Macrophages in Glioblastoma and How Non-Coding RNAs Impact Their Differentiation
Abstract
1. Introduction
2. Analysis of Current Research
2.1. Macrophages in the Progression of GBM
2.2. ncRNAs in the Progression of GBM
2.3. ncRNAs and Their Roles in Macrophage Polarization
2.4. Macrophage-Modulating ncRNAs Identified Within GBM
2.5. Connections Between ncRNAs in Macrophages and How They Impacts GBM Progression
2.6. Clinical Relevance
3. Discussion and Future Avenues of Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma multiforme |
| TME | Tumor microenvironment |
| RNA | Ribonucleic acid |
| ncRNA | Non-coding RNA |
| miRNA | MicroRNA |
| miR | MicroRNA |
| piwiRNA | Piwi-interacting RNA |
| snRNA | Small nuclear RNA |
| snoRNA | Small nucleolar RNA |
| lncRNA | Long non-coding RNA |
| lincRNA | Long intergenic non-coding RNA |
| circRNAs | Circular RNA |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| iNOS | Inducible nitric oxide synthase |
| IFNγ | Interferon—gamma |
| TAMs | Tumor-associated macrophages |
| Teff | Effector T cells |
| TGF-β | Transforming growth factor beta |
| Treg | T regulatory cells |
| MDSCs | Myeloid-derived suppressor cells |
| CAR-T | Chimeric antigen receptor T cell therapy |
| nt | Nucleotides |
| Let7a | Lethal-7 family microRNA A |
| BBB | Blood–brain barrier |
| HOTAIR | HOX transcript antisense RNA |
| PD-L1 | Programed cell death ligand 1 |
| IL-6 | Interleukin 6 |
| Let7b | Lethal-7 family microRNA B |
| lncRNA ANCR | Antisense non-coding RNA in the INK4 locus |
| CCAT1 | Colon cancer-associated transcript 1 |
| Cox2 | Cyclooxygenase 2 |
| GAS5 | Growth arrest-specific transcript 5 |
| GNAS-AS1 | GNAS-antisense transcript 1 |
| Linc00662 | Long intergenic non-coding RNA 00662 |
| lincRNA p21 | Long intergenic non-coding RNA p21 |
| lncRNA RP11-361F15.2 | Long non-coding RNA RP11-361F15.2 |
| MEG3 | Maternally expressed gene 3 |
| lncRNA-MM2P | Long non-coding RNA macrophage m2 polarization |
| NIFK-AS1 | NIFK-antisense 1 |
| PACERR | P50-associated cyclooxygenase-2 extragenic RNA |
| PVT1 | Plasmacytoma variant translocation 1 |
| RPPH1 | RNA component of ribonuclease P |
| TUC339 | Transcribed non-coding RNA from ultra conserved element 339 |
| XIST | X-inactive-specific transcript |
| DHX9 | Dexh-box helicase 9 |
| TERF2IP | Telomeric repeat binding factor 2 interacting protein |
| LTBP1 | Latent transforming growth factor beta binding protein 1 |
| siRNA | Small interfering RNA |
References
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro Oncol. 2024, 26, vi1–vi85. [Google Scholar] [CrossRef]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and Other Central Nervous System Tumor Statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; ISBN 978-0-9944381-2-6. [Google Scholar]
- Liu, Y.; Zhou, F.; Ali, H.; Lathia, J.D.; Chen, P. Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives. Cell. Mol. Immunol. 2024, 21, 1354–1375. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.L.; Hughes, J.T.; Esiri, M.M.; Coakham, H.B.; Brownell, D.B. Immunohistological Study of Mononuclear Cell Infiltrate in Malignant Gliomas. Acta Neuropathol. 1987, 74, 269–277. [Google Scholar] [CrossRef]
- Wood, G.W.; Morantz, R.A. Immunohistologic Evaluation of the Lymphoreticular Infiltrate of Human Central Nervous System Tumors2. J. Natl. Cancer Inst. 1979, 62, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Badie, B.; Schartner, J.M. Flow Cytometric Characterization of Tumor-Associated Macrophages in Experimental Gliomas. Neurosurgery 2000, 46, 957–962. [Google Scholar] [CrossRef]
- Chen, H.-R.; Sun, Y.-Y.; Chen, C.-W.; Kuo, Y.-M.; Kuan, I.S.; Tiger Li, Z.-R.; Short-Miller, J.C.; Smucker, M.R.; Kuan, C.-Y. Fate Mapping via CCR2-CreER Mice Reveals Monocyte-to-Microglia Transition in Development and Neonatal Stroke. Sci. Adv. 2020, 6, eabb2119. [Google Scholar] [CrossRef]
- De, S.; Van Deren, D.; Peden, E.; Hockin, M.; Boulet, A.; Titen, S.; Capecchi, M.R. Two Distinct Ontogenies Confer Heterogeneity to Mouse Brain Microglia. Development 2018, 145, dev152306. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, X.; Herting, C.J.; Garcia, V.A.; Nie, K.; Pong, W.W.; Rasmussen, R.; Dwivedi, B.; Seby, S.; Wolf, S.A.; et al. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res. 2017, 77, 2266–2278. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thomas, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-Infiltrated Innate Immune Cells Resemble M0 Macrophage Phenotype. JCI Insight 2016, 1, e85841. [Google Scholar] [CrossRef]
- Klemm, F.; Maas, R.R.; Bowman, R.L.; Kornete, M.; Soukup, K.; Nassiri, S.; Brouland, J.-P.; Iacobuzio-Donahue, C.A.; Brennan, C.; Tabar, V.; et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell 2020, 181, 1643–1660.e17. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-Inflammatory Properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.R. The Dark Side of the Human Genome. Nature 2016, 538, 275–277. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The Complete Sequence of a Human Genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations—Nature Reviews Molecular Cell Biology. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet. Pathol. 2014, 51, 759–774. [Google Scholar] [CrossRef]
- Wei, J.; Nduom, E.K.; Kong, L.-Y.; Hashimoto, Y.; Xu, S.; Gabrusiewicz, K.; Ling, X.; Huang, N.; Qiao, W.; Zhou, S.; et al. MiR-138 Exerts Anti-Glioma Efficacy by Targeting Immune Checkpoints. Neuro Oncol. 2016, 18, 639–648. [Google Scholar] [CrossRef]
