Mitochondrial ROS–ER Stress Axis Governs IL-10 Production in Neutrophils and Regulates Inflammation in Murine Chlamydia pneumoniae Lung Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Chlamydia and Infection
2.3. Depletion of Neutrophils In Vivo
2.4. Cells and Reagents
2.5. In Vitro and In Vivo Treatment with 4-PBA
2.6. Cytokine ELISA
2.7. LDH Cell Cytotoxicity Assay
2.8. Flow Cytometric Determination of Mitochondrial ROS, Mitochondrial Membrane Potential, and XBP1s Protein in Neutrophils
2.9. Isolation of Mononuclear Cells from the Lung Tissues of Mice
2.10. Flow Cytometric Analysis and Intracellular Cytokine
2.11. Immunofluorescence
2.12. Immunoblot Analysis
2.13. Histology and Immunochemistry
2.14. Quantitative RT-PCR
2.15. Xbp1 Splicing Assay
2.16. Statistical Analysis
3. Results
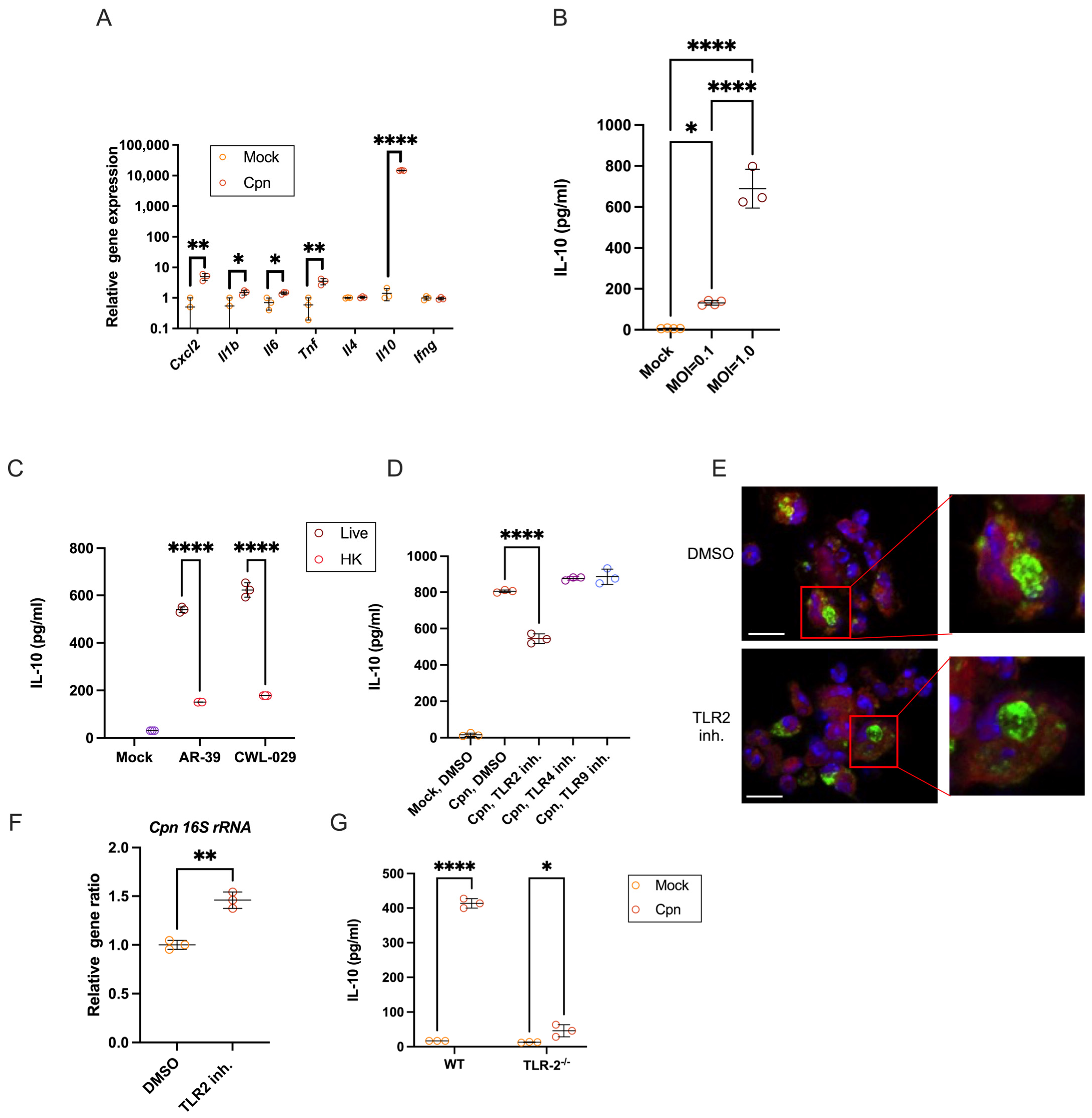
3.1. C. pneumoniae Live Infection in Mouse Neutrophils Triggers Robust IL-10 Production via TLR2
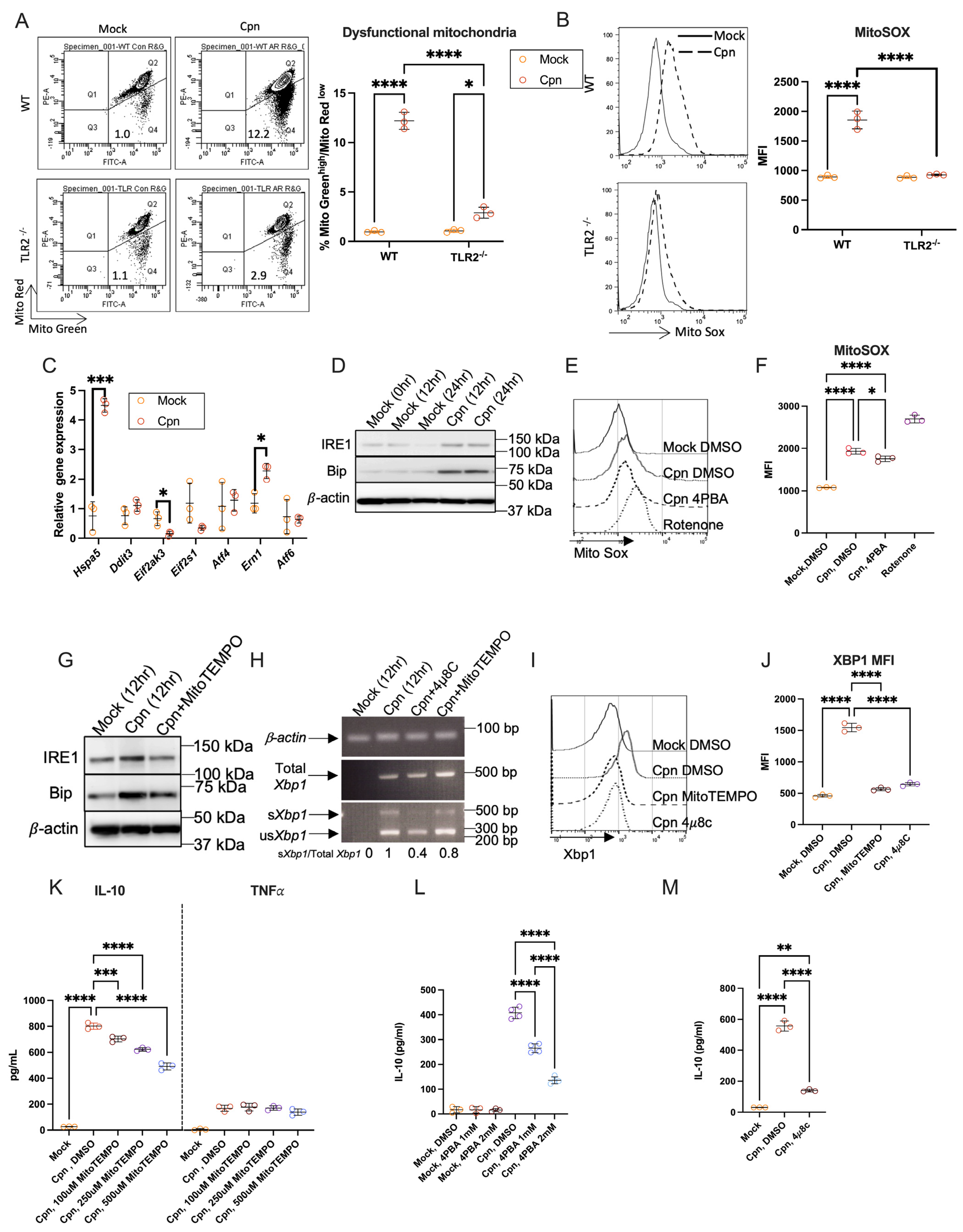
3.2. Mitochondrial ROS and ER Stress–IRE1α Axis Are Essential for Neutrophil IL-10 Production After C. pneumoniae Infection
3.3. C. muridarum Infection Neither Increases mtROS, Provokes ER Stress, nor Causes Neutrophils to Produce IL-10
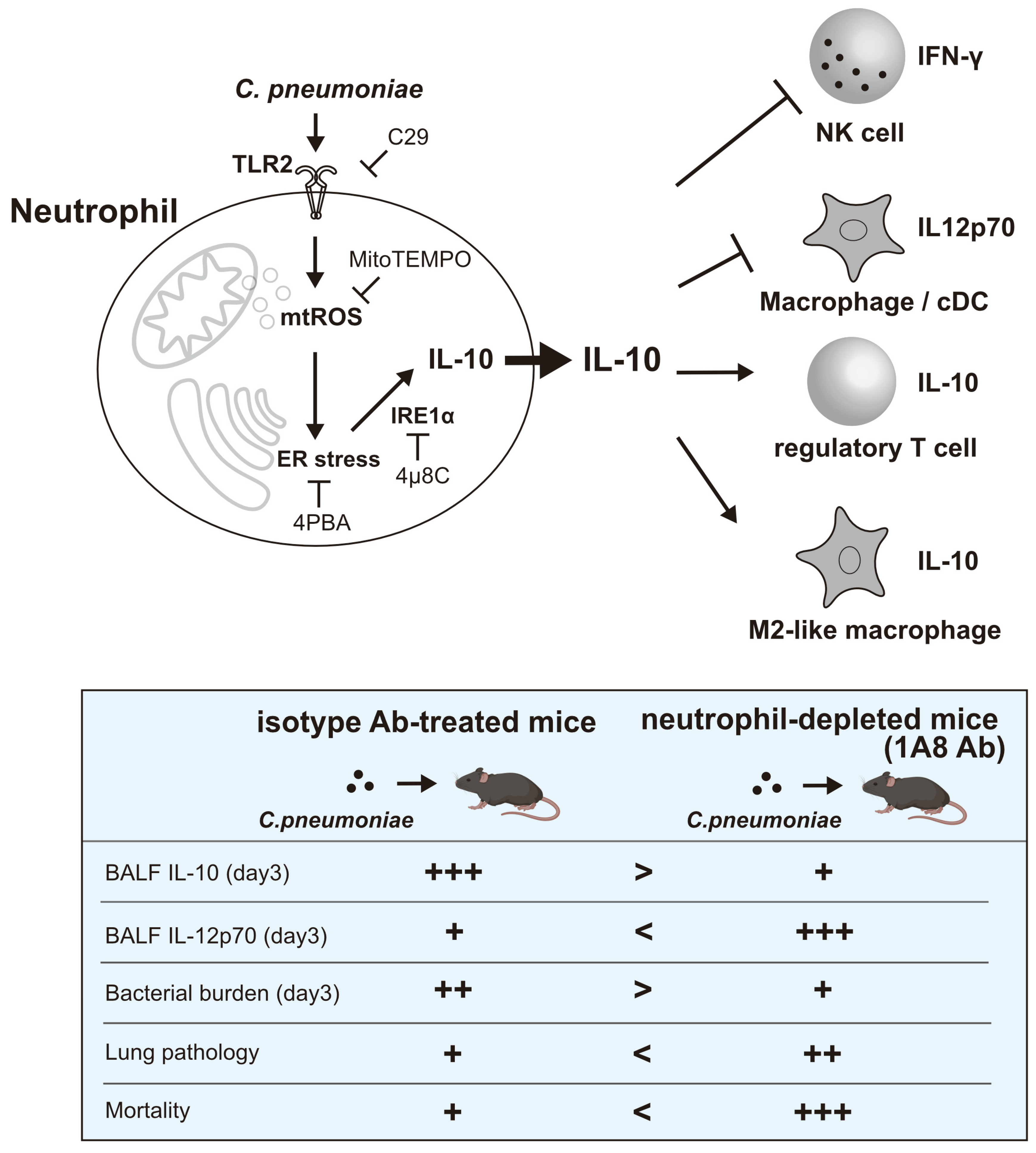
3.4. In Vivo, Neutrophil-Derived IL-10 Shapes Immune Responses in the Lungs of Mice Infected with C. pneumoniae
3.5. ER Stress Inhibition Recapitulates the Neutrophil IL-10 Depletion Phenotype
3.6. In Vivo, Neutrophil-Derived IL-10 Mitigates Tissue Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grayston, J.T. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J. Infect. Dis. 2000, 181 (Suppl. S3), S402–S410. [Google Scholar] [CrossRef]
