TGF-beta Increases Permeability of 70 kDa Molecular Tracer from the Heart to Cells of the Osteoarthritic Guinea Pig Knee Joint
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Methods
2.2. Imaging
2.2.1. Confocal Laser Scanning Microscopy
2.2.2. Backscattered Electron Scanning Electron Microscopy
2.3. Statistical Analysis
2.4. Sample Size Calculations
2.5. Specimen Fixation and Preparation for Imaging
3. Results
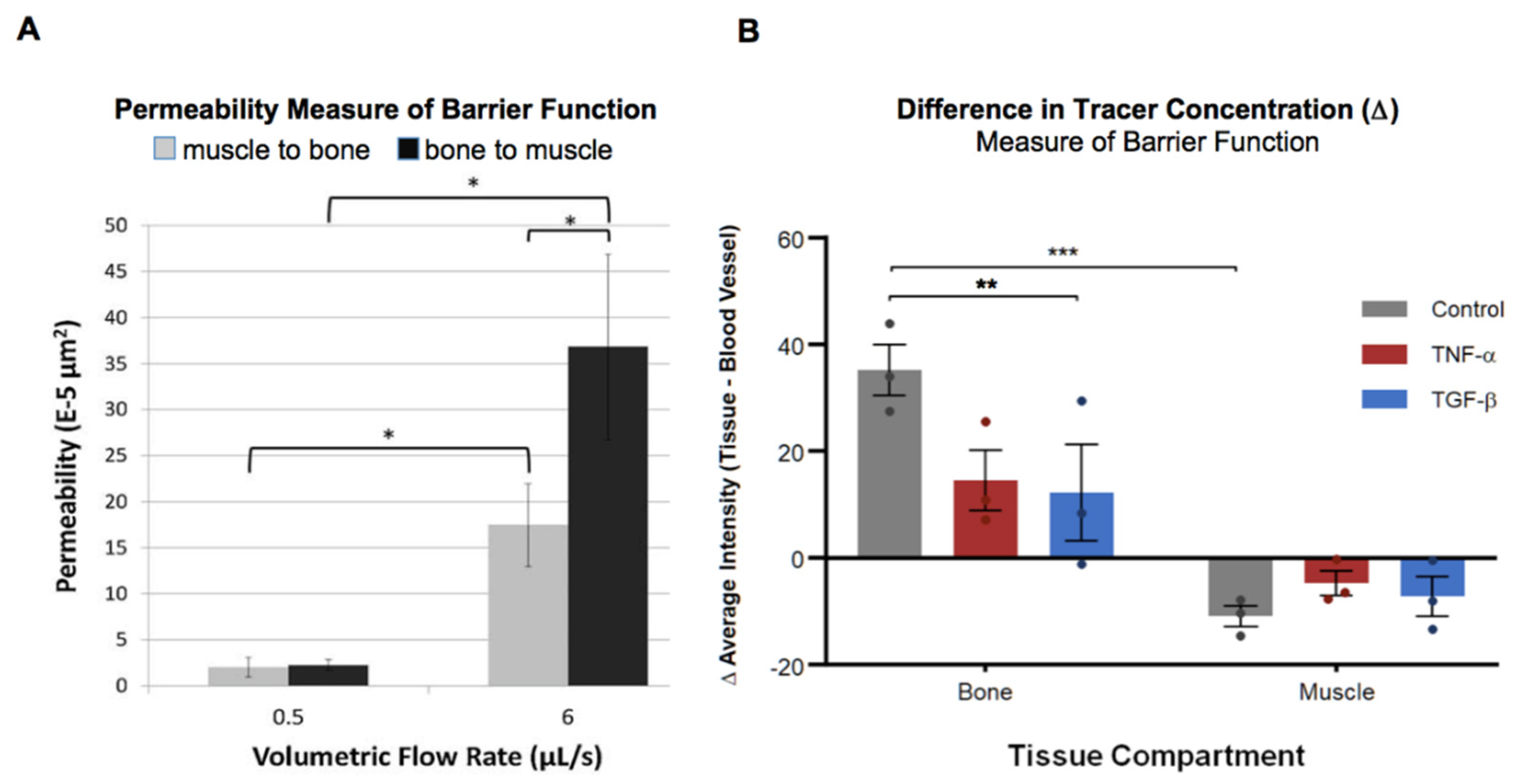
3.1. Immediate Cytokine-Modulated Transport Within the Joint
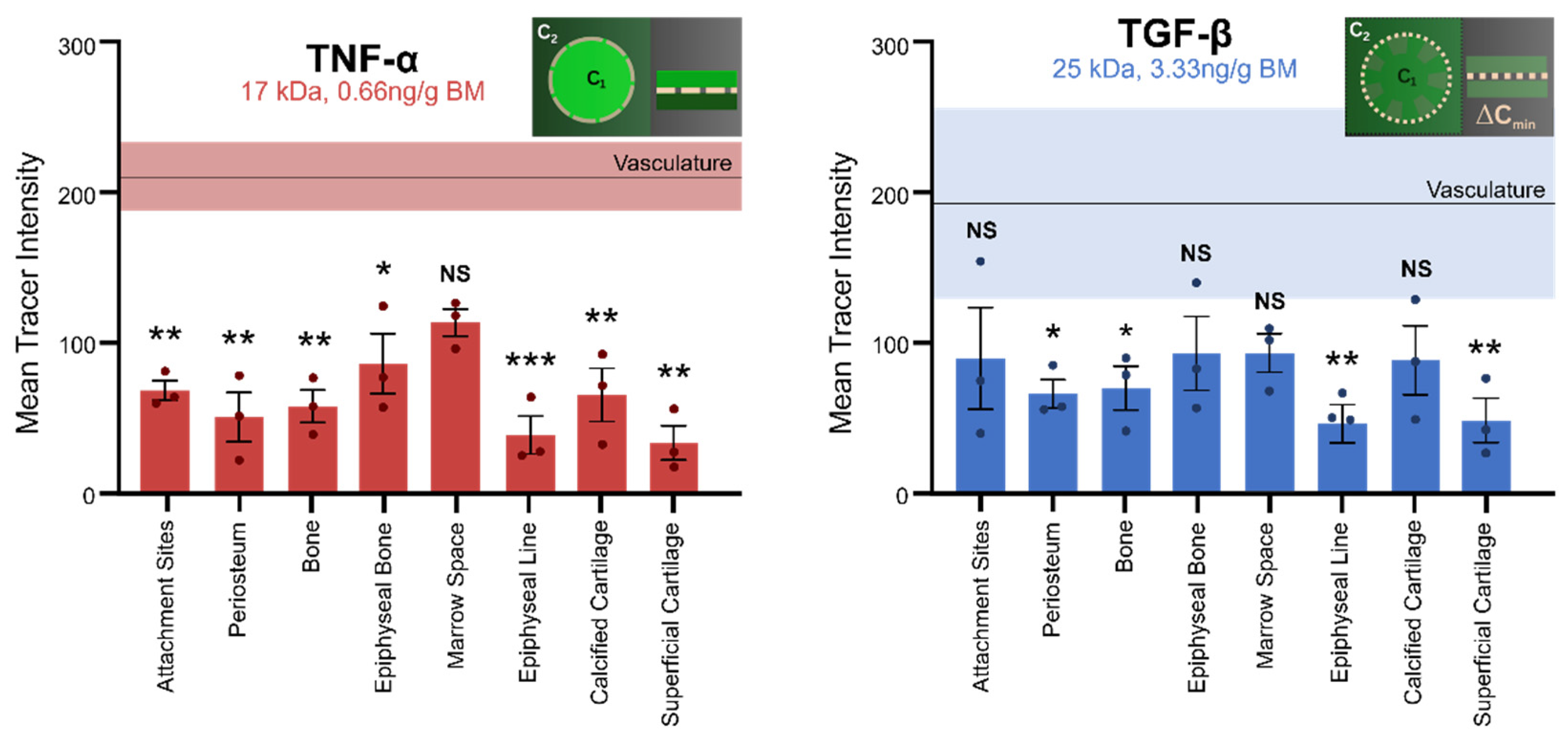
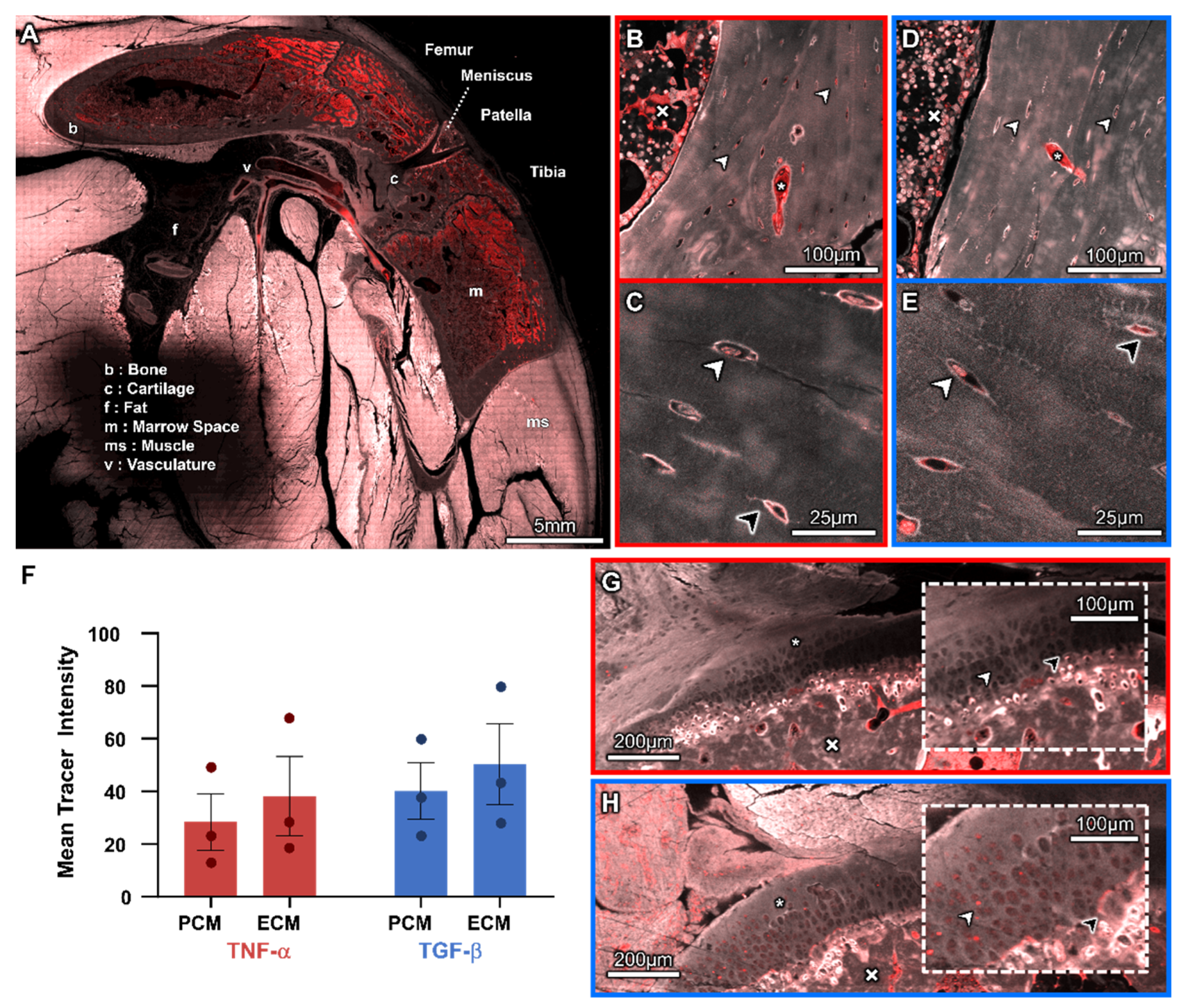
3.2. Molecular Transport from the Heart to the Cellular Inhabitants of the Musculoskeletal System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| ZO-1 | Zonula occludens-1 |
| ARRIVE | Animal research: reporting of in vivo experiments |
| IACUC | Institutional animal care and use committee |
| PMMA | Poly(methyl methacrylate) |
| CNC | Computer numerical control |
| CLSM | Confocal laser scanning microscopy |
| SEM | Scanning electron microscopy |
| BSE-SEM | Backscattered electron scanning electron microscopy |
| API | Application programming interface |
| PCM | Pericellular matrix |
| ECM | Extracellular matrix |
References
- Ngo, L.; Knothe Tate, M.L. A Spike in Circulating Cytokines TNF-α and TGF-β Alters Barrier Function between Vascular and Musculoskeletal Tissues. Sci. Rep. 2023, 13, 9119. [Google Scholar] [CrossRef]
- Knothe Tate, M.L.; Niederer, P.; Knothe, U.R. In Vivo Tracer Transport through the Lacunocanalicular System of Rat Bone in an Environment Devoid of Mechanical Loading. Bone 1998, 22, 107–117. [Google Scholar] [CrossRef]
- Evans, S.F.; Parent, J.B.; Lasko, C.E.; Zhen, X.; Knothe, U.R.; Lemaire, T.; Knothe Tate, M.L. Periosteum, Bone’s “Smart” Bounding Membrane, Exhibits Direction-Dependent Permeability. J. Bone Miner. Res. 2013, 28, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.; Knothe Tate, M.L. Osteoarthritis: New Strategies for Transport and Drug Delivery Across Length Scales. ACS Biomater. Sci. Eng. 2020, 6, 6009–6020. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of Inflammation in the Pathogenesis of Osteoarthritis: Latest Findings and Interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory Mediators in Osteoarthritis: A Critical Review of the State-of-the-Art, Current Prospects, and Future Challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an Inflammatory Disease (Osteoarthritis Is Not Osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M. Osteoarthritis Year in Review 2015: Biology. Osteoarthr. Cartil. 2016, 24, 21–26. [Google Scholar] [CrossRef]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.; Stanciu, G.-D.; Dima, N.; Bădescu, C.; Rezuș, C. The Link Between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef]
- Muñoz-Valle, J.F.; Oregón-Romero, E.; Rangel-Villalobos, H.; Martínez-Bonilla, G.E.; Castañeda-Saucedo, E.; Salgado-Goytia, L.; Leyva-Vázquez, M.A.; Illades-Aguiar, B.; Alarcón-Romero Ldel, C.; Espinoza-Rojo, M.; et al. High Expression of TNF Alpha Is Associated with −308 and −238 TNF Alpha Polymorphisms in Knee Osteoarthritis. Clin. Exp. Med. 2014, 14, 61–67. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, H.-S.; Youn, J.-C.; Shin, E.-C.; Park, S. Serum Cytokine Profiles in Healthy Young and Elderly Population Assessed Using Multiplexed Bead-Based Immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Zhang, L.; Zhou, T.; Li, Y.; Zhou, G.; Miao, Z.; Shang, M.; He, J.; Ding, N.; et al. Duality of Interactions Between TGF-β and TNF-α During Tumor Formation. Front. Immunol. 2022, 12, 810286. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-α Modulation of Intestinal Epithelial Tight Junction Barrier Is Regulated by ERK1/2 Activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Fritton, S.P.; Weinbaum, S. Fluid and solute transport in bone: Flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Zhou, X.; Li, W.; Wang, L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: Direct evidence for load-induced fluid flow. J. Bone Miner. Res. 2011, 26, 277–285. [Google Scholar] [CrossRef]
- Culliton, K.N.; Speirs, A.D. Sliding contact accelerates solute transport into the cartilage surface compared to axial loading. Osteoarthr. Cartil. 2021, 29, 1362–1369. [Google Scholar] [CrossRef]
- Chary, S.R.; Jain, R.K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl. Acad. Sci. USA 1989, 86, 5385–5389. [Google Scholar] [CrossRef]
- Priam, S.; Bougault, C.; Houard, X.; Gosset, M.; Salvat, C.; Berenbaum, F.; Jacques, C. Identification of soluble 14-3-3eta as a novel subchondral bone mediator involved in cartilage degradation in osteoarthritis. Arthritis Rheum. 2013, 65, 1831–1842. [Google Scholar] [CrossRef]