- Martinez-Terroba, E.; Plasek-Hegde, L.M.; Chiotakakos, I.; Li, V.; de Miguel, F.J.; Robles-Oteiza, C.; Tyagi, A.; Politi, K.; Zamudio, J.R.; Dimitrova, N. Overexpression of Malat1 Drives Metastasis through Inflammatory Reprogramming of the Tumor Microenvironment. Sci. Immunol. 2024, 9, eadh5462. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e5. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, Z.; Kang, Z.; Li, M.; Lv, Y.; Li, S.; Tong, L.; Feng, F.; Li, Y.; Zhang, M.; et al. Preclinical and Early Clinical Studies of a Novel Compound SYHA1813 That Efficiently Crosses the Blood–Brain Barrier and Exhibits Potent Activity against Glioblastoma. Acta Pharm. Sin. B 2023, 13, 4748–4764. [Google Scholar] [CrossRef] [PubMed]
- Kratochvill, F.; Neale, G.; Haverkamp, J.M.; van de Velde, L.-A.; Smith, A.M.; Kawauchi, D.; McEvoy, J.; Roussel, M.F.; Dyer, M.A.; Qualls, J.E.; et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015, 12, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Awad, F.; Assrawi, E.; Jumeau, C.; Georgin-Lavialle, S.; Cobret, L.; Duquesnoy, P.; Piterboth, W.; Thomas, L.; Stankovic-Stojanovic, K.; Louvrier, C.; et al. Impact of Human Monocyte and Macrophage Polarization on NLR Expression and NLRP3 Inflammasome Activation. PLoS ONE 2017, 12, e0175336. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 Potently Enhances Murine Macrophage Mannose Receptor Activity: A Marker of Alternative Immunologic Macrophage Activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Komohara, Y.; Ohnishi, K.; Kuratsu, J.; Takeya, M. Possible Involvement of the M2 Anti-Inflammatory Macrophage Phenotype in Growth of Human Gliomas. J. Pathol. 2008, 216, 15–24. [Google Scholar] [CrossRef]
- Nduom, E.K.; Weller, M.; Heimberger, A.B. Immunosuppressive Mechanisms in Glioblastoma. Neuro Oncol. 2015, 17, vii9–vii14. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Pan, Y.; Gutmann, D.H. Genetic and Genomic Alterations Differentially Dictate Low-Grade Glioma Growth through Cancer Stem Cell–Specific Chemokine Recruitment of T Cells and Microglia. Neuro Oncol. 2019, 21, 1250–1262. [Google Scholar] [CrossRef]
- Zhang, G.; Tao, X.; Ji, B.; Gong, J. Hypoxia-Driven M2-Polarized Macrophages Facilitate Cancer Aggressiveness and Temozolomide Resistance in Glioblastoma. Oxid. Med. Cell. Longev. 2022, 2022, 1614336. [Google Scholar] [CrossRef]
- Beury, D.W.; Parker, K.H.; Nyandjo, M.; Sinha, P.; Carter, K.A.; Ostrand-Rosenberg, S. Cross-Talk among Myeloid-Derived Suppressor Cells, Macrophages, and Tumor Cells Impacts the Inflammatory Milieu of Solid Tumors. J. Leukoc. Biol. 2014, 96, 1109–1118. [Google Scholar] [CrossRef]
- Kwak, T.; Wang, F.; Deng, H.; Condamine, T.; Kumar, V.; Perego, M.; Kossenkov, A.; Montaner, L.J.; Xu, X.; Xu, W.; et al. Distinct Populations of Immune-Suppressive Macrophages Differentiate from Monocytic Myeloid-Derived Suppressor Cells in Cancer. Cell Rep. 2020, 33, 108571. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current State of Immunotherapy for Glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Brandes, A.A.; Carpentier, A.F.; Idbaih, A.; Reardon, D.A.; Cloughesy, T.; Sumrall, A.; Baehring, J.; van den Bent, M.; Bähr, O.; et al. Radiotherapy Combined with Nivolumab or Temozolomide for Newly Diagnosed Glioblastoma with Unmethylated MGMT Promoter: An International Randomized Phase III Trial. Neuro Oncol. 2023, 25, 123–134. [Google Scholar] [CrossRef]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III Trial of Chemoradiotherapy with Temozolomide plus Nivolumab or Placebo for Newly Diagnosed Glioblastoma with Methylated MGMT Promoter. Neuro Oncol. 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Reardon, D.A.; Kim, T.M.; Frenel, J.-S.; Simonelli, M.; Lopez, J.; Subramaniam, D.S.; Siu, L.L.; Wang, H.; Krishnan, S.; Stein, K.; et al. Treatment with Pembrolizumab in Programmed Death Ligand 1–Positive Recurrent Glioblastoma: Results from the Multicohort Phase 1 KEYNOTE-028 Trial. Cancer 2021, 127, 1620–1629. [Google Scholar] [CrossRef]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab Plus Bevacizumab Versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.F.; Ng, S.M.; Brooks, C.; Coutts, T.; Holmes, J.; Roberts, C.; Elhussein, L.; Hoskin, P.; Maughan, T.; Blagden, S.; et al. A Phase II Open Label, Randomised Study of Ipilimumab with Temozolomide Versus Temozolomide Alone after Surgery and Chemoradiotherapy in Patients with Recently Diagnosed Glioblastoma: The Ipi-Glio Trial Protocol. BMC Cancer 2020, 20, 198. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or Without Ipilimumab in Patients with Recurrent Glioblastoma: Results from Exploratory Phase I Cohorts of CheckMate 143. Neuro Oncol. 2018, 20, 674–686. [Google Scholar] [CrossRef]
- de Groot, J.; Penas-Prado, M.; Alfaro-Munoz, K.; Hunter, K.; Pei, B.L.; O’Brien, B.; Weathers, S.-P.; Loghin, M.; Kamiya Matsouka, C.; Yung, W.K.A.; et al. Window-of-Opportunity Clinical Trial of Pembrolizumab in Patients with Recurrent Glioblastoma Reveals Predominance of Immune-Suppressive Macrophages. Neuro Oncol. 2020, 22, 539–549. [Google Scholar] [CrossRef]
- Hunter, T.L.; Bao, Y.; Zhang, Y.; Matsuda, D.; Riener, R.; Wang, A.; Li, J.J.; Soldevila, F.; Chu, D.S.H.; Nguyen, D.P.; et al. In Vivo CAR T Cell Generation to Treat Cancer and Autoimmune Disease. Science 2025, 388, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, E.; et al. Intrathecal Bivalent CAR T Cells Targeting EGFR and IL13Rα2 in Recurrent Glioblastoma: Phase 1 Trial Interim Results. Nat. Med. 2024, 30, 1320–1329. [Google Scholar] [CrossRef]
- Waibl Polania, J.; Hoyt-Miggelbrink, A.; Tomaszewski, W.H.; Wachsmuth, L.P.; Lorrey, S.J.; Wilkinson, D.S.; Lerner, E.; Woroniecka, K.; Finlay, J.B.; Ayasoufi, K.; et al. Antigen Presentation by Tumor-Associated Macrophages Drives T Cells from a Progenitor Exhaustion State to Terminal Exhaustion. Immunity 2025, 58, 232–246.e6. [Google Scholar] [CrossRef]
