Mistletoe Extracts Inhibit Progressive Growth of Prostate Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Mistletoe Extracts
| Iscucin® Tiliae | Iscucin® Populi | Iscucin® Salicis | Iscucin® Crataegi |
| 1:8 × 106 | 1:8 × 105 | 1:8 × 105 | 1:8 × 105 |
| 1:1.6 × 105 (E) | 1:1.6 × 105 (E) | 1:1.6 × 105 (E) | 1:1.6 × 105 (E) |
| 1:8 × 104 | 1:8 × 104 | 1:8 × 104 | 1:8 × 104 |
| 1:8 × 103 (F) | 1:8 × 103 (F) | 1:8 × 103 (F) | 1:8 × 103 (F) |
2.3. Tumor Cell Growth
2.4. Tumor Cell Proliferation
2.5. Clonogenic Growth
2.6. Cell-Cycle Analysis
2.7. Apoptosis
2.8. CD44 Expression
2.9. Integrin Surface Expression
2.10. Western Blot Analysis
2.11. Blocking Studies
2.12. Statistics
3. Results
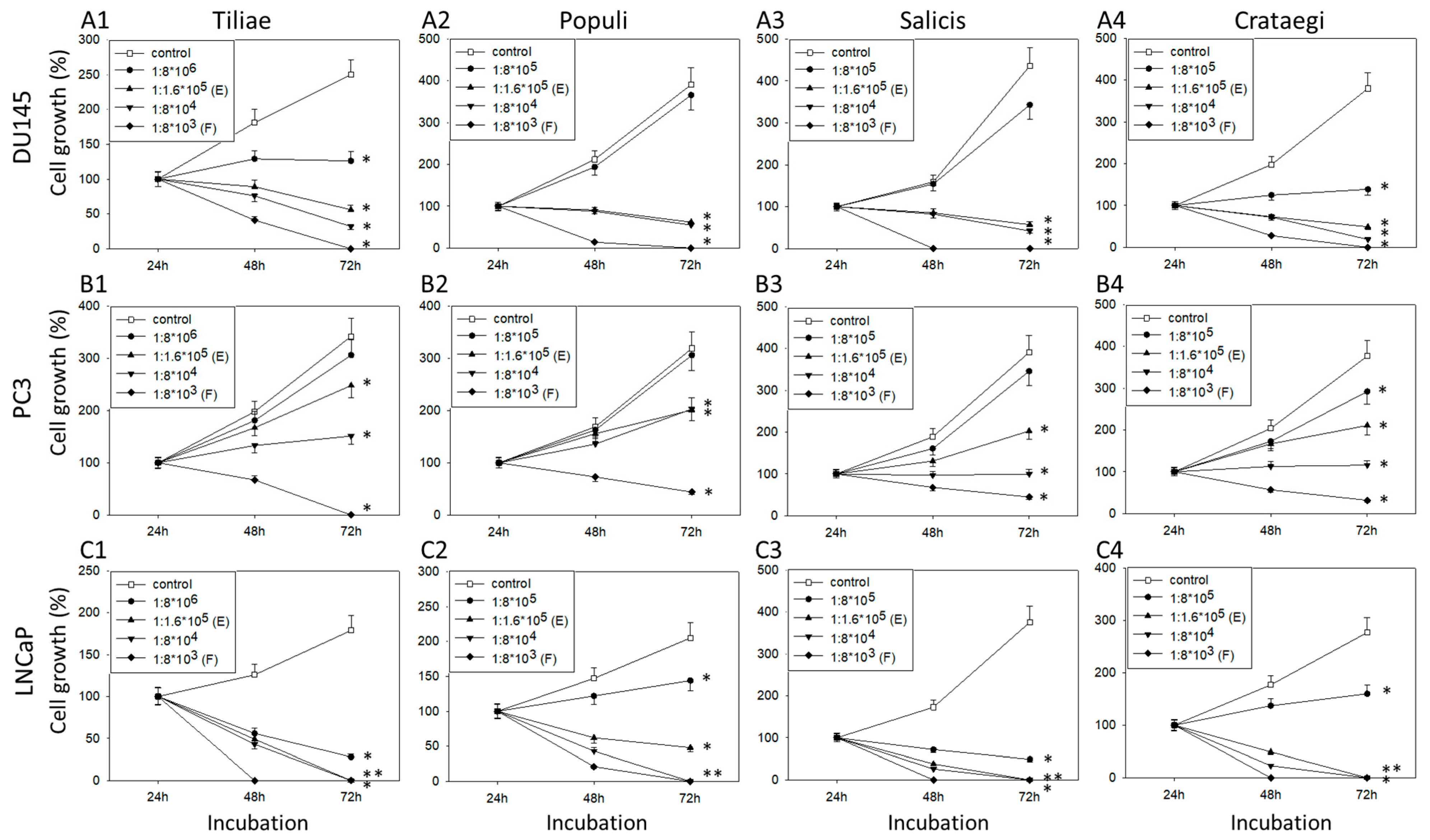
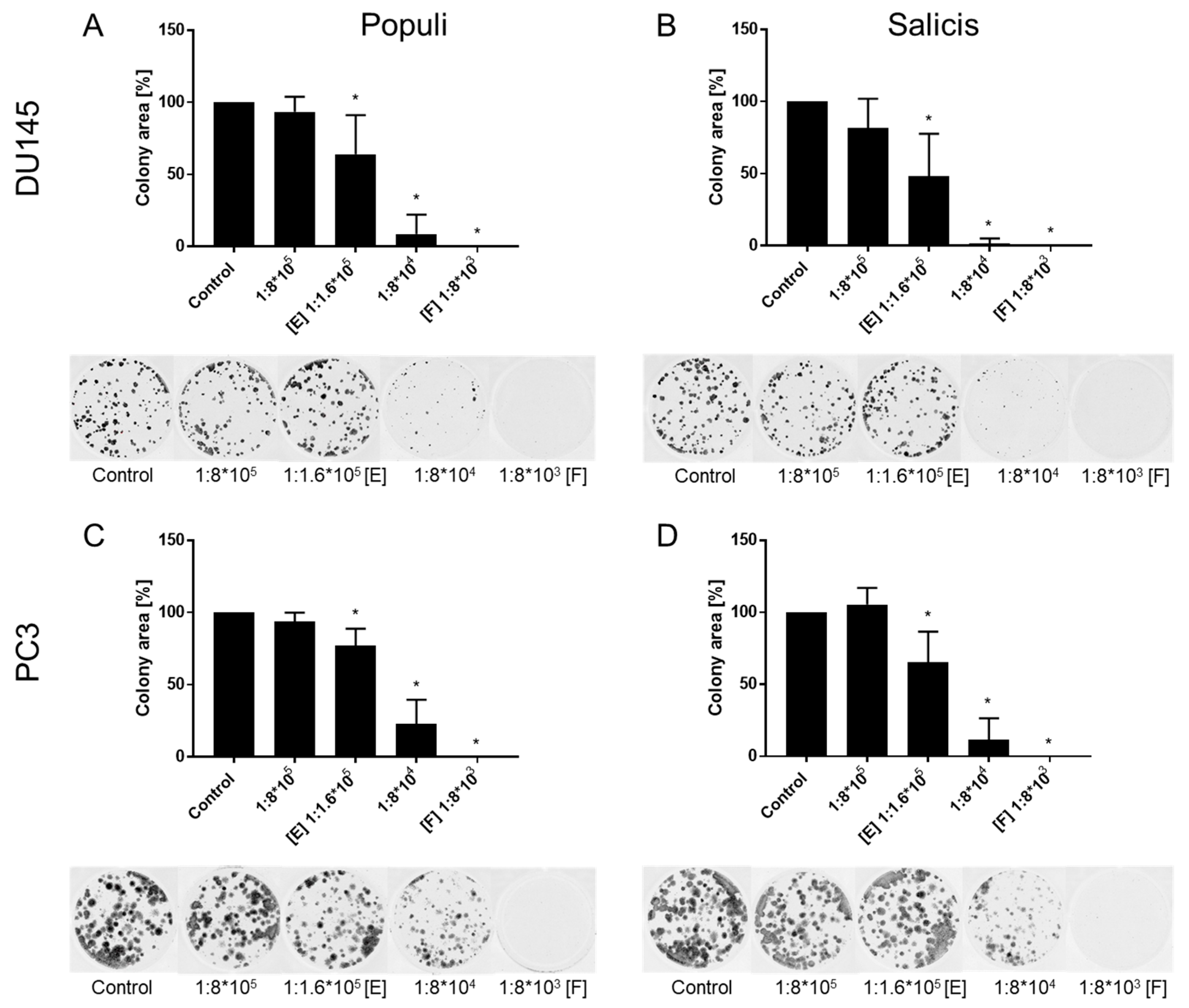
3.1. Extracts from Mistletoe Inhibit Tumor Cell Growth and Proliferation of PCa Cells
3.2. Mistletoe Extracts Inhibit the Cell Cycle of DU145 Cells
3.3. Mistletoe Extracts Induce Apoptosis in DU145 Cells
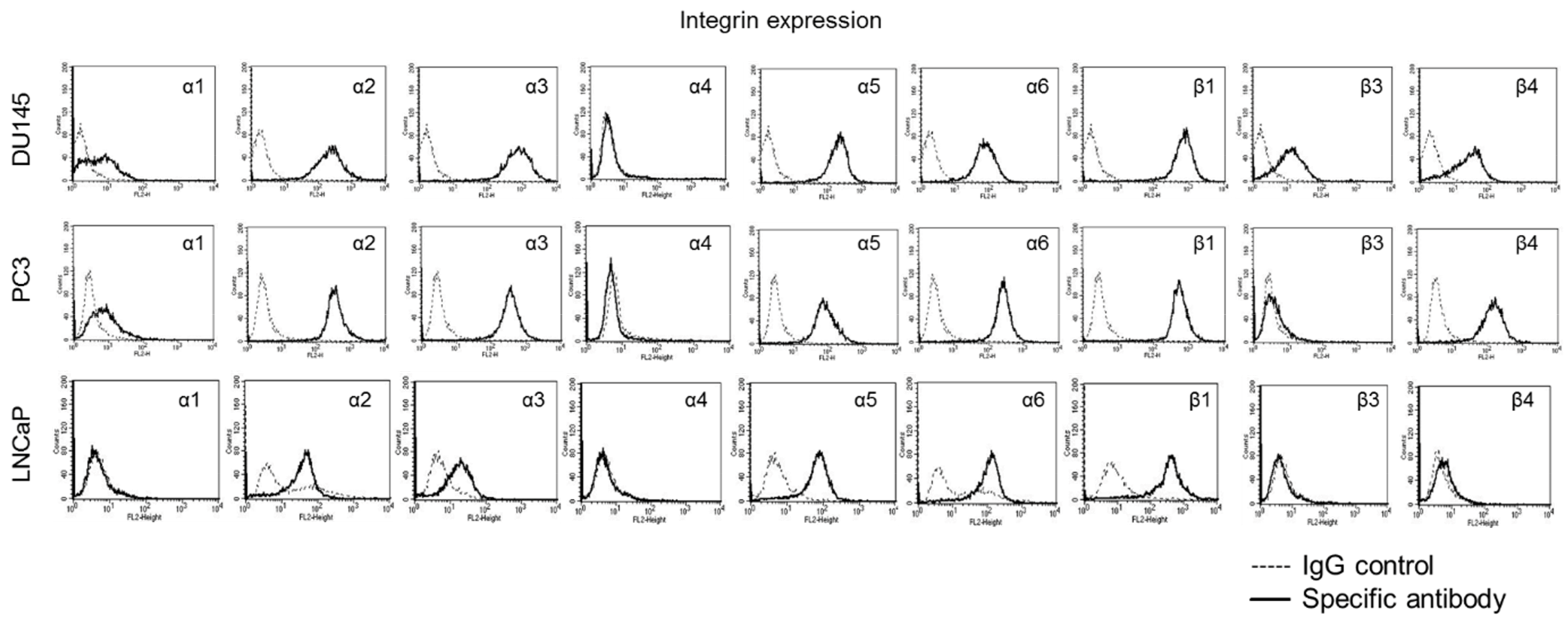
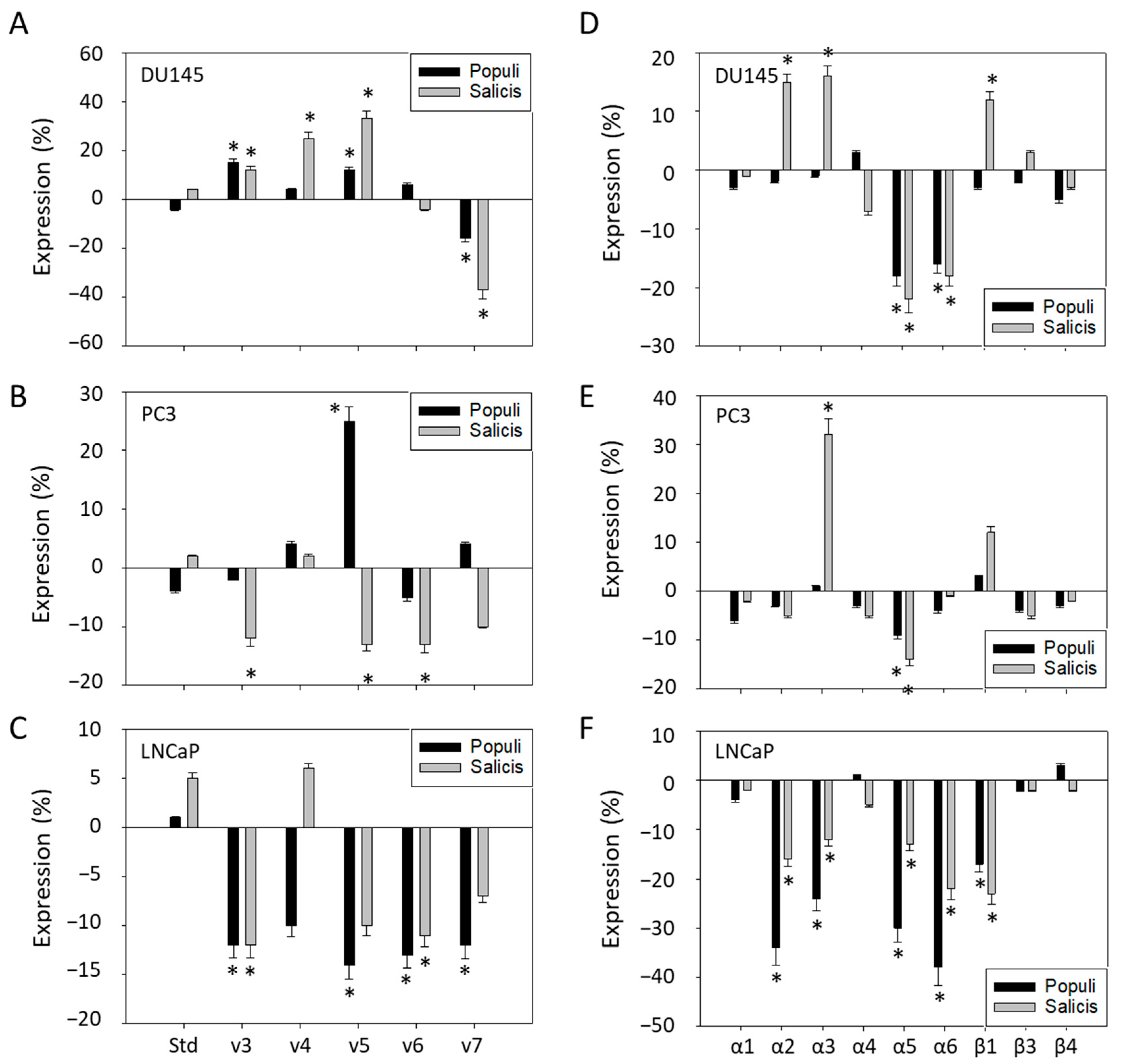
3.4. Mistletoe Extracts Modulate CD44 and Integrin Expression in PCa Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and Therapeutic Strategies for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 484–490. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Knipper, S.; Ott, S.; Schlemmer, H.P.; Grimm, M.O.; Graefen, M.; Wiegel, T. Options for Curative Treatment of Localized Prostate Cancer. Dtsch. Arztebl. Int. 2021, 118, 228–236. [Google Scholar] [CrossRef]
- de Vos, I.I.; Rosenstand, C.; Hogenhout, R.; van den Bergh, R.C.N.; Remmers, S.; Roobol, M.J. Reducing Overtreatment of Prostate Cancer Patients: Revisiting the European Association of Urology Pretreatment Risk Group Classification Using Long-term Follow-up Data from the European Randomized Study of Screening for Prostate Cancer Rotterdam. Eur. Urol. Oncol. 2024, 8, 747–754. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef]
- Kadeerhan, G.; Xue, B.; Wu, X.L.; Chen, W.N.; Wang, D.W. Incidence trends and survival of metastatic prostate cancer with bone and visceral involvement: 2010-2019 surveillance, epidemiology, and end results. Front. Oncol. 2023, 13, 1201753. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Papatsoris, A.; Sryropoulou, D.; Chakra, M.A.; Dellis, A.; Tzelves, L. A pharmacoeconomic evaluation of pharmaceutical treatment options for prostate cancer. Expert Opin. Pharmacother. 2021, 22, 1685–1728. [Google Scholar] [CrossRef] [PubMed]