- Wei, F.; Flowerdew, K.; Kinzel, M.; Perotti, L.E.; Asiatico, J.; Omer, M.; Hovell, C.; Reumers, V.; Coathup, M.J. Changes in interstitial fluid flow, mass transport and the bone cell response in microgravity and normogravity. Bone Res. 2022, 10, 65. [Google Scholar] [CrossRef]
- Ngo, L.; Knothe, L.E.; Knothe Tate, M.L. Knee Joint Tissues Effectively Separate Mixed Sized Molecules Delivered in a Single Bolus to the Heart. Sci. Rep. 2018, 8, 10254. [Google Scholar] [CrossRef]
- Ngo, L.; Tate, M.L.K. Multi-Modal Sample Preparation and Imaging Protocol for Nano-to-Mesoscopic Mapping of Cellular Inhabitants in Diverse Tissue Compartments, Across Organ Systems. Protoc. Exch. 2023. [Google Scholar] [CrossRef]
- Tami, A.E.; Schaffler, M.B.; Knothe Tate, M.L. Probing the Tissue to Subcellular Level Structure Underlying Bone’s Molecular Sieving Function. Biorheology 2003, 40, 577–590. [Google Scholar] [CrossRef]
- Tami, A.E.; Nasser, P.; Schaffler, M.B.; Tate, M.L.K. Noninvasive Fatigue Fracture Model of the Rat Ulna. J. Orthop. Res. 2003, 21, 1018–1024. [Google Scholar] [CrossRef]
- Clark, P.R.; Kim, R.K.; Pober, J.S.; Kluger, M.S. Tumor Necrosis Factor Disrupts Claudin-5 Endothelial Tight Junction Barriers in Two Distinct NF-ΚB-Dependent Phases. PLoS ONE 2015, 10, e0120075. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; DeMarco, K.M.; Sanchez-Covarrubias, L.; Solinsky, C.M.; Davis, T.P. Transforming Growth Factor-β Signaling Alters Substrate Permeability and Tight Junction Protein Expression at the Blood-Brain Barrier during Inflammatory Pain. J. Cereb. Blood Flow Metab. 2009, 29, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming Growth Factor-Beta Regulation of Epithelial Tight Junction Proteins Enhances Barrier Function and Blocks Enterohemorrhagic Escherichia coli O157:H7-Induced Increased Permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.F.; Docheva, D.; Bernecker, A.; Colnot, C.; Richter, R.P.; Knothe Tate, M.L. Solid-Supported Lipid Bilayers to Drive Stem Cell Fate and Tissue Architecture Using Periosteum Derived Progenitor Cells. Biomaterials 2013, 34, 1878–1887. [Google Scholar] [CrossRef]
