Heat Shock Proteins in Gastrointestinal and Lung Neuroendocrine Neoplasm: Diagnostic and Therapeutic Perspectives
Abstract
1. Introduction
2. The Aim of the Study
3. The Significance of HSPs in the Diagnosis and Therapy of Well-Differentiated Neuroendocrine Tumors of the Lung and Small Cell Lung Cancer
3.1. Typical and Atypical Carcinoid Tumors
3.2. Small Cell Lung Cancer
3.2.1. The Role of HSP70 and HSP90 in the Proliferation, Invasiveness and Chemo-Resistance of SCLCs
3.2.2. HSP70 as a Diagnostic and Prognostic Marker for SCLC
3.2.3. The Potential Utility of Heat Shock Proteins
3.3. Neuroendocrine Tumors of the Gastrointestinal Tract (GEP-NET) and Pancreas (Pan-NET)
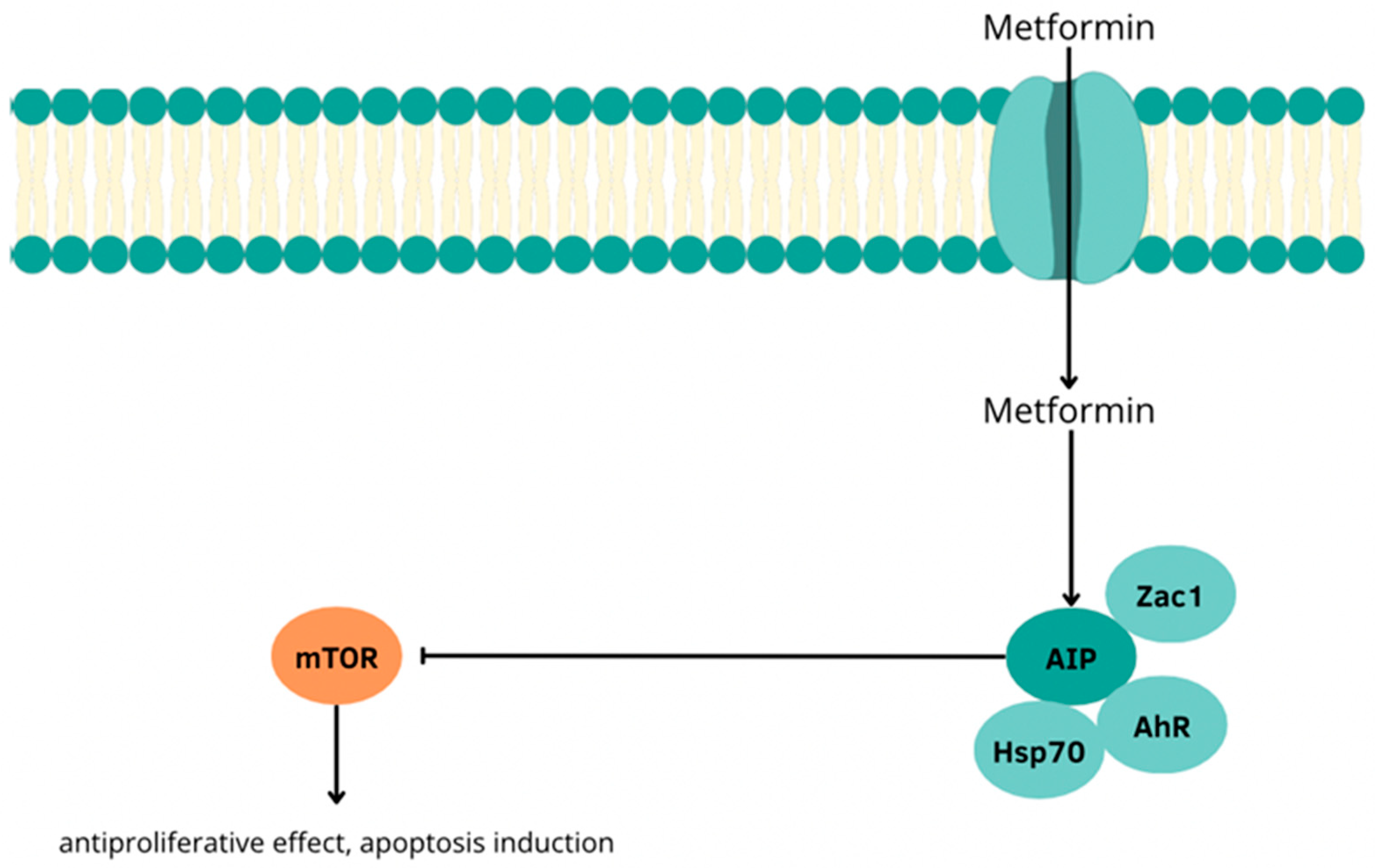
3.4. The Use of Metformin in the Treatment of P-NETs and Its Interactions with Heat Shock Proteins
3.5. Application of HSP90 in Positron Emission Tomography (PET)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsushima-Nishiwaki, R.; Toyoda, H.; Nagasawa, T.; Yasuda, E.; Chiba, N.; Okuda, S.; Maeda, A.; Kaneoka, Y.; Kumada, T.; Kozawa, O. Phosphorylated Heat Shock Protein 20 (HSPB6) Regulates Transforming Growth Factor-α-Induced Migration and Invasion of Hepatocellular Carcinoma Cells. PLoS ONE 2016, 11, e0151907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edwards, H.V.; Cameron, R.T.; Baillie, G.S. The emerging role of HSP20 as a multifunctional protective agent. Cell. Signal. 2011, 23, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Matsushima-Nishiwaki, R.; Toyoda, H.; Matsuura, J.; Kumada, T.; Kozawa, O. Heat shock protein 20 (HSPB6) regulates apoptosis in human hepatocellular carcinoma cells: Direct association with Bax. Oncol. Rep. 2014, 32, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qian, X.; Sha, B. Heat shock protein 40: Structural studies and their functional implications. Protein Pept. Lett. 2009, 16, 606–612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, B.X.; Shen, F.M.; Chen, J.K. The role of HSP40 in cancer: Recent advances. Histol. Histopathol. 2024, 39, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Shin, Y.; Han, S.; Ha, J.; Tiwari, P.K.; Kim, S.S.; Kang, I. Molecular Chaperonin HSP60: Current Understanding and Future Prospects. Int. J. Mol. Sci. 2024, 25, 5483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.; Xiao, H.; Cao, L. Recent advances in heat shock proteins in cancer diagnosis, prognosis, metabolism and treatment. Biomed. Pharmacother. 2021, 142, 112074. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Fan, S.; Wen, Q. The multiple roles and therapeutic potential of HSP60 in cancer. Biochem. Pharmacol. 2022, 201, 115096. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfister, K.; Radons, J.; Busch, R.; Tidball, J.G.; Pfeifer, M.; Freitag, L.; Feldmann, H.J.; Milani, V.; Issels, R.; Multhoff, G. Patient survival by Hsp70 membrane phenotype: Association with different routes of metastasis. Cancer 2007, 110, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Lampros, M.; Vlachos, N.; Voulgaris, S.; Alexiou, G.A. The Role of Hsp27 in Chemotherapy Resistance. Biomedicines 2022, 10, 897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santiago-O’Farrill, J.M.; Kleinerman, E.S.; Hollomon, M.G.; Livingston, A.; Wang, W.L.; Tsai, J.W.; Gordon, N.B. Phosphorylated heat shock protein 27 as a potential biomarker to predict the role of chemotherapy-induced autophagy in osteosarcoma response to therapy. Oncotarget 2017, 9, 1602–1616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Murshid, A.; Prince, T.L.; Lang, B.; Calderwood, S.K. Role of Heat Shock Factors in Stress-Induced Transcription. Methods Mol. Biol. 2018, 1709, 23–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pfister, J.A.; Mallick, S.; D’Mello, S.R. HSF1 protects neurons through a novel trimerization- and HSP-independent mechanism. J. Neurosci. 2014, 34, 1599–1612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujimoto, M.; Nakai, A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010, 277, 4112–4125. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pawlikowska, M.; Deptuła, W. Udział i rola białek szoku termicznego (HSP) w patogenezie chlamydioz i chlamydofiloz u ludzi i zwierząt. Postępy Biol. Komórki 2009, 36, 419–428. [Google Scholar]
- Boliukh, I.; Rombel-Bryzek, A.; Radecka, B. Immunological aspects of heat shock protein functions and their significance in the development of cancer vaccines. Nowotw. J. Oncol. 2022, 72, 174–183. [Google Scholar] [CrossRef]
- Fontana, J.; Fulton, D.; Chen, Y.; Fairchild, T.A.; McCabe, T.J.; Fujita, N.; Tsuruo, T.; Sessa, W.C. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 2002, 90, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Meyrick, B. Hypoxia increases Hsp90 binding to eNOS via PI3K-Akt in porcine coronary artery endothelium. Lab. Investig. 2004, 84, 182–190. [Google Scholar] [CrossRef]
- Milani, V.; Noessner, E.; Ghose, S.; Kuppner, M.; Ahrens, B.; Scharner, A.; Gastpar, R.; Issels, R.D. Heat shock protein 70: Role in antigen presentation and immune stimulation. Int. J. Hyperth. 2002, 18, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, M.K.; Shin, Y.; Ju, S.; Han, S.; Choe, W.; Yoon, K.-S.; Kim, S.S.; Kang, I. Heat Shock Response and Heat Shock Proteins: Current Understanding and Future Opportunities in Human Diseases. Int. J. Mol. Sci. 2024, 25, 4209. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Charagh, S.; Dong, N.; Lu, F.; Wang, Y.; Cao, R.; Ma, L.; Wang, S.; Jiao, G.; Xie, L.; et al. Genome-Wide Analysis of Heat Shock Protein Family and Identification of Their Functions in Rice Quality and Yield. Int. J. Mol. Sci. 2024, 25, 11931. [Google Scholar] [CrossRef]