- Bryce, A.H.; Agarwal, N.; Beltran, H.; Hussain, M.H.; Sartor, O.; Shore, N.; Antonarakis, E.S.; Armstrong, A.J.; Calais, J.; Carducci, M.A.; et al. Implementing evidence-based strategies for men with biochemically recurrent and advanced prostate cancer: Consensus recommendations from the US Prostate Cancer Conference 2024. Cancer 2025, 131, e35612. [Google Scholar] [CrossRef] [PubMed]
- Murillo, R.; Pinto-Martínez, N.; Serrano, N.; Uribe, C.; Navarro, E.; Duque, J.; Yepes, A.; Olaya, L.; Mariño, C.; Morales, O.L.; et al. Use of complementary and alternative medicine by cancer patients in Colombia. BMC Complement. Med. Ther. 2023, 23, 321. [Google Scholar] [CrossRef]
- Lam, C.S.; Koon, H.K.; Ma, C.T.; Au, K.Y.; Zuo, Z.; Chung, V.C.; Cheung, Y.T. Real-world data on herb-drug interactions in oncology: A scoping review of pharmacoepidemiological studies. Phytomedicine 2022, 103, 154247. [Google Scholar] [CrossRef]
- Bonucci, M.; Geraci, A.; Pero, D.; Villivà, C.; Cordella, D.; Condello, M.; Meschini, S.; Del Campo, L.; Tomassi, F.; Porcu, A.; et al. Complementary and Integrative Approaches to Cancer: A Pilot Survey of Attitudes and Habits among Cancer Patients in Italy. Evid. Based Complement. Altern. Med. 2022, 2022, 2923967. [Google Scholar] [CrossRef]
- Kanimozhi, T.; Hindu, K.; Maheshvari, Y.; Khushnidha, Y.G.; Kumaravel, M.; Srinivas, K.S.; Manickavasagam, M.; Mangathayaru, K. Herbal supplement usage among cancer patients: A questionnaire-based survey. J. Cancer Res. Ther. 2021, 17, 136–141. [Google Scholar] [CrossRef]
- Kus, F.; Can Guven, D.; Yildirim, H.C.; Akagunduz, B.; Karakaya, S.; Sutcuoglu, O.; Chalabiyev, E.; Akyildiz, A.; Koksal, B.; Sahin, Y.B.; et al. The Use of Herbal Medicine and Dietary Supplements in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Multicenter Cross-Sectional Study. Integr. Cancer Ther. 2024, 23, 15347354241280273. [Google Scholar] [CrossRef]
- Steigenberger, C.; Schnell-Inderst, P.; Flatscher-Thöni, M.; Plank, L.M.; Siebert, U. Patient’ and social aspects related to complementary mistletoe therapy in patients with breast cancer: A systematic review commissioned by the German agency for Health Technology Assessment. Eur. J. Oncol. Nurs. 2023, 65, 102338. [Google Scholar] [CrossRef]
- Wode, K.; Kienle, G.S.; Björ, O.; Fransson, P.; Sharp, L.; Elander, N.O.; Bernhardson, B.M.; Johansson, B.; Edwinsdotter Ardnor, C.; Scheibling, U.; et al. Mistletoe Extract in Patients With Advanced Pancreatic Cancer: A Double-Blind, Randomized, Placebo-Controlled Tial (MISTRAL). Dtsch. Arztebl. Int. 2024, 121, 347–354. [Google Scholar] [CrossRef]
- Schad, F.; Steinmann, D.; Oei, S.L.; Thronicke, A.; Grah, C. Evaluation of quality of life in lung cancer patients receiving radiation and Viscum album L.: A real-world data study. Radiat. Oncol. 2023, 18, 47. [Google Scholar] [CrossRef]