- Oliveira Silva, M.; Gregory, J.L.; Ansari, N.; Stok, K.S. Molecular Signaling Interactions and Transport at the Osteochondral Interface: A Review. Front. Cell Dev. Biol. 2020, 8, 750. [Google Scholar] [CrossRef]
- DiDomenico, C.D.; Lintz, M.; Bonassar, L.J. Molecular transport in articular cartilage—What have we learned from the past 50 years? Nat. Rev. Rheumatol. 2018, 14, 393–403. [Google Scholar] [CrossRef]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone–cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, X.; Li, W.; Novotny, J.E.; Doty, S.B.; Wang, L. In Situ Measurement of Transport between Subchondral Bone and Articular Cartilage. J. Orth. Res. 2009, 27, 1347–1352. [Google Scholar] [CrossRef]
- Arkill, K.P.; Winlove, C.P. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthr. Cartil. 2007, 16, 708–714. [Google Scholar] [CrossRef]
- Maroudas, A. Transport of solutes through cartilage: Permeability to large molecules. J. Anat. 1976, 122, 335–347. [Google Scholar]
- Knothe Tate, M.L.; Tami, A.E.E.; Netrebko, P.; Milz, S.; Docheva, D. Multiscale Computational and Experimental Approaches to Elucidate Bone and Ligament Mechanobiology Using the Ulna-Radius-Interosseous Membrane Construct as a Model System. Technol. Health Care 2012, 20, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Ferdowsian, H.R.; Beck, N. Ethical and scientific considerations regarding animal testing and research. PLoS ONE 2011, 6, e24059. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255–E266. [Google Scholar] [CrossRef] [PubMed]
- Leddy, H.A.; Guilak, F. Site-Specific Molecular Diffusion in Articular Cartilage Measured Using Fluorescence Recovery after Photobleaching. Ann. Biomed. Eng. 2003, 31, 753–760. [Google Scholar] [CrossRef]
- Ngo, L.; Nathanson, A.; Garbowski, T.; Knothe, U.R.; Zeidler, D.; Knothe Tate, M.L. Electron Microscopy Sample Preparation Protocol Enabling Nano-to-Mesoscopic Mapping of Cellular Connectomes and Their Habitats in Human Tissues and Organs. Bio. Protoc. 2019, 9, e3298. [Google Scholar] [CrossRef]
- Hageman, D.; Pereira, A.F.; Zeidler, D.; Knothe, U.R.; Gardner, L.; Knothe Tate, M.L. Cellular Epidemiology of Human Disease Using Biogeographic and Google Maps Approaches—Towards Definition of Cell Network Indices for Rapid Diagnostics. Ann. Biomed. Eng. 2016, 44, 3719. [Google Scholar]