- Dutta, S.K.; Girotra, M.; Singla, M.; Dutta, A.; Otis Stephen, F.; Nair, P.P.; Merchant, N.B. Serum HSP70: A novel biomarker for early detection of pancreatic cancer. Pancreas 2012, 41, 530–534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuramitsu, Y.; Wang, Y.; Taba, K.; Suenaga, S.; Ryozawa, S.; Kaino, S.; Sakaida, I.; Nakamura, K. Heat-shock protein 27 plays the key role in gemcitabine-resistance of pancreatic cancer cells. Anticancer Res. 2012, 32, 2295–2299. [Google Scholar] [PubMed]
- Somu, P.; Mohanty, S.; Basavegowda, N.; Yadav, A.K.; Paul, S.; Baek, K.-H. The Interplay between Heat Shock Proteins and Cancer Pathogenesis: A Novel Strategy for Cancer Therapeutics. Cancers 2024, 16, 638. [Google Scholar] [CrossRef]
- Giri, B.; Sethi, V.; Modi, S.; Garg, B.; Banerjee, S.; Saluja, A.; Dudeja, V. Heat shock protein 70 in pancreatic diseases: Friend or foe. J. Surg. Oncol. 2017, 116, 114–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sultana, Q.; Kar, J.; Verma, A.; Sanghvi, S.; Kaka, N.; Patel, N.; Sethi, Y.; Chopra, H.; Kamal, M.A.; Greig, N.H. A Comprehensive Review on Neuroendocrine Neoplasms: Presentation, Pathophysiology and Management. J. Clin. Med. 2023, 12, 5138. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, D.S.; Beltran, H.; Lilenbaum, R.; Lilenbaum, R.; Bergsland, E.K. The Spectrum of Neuroendocrine Tumors: Histologic Classification, Unique Features and Areas of Overlap. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 92–103. [Google Scholar] [CrossRef]
- Rothenstein, J.; Cleary, S.P.; Pond, G.R.; Dale, D.; Gallinger, S.; Moore, M.J.; Brierley, J.D.; Siu, L.L. Neuroendocrine Tumors of the Gastrointestinal Tract: A Decade of Experience at the Princess Margaret Hospital. Am. J. Clin. Oncol. 2008, 31, 64–70. [Google Scholar] [CrossRef]
- Silveira, F.; Basile, M.L.; Kuga, F.S.; Próspero, J.D.; Paes, R.A.P.; Paes, R.P.; Bernardi, F.D.C. Neuroendocrine Tumors: An Epidemiological Study of 250 Cases at a Tertiary Hospital. Rev. Assoc. Med. Bras. 2017, 63, 856–861. [Google Scholar] [CrossRef]