- Paller, C.J.; Wang, L.; Fu, W.; Kumar, R.; Durham, J.N.; Azad, N.S.; Laheru, D.A.; Browner, I.; Kachhap, S.K.; Boyapati, K.; et al. Phase I Trial of Intravenous Mistletoe Extract in Advanced Cancer. Cancer Res. Commun. 2023, 3, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M. The Anti-Inflammatory Activity of Viscum album. Plants 2023, 12, 1460. [Google Scholar] [CrossRef]
- Cogo, E.; Elsayed, M.; Bhardwaj, S.; Cooley, K.; Aycho, C.; Liang, V.; Papadogianis, P.; Psihogios, A.; Seely, D. Mistletoe Extracts during the Oncological Perioperative Period: A Systematic Review and Meta-Analysis of Human Randomized Controlled Trials. Curr. Oncol. 2023, 30, 8196–8219. [Google Scholar] [CrossRef] [PubMed]
- Pdq Integrative, A.; Complementary Therapies Editorial, B. Mistletoe Extracts (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Marvibaigi, M.; Supriyanto, E.; Amini, N.; Abdul Majid, F.A.; Jaganathan, S.K. Preclinical and clinical effects of mistletoe against breast cancer. BioMed Res. Int. 2014, 2014, 785479. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Chun, S.Y.; Kim, M.K.; Nan, H.Y.; Lee, C.; Kim, S. Mistletoe Extract Targets the STAT3-FOXM1 Pathway to Induce Apoptosis and Inhibits Metastasis in Breast Cancer Cells. Am. J. Chin. Med. 2021, 49, 487–504. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, Y.; Pan, Y.; Ding, X.; Rhea, P.; Ding, J.; Hawke, D.H.; Felsher, D.; Narla, G.; Lu, Z.; et al. Mistletoe extract Fraxini inhibits the proliferation of liver cancer by down-regulating c-Myc expression. Sci. Rep. 2019, 9, 6428. [Google Scholar] [CrossRef]
- Baek, J.H.; Jeon, Y.; Han, K.W.; Jung, D.H.; Kim, K.O. Effect of mistletoe extract on tumor response in neoadjuvant chemoradiotherapy for rectal cancer: A cohort study. World J. Surg. Oncol. 2021, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Felenda, J.E.; Turek, C.; Stintzing, F.C. Antiproliferative potential from aqueous Viscum album L. preparations and their main constituents in comparison with ricin and purothionin on human cancer cells. J. Ethnopharmacol. 2019, 236, 100–107. [Google Scholar] [CrossRef]
- Szurpnicka, A.; Kowalczuk, A.; Szterk, A. Biological activity of mistletoe: In vitro and in vivo studies and mechanisms of action. Arch. Pharmacal Res. 2020, 43, 593–629. [Google Scholar] [CrossRef]
- Juengel, E.; Rutz, J.; Meiborg, M.; Markowitsch, S.D.; Maxeiner, S.; Grein, T.; Thomas, A.; Chun, F.K.; Haferkamp, A.; Tsaur, I.; et al. Mistletoe Extracts from Different Host Trees Disparately Inhibit Bladder Cancer Cell Growth and Proliferation. Cancers 2023, 15, 4849. [Google Scholar] [CrossRef] [PubMed]