- Knothe Tate, M.L.; Zeidler, D.; Pereira, A.F.; Hageman, D.; Garbowski, T.; Mishra, S.; Gardner, L.; Knothe, U.R. Organ-to-Cell-Scale Health Assessment Using Geographical Information System Approaches with Multibeam Scanning Electron Microscopy. Adv. Healthc. Mater. 2016, 5, 1581–1587. [Google Scholar] [CrossRef]
- Pereira, A.F.; Hageman, D.J.; Garbowski, T.; Riedesel, C.; Knothe, U.R.; Zeidler, D.; Knothe Tate, M.L. Creating High-Resolution Multiscale Maps of Human Tissue Using Multi-Beam SEM. PLoS Comput. Biol. 2016, 12, e1005217. [Google Scholar] [CrossRef]
- Anastopolous, S.; Ngo, L.; Ng, J.; Putra, V.; Knothe Tate, M.L. Interface Tissues of the Mesoderm: Periosteum, Ligament, Interosseous Membrane, & Myofascial Tissues, an Inspiration for next Generation Medical Textiles. Curr. Opin. Biomed. Eng. 2024, 31, 100543. [Google Scholar] [CrossRef]
- Boyde, A. Staining Plastic Blocks with Triiodide to Image Cells and Soft Tissues in Backscattered Electron SEM of Skeletal and Dental Tissues. Eur. Cell Mater. 2012, 24, 154–161. [Google Scholar] [CrossRef]
- Aho, O.-M.; Finnilä, M.; Thevenot, J.; Saarakkala, S.; Lehenkari, P. Subchondral Bone Histology and Grading in Osteoarthritis. PLoS ONE 2017, 12, e0173726. [Google Scholar] [CrossRef]
- Anderson, E.J.; Kreuzer, S.M.; Small, O.; Knothe Tate, M.L. Pairing Computational and Scaled Physical Models to Determine Permeability as a Measure of Cellular Communication in Micro- and Nano-Scale Pericellular Spaces. Microfluid. Nanofluid. 2008, 4, 193–204. [Google Scholar] [CrossRef]
- Sun, Y.; Scannell, B.P.; Honeycutt, P.R.; Mauerhan, D.R.; Norton, J.; Hanley, E.N., Jr. Cartilage Degeneration, Subchondral Mineral and Meniscal Mineral Densities in Hartley and Strain 13 Guinea Pigs. Open Rheumatol. J. 2015, 9, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Salamanna, F.; Martini, L.; Fini, M. Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model. Int. J. Mol. Sci. 2022, 23, 7309. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Caldito, N. Role of tumor necrosis factor-alpha in the central nervous system: A focus on autoimmune disorders. Front. Immun. 2023, 14, 1213448. [Google Scholar] [CrossRef] [PubMed]