- Gieffers, J.; van Zandbergen, G.; Rupp, J.; Sayk, F.; Krüger, S.; Ehlers, S.; Solbach, W.; Maass, M. Phagocytes transmit Chlamydia pneumoniae from the lungs to the vasculature. Eur. Respir. J. 2004, 23, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Belland, R.; Ojcius, D.M.; Byrne, G.I. Focus: Chlamydia. Nat. Rev. Microbiol. 2004, 2, 530. [Google Scholar] [CrossRef]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Mocsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef]
- Lehman, H.K.; Segal, B.H. The role of neutrophils in host defense and disease. J. Allergy Clin. Immunol. 2020, 145, 1535–1544. [Google Scholar] [CrossRef]
- Lewkowicz, N.; Mycko, M.P.; Przygodzka, P.; Ćwiklińska, H.; Cichalewska, M.; Matysiak, M.; Selmaj, K.; Lewkowicz, P. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2016, 9, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Majlessi, L.; Deriaud, E.; Leclerc, C.; Lo-Man, R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 2009, 31, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Doz, E.; Lombard, R.; Carreras, F.; Buzoni-Gatel, D.; Winter, N. Mycobacteria-infected dendritic cells attract neutrophils that produce IL-10 and specifically shut down Th17 CD4 T cells through their IL-10 receptor. J. Immunol. 2013, 191, 3818–3826. [Google Scholar] [CrossRef]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004, 21, 215–226. [Google Scholar] [CrossRef]
- Ocuin, L.M.; Bamboat, Z.M.; Balachandran, V.P.; Cavnar, M.J.; Obaid, H.; Plitas, G.; DeMatteo, R.P. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J. Leukoc. Biol. 2011, 89, 423–432. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Pillich, H.; Loose, M.; Zimmer, K.P.; Chakraborty, T. Diverse roles of endoplasmic reticulum stress sensors in bacterial infection. Mol. Cell. Pediatr. 2016, 3, 9. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Chaudhary, M.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum (ER) Stress Response Failure in Diseases. Trends Cell Biol. 2020, 30, 672–675. [Google Scholar] [CrossRef]