- Ponholzer, A.; Struhal, G.; Madersbacher, S. Frequent use of complementary medicine by prostate cancer patients. Eur. Urol. 2003, 43, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Büssing, A.; Stumpf, C.; Tröger, W.; Schietzel, M. Course of mitogen-stimulated T lymphocytes in cancer patients treated with Viscum album extracts. Anticancer Res. 2007, 27, 2903–2910. [Google Scholar] [PubMed]
- Weissenstein, U.; Kunz, M.; Urech, K.; Baumgartner, S. Interaction of standardized mistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement. Altern. Med. 2014, 14, 6. [Google Scholar] [CrossRef]
- Huber, R.; Schlodder, D.; Effertz, C.; Rieger, S.; Tröger, W. Safety of intravenously applied mistletoe extract—Results from a phase I dose escalation study in patients with advanced cancer. BMC Complement. Altern. Med. 2017, 17, 465. [Google Scholar] [CrossRef]
- Samaržija, I. The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery. Biomedicines 2024, 12, 79. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, Z.; Lan, T.; Fu, C.; Cheng, P. CD44 and its implication in neoplastic diseases. MedComm 2024, 5, e554. [Google Scholar] [CrossRef] [PubMed]
- Zuzak, T.; Rist, L.; Viviani, A.; Pally, J.; Mol, C.; Riegert, U.; Meyer, U. Das Mistelpräparat Iscucin-Herstellung, Analytik, Wirkung in vitro. Merkurstab-Z. Anthropos. Med. 2005, 57, 467–473. [Google Scholar]
- Felenda, J.E.; Gruber, K.; Pacifico, S.; Turek, C.; Beckmann, C.; Stintzing, F.C. In-vitro-Untersuchungen zur Weidenmistel an verschiedenen humanen Tumorzelllinien. Z. Phytother. 2019, 40, 112–119. [Google Scholar] [CrossRef]
- Markowitsch, S.D.; Schupp, P.; Lauckner, J.; Vakhrusheva, O.; Slade, K.S.; Mager, R.; Efferth, T.; Haferkamp, A.; Juengel, E. Artesunate Inhibits Growth of Sunitinib-Resistant Renal Cell Carcinoma Cells through Cell Cycle Arrest and Induction of Ferroptosis. Cancers 2020, 12, 3150. [Google Scholar] [CrossRef]
- Siech, C.; Rutz, J.; Maxeiner, S.; Grein, T.; Sonnenburg, M.; Tsaur, I.; Chun, F.K.-H.; Blaheta, R.A. Insulin-like Growth Factor-1 Influences Prostate Cancer Cell Growth and Invasion through an Integrin α3, α5, αV, and β1 Dependent Mechanism. Cancers 2022, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Urech, K.; Buessing, A.; Thalmann, G.; Schaefermeyer, H.; Heusser, P. Antiproliferative Effects of Mistletoe (Viscum album L.) Extract in Urinary Bladder Carcinoma Cell Lines. Anticancer Res. 2006, 26, 3049–3055. [Google Scholar] [PubMed]
- Park, Y.R.; Jee, W.; Park, S.M.; Kim, S.W.; Bae, H.; Jung, J.H.; Kim, H.; Kim, S.; Chung, J.S.; Jang, H.J. Viscum album Induces Apoptosis by Regulating STAT3 Signaling Pathway in Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 11988. [Google Scholar] [CrossRef]