- Saadatpour, L.; Fadaee, E.; Fadaei, S.; Nassiri Mansour, R.; Mohammadi, M.; Mousavi, S.M.; Goodarzi, M.; Verdi, J.; Mirzaei, H. Glioblastoma: Exosome and microRNA as Novel Diagnosis Biomarkers. Cancer Gene Ther. 2016, 23, 415–418. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Meneely, P.M.; Herman, R.K. Lethals, Steriles and Deficiencies in a Region of the X Chromosome of Caenorhabditis elegans. Genetics 1979, 92, 99–115. [Google Scholar] [CrossRef]
- Song, J.; Oh, Y.; Lee, J.E. miR-Let7A Modulates Autophagy Induction in LPS-Activated Microglia. Exp. Neurobiol. 2015, 24, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yoon, S.R.; Kim, O.Y. miR-Let7A Controls the Cell Death and Tight Junction Density of Brain Endothelial Cells Under High Glucose Condition. Oxid. Med. Cell. Longev. 2017, 2017, 6051874. [Google Scholar] [CrossRef]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long Non-Coding RNA HOTAIR Reprograms Chromatin State to Promote Cancer Metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, C.; Zhang, Y.; Weng, M.; Zhang, M.; Qin, Y.; Gong, W.; Quan, Z. Long Non-Coding RNA HOTAIR, a c-Myc Activated Driver of Malignancy, Negatively Regulates miRNA-130a in Gallbladder Cancer. Mol. Cancer 2014, 13, 156. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhang, B.; Zhang, T.; Zhang, Y.; Wang, F. LncRNA GATA6-AS Promotes Cancer Cell Proliferation and Inhibits Apoptosis in Glioma by Downregulating lncRNA TUG1. Cancer Biother. Radiopharm. 2019, 34, 660–665. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Tian, Y.F.; Wu, H.; Ouyang, S.Y.; Kuang, W.L. LncRNA H19 Promotes Glioma Angiogenesis through miR-138/HIF-1α/VEGF Axis. Neoplasma 2020, 67, 111–118. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Zheng, J.; Song, J.; Liu, Y.; Ruan, X.; Shen, S.; Shao, L.; Yang, C.; Wang, D.; et al. Lin28A Promotes IRF6-Regulated Aerobic Glycolysis in Glioma Cells by Stabilizing SNHG14. Cell Death Dis. 2020, 11, 447. [Google Scholar] [CrossRef]
- Du, P.; Liao, Y.; Zhao, H.; Zhang, J.; Muyiti; Keremu; Mu, K. ANXA2P2/miR-9/LDHA Axis Regulates Warburg Effect and Affects Glioblastoma Proliferation and Apoptosis. Cell. Signal. 2020, 74, 109718. [Google Scholar] [CrossRef]
- Gao, X.F.; He, H.Q.; Zhu, X.B.; Xie, S.L.; Cao, Y. LncRNA SNHG20 Promotes Tumorigenesis and Cancer Stemness in Glioblastoma via Activating PI3K/Akt/mTOR Signaling Pathway. Neoplasma 2019, 66, 532–542. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.; Xiao, J. Long Noncoding RNA MALAT1 Knockdown Reverses Chemoresistance to Temozolomide via Promoting microRNA-101 in Glioblastoma. Cancer Med. 2018, 7, 1404–1415. [Google Scholar] [CrossRef]
- Bian, E.-B.; Chen, E.-F.; Xu, Y.-D.; Yang, Z.-H.; Tang, F.; Ma, C.-C.; Wang, H.-L.; Zhao, B. Exosomal lncRNA—ATB Activates Astrocytes That Promote Glioma Cell Invasion. Int. J. Oncol. 2019, 54, 713–721. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, W.; Mu, M.; Hu, S.; Niu, C. Long Non-Coding RNA LPP-AS2 Promotes Glioma Tumorigenesis via miR-7-5p/EGFR/PI3K/AKT/c-MYC Feedback Loop. J. Exp. Clin. Cancer Res. 2020, 39, 196. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, G.; Luo, Y. Long Non-coding RNA PVT1 Promotes Glioma Cell Proliferation and Invasion by Targeting miR-200a. Exp. Ther. Med. 2019, 17, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gao, Z.; Xu, J.; Wang, H.; Guo, Q.; Li, B.; Li, M.; Xu, H.; Qi, Y.; Zhao, S.; et al. SPI1-Mediated MIR222HG Transcription Promotes Proneural-to-Mesenchymal Transition of Glioma Stem Cells and Immunosuppressive Polarization of Macrophages. Theranostics 2023, 13, 3310–3329. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.-H.; Shih, C.-M.; Liu, A.-J.; Chen, K.-C. Hypoxia-inducible lncRNA MIR210HG Interacting with OCT1 Is Involved in Glioblastoma Multiforme Malignancy. Cancer Sci. 2021, 113, 540–552. [Google Scholar] [CrossRef]
- Cui, B.; Li, B.; Liu, Q.; Cui, Y. lncRNA CCAT1 Promotes Glioma Tumorigenesis by Sponging miR-181b. J. Cell. Biochem. 2017, 118, 4548–4557. [Google Scholar] [CrossRef]
- Jin, Z.; Piao, L.; Sun, G.; Lv, C.; Jing, Y.; Jin, R. Long Non-Coding RNA PART1 Exerts Tumor Suppressive Functions in Glioma via Sponging miR-190a-3p and Inactivation of PTEN/AKT Pathway. OncoTargets Ther. 2020, 13, 1073–1086. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Fu, C.; Wang, C.; Duan, X.; Zou, W.; Zhao, T. Knockdown of Long Non-Coding RNA PCAT1 in Glioma Stem Cells Promotes Radiation Sensitivity. Med. Mol. Morphol. 2019, 52, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum Long Noncoding RNA HOTAIR as a Novel Diagnostic and Prognostic Biomarker in Glioblastoma Multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef]
- Han, N.; Yang, L.; Zhang, X.; Zhou, Y.; Chen, R.; Yu, Y.; Dong, Z.; Zhang, M. LncRNA MATN1-AS1 Prevents Glioblastoma Cell from Proliferation and Invasion via RELA Regulation and MAPK Signaling Pathway. Ann. Transl. Med. 2019, 7, 784. [Google Scholar] [CrossRef]
- Li, Q.; Dong, C.; Cui, J.; Wang, Y.; Hong, X. Over-Expressed lncRNA HOTAIRM1 Promotes Tumor Growth and Invasion through up-Regulating HOXA1 and Sequestering G9a/EZH2/Dnmts Away from the HOXA1 Gene in Glioblastoma Multiforme. J. Exp. Clin. Cancer Res. 2018, 37, 265. [Google Scholar] [CrossRef]
- Yi, K.; Cui, X.; Liu, X.; Wang, Y.; Zhao, J.; Yang, S.; Xu, C.; Yang, E.; Xiao, M.; Hong, B.; et al. PTRF/Cavin-1 as a Novel RNA-Binding Protein Expedites the NF-κB/PD-L1 Axis by Stabilizing lncRNA NEAT1, Contributing to Tumorigenesis and Immune Evasion in Glioblastoma. Front. Immunol. 2022, 12, 802795. [Google Scholar] [CrossRef]
- Liang, J.; Liu, C.; Xu, D.; Xie, K.; Li, A. LncRNA NEAT1 Facilitates Glioma Progression via Stabilizing PGK1. J. Transl. Med. 2022, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 31 October 2024).