- Centonze, G.; Maisonneuve, P.; Simbolo, M.; Lagano, V.; Grillo, F.; Fabbri, A.; Prinzi, N.; Garzone, G.; Filugelli, M.; Pardo, C.; et al. Lung carcinoid tumours: Histology and Ki-67, the eternal rivalry. Histopathology 2023, 82, 324–339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granberg, D.; Juhlin, C.C.; Falhammar, H.; Hedayati, E. Lung Carcinoids: A Comprehensive Review for Clinicians. Cancers 2023, 15, 5440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niinimäki, J.; Sihto, H.; Arola, J.; Vesterinen, T. HSP90 expression is associated with outcome in pulmonary carcinoid tumor patients. Transl. Lung Cancer Res. 2023, 12, 1876–1886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zitzmann, K.; Ailer, G.; Vlotides, G.; Spoettl, G.; Maurer, J.; Göke, B.; Beuschlein, F.; Auernhammer, C.J. Potent antitumor activity of the novel HSP90 inhibitors AUY922 and HSP990 in neuroendocrine carcinoid cells. Int. J. Oncol. 2013, 43, 1824–1832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, Y.; Wu, J.; Luo, L. Secreted Heat Shock Protein 90α Attenuated the Effect of Anticancer Drugs in Small-Cell Lung Cancer Cells Through AKT/GSK3β/β-Catenin Signaling. Cancer Control 2018, 25, 1073274818804489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balázs, M.; Zsolt, H.; László, G.; Gabriella, G.; Lilla, T.; Gyula, O.; Balázs, D.; Éva, M.; Zoltán, B.; Zoltán, P.; et al. Serum Heat Shock Protein 70, as a Potential Biomarker for Small Cell Lung Cancer. Pathol. Oncol. Res. 2017, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, M.H.; Park, I.; Jeon, H.; Choi, J.; Seo, S.; Kim, S.W.; Koh, G.Y.; Park, K.S.; Lee, D.H. HSP90 inhibitor (NVP-AUY922) enhances the anti-cancer effect of BCL-2 inhibitor (ABT-737) in small cell lung cancer expressing BCL-2. Cancer Lett 2017, 411, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.S.; Warner, E.A.; Giaccone, G. Ganetespib for small cell lung cancer. Expert Opin. Investig. Drugs 2016, 26, 103–108. [Google Scholar] [CrossRef]
- Pillai, R.N.; Fennell, D.A.; Kovcin, V.; Ciuleanu, T.E.; Ramlau, R.; Kowalski, D.; Schenker, M.; Yalcin, I.; Teofilovici, F.; Vukovic, V.M.; et al. Randomized Phase III Study of Ganetespib, a Heat Shock Protein 90 Inhibitor, With Docetaxel Versus Docetaxel in Advanced Non-Small-Cell Lung Cancer (GALAXY-2). J. Clin. Oncol. 2020, 38, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Goss, G.; Rosell, R.; Schmid-Bindert, G.; Zaric, B.; Andric, Z.; Bondarenko, I.; Komov, D.; Ceric, T.; Khuri, F.; et al. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1). Ann. Oncol. 2015, 26, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.S.; Liu, S.V.; Crawford, J.; Kramer, J.; Thompson, J.; Wang, H.; Giaccone, G. A Phase Ib/II Study of Ganetespib with Doxorubicin in Advanced Solid Tumors Including Relapsed-Refractory Small Cell Lung Cancer. Front. Oncol. 