- Beuth, J.; Ko, H.L.; Schneider, H.; Tawadros, S.; Kasper, H.U.; Zimst, H.; Schierholz, J.M. Intratumoral application of standardized mistletoe extracts down regulates tumor weight via decreased cell proliferation, increased apoptosis and necrosis in a murine model. Anticancer Res. 2006, 26, 4451–4456. [Google Scholar]
- Wu, X.; Gong, S.; Roy-Burman, P.; Lee, P.; Culig, Z. Current mouse and cell models in prostate cancer research. Endocr. Relat. Cancer 2013, 20, R155–R170. [Google Scholar] [CrossRef]
- Hunziker-Basler, N.; Zuzak, T.J.; Eggenschwiler, J.; Rist, L.; Simões-Wüst, A.P.; Viviani, A. Prolonged cytotoxic effect of aqueous extracts from dried viscum album on bladder cancer cells. Pharmazie 2007, 62, 237–238. [Google Scholar]
- Iliev, I.; Tsoneva, I.; Nesheva, A.; Staneva, G.; Robev, B.; Momchilova, A.; Nikolova, B. Complementary Treatment of Breast Cancer Cells with Different Metastatic Potential with Iscador Qu in the Presence of Clinically Approved Anticancer Drugs. Curr. Issues Mol. Biol. 2024, 46, 12457–12480. [Google Scholar] [CrossRef]
- Ostermann, T.; Raak, C.; Büssing, A. Survival of cancer patients treated with mistletoe extract (Iscador): A systematic literature review. BMC Cancer 2009, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, T.; Appelbaum, S.; Poier, D.; Boehm, K.; Raak, C.; Büssing, A. A Systematic Review and Meta-Analysis on the Survival of Cancer Patients Treated with a Fermented Viscum album L. Extract (Iscador): An Update of Findings. Complement. Med. Res. 2020, 27, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.N.O.; Ochioni, A.C.; Zancan, P.; Oliveira, A.P.; Grazi, M.; Garrett, R.; Holandino, C.; Baumgartner, S. Viscum album mother tinctures: Harvest conditions and host trees influence the plant metabolome and the glycolytic pathway of breast cancer cells. Front. Pharmacol. 2022, 13, 1027931. [Google Scholar] [CrossRef] [PubMed]
- Holandino, C.; Melo, M.N.d.O.; Oliveira, A.P.; Batista, J.V.d.C.; Capella, M.A.M.; Garrett, R.; Grazi, M.; Ramm, H.; Torre, C.D.; Schaller, G.; et al. Phytochemical analysis and in vitro anti-proliferative activity of Viscum album ethanolic extracts. BMC Complement. Med. Ther. 2020, 20, 215. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lou, W.; Nadiminty, N.; Chen, X.; Zhou, Q.; Shi, X.B.; deVere White, R.W.; Gao, A.C. Functional p53 determines docetaxel sensitivity in prostate cancer cells. Prostate 2013, 73, 418–427. [Google Scholar] [CrossRef]
- Hoang, T.; Sutera, P.; Nguyen, T.; Chang, J.; Jagtap, S.; Song, Y.; Shetty, A.C.; Chowdhury, D.D.; Chan, A.; Carrieri, F.A.; et al. TP53 structure-function relationships in metastatic castrate-sensitive prostate cancer and the impact of APR-246 treatment. Prostate 2024, 84, 87–99. [Google Scholar] [CrossRef]