- Liu, D.; Wei, Y.; Liu, Y.; Wu, T.; Hu, J.; Lu, H. The Long Non-Coding RNA NEAT1/miR-224-5p/IL-33 Axis Modulates Macrophage M2a Polarization and A1 Astrocyte Activation. Mol. Neurobiol. 2021, 58, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Toker, J.; Iorgulescu, J.B.; Ling, A.L.; Villa, G.R.; Gadet, J.A.M.A.; Parida, L.; Getz, G.; Wu, C.J.; Reardon, D.A.; Chiocca, E.A.; et al. Clinical Importance of the lncRNA NEAT1 in Cancer Patients Treated with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2023, 29, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, R.; Madhyastha, H.; Nurrahmah, Q.I.; Purbasari, B.; Maruyama, M.; Nakajima, Y. MicroRNA 21 Elicits a Pro-Inflammatory Response in Macrophages, with Exosomes Functioning as Delivery Vehicles. Inflammation 2021, 44, 1274–1287. [Google Scholar] [CrossRef]
- Wang, A.; Dai, H.; Gong, Y.; Zhang, C.; Shu, J.; Luo, Y.; Jiang, Y.; Liu, W.; Bie, P. ANLN-Induced EZH2 Upregulation Promotes Pancreatic Cancer Progression by Mediating miR-218-5p/LASP1 Signaling Axis. J. Exp. Clin. Cancer Res. 2019, 38, 347. [Google Scholar] [CrossRef]
- Van Der Vos, K.E.; Abels, E.R.; Zhang, X.; Lai, C.; Carrizosa, E.; Oakley, D.; Prabhakar, S.; Mardini, O.; Crommentuijn, M.H.W.; Skog, J.; et al. Directly Visualized Glioblastoma-Derived Extracellular Vesicles Transfer RNA to Microglia/Macrophages in the Brain. Neuro Oncol. 2016, 18, 58–69. [Google Scholar] [CrossRef]
- Tankov, S.; Petrovic, M.; Lecoultre, M.; Espinoza, F.; El-Harane, N.; Bes, V.; Chliate, S.; Bedoya, D.M.; Jordan, O.; Borchard, G.; et al. Hypoxic Glioblastoma-Cell-Derived Extracellular Vesicles Impair cGAS-STING Activity in Macrophages. Cell Commun. Signal. 2024, 22, 144. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B.; Zhu, J.; Huang, W.; Han, B.; Wang, Q.; Qi, C.; Wang, M.; Liu, F. miR-106b-5p Inhibits IRF1/IFN-β Signaling to Promote M2 Macrophage Polarization of Glioblastoma. OncoTargets Ther. 2020, 13, 7479–7492. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; You, J.Y.; Paek, K.; Park, J.; Kang, S.J.; Han, E.H.; Choi, N.; Chung, S.; Rhee, W.J.; Kim, J.A. Inhibition of Tumor Progression and M2 Microglial Polarization by Extracellular Vesicle-Mediated microRNA-124 in a 3D Microfluidic Glioblastoma Microenvironment. Theranostics 2021, 11, 9687–9704. [Google Scholar] [CrossRef] [PubMed]
- da Silva, K.C.; Lima, I.S.; Santos, C.C.d.; Nonaka, C.K.V.; Souza, B.S.d.F.; David, J.M.; Ulrich, H.; Nascimento, R.P.d.; Costa, M.d.F.D.; dos Santos, B.L.; et al. Agathisflavone Inhibits Viability and Modulates the Expression of miR-125b, miR-155, IL-6, and Arginase in Glioblastoma Cells and Microglia/Macrophage Activation. Molecules 2025, 30, 158. [Google Scholar] [CrossRef]
- Sonda, N.; Simonato, F.; Peranzoni, E.; Calì, B.; Bortoluzzi, S.; Bisognin, A.; Wang, E.; Marincola, F.M.; Naldini, L.; Gentner, B.; et al. miR-142-3p Prevents Macrophage Differentiation during Cancer-Induced Myelopoiesis. Immunity 2013, 38, 1236–1249. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, Y.; Wang, H.; Rong, X.; Peng, J.; He, L.; Peng, Y. An miR-340-5p-Macrophage Feedback Loop Modulates the Progression and Tumor Microenvironment of Glioblastoma Multiforme. Oncogene 2019, 38, 7399–7415. [Google Scholar] [CrossRef]
- Qian, M.; Wang, S.; Guo, X.; Wang, J.; Zhang, Z.; Qiu, W.; Gao, X.; Chen, Z.; Xu, J.; Zhao, R.; et al. Hypoxic Glioma-Derived Exosomes Deliver microRNA-1246 to Induce M2 Macrophage Polarization by Targeting TERF2IP via the STAT3 and NF-κB Pathways. Oncogene 2020, 39, 428–442. [Google Scholar] [CrossRef]
- Huang, S.; Liu, L.; Xu, Z.; Liu, X.; Wu, A.; Zhang, X.; Li, Z.; Li, S.; Li, Y.; Yuan, J.; et al. Exosomal miR-6733-5p Mediates Cross-Talk between Glioblastoma Stem Cells and Macrophages and Promotes Glioblastoma Multiform Progression Synergistically. CNS Neurosci. Ther. 2023, 29, 3756–3773. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Zhong, J.; Wu, X.; Leng, Z.; Liu, M.; Wang, Y.; Wang, Y.; Yang, X.; Huang, N.; et al. Lnc-H19-Derived Protein Shapes the Immunosuppressive Microenvironment of Glioblastoma. Cell Rep. Med. 2024, 5, 101806. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.; Liao, C.; Zhao, Z.; Gao, H.; Huang, R.; Chen, J.; Wu, F.; Zeng, F.; Zhang, Y.; et al. PVT1 Promotes Proliferation and Macrophage Immunosuppressive Polarization through STAT1 and CX3CL1 Regulation in Glioblastoma Multiforme. CNS Neurosci. Ther. 2024, 30, e14566. [Google Scholar] [CrossRef]