- Atis, M.; Akcan, U.; Altunsu, D.; Ayvaz, E.; Yılmaz, C.U.; Sarıkaya, D.; Temizyürek, A.; Ahıshalı, B.; Girouard, H.; Kaya, M. Targeting the blood–brain barrier disruption in hypertension by ALK5/TGF-Β type I receptor inhibitor SB-431542 and dynamin inhibitor dynasore. Brain Res. 2022, 1794, 148071. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.L.; Hwang, J.B.; Park, J.J.; Kim, S.G. Expression of transforming growth factor β1, transforming growth factor type I and II receptors, and TNF-α in the mucosa of the small intestine in infants with food protein–induced enterocolitis syndrome. J. Allergy Clin. Immunol. 2002, 109, 150–154. [Google Scholar] [CrossRef]
- Raschick, M.; Richter, A.; Fischer, L.; Knopf, L.; Schult, A.; Yakupov, R.; Behnisch, G.; Guttek, K.; Düzel, E.; Dunay, I.R.; et al. Plasma concentrations of anti-inflammatory cytokine TGF-β are associated with hippocampal structure related to explicit memory performance in older adults. J. Neural Transm. 2023, 130, 989–1002. [Google Scholar] [CrossRef]
- Masouredis, S.P.; Melcher, L.R. Plasma and “Globulin” Space of Guinea Pigs Determined with I131 Rabbit Globulin. Blood 1951, 78, 264–266. [Google Scholar] [CrossRef]
- Jia, F.; Wang, G.; Xu, J.; Long, J.; Deng, F.; Jiang, W. Role of tumor necrosis factor-α in the mortality of hospitalized patients with severe and critical COVID-19 pneumonia. Aging 2021, 13, 23895–23912. [Google Scholar] [CrossRef]
- Zivancevic-Simonovic, S.; Minic, R.; Cupurdija, V.; Stanojevic-Pirkovic, M.; Milosevic-Djordjevic, O.; Jakovljevic, V.; Mihaljevic, O. Transforming growth factor beta 1 (TGF-β1) in COVID-19 patients: Relation to platelets and association with the disease outcome. Mol. Cell Biochem. 2023, 478, 2461–2471. [Google Scholar] [CrossRef]
- Worrall, N.K.; Chang, K.; Lejeune, W.S.; Misko, T.P.; Sullivan, P.M.; Ferguson, T.B.; Williamson, J.R. TNF-α Causes Reversible in Vivo Systemic Vascular Barrier Dysfunction via NO-Dependent and -Independent Mechanisms. Am. J. Physiol.-Heart Circ. Physiol. 1997, 273, H2565–H2574. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Crum, J.; Jones, Y.Z.; Lanman, J.; Smarr, B.; Terada, M.; Martone, M.E.; Deerinck, T.J.; Johnson, J.E.; Ellisman, M.H. The Combination of Chemical Fixation Procedures with High Pressure Freezing and Freeze Substitution Preserves Highly Labile Tissue Ultrastructure for Electron Tomography Applications. J. Struct. Biol. 2008, 161, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Pryor, P.R. Analyzing Lysosomes in Live Cells. Methods Enzymol. 