- Nogueira, V.; Patra, K.C.; Hay, N. Selective eradication of cancer displaying hyperactive Akt by exploiting the metabolic consequences of Akt activation. Elife 2018, 7, e32213. [Google Scholar] [CrossRef]
- Klingbeil, M.F.; Xavier, F.C.; Sardinha, L.R.; Severino, P.; Mathor, M.B.; Rodrigues, R.V.; Pinto, D.S., Jr. Cytotoxic effects of mistletoe (Viscum album L.) in head and neck squamous cell carcinoma cell lines. Oncol. Rep. 2013, 30, 2316–2322. [Google Scholar] [CrossRef]
- Schötterl, S.; Miemietz, J.T.; Ilina, E.I.; Wirsik, N.M.; Ehrlich, I.; Gall, A.; Huber, S.M.; Lentzen, H.; Mittelbronn, M.; Naumann, U. Mistletoe-Based Drugs Work in Synergy with Radio-Chemotherapy in the Treatment of Glioma In Vitro and In Vivo in Glioblastoma Bearing Mice. Evid. Based Complement. Altern. Med. 2019, 2019, 1376140. [Google Scholar] [CrossRef]
- Weissenstein, U.; Kunz, M.; Urech, K.; Regueiro, U.; Baumgartner, S. Interaction of a standardized mistletoe (Viscum album) preparation with antitumor effects of Trastuzumab in vitro. BMC Complement. Altern. Med. 2016, 16, 271. [Google Scholar] [CrossRef]
- Lyu, S.-Y.; Meshesha, S.M.; Hong, C.-E. Synergistic Effects of Mistletoe Lectin and Cisplatin on Triple-Negative Breast Cancer Cells: Insights from 2D and 3D In Vitro Models. Int. J. Mol. Sci. 2025, 26, 366. [Google Scholar] [CrossRef]
- dela Cruz, J.F.; Kim, Y.S.; Lumbera, W.M.; Hwang, S.G. Viscum Album Var Hot Water Extract Mediates Anti-cancer Effects through G1 Phase Cell Cycle Arrest in SK-Hep1 Human Hepatocarcinoma cells. Asian Pac. J. Cancer Prev. 2015, 16, 6417–6421. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Goda, M.S.; Nafie, M.S.; Abu-Elsaoud, A.M.; Hareeri, R.H.; Aldurdunji, M.M.; Elhady, S.S.; Badr, J.M.; Eltahawy, N.A. Comparative Assessment of the Antioxidant and Anticancer Activities of Plicosepalus acacia and Plicosepalus curviflorus: Metabolomic Profiling and In Silico Studies. Antioxidants 2022, 11, 1249. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef]
- Zeng, T.; Zhu, L.; Liao, M.; Zhuo, W.; Yang, S.; Wu, W.; Wang, D. Knockdown of PYCR1 inhibits cell proliferation and colony formation via cell cycle arrest and apoptosis in prostate cancer. Med. Oncol. 2017, 34, 27. [Google Scholar] [CrossRef] [PubMed]
- Zaiden, M.; Feinshtein, V.; David, A. Inhibition of CD44v3 and CD44v6 function blocks tumor invasion and metastatic colonization. J. Control. Release 2017, 257, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Aaltomaa, S.; Lipponen, P.; Ala-Opas, M.; Kosma, V.M. Expression and prognostic value of CD44 standard and variant v3 and v6 isoforms in prostate cancer. Eur. Urol. 2001, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.M.; Davies, G.; Martin, T.A.; Mason, M.D.; Jiang, W.G. The influence of CD44v3-v10 on adhesion, invasion and MMP-14 expression in prostate cancer cells. Oncol. Rep. 2006, 15, 199–206. [Google Scholar] [CrossRef][Green Version]