- Wang, Z.; Brandt, S.; Medeiros, A.; Wang, S.; Wu, H.; Dent, A.; Serezani, C.H. MicroRNA 21 Is a Homeostatic Regulator of Macrophage Polarization and Prevents Prostaglandin E2-Mediated M2 Generation. PLoS ONE 2015, 10, e0115855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tian, Y.; Guo, F.; Yu, B.; Li, J.; Xu, H.; Su, Z. LincRNA-P21 Knockdown Reversed Tumor-Associated Macrophages Function by Promoting MDM2 to Antagonize* P53 Activation and Alleviate Breast Cancer Development. Cancer Immunol. Immunother. 2020, 69, 835–846. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of Novel Genes Coding for Small Expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs Are Processed from Capped, Polyadenylated Transcripts That Can Also Function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef]
- Qu, K.; Lin, T.; Pang, Q.; Liu, T.; Wang, Z.; Tai, M.; Meng, F.; Zhang, J.; Wan, Y.; Mao, P.; et al. Extracellular miRNA-21 as a Novel Biomarker in Glioma: Evidence from Meta-Analysis, Clinical Validation and Experimental Investigations. Oncotarget 2016, 7, 33994–34010. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, N.; Segit, P.; Walentynowicz, K.A.; Wojnicki, K.; Cyranowski, S.; Swatler, J.; Mieczkowski, J.; Kaminska, B. Single-Cell RNA Sequencing Reveals Functional Heterogeneity of Glioma-Associated Brain Macrophages. Nat. Commun. 2021, 12, 1151. [Google Scholar] [CrossRef]
- Pombo Antunes, A.R.; Scheyltjens, I.; Lodi, F.; Messiaen, J.; Antoranz, A.; Duerinck, J.; Kancheva, D.; Martens, L.; De Vlaminck, K.; Van Hove, H.; et al. Single-Cell Profiling of Myeloid Cells in Glioblastoma across Species and Disease Stage Reveals Macrophage Competition and Specialization. Nat. Neurosci. 2021, 24, 595–610. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.-C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of Tumor-Associated Macrophages and T Cells in Glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Bowman, R.L.; Akkari, L.; Quick, M.L.; Schuhmacher, A.J.; Huse, J.T.; Holland, E.C.; Sutton, J.C.; Joyce, J.A. The Tumor Microenvironment Underlies Acquired Resistance to CSF-1R Inhibition in Gliomas. Science 2016, 352, aad3018. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Kowal, J.; Akkari, L.; Schuhmacher, A.J.; Huse, J.T.; West, B.L.; Joyce, J.A. Inhibition of Colony Stimulating Factor-1 Receptor Abrogates Microenvironment-Mediated Therapeutic Resistance in Gliomas. Oncogene 2017, 36, 6049–6058. [Google Scholar] [CrossRef]
- Assistance Publique—Hôpitaux de Paris. Establishment of a Signature of Circulating MicroRNA as a Tool to Aid Diagnosis of Primary Brain Tumors in Adults. 2018. Available online: https://clinicaltrials.gov/study/NCT03630861?cond=Establishment%20of%20a%20Signature%20of%20Circulating%20microRNA%20as%20a%20Tool%20to%20Aid%20Diagnosis%20of%20Primary%20Brain%20Tumors%20in%20Adults&rank=1 (accessed on 7 July 2025).
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A First-in-Human Phase 0 Clinical Study of RNA Interference-Based Spherical Nucleic Acids in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Alnylam Pharmaceuticals. A Study to Investigate the Safety and Efficacy of Intranasal ALN-RSV01 Administered to Adult Volunteers Experimentally Inoculated with Respiratory Syncytial Virus. 2007. Available online: https://clinicaltrials.gov/study/NCT00496821?term=AREA%5BBasicSearch%5D(Alnylam%20pharmaceuticals)&rank=1 (accessed on 7 July 2025).
- ProQR Therapeutics. Phase 1b, Randomized, Double-Blind, Placebo-Controlled, Dose Escalation Study to Evaluate the Safety, Tolerability and Pharmacokinetics of QR-010 in Subjects with Homozygous ΔF508 Cystic Fibrosis. 2017. Available online: https://cdn.clinicaltrials.gov/large-docs/64/NCT02532764/SAP_001.pdf (accessed on 7 July 2025).