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaponova, A.V.; Nikonova, A.S.; Deneka, A.; Kopp, M.C.; Kudinov, A.E.; Skobeleva, N.; Khazak, V.; Ogawa, L.S.; Cai, K.Q.; Duncan, K.E.; et al. A Novel HSP90 Inhibitor-Drug Conjugate to SN38 Is Highly Effective in Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 5120–5129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Gong, Y.F.; Zhuang, H.K.; Zhou, Z.X.; Huang, S.Z.; Zou, Y.P.; Huang, B.W.; Sun, Z.H.; Zhang, C.Z.; Tang, Y.Q.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mpilla, G.B.; Philip, P.A.; El-Rayes, B.; Azmi, A.S. Pancreatic neuroendocrine tumors: Therapeutic challenges and research limitations. World J. Gastroenterol. 2020, 26, 4036–4054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, H.L.; Wang, W.Q.; Xu, H.X.; Wu, C.T.; Li, H.; Ni, Q.X.; Yu, X.J.; Liu, L. Active surveillance in metastatic pancreatic neuroendocrine tumors: A 20-year single-institutional experience. World J. Clin. Cases 2020, 8, 3751–3762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gamboa, A.C.; Ethun, C.G.; Postlewait, L.M.; Lopez-Aguiar, A.G.; Zhelnin, K.; Krasinskas, A.; El-Rayes, B.F.; Russell, M.C.; Kooby, D.A.; Staley, C.A.; et al. HSP90 expression and early recurrence in gastroenteropancreatic neuroendocrine tumors: Potential for a novel therapeutic target. Surg. Oncol. 2020, 35, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Lundsten, S.; Spiegelberg, D.; Stenerlöw, B.; Nestor, M. The HSP90 inhibitor onalespib potentiates 177Lu-DOTATATE therapy in neuroendocrine tumor cells. Int. J. Oncol. 2019, 55, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lundsten, S.; Spiegelberg, D.; Raval, N.R.; Nestor, M. The radiosensitizer Onalespib increases complete remission in 177Lu-DOTATATE-treated mice bearing neuroendocrine tumor xenografts. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 980–990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gloesenkamp, C.; Nitzsche, B.; Lim, A.R.; Normant, E.; Vosburgh, E.; Schrader, M.; Ocker, M.; Scherubl, H.; Hopfner, M. Heat shock protein 90 is a promising target for effective growth inhibition of gastrointestinal neuroendocrine tumours. Int. J. Oncol. 2012, 40, 1659–1667. [Google Scholar] [PubMed][Green Version]
- Aristizabal Prada, E.T.; Auernhammer, C.J. Targeted therapy of gastroenteropancreatic neuroendocrine tumours: Preclinical strategies and future targets. Endocr. Connect. 