- So, J.S. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef]
- Shan, B.; Wang, X.; Wu, Y.; Xu, C.; Xia, Z.; Dai, J.; Shao, M.; Zhao, F.; He, S.; Yang, L.; et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 2017, 18, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Ren, H.; Zhou, G.; Yuan, X.; Shi, X. ER-stress regulates macrophage polarization through pancreatic EIF-2alpha kinase. Cell. Immunol. 2019, 336, 40–47. [Google Scholar] [CrossRef] [PubMed]
- LaMarche, N.M.; Kane, H.; Kohlgruber, A.C.; Dong, H.; Lynch, L.; Brenner, M.B. Distinct iNKT Cell Populations Use IFNgamma or ER Stress-Induced IL-10 to Control Adipose Tissue Homeostasis. Cell Metab. 2020, 32, 243–258.e6. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Chen, Z.F.; Yan, J.; Li, Q.F.; Huang, Y.; Xu, H.; Zhang, X.P.; Jiang, H. Endoplasmic Reticulum Stress of Neutrophils Is Required for Ischemia/Reperfusion-Induced Acute Lung Injury. J. Immunol. 2015, 195, 4802–4809. [Google Scholar] [CrossRef]
- Garcia-Navas, R.; Gajate, C.; Mollinedo, F. Neutrophils drive endoplasmic reticulum stress-mediated apoptosis in cancer cells through arginase-1 release. Sci. Rep. 2021, 11, 12574. [Google Scholar] [CrossRef]
- Sule, G.; Abuaita, B.H.; Steffes, P.A.; Fernandes, A.T.; Estes, S.K.; Dobry, C.; Pandian, D.; Gudjonsson, J.E.; Kahlenberg, J.M.; O’Riordan, M.X.; et al. Endoplasmic reticulum stress sensor IRE1alpha propels neutrophil hyperactivity in lupus. J. Clin. Investig. 2021, 131, e137866. [Google Scholar] [CrossRef] [PubMed]