- Haque, A.K.M.A.; Dewerth, A.; Antony, J.S.; Riethmüller, J.; Schweizer, G.R.; Weinmann, P.; Latifi, N.; Yasar, H.; Pedemonte, N.; Sondo, E.; et al. Chemically Modified hCFTR mRNAs Recuperate Lung Function in a Mouse Model of Cystic Fibrosis. Sci. Rep. 2018, 8, 16776. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of microRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Sankowski, R.; Böttcher, C.; Masuda, T.; Geirsdottir, L.; Sagar; Sindram, E.; Seredenina, T.; Muhs, A.; Scheiwe, C.; Shah, M.J.; et al. Mapping Microglia States in the Human Brain through the Integration of High-Dimensional Techniques. Nat. Neurosci. 2019, 22, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Wieghofer, P.; Jordão, M.J.C.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M.; et al. Origin, Fate and Dynamics of Macrophages at Central Nervous System Interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Salami, R.; Salami, M.; Mafi, A.; Vakili, O.; Asemi, Z. Circular RNAs and Glioblastoma Multiforme: Focus on Molecular Mechanisms. Cell Commun. Signal. 2022, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Son, C.J.; Carnino, J.M.; Lee, H.; Jin, Y. Emerging Roles of Circular RNA in Macrophage Activation and Inflammatory Lung Responses. Cells 2024, 13, 1407. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Zhao, Z.; Lu, J.; Chen, H.; Ding, N.; Wang, G.; Xu, J.; Li, X. Identifying and Functionally Characterizing Tissue-Specific and Ubiquitously Expressed Human lncRNAs. Oncotarget 2016, 7, 7120–7133. [Google Scholar] [CrossRef]
- Mattioli, K.; Volders, P.-J.; Gerhardinger, C.; Lee, J.C.; Maass, P.G.; Melé, M.; Rinn, J.L. High-Throughput Functional Analysis of lncRNA Core Promoters Elucidates Rules Governing Tissue Specificity. Genome Res. 2019, 29, 344–355. [Google Scholar] [CrossRef]
- Khayati, S.; Dehnavi, S.; Sadeghi, M.; Tavakol Afshari, J.; Esmaeili, S.-A.; Mohammadi, M. The Potential Role of miRNA in Regulating Macrophage Polarization. Heliyon 2023, 9, e21615. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Pioppini, C.; Ozpolat, B.; Calin, G.A. Non-Coding RNAs Regulation of Macrophage Polarization in Cancer. Mol. Cancer 2021, 20, 24. [Google Scholar] [CrossRef]
- Moghaddasnejad, M.R.; Keshavarz, A.; Mardi, A.; Sherafat, N.S.; Aghebati-Maleki, L.; Mohammadi, M.H. LncRNAs as Behind-the-Scenes Molecules in Cancer Progression through Regulating Tumor-Associated Innate Immune System Cells. Mol. Biol. Rep. 2025, 52, 449. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Lee, J.-K.; Jeon, Y.-K.; Kim, C.-W. Exosome Derived from Epigallocatechin Gallate Treated Breast Cancer Cells Suppresses Tumor Growth by Inhibiting Tumor-Associated Macrophage Infiltration and M2 Polarization. BMC Cancer 2013, 13, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Chen, C.; Liu, Y.; Si, Q.; Chuang, T.-H.; Li, N.; Gomez-Cabrero, A.; Reisfeld, R.A.; Xiang, R.; et al. MicroRNA-19a-3p Inhibits Breast Cancer Progression and Metastasis by Inducing Macrophage Polarization through Downregulated Expression of Fra-1 Proto. Oncogene 2014, 33, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Fordham, J.B.; Naqvi, A.R.; Nares, S. miR-24 Regulates Macrophage Polarization and Plasticity. J. Clin. Cell. Immunol. 2015, 6, 362. [Google Scholar] [CrossRef]
- Cai, J.; Qiao, B.; Gao, N.; Lin, N.; He, W. Oral Squamous Cell Carcinoma-Derived Exosomes Promote M2 Subtype Macrophage Polarization Mediated by Exosome-Enclosed miR-29a-3p. Am. J. Physiol.-Cell Physiol. 2019, 316, C731–C740. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, D.; Luo, Y.; Sun, Y.; Duan, C.; Yang, J.; Wei, J.; Li, X.; Lu, Y.; Lai, X. miR-34a Promotes the Immunosuppressive Function of Multiple Myeloma-Associated Macrophages by Dampening the TLR-9 Signaling. Cancer Med. 2024, 13, e7387. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, Y.; Wang, Y.; Li, T.; Liu, Q.; Hou, Z. Exosomal miR-92a-3p Modulates M2 Macrophage Polarization in Colorectal Cancer: Implications for Tumor Migration and Angiogenesis. Med. Oncol. 2025, 42, 1–15. [Google Scholar] [CrossRef]
- Banerjee, S.; Cui, H.; Xie, N.; Tan, Z.; Yang, S.; Icyuz, M.; Thannickal, V.J.; Abraham, E.; Liu, G. miR-125a-5p Regulates Differential Activation of Macrophages and Inflammation*. J. Biol. Chem. 2013, 288, 35428–35436. [Google Scholar] [CrossRef]
- Ying, H.; Kang, Y.; Zhang, H.; Zhao, D.; Xia, J.; Lu, Z.; Wang, H.; Xu, F.; Shi, L. MiR-127 Modulates Macrophage Polarization and Promotes Lung Inflammation and Injury by Activating the JNK Pathway. J. Immunol. 2015, 194, 1239–1251. [Google Scholar] [CrossRef]
- Huang, Y.; Du, K.L.; Guo, P.Y.; Zhao, R.M.; Wang, B.; Zhao, X.L.; Zhang, C.Q. IL-16 Regulates Macrophage Polarization as a Target Gene of Mir-145-3p. Mol. Immunol. 2019, 107, 1–9. [Google Scholar] [CrossRef]