- Moura, C.M.; Pontes, J., Jr.; Reis, S.T.; Viana, N.I.; Morais, D.R.; Dip, N.; Katz, B.; Srougi, M.; Leite, K.R. Expression profile of standard and variants forms of CD44 related to prostate cancer behavior. Int. J. Biol. Markers 2015, 30, e49–e55. [Google Scholar] [CrossRef]
- Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.K.; Blaheta, R.A. Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, I.; Thomas, A.; Juengel, E.; Maxeiner, S.; Grein, T.; Le, Q.C.; Muschta, V.; Rutz, J.; Chun, F.K.H.; Blaheta, R.A. Deciphering the Molecular Machinery-Influence of sE-Cadherin on Tumorigenic Traits of Prostate Cancer Cells. Biology 2021, 10, 1007. [Google Scholar] [CrossRef]
- Muys, B.R.; Anastasakis, D.G.; Claypool, D.; Pongor, L.; Li, X.L.; Grammatikakis, I.; Liu, M.; Wang, X.; Prasanth, K.V.; Aladjem, M.I.; et al. The p53-induced RNA-binding protein ZMAT3 is a splicing regulator that inhibits the splicing of oncogenic CD44 variants in colorectal carcinoma. Genes Dev. 2021, 35, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Song, A.; Gui, P.; Wang, J.; Pan, Y.; Li, C.; Li, S.; Zhang, Y.; Jiang, T.; Xu, Y.; et al. Long noncoding RNA LINC01594 inhibits the CELF6-mediated splicing of oncogenic CD44 variants to promote colorectal cancer metastasis. Cell Death Dis. 2023, 14, 427. [Google Scholar] [CrossRef]
- Zhang, J.; Gan, W.; Peng, R.; Lu, L.; Lu, W.; Liu, J. Activation of the mTOR pathway promotes neurite growth through upregulation of CD44 expression. J. Int. Med. Res. 2023, 51, 3000605231178510. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Mao, S.; Mo, Z.; Cao, Z.; Luo, J.; Zhou, M.; Liu, X.; Zhang, S.; Yu, L. Exosome CTLA-4 Regulates PTEN/CD44 Signal Pathway in Spleen Deficiency Internal Environment to Promote Invasion and Metastasis of Hepatocellular Carcinoma. Front. Pharmacol. 2021, 12, 757194. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z. p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb. Perspect. Biol. 2010, 2, a001057. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H.; Luo, C.; Xiao, H.; Zou, X.; Zou, J.; Zhang, G. Targeting integrin α5β1 in urological tumors: Opportunities and challenges. Front. Oncol. 2023, 13, 1165073. [Google Scholar] [CrossRef]
- Connell, B.; Kopach, P.; Ren, W.; Joshi, R.; Naber, S.; Zhou, M.; Mathew, P. Aberrant integrin αv and α5 expression in prostate adenocarcinomas and bone-metastases is consistent with a bone-colonizing phenotype. Transl. Androl. Urol. 2020, 9, 1630–1638. [Google Scholar] [CrossRef]
