; et al. Basal Left Ventricular Dilatation and Reduced Contraction in Patients with Mitral Valve Prolapse Can Be Secondary to Annular Dilatation: Preoperative and Postoperative Speckle-Tracking Echocardiographic Study on Left Ventricle and Mitral Valve Annulus Interaction. Circ. Cardiovasc. Imaging 2016, 9, e005113. [Google Scholar] [CrossRef] [PubMed]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients with Primary Mitral Regurgitation with and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Sriram, C.S.; Syed, F.F.; Ferguson, M.E.; Johnson, J.N.; Enriquez-Sarano, M.; Cetta, F.; Cannon, B.C.; Asirvatham, S.J.; Ackerman, M.J. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2013, 62, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Perazzolo Marra, M. Mitral Annulus Disjunction: Emerging Role of Myocardial Mechanical Stretch in Arrhythmogenesis. J. Am. Coll. Cardiol. 2018, 72, 1610–1612. [Google Scholar] [CrossRef]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Skjolsvik, E.T.; Lie, O.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Imbrie-Moore, A.M.; Paulsen, M.J.; Zhu, Y.; Wang, H.; Lucian, H.J.; Farry, J.M.; MacArthur, J.W.; Ma, M.; Woo, Y.J. A novel cross-species model of Barlow’s disease to biomechanically analyze repair techniques in an ex vivo left heart simulator. J. Thorac. Cardiovasc. Surg. 2021, 161, 1776–1783. [Google Scholar] [CrossRef]
- Petitjean, L.; Chen, L.; Zhang, X.; Schiano, T.; Petitjean, M.; Sanyal, A.J.; Fiel, M. Digital Pathology Quantification of the Continuum of Cirrhosis Severity in Human Liver Biopsies. Liver Int. 2025, 45, e70166. [Google Scholar] [CrossRef]
- Watson, A.; Petitjean, L.; Petitjean, M.; Pavlides, M. Liver fibrosis phenotyping and severity scoring by quantitative image analysis of biopsy slides. Liver Int. 2024, 44, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Hompesch, M.; Petitjean, M.; Serdjebi, C.; Iyer, J.S.; Parwani, A.V.; Tai, D.; Bugianesi, E.; Cusi, K.; Friedman, S.L.; et al. Artificial intelligence-assisted digital pathology for non-alcoholic steatohepatitis: Current status and future directions. J. Hepatol. 2024, 80, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Francque, S.; Behling, C.A.; Cejvanovic, V.; Cortez-Pinto, H.; Iyer, J.S.; Krarup, N.; Le, Q.; Sejling, A.S.; Tiniakos, D.; et al. Artificial intelligence scoring of liver biopsies in a phase II trial of semaglutide in nonalcoholic steatohepatitis. Hepatology 2024, 80, 173–185. [Google Scholar] [CrossRef]
- Fukushima, M.; Miyaaki, H.; Nakao, Y.; Sasaki, R.; Haraguchi, M.; Takahashi, K.; Ozawa, E.; Miuma, S.; Akazawa, Y.; Soyama, A.; et al. Characterizing alcohol-related and metabolic dysfunction-associated steatotic liver disease cirrhosis via fibrotic pattern analysis. Sci. Rep. 2024, 14, 23679. [Google Scholar] [CrossRef]
- Kostadinova, R.; Strobel, S.; Chen, L.; Fiaschetti-Egli, K.; Gadient, J.; Pawlowska, A.; Petitjean, L.; Bieri, M.; Thoma, E.; Petitjean, M. Digital pathology with artificial intelligence analysis provides insight to the efficacy of anti-fibrotic compounds in human 3D MASH model. Sci. Rep. 2024, 14, 5885. [Google Scholar] [CrossRef]
- Quintana, E.; Suri, R.M.; Thalji, N.M.; Daly, R.C.; Dearani, J.A.; Burkhart, H.M.; Li, Z.; Enriquez-Sarano, M.; Schaff, H.V. Left ventricular dysfunction after mitral valve repair—The fallacy of “normal” preoperative myocardial function. J. Thorac. Cardiovasc. Surg. 2014, 148, 2752–2760. [Google Scholar] [CrossRef]
- Wang, S.; Li, K.; Pickholz, E.; Dobie, R.; Matchett, K.P.; Henderson, N.C.; Carrico, C.; Driver, I.; Borch Jensen, M.; Chen, L.; et al. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis. Sci. Transl. Med. 2023, 15, eadd3949. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phookan, R.; Morningstar, J.E.; Loizzi, B.; Van Kampen, A.; Gensemer, C.; Dieterlen, M.-T.; Spampinato, R.; Petitjean, L.; Petitjean, M.; Petrucci, T.; et al. Collagen Fiber Maturity and Architecture in MVP-Associated Fibrosis Quantified by Digital Pathology. Cells 2025, 14, 1536. https://doi.org/10.3390/cells14191536
Phookan R, Morningstar JE, Loizzi B, Van Kampen A, Gensemer C, Dieterlen M-T, Spampinato R, Petitjean L, Petitjean M, Petrucci T, et al. Collagen Fiber Maturity and Architecture in MVP-Associated Fibrosis Quantified by Digital Pathology. Cells. 2025; 14(19):1536. https://doi.org/10.3390/cells14191536
Chicago/Turabian StylePhookan, Ranan, Jordan E. Morningstar, Brian Loizzi, Antonia Van Kampen, Cortney Gensemer, Maja-Theresa Dieterlen, Ricardo Spampinato, Louis Petitjean, Mathieu Petitjean, Taylor Petrucci, and et al. 2025. "Collagen Fiber Maturity and Architecture in MVP-Associated Fibrosis Quantified by Digital Pathology" Cells 14, no. 19: 1536. https://doi.org/10.3390/cells14191536
APA StylePhookan, R., Morningstar, J. E., Loizzi, B., Van Kampen, A., Gensemer, C., Dieterlen, M.-T., Spampinato, R., Petitjean, L., Petitjean, M., Petrucci, T., Fenner, R., Griner, J., Byerly, K., Levine, R. A., Borger, M. A., & Norris, R. A. (2025). Collagen Fiber Maturity and Architecture in MVP-Associated Fibrosis Quantified by Digital Pathology. Cells, 14(19), 1536. https://doi.org/10.3390/cells14191536