2018, 7, R1–R25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilbert, J.A.; Adhikari, L.J.; Lloyd, R.V.; Halfdanarson, T.R.; Muders, M.H.; Ames, M.M. Markery molekularne dla nowych strategii terapeutycznych w nowotworach endokrynnych trzustki. Pancreas 2013, 42, 411–421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofving, T.; Sandblom, V.; Arvidsson, Y.; Shubbar, E.; Altiparmak, G.; Swanpalmer, J.; Almobarak, B.; Elf, A.K.; Johanson, V.; Elias, E.; et al. 177Lu-octreotate therapy for neuroendocrine tumours is enhanced by Hsp90 inhibition. Endocr. Relat. Cancer 2019, 26, 437–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitali, E.; Boemi, I.; Piccini, S.; Tarantola, G.; Smiroldo, V.; Lavezzi, E.; Brambilla, T.; Zerbi, A.; Carnaghi, C.; Mantovani, G.; et al. A novel insight into the anticancer mechanism of metformin in pancreatic neuroendocrine tumor cells. Mol. Cell. Endocrinol. 2020, 509, 110803. [Google Scholar] [CrossRef] [PubMed]
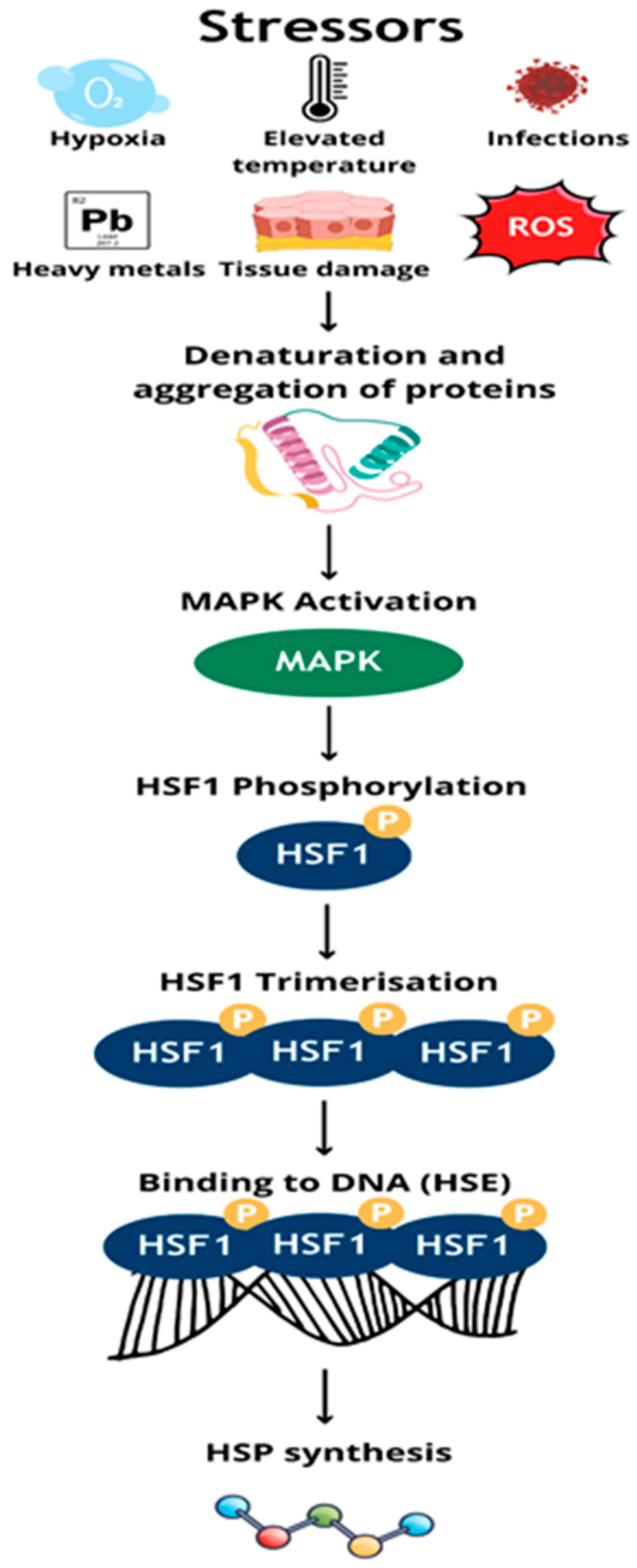
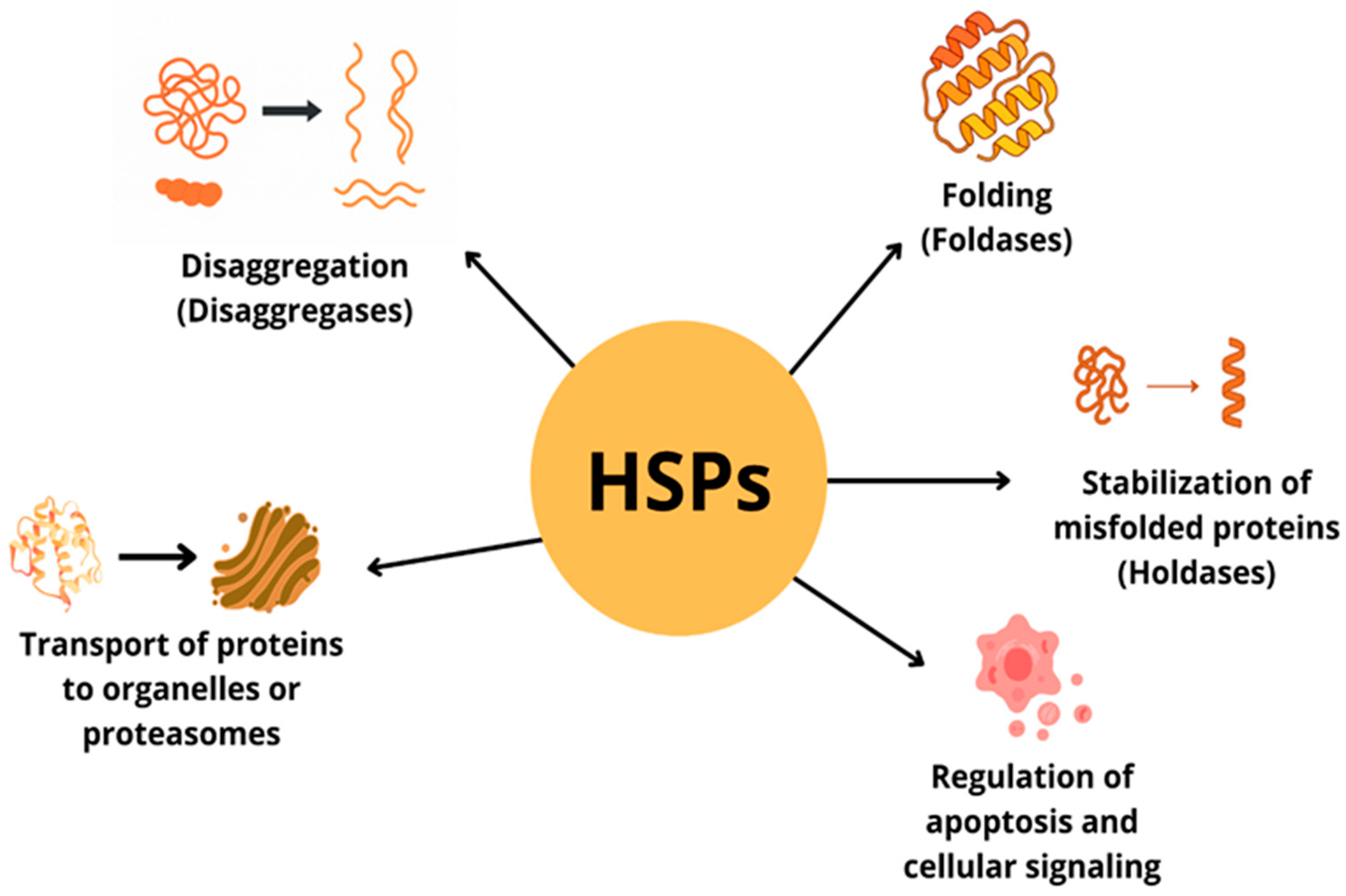
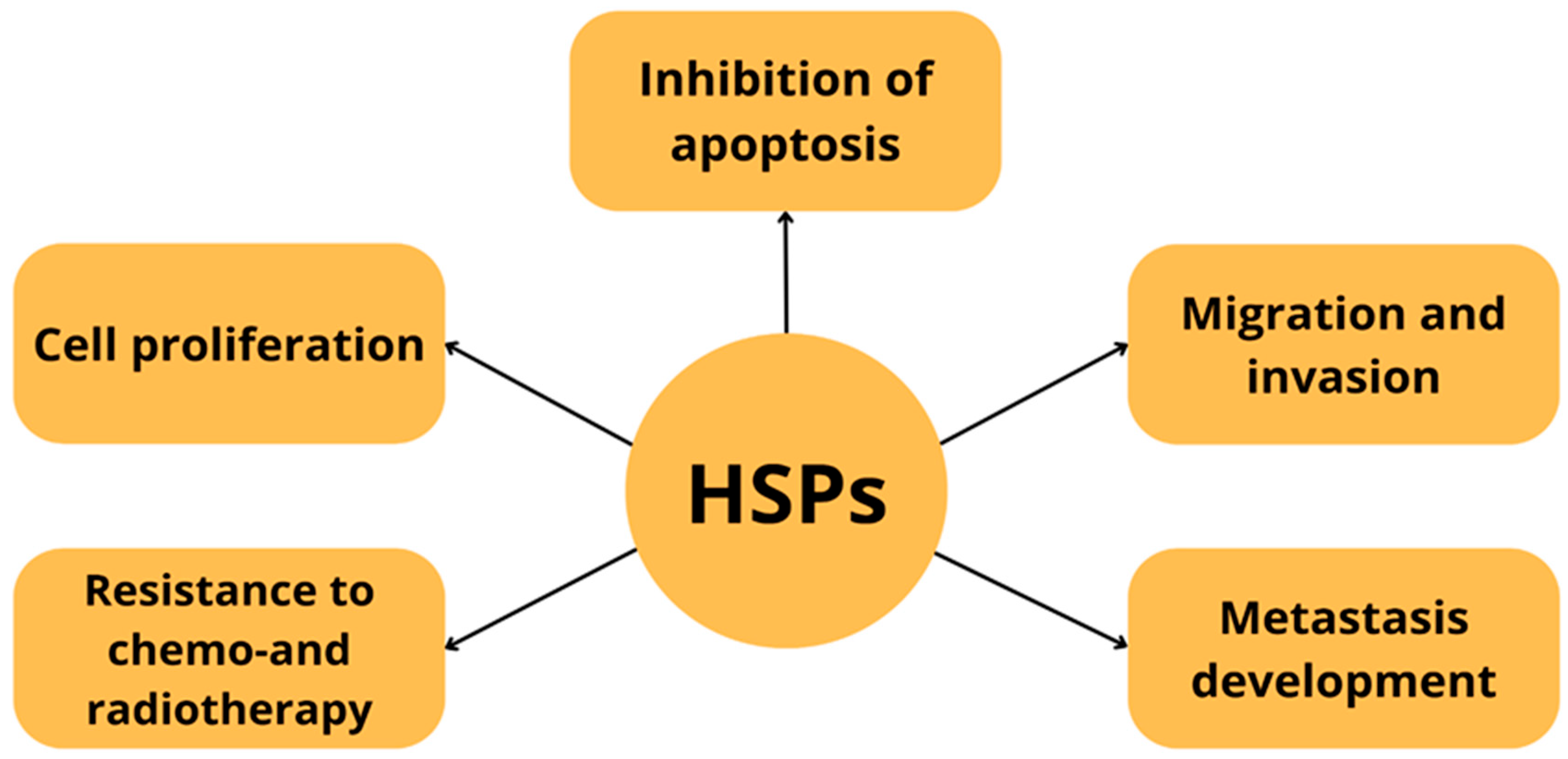
| Protein Family Name | Function Performed | Role in Carcinogenesis |
|---|---|---|
| HSP20 | Hsp20 is expressed in many tissues, but is most abundant in muscle including heart muscle In the heart, it protects against ischemia-induced damage and β-agonist-dependent remodeling, as well as apoptosis. In addition, it inhibits aggregation and activation of human platelets outside the cell. It is an inhibitor of apoptosis [3,4]. | Expression of the HSP20 protein can inhibit HCC cell growth by both reducing cell proliferation signals and activating the apoptosis pathway [5]. |
| HSP40 (family DnaJ) | It is the largest and most diverse subgroup of the HSP family. The Hsp40 protein acts in specific pairing with the Hsp70 protein to regulate the ATPase activity of HSP70. It is involved in promoting protein folding, translocation and degradation [1,6]. | The HSP40 family plays an important role in the development, progression, metastasis and chemoresistance of various malignancies [7]. Overexpression of the HSP40 family has been demonstrated in various cancers including colorectal, breast, prostate, ovarian, liver, head and neck cancers. HSP40 acts not only in tandem with HSP70 but also as an indirect regulator of the HSP90 complex, with roles in both pro- and anti-tumor processes [7]. |
| HSP60 (chaperonin\Cpn60) | Localized mainly in mitochondria (80–85%), HSP60 is responsible for ATP-dependent protein folding. In addition, it is involved in the refolding and degradation of mitochondrial proteins. The cytoplasmic form of HSP60 is involved in cell signaling in various cell types, such as cardiomyocytes and hepatocytes [8]. | HSP60 is overexpressed in cancers such as colorectal cancer, non-small cell lung cancer (NSCLC), breast cancer and hepatocellular carcinoma (HCC), while at the same time HSP60 levels are lower in bladder cancer and clear cell renal cell carcinoma [8,9,10]. HSP60 has been linked to cancer pathogenesis and progression at the level of various mechanisms. The cytoplasmic form of HSP60 is involved in cell signaling in various cell types, such as cardiomyocytes and hepatocytes [8]. |