- Peng, X.; He, F.; Mao, Y.; Lin, Y.; Fang, J.; Chen, Y.; Sun, Z.; Zhuo, Y.; Jiang, J. miR-146a Promotes M2 Macrophage Polarization and Accelerates Diabetic Wound Healing by Inhibiting the TLR4/NF-κB Axis. J. Mol. Endocrinol. 2022, 69, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, C.; Cai, D.; Zhu, R.; Cao, Y. Exosomal miR-155-5p Drives Widespread Macrophage M1 Polarization in Hypervirulent Klebsiella Pneumoniae-Induced Acute Lung Injury via the MSK1/P38-MAPK Axis. Cell. Mol. Biol. Lett. 2023, 28, 92. [Google Scholar] [CrossRef]
- Bras, J.P.; Silva, A.M.; Calin, G.A.; Barbosa, M.A.; Santos, S.G.; Almeida, M.I. miR-195 Inhibits Macrophages pro-Inflammatory Profile and Impacts the Crosstalk with Smooth Muscle Cells. PLoS ONE 2017, 12, e0188530. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, L.; Li, H.; Ye, J.; Lin, N.; Chen, M.; Pan, D.; Chen, Z. Endometriosis Derived Exosomal miR-301a-3p Mediates Macrophage Polarization via Regulating PTEN-PI3K Axis. Biomed. Pharmacother. 2022, 147, 112680. [Google Scholar] [CrossRef]
- Do, D.C.; Mu, J.; Zhou, Y.; Gao, P. miR-511-3p Limits Allergic Inflammation through M2 Macrophage Polarization and Modulating CCL2 Expression. J. Allergy Clin. Immunol. 2018, 141, AB80. [Google Scholar] [CrossRef]
- Zhong, Y.; Yi, C. MicroRNA-720 Suppresses M2 Macrophage Polarization by Targeting GATA3. Biosci. Rep. 2016, 36, e00363. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, X.; Zhang, J.; Huang, X.; Zhang, X.; Wang, J.; Shi, H.; Yu, A. Let-7a Promotes Microglia M2 Polarization by Targeting CKIP-1 Following ICH. Immunol. Lett. 2018, 202, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Hu, Y.; Huang, Y.; Zhang, Y.; Zheng, X.; Wang, S.; Wang, Y.; Yu, Y.; Zhang, M.; et al. miRNA Let-7b Modulates Macrophage Polarization and Enhances Tumor-Associated Macrophages to Promote Angiogenesis and Mobility in Prostate Cancer. Sci. Rep. 2016, 6, 25602. [Google Scholar] [CrossRef]
- Xie, C.; Guo, Y.; Lou, S. LncRNA ANCR Promotes Invasion and Migration of Gastric Cancer by Regulating FoxO1 Expression to Inhibit Macrophage M1 Polarization. Dig. Dis. Sci. 2020, 65, 2863–2872. [Google Scholar] [CrossRef]
- Liu, J.; Ding, D.; Jiang, Z.; Du, T.; Liu, J.; Kong, Z. Long Non-Coding RNA CCAT1/miR-148a/PKCζ Prevents Cell Migration of Prostate Cancer by Altering Macrophage Polarization. The Prostate 2019, 79, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, Y.; Lai, Y.; He, W.; Li, Y.; Wang, R.; Luo, X.; Chen, R.; Chen, T. Long Non-Coding RNA Cox-2 Prevents Immune Evasion and Metastasis of Hepatocellular Carcinoma by Altering M1/M2 Macrophage Polarization. J. Cell. Biochem. 2018, 119, 2951–2963. [Google Scholar] [CrossRef]
- Sun, D.; Yu, Z.; Fang, X.; Liu, M.; Pu, Y.; Shao, Q.; Wang, D.; Zhao, X.; Huang, A.; Xiang, Z.; et al. LncRNA GAS5 Inhibits Microglial M2 Polarization and Exacerbates Demyelination. EMBO Rep. 2017, 18, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, C.; Guo, J.; Hu, X.; Xie, D. GNAS-AS1/miR-4319/NECAB3 Axis Promotes Migration and Invasion of Non-Small Cell Lung Cancer Cells by Altering Macrophage Polarization. Funct. Integr. Genomics 2020, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, N.; Zheng, Z.; Che, Y.; Suzuki, M.; Kano, S.; Lu, J.; Wang, P.; Sun, Y.; Homma, A. Exosomal lncRNA HOTAIR Induce Macrophages to M2 Polarization via PI3K/p-AKT/AKT Pathway and Promote EMT and Metastasis in Laryngeal Squamous Cell Carcinoma. BMC Cancer 2022, 22, 1208. [Google Scholar] [CrossRef]
- Tian, X.; Wu, Y.; Yang, Y.; Wang, J.; Niu, M.; Gao, S.; Qin, T.; Bao, D. Long Noncoding RNA LINC00662 Promotes M2 Macrophage Polarization and Hepatocellular Carcinoma Progression via Activating Wnt/β-Catenin Signaling. Mol. Oncol. 2020, 14, 462–483. [Google Scholar] [CrossRef]
- Yang, D.; Liu, K.; Fan, L.; Liang, W.; Xu, T.; Jiang, W.; Lu, H.; Jiang, J.; Wang, C.; Li, G.; et al. LncRNA RP11-361F15.2 Promotes Osteosarcoma Tumorigenesis by Inhibiting M2-Like Polarization of Tumor-Associated Macrophages of CPEB4. Cancer Lett. 2020, 473, 33–49. [Google Scholar] [CrossRef]
- Ahmad, I.; Naqvi, R.A.; Valverde, A.; Naqvi, A.R. LncRNA MALAT1/microRNA-30b Axis Regulates Macrophage Polarization and Function. Front. Immunol. 2023, 14, 1214810. [Google Scholar] [CrossRef]
- Wei, H.; Wu, X.; Huang, L.; Long, C.; Lu, Q.; Huang, Z.; Huang, Y.; Li, W.; Pu, J. LncRNA MEG3 Reduces the Ratio of M2/M1 Macrophages Through the HuR/CCL5 Axis in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 543–562. [Google Scholar] [CrossRef]