- Monteiro, A.V.O.; dos Santos, N.N.d.C.; da Silva, J.P.R.; Brasileiro, S.A.; Botelho, J.C.; Sobreira, L.E.R.; Leal, A.L.A.B.; Pereira, A.L.; de Oliveira, A.C.A.; Monteiro, J.R.S.; et al. Genetic variations related to the prostate cancer risk: A field synopsis and revaluation by Bayesian approaches of genome-wide association studies. Urol. Oncol. Semin. Orig. Investig. 2025, 43, e219–e270. [Google Scholar] [CrossRef]
- Rubenstein, C.S.; Gard, J.M.C.; Wang, M.; McGrath, J.E.; Ingabire, N.; Hinton, J.P.; Marr, K.D.; Simpson, S.J.; Nagle, R.B.; Miranti, C.K.; et al. Gene Editing of α6 Integrin Inhibits Muscle Invasive Networks and Increases Cell–Cell Biophysical Properties in Prostate Cancer. Cancer Res. 2019, 79, 4703–4714. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Kaakinen, M.; Wenta, T.; Manninen, A. Loss of α6β4 Integrin-Mediated Hemidesmosomes Promotes Prostate Epithelial Cell Migration by Stimulating Focal Adhesion Dynamics. Front. Cell Dev. Biol. 2022, 10, 886569. [Google Scholar] [CrossRef]
- Wenta, T.; Schmidt, A.; Zhang, Q.; Devarajan, R.; Singh, P.; Yang, X.; Ahtikoski, A.; Vaarala, M.; Wei, G.-H.; Manninen, A. Disassembly of α6β4-mediated hemidesmosomal adhesions promotes tumorigenesis in PTEN-negative prostate cancer by targeting plectin to focal adhesions. Oncogene 2022, 41, 3804–3820. [Google Scholar] [CrossRef]
- Koivusalo, S.; Schmidt, A.; Manninen, A.; Wenta, T. Regulation of Kinase Signaling Pathways by α6β4-Integrins and Plectin in Prostate Cancer. Cancers 2022, 15, 149. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowitsch, S.D.; Albrecht, L.; Meiborg, M.; Rutz, J.; Thomas, A.; Chun, F.K.-H.; Haferkamp, A.; Juengel, E.; Blaheta, R.A. Mistletoe Extracts Inhibit Progressive Growth of Prostate Cancer Cells. Cells 2025, 14, 1535. https://doi.org/10.3390/cells14191535
Markowitsch SD, Albrecht L, Meiborg M, Rutz J, Thomas A, Chun FK-H, Haferkamp A, Juengel E, Blaheta RA. Mistletoe Extracts Inhibit Progressive Growth of Prostate Cancer Cells. Cells. 2025; 14(19):1535. https://doi.org/10.3390/cells14191535
Chicago/Turabian StyleMarkowitsch, Sascha D., Larissa Albrecht, Moritz Meiborg, Jochen Rutz, Anita Thomas, Felix K.-H. Chun, Axel Haferkamp, Eva Juengel, and Roman A. Blaheta. 2025. "Mistletoe Extracts Inhibit Progressive Growth of Prostate Cancer Cells" Cells 14, no. 19: 1535. https://doi.org/10.3390/cells14191535
APA StyleMarkowitsch, S. D., Albrecht, L., Meiborg, M., Rutz, J., Thomas, A., Chun, F. K.-H., Haferkamp, A., Juengel, E., & Blaheta, R. A. (2025). Mistletoe Extracts Inhibit Progressive Growth of Prostate Cancer Cells. Cells, 14(19), 1535. https://doi.org/10.3390/cells14191535