| HSP70 | Responsible for the folding of non-native proteins, prevents their aggregation [11]. Controls the quality of misfolded proteins and is responsible for co-translational and post-translational folding of de novo nascent proteins [11]. | Stimulates both innate and adaptive immune response expression correlates with better prognosis in gastric and colorectal cancer and poor prognosis in lower rectal and squamous cell carcinoma [12]. |
| HSP90 | Key regulator of proteostasis under both physiological and stress conditions in eukaryotic cells. Participates in DNA repair, immune response. It has a large number of co-chaperones [13]. | Associated with tumor cell invasion and migration. By increasing transcription and expression of vascular endothelial growth factor receptor (VEGFR), it is involved in angiogenesis of cell proliferation, migration and invasion. The expression level of HSP90 has been recognized as a potential biomarker of poor prognosis in lung cancer or esophageal cancer, gastrointestinal neuroendocrine tumors [9]. |
| HSP27 | ATP-independent, small molecule chaperone. Hsp27 phosphorylation is involved in cell migration, modulation of cell cycle progression through inhibition of the MEK/ERK signaling pathway or interaction with p53 and inhibition of apoptosis [14]. | Overexpressed in many cancer cell types Increased Hsp27 expression or phosphorylation is associated with chemotherapeutic resistance, tumor progression and metastatic potential. Associated with poor prognosis in gastric, liver, prostate cancer and osteosarcoma [2,15]. |
| Authors of the Study | Trial Model | Results |
|---|---|---|
| Niinimäki J et al. [42] | Patients with pulmonary carcinoid tumor | HSP90AB1 expression was significantly elevated in metastatic PC tumors (p < 0.0001) HSP90 protein expression was associated with shorter disease-specific survival (DSS) (p = 0.009) and increased risk of disease-specific death |
| Zitzmann et al. [43] | Neuroendocrine tumor cells of pancreatic (BON1), midgut (GOT1) and bronchopulmonary origin (NCI-H727). | The HSP90 inhibitors AUY922 and HSP990 inhibited the viability of BON1 cells, NCI-H727 and GOT1 cells. Inhibition of HSP90 induces apoptosis in neuroendocrine tumor cells. Inhibition of HSP90 in human BON1 pancreatic cells was associated with a significant increase in the number of cells in the G2/M phase, while no effect on cell cycle distribution was observed in NCI-H727 and GOT1 cells. |