- Beckett, E.L.; Phipps, S.; Starkey, M.R.; Horvat, J.C.; Beagley, K.W.; Foster, P.S.; Hansbro, P.M. TLR2, but not TLR4, is required for effective host defence against Chlamydia respiratory tract infection in early life. PLoS ONE 2012, 7, e39460. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Arditi, M. Innate immune responses to Chlamydia pneumoniae infection: Role of TLRs, NLRs, and the inflammasome. Microbes Infect. 2012, 14, 1301–1307. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Chandel, N.S. Mitochondria reactive oxygen species signaling in immune responses. Immunity 2025, 58, 1904–1921. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.-R.; Chae, H.-J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Itoh, R.; Murakami, I.; Chou, B.; Ishii, K.; Soejima, T.; Suzuki, T.; Hiromatsu, K. Chlamydia pneumoniae harness host NLRP3 inflammasome-mediated caspase-1 activation for optimal intracellular growth in murine macrophages. Biochem. Biophys. Res. Commun. 2014, 452, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, N.; Matsumoto, A. Establishment of a particle-counting method for purified elementary bodies of chlamydiae and evaluation of sensitivities of the IDEIA Chlamydia kit and DNA probe by using the purified elementary bodies. J. Clin. Microbiol. 1992, 30, 2911–2916. [Google Scholar] [CrossRef]
- Ishii, K.; Kurihara, Y.; Yoshimura, M.; Walenna, N.F.; Shimizu, A.; Ozuru, R.; Hiromatsu, K. FABP4-dependent fatty acid oxidation-fueled mitochondrial ROS induces the mobilization of cellular iron and facilitates Trypanosoma cruzi proliferation in murine adipocytes. mBio 2025, e0218025. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.A.; Melo-Gonzalez, F.; Sebastian, V.P.; Vallejos, O.P.; Noguera, L.P.; Suazo, I.D.; Schultz, B.M.; Manosalva, A.H.; Penaloza, H.F.; Soto, J.A.; et al. Characterization of the Anti-Inflammatory Capacity of IL-10-Producing Neutrophils in Response to Streptococcus pneumoniae Infection. Front. Immunol. 2021, 12, 638917. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Nieto, P.A.; Muñoz-Durango, N.; Salazar-Echegarai, F.J.; Torres, J.; Parga, M.J.; Alvarez-Lobos, M.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. Interleukin-10 plays a key role in the modulation of neutrophils recruitment and lung inflammation during infection by Streptococcus pneumoniae. Immunology 2015, 146, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Walenna, N.F.; Kurihara, Y.; Chou, B.; Ishii, K.; Soejima, T.; Hiromatsu, K. Chlamydia pneumoniae infection-induced endoplasmic reticulum stress causes fatty acid-binding protein 4 secretion in murine adipocytes. J. Biol. Chem. 2020, 295, 2713–2723. [Google Scholar] [CrossRef]
- Shekhova, E. Mitochondrial reactive oxygen species as major effectors of antimicrobial immunity. PLoS Pathog. 2020, 16, e1008470. [Google Scholar] [CrossRef]
- Silwal, P.; Kim, J.K.; Kim, Y.J.; Jo, E.K. Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection. Front. Immunol. 2020, 11, 1649. [Google Scholar] [CrossRef]
- Monteiro, L.B.; Davanzo, G.G.; de Aguiar, C.F.; Moraes-Vieira, P.M.M. Using flow cytometry for mitochondrial assays. MethodsX 2020, 7, 100938. [Google Scholar] [CrossRef]
- Navid, F.; Colbert, R.A. Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 25–40. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Z.; Dress, R.J.; Zhu, Y.; Zhang, S.; De Feo, D.; Kong, W.T.; Cai, P.; Shin, A.; et al. Dendritic cell type 3 arises from Ly6C(+) monocyte-dendritic cell progenitors. Immunity 2023, 56, 1761–1777.e6. [Google Scholar] [CrossRef]
- Lutz, M.B.; Ali, S.; Audiger, C.; Autenrieth, S.E.; Berod, L.; Bigley, V.; Cyran, L.; Dalod, M.; Dörrie, J.; Dudziak, D.; et al. Guidelines for mouse and human DC generation. Eur. J. Immunol. 2023, 53, e2249816. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Tran, K.A.; Chevre, R.; Locher, V.; Richter, M.; Sun, S.; Sadeghi, M.; Pernet, E.; Herrero-Cervera, A.; Grant, A.; et al. β-Glucan reprograms neutrophils to promote disease tolerance against influenza A virus. Nat. Immunol. 2025, 26, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.P.; Teixeira, L.; Moita, L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017, 17, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.S. The Biology of Physiological Health. Cell 2020, 181, 250–269. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, B.; Ishii, K.; Kurihara, Y.; Shimizu, A.; Yoshimura, M.; Ozuru, R.; Itoh, R.; Sakamoto, A.; Hiromatsu, K. Mitochondrial ROS–ER Stress Axis Governs IL-10 Production in Neutrophils and Regulates Inflammation in Murine Chlamydia pneumoniae Lung Infection. Cells 2025, 14, 1523. https://doi.org/10.3390/cells14191523
Chou B, Ishii K, Kurihara Y, Shimizu A, Yoshimura M, Ozuru R, Itoh R, Sakamoto A, Hiromatsu K. Mitochondrial ROS–ER Stress Axis Governs IL-10 Production in Neutrophils and Regulates Inflammation in Murine Chlamydia pneumoniae Lung Infection. Cells. 2025; 14(19):1523. https://doi.org/10.3390/cells14191523
Chicago/Turabian StyleChou, Bin, Kazunari Ishii, Yusuke Kurihara, Akinori Shimizu, Michinobu Yoshimura, Ryo Ozuru, Ryota Itoh, Atsuhiko Sakamoto, and Kenji Hiromatsu. 2025. "Mitochondrial ROS–ER Stress Axis Governs IL-10 Production in Neutrophils and Regulates Inflammation in Murine Chlamydia pneumoniae Lung Infection" Cells 14, no. 19: 1523. https://doi.org/10.3390/cells14191523
APA StyleChou, B., Ishii, K., Kurihara, Y., Shimizu, A., Yoshimura, M., Ozuru, R., Itoh, R., Sakamoto, A., & Hiromatsu, K. (2025). Mitochondrial ROS–ER Stress Axis Governs IL-10 Production in Neutrophils and Regulates Inflammation in Murine Chlamydia pneumoniae Lung Infection. Cells, 14(19), 1523. https://doi.org/10.3390/cells14191523