2012, 505, 145–157. [Google Scholar]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A Model for the Excitation of Osteocytes by Mechanical Loading-Induced Bone Fluid Shear Stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Wang, L.; Ciani, C.; Doty, S.B.; Fritton, S.P. Delineating Bone’s Interstitial Fluid Pathway in Vivo. Bone 2004, 34, 499–509. [Google Scholar] [CrossRef]
- Knothe Tate, M.L.; Steck, R.; Forwood, M.R.; Niederer, P. In Vivo Demonstration of Load-Induced Fluid Flow in the Rat Tibia and Its Potential Implications for Processes Associated with Functional Adaptation. J. Exp. Biol. 2000, 203, 2737–2745. [Google Scholar] [CrossRef]
- Maroudas, A.; Bayliss, M.T.; Venn, M.F. Further Studies on the Composition of Human Femoral Head Cartilage. Ann. Rheum. Dis. 1980, 39, 514–523. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, C.D.; Goodearl, A.; Yarilina, A.; Sun, V.; Mitra, S.; Sterman, A.S.; Bonassar, L.J. The Effect of Antibody Size and Mechanical Loading on Solute Diffusion Through the Articular Surface of Cartilage. J. Biomech. Eng. 2017, 139, 091005. [Google Scholar] [CrossRef] [PubMed]
- Leddy, H.A.; Christensen, S.E.; Guilak, F. Microscale Diffusion Properties of the Cartilage Pericellular Matrix Measured Using 3D Scanning Microphotolysis. J Biomech Eng 2008, 130, 061002. [Google Scholar] [CrossRef] [PubMed]
- Findlay, D.M. Vascular Pathology and Osteoarthritis. Rheumatology 2007, 46, 1763–1768. [Google Scholar] [CrossRef]
- Walshe, T.E.; Saint-Geniez, M.; Maharaj, A.S.R.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. TGF-β Is Required for Vascular Barrier Function, Endothelial Survival and Homeostasis of the Adult Microvasculature. PLoS ONE 2009, 4, e5149. [Google Scholar] [CrossRef]
- Behzadian, M.A.; Wang, X.L.; Windsor, L.J.; Ghaly, N.; Caldwell, R.B. TGF-β Increases Retinal Endothelial Cell Permeability by Increasing MMP-9: Possible Role of Glial Cells in Endothelial Barrier Function. Investig. Ophthalmol. Vis. Sci. 2001, 42, 853–859. [Google Scholar]
- Mark, K.S.; Miller, D.W. Increased Permeability of Primary Cultured Brain Microvessel Endothelial Cell Monolayers Following TNF-α Exposure. Life Sci. 1999, 64, 1941–1953. [Google Scholar] [CrossRef]
- Petrache, I.; Birukova, A.; Ramirez, S.I.; Garcia, J.G.N.; Verin, A.D. The Role of the Microtubules in Tumor Necrosis Factor-α–Induced Endothelial Cell Permeability. Am. J. Respir. Cell Mol. Biol. 2003, 28, 574–581. [Google Scholar] [CrossRef]