| A.C. Gamboa et al. [59] | Patients with non-metastatic GEP NETs and GEP NETs with liver metastases. | Among patients who underwent resection (R0 or R1) due to primary, non-metastatic GEP-NET patients with high HSP90 expression had lower 1- and 3-year survival rates compared to patients with low HSP90 expression. High HSP90 expression is associated with poorer recurrence-free survival. Patients with high HSP90 expression and liver metastases had lower 1- and 3-year survival rates compared to patients with low HSP90 expression. |
| Lundsten et al. [60] | NET cell lines BON (cells from lymph node metastasis of a carcinoid tumor of the pancreas), NCI-H727 (neuroendocrine cell line derived from a human lung carcinoidand), NCI-H460 (large cell lung carcinoma human cell line with neuroendocrine features). | Onalespib was able to synergistically enhance the treatment of 177 Lu-DOTATATE in a manner specific to SSTR. The mechanisms of radiosensitivity of onalespib included a reduction in EGFR expression and the induction of apoptosis. |
| Lundsten S et al. [61] | NET cell lines BON, established from a lymph node metastasis of a pancreatic carcinoid tumor Squamous cell carcinoma cell line UM-SCC-74B | Potentiation of 177 Lu-DOTATATE in a neuroendocrine tumor xenograft model, resulting in delayed tumor growth, increased total remission rates, and reduced renal toxicity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabut, J.; Sokołowski, J.; Żelazna, W.; Stępień, M.; Strauchman, M.; Jaworska, N.; Gorzelak-Magiera, A.; Wnuk, J.; Gisterek-Grocholska, I. Heat Shock Proteins in Gastrointestinal and Lung Neuroendocrine Neoplasm: Diagnostic and Therapeutic Perspectives. Cells 2025, 14, 1501. https://doi.org/10.3390/cells14191501
Kabut J, Sokołowski J, Żelazna W, Stępień M, Strauchman M, Jaworska N, Gorzelak-Magiera A, Wnuk J, Gisterek-Grocholska I. Heat Shock Proteins in Gastrointestinal and Lung Neuroendocrine Neoplasm: Diagnostic and Therapeutic Perspectives. Cells. 2025; 14(19):1501. https://doi.org/10.3390/cells14191501
Chicago/Turabian StyleKabut, Jacek, Jakub Sokołowski, Wiktoria Żelazna, Mateusz Stępień, Marta Strauchman, Natalia Jaworska, Anita Gorzelak-Magiera, Jakub Wnuk, and Iwona Gisterek-Grocholska. 2025. "Heat Shock Proteins in Gastrointestinal and Lung Neuroendocrine Neoplasm: Diagnostic and Therapeutic Perspectives" Cells 14, no. 19: 1501. https://doi.org/10.3390/cells14191501
APA StyleKabut, J., Sokołowski, J., Żelazna, W., Stępień, M., Strauchman, M., Jaworska, N., Gorzelak-Magiera, A., Wnuk, J., & Gisterek-Grocholska, I. (2025). Heat Shock Proteins in Gastrointestinal and Lung Neuroendocrine Neoplasm: Diagnostic and Therapeutic Perspectives. Cells, 14(19), 1501. https://doi.org/10.3390/cells14191501