- Cao, J.; Dong, R.; Jiang, L.; Gong, Y.; Yuan, M.; You, J.; Meng, W.; Chen, Z.; Zhang, N.; Weng, Q.; et al. LncRNA-MM2P Identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol. Res. 2019, 7, 292–305. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, W.; Mao, L.; Wang, Y.; Xia, L.; Cao, M.; Shen, J.; Chen, J. Long Non-Coding RNA NIFK-AS1 Inhibits M2 Polarization of Macrophages in Endometrial Cancer Through Targeting miR-146a. Int. J. Biochem. Cell Biol. 2018, 104, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, M.; He, X.; Cao, Y.; Liu, P.; Li, F.; Zou, S.; Wen, C.; Zhan, Q.; Xu, Z.; et al. LncRNA-PACERR Induces pro-Tumour Macrophages via Interacting with miR-671-3p and m6A-Reader IGF2BP2 in Pancreatic Ductal Adenocarcinoma. J. Hematol. Oncol. J Hematol Oncol 2022, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, H.; Wang, F.; Xiong, L.; Zhou, C.; Hu, T.; He, X.; Wu, X.; Xie, D.; Wu, X.; et al. LncRNA RPPH1 Promotes Colorectal Cancer Metastasis by Interacting with TUBB3 and by Promoting Exosomes-Mediated Macrophage M2 Polarization. Cell Death Dis. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lei, Y.; Wu, M.; Li, N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal lncRNA TUC339. Int. J. Mol. Sci. 2018, 19, 2958. [Google Scholar] [CrossRef]
- Pi, L.; Fang, B.; Meng, X.; Qian, L. LncRNA XIST Accelerates Burn Wound Healing by Promoting M2 Macrophage Polarization through Targeting IL-33 via miR-19b. Cell Death Discov. 2022, 8, 220. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Z.; Dong, J.; Bi, H.; Wang, B.; Du, K.; Zhang, C.; Chen, L. lncRNA XIST Inhibition Promotes M2 Polarization of Microglial and Aggravates the Spinal Cord Injury via Regulating miR-124–3p/IRF1 Axis. Heliyon 2023, 9, e17852. [Google Scholar] [CrossRef]

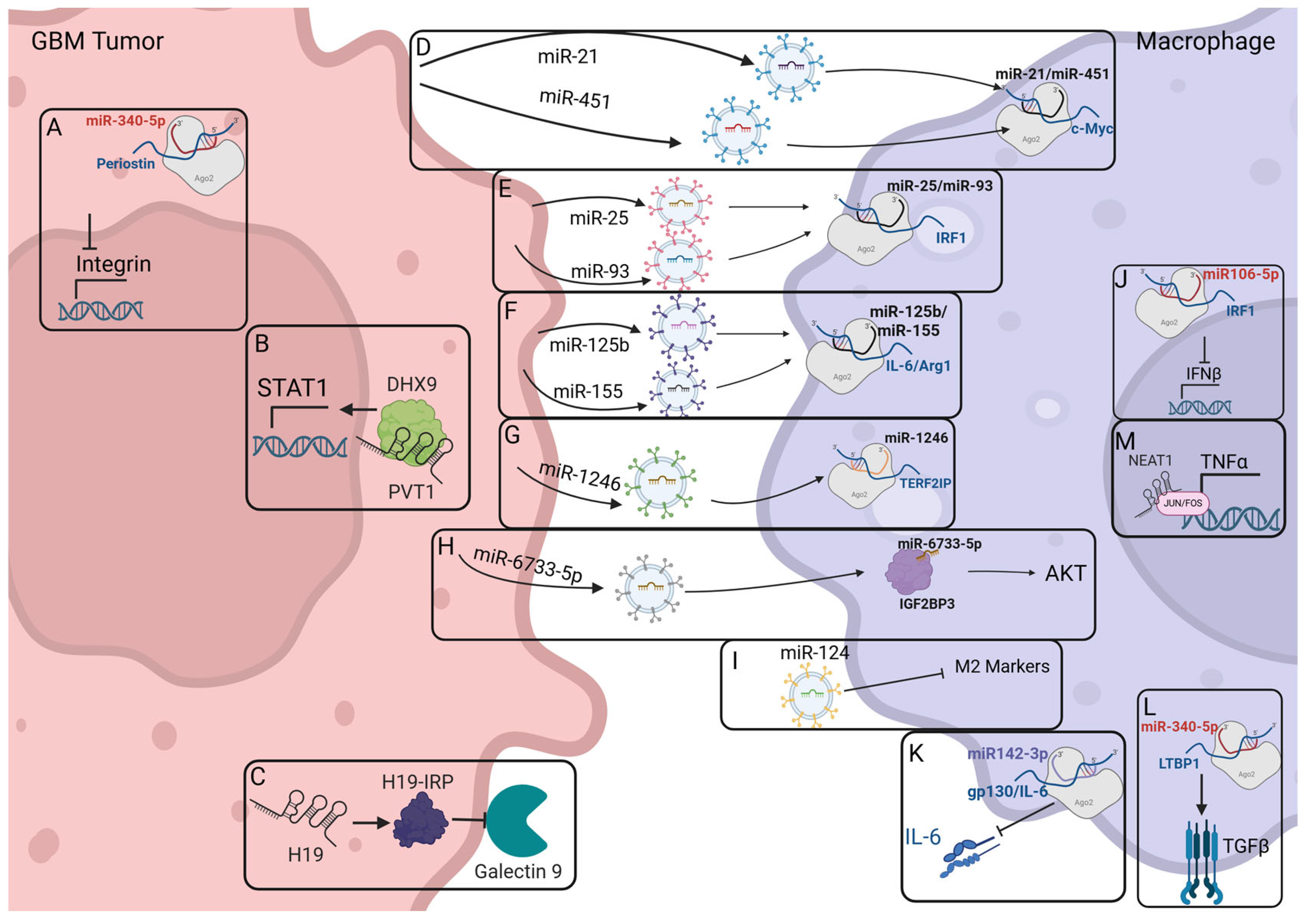
| ncRNA Acronym | Full Name | Polarization | Targets/Function | Citation |
|---|---|---|---|---|
| Mir21 | Increases M2 Polarization | Loaded into exosomes, targets c-Myc in microglia | van der Vos K, et al. [81] | |
| Mir25 | Decreases M1 Polarization | Mir25 and mir93 are loaded into exosomes, where they can target the cGAS-STING pathway in macrophages | Tankov S, et al. [82] | |
| Mir93 | Decreases M1 Polarization | Mir93 and mir25 are loaded into exosomes, where they can target the cGAS-STING pathway in macrophages | Tankov S, et al. [82] | |
| mir106-5p | Increases M2 Polarization | Targets the IFNβ pathway, inhibiting IRF1 signaling | Shi Y, et al. [83] | |
| miR124 | Decreases M2 Polarization | Loaded into exosomes and decreases STAT3 signaling, preventing M2 recruitment and tumor progression | Hong S, et al. [84] | |
| Mir125b | Increases M2 Polarization | Targets IL-6 and Arginase-1, decreasing immune stimulation | Da Silva KC, et al. [85] | |
| mir142-3p | Decreases Macrophage Polarization | blocks translation of gp130 subunit of IL-6 | Sonda N, et al. [86] | |
| mir155 | Increases M2 Polarization | Targets IL-6 and Arginase-1, decreasing immune stimulation | Da Silva KC, et al. [85] | |
| Mir340-5p | Decreases M2 Polarization | Through its interactions with Periostin, it prevents M2-like TAM recruitment to the TME | Liu Y, et al. [87] | |
| mir451 | Increase M2 Polarization | Loaded into Exosomes, targets c-Myc in microglia | van der Vos K, et al. [81] | |
| mir1246 | Increases M2 Polarization | Loaded into exosomes and delivered to macrophages, targeting TERF2IP, activating the STAT3 Pathway | Qian M, et al. [88] | |
| miR6733-5p | Increase M2 Polarization | Loaded into exosomes and delivered to macrophages, targeting the AKT pathway via IGF2BP3 | Huang S, et al. [89] | |
| H19 | Increases M2 Polarization | Encodes a small immune protein, H19-IRP, which prevents transcription of CCL2 and Galectin-9 | Chen J, et al. [90] | |
| NEAT1 | Nuclear Paraspeckle Assembly Transcript 1 | Increases M1 Polarization | Enriches TNFα and NF-κB pathways and downstream genes while decreasing inflammatory cytokine expression | Toker J, et al. [78] |
| PVT1 | Plasmacytoma variant translocation 1 | Increases M2 Polarization | Stabilizes DHX9, which acts as a transcription factor for STAT1 and CX3CL1 | Huang L, et al. [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westemeier-Rice, E.S.; Nduom, E.K. Macrophages in Glioblastoma and How Non-Coding RNAs Impact Their Differentiation. Cells 2025, 14, 1528. https://doi.org/10.3390/cells14191528
Westemeier-Rice ES, Nduom EK. Macrophages in Glioblastoma and How Non-Coding RNAs Impact Their Differentiation. Cells. 2025; 14(19):1528. https://doi.org/10.3390/cells14191528
Chicago/Turabian StyleWestemeier-Rice, Emily S., and Edjah K. Nduom. 2025. "Macrophages in Glioblastoma and How Non-Coding RNAs Impact Their Differentiation" Cells 14, no. 19: 1528. https://doi.org/10.3390/cells14191528
APA StyleWestemeier-Rice, E. S., & Nduom, E. K. (2025). Macrophages in Glioblastoma and How Non-Coding RNAs Impact Their Differentiation. Cells, 14(19), 1528. https://doi.org/10.3390/cells14191528