- Findlay, D.M.; Kuliwaba, J.S. Bone–Cartilage Crosstalk: A Conversation for Understanding Osteoarthritis. Bone Res. 2016, 4, 16028. [Google Scholar] [CrossRef]
- Leduc, M.; Guay, D.; Leask, R.L.; Coulombe, S. Cell permeabilization using a non-thermal plasma. New J. Phys. 2009, 11, 115021. [Google Scholar] [CrossRef]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Ren. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef] [PubMed]
- Knothe Tate, M.L.; Srikantha, A.; Wojek, C.; Zeidler, D. Connectomics of Bone to Brain—Probing Physical Renderings of Cellular Experience. Front. Physiol. 2021, 12, 647603. [Google Scholar] [CrossRef]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Biomechanical, Biophysical and Biochemical Modulators of Cytoskeletal Remodelling and Emergent Stem Cell Lineage Commitment. Commun. Biol. 2023, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Stem cell mechanoadaptation. I. Effect of microtubule stabilization and volume changing stresses on cytoskeletal remodeling. APL Bioeng. 2025, 9, 016102. [Google Scholar] [CrossRef]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Stem cell mechanoadaptation. II. Microtubule stabilization and substrate compliance effects on cytoskeletal remodeling. APL Bioeng. 2025, 9, 016103. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, L.; Tate, M.L.K. TGF-beta Increases Permeability of 70 kDa Molecular Tracer from the Heart to Cells of the Osteoarthritic Guinea Pig Knee Joint. Cells 2025, 14, 1524. https://doi.org/10.3390/cells14191524
Ngo L, Tate MLK. TGF-beta Increases Permeability of 70 kDa Molecular Tracer from the Heart to Cells of the Osteoarthritic Guinea Pig Knee Joint. Cells. 2025; 14(19):1524. https://doi.org/10.3390/cells14191524
Chicago/Turabian StyleNgo, Lucy, and Melissa L. Knothe Tate. 2025. "TGF-beta Increases Permeability of 70 kDa Molecular Tracer from the Heart to Cells of the Osteoarthritic Guinea Pig Knee Joint" Cells 14, no. 19: 1524. https://doi.org/10.3390/cells14191524
APA StyleNgo, L., & Tate, M. L. K. (2025). TGF-beta Increases Permeability of 70 kDa Molecular Tracer from the Heart to Cells of the Osteoarthritic Guinea Pig Knee Joint. Cells, 14(19), 1524. https://doi.org/10.3390/cells14191524





