Pericyte Expression of VEGF-A Minimally Impacts Ocular Vascular Development and Neovascularization
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals
2.3. Trypsin-Digested Retinal Vessel Preparation
2.4. Visualization of Retinal Vasculature
2.5. Fundus Imaging
2.6. Laser-Induced Choroidal Neovascularization (CNV) and OIR
2.7. RNA Purification and Real-Time qPCR Analysis
2.8. Data Analysis
| Gene | Forward | Reverse |
|---|---|---|
| Vegfa | 5′-GGAGAGCAGAAGTCCCATGA-3′ | 5′-ACTCCAGGGCTTCATCGTTA-3′ |
| Vegfb | 5′-TGGTGCCATGGATAGACGTT-3′ | 5′-TTGTTTGACCACATTGCCCA-3′ |
| Vegfc | 5′-GTGTGCGAATCGACTGAAGC-3′ | 5′-GTTCAGATGTGGCCTTTTCCA-3′ |
| Vegfr1 | 5′-GGCCCGGGATATTTATAAGAAC-3′ | 5′-CCATCCATTTTAGGGGAAGTC-3′ |
| Vegfr2 | 5′-CCCCAAATTCCATTATGACAA-3′ | 5′-CGGCTCTTTCGCTTACTGTT-3′ |
| Vegfr3 | 5′-CCATCTCAACGTGGTCAACC-3′ | 5′-AAGTTGGAGAGGTTGCCGTA-3′ |
| Pdgfb | 5′-CTCGGCCTGTGACTAGAAGT-3′ | 5′-GGATTCTCACCGTCCGAATG-3′ |
| Pdgfrb | 5′-ATCGCGCCACCTTAATCAAC-3′ | 5′-GCTAAGAAGTCCATGCCGTT-3′ |
| Angpt1 | 5′-GTGCAGCAACCAGCGCCGAA-3′ | 5′-CGCACTCTCACGGCAGTTCCC-3′ |
| Angpt2 | 5′-CCGGTCAGCACCGCTACGTG-3′ | 5′-ATGCGCCTCGTTGCCTTCCC-3′ |
| Tek/Tie2 | 5′-TCCAACATCACTGACTCCACA-3′ | 5′-GCCCTGAACCTTATACCGGA-3′ |
| Acta2/Sma | 5′-GGCATCCACGAAACCACC-3′ | 5′-CATGGTGGTACCCCCTGAC-3′ |
| Cspg4/Ng2 | 5′-CTCACGAGCCCCTGTATCTC-3′ | 5′-GGGTGCCCTCTGTACTTCAT-3′ |
| Mmrn2 | 5′-CATCACCGGGTTCCAGTCTA-3′ | 5′-CACGCTCTCTCACCCTTTTG-3′ |
| Cd93 | 5′-ACAACAGGTCTCTTCGTCCA-3′ | 5′-GTATGTGCCCAACTCGAACC-3′ |
| Kc/Cxcl1 | 5′-ACAGGGGCGCCTATCGCCAA-3′ | 5′-CGGTTTGGGTGCAGTGGGGC-3′ |
| Cxcl11 | 5′-TGGCAGAGATCGAGAAAGCT-3′ | 5′-GCACCTTTGTCGTTTATGAGC-3′ |
| Cxcr3 | 5′-TCTGCTGGTGTTAACTCTTCCA-3′ | 5′-GTTGATGTTGAACAAGGCGC-3′ |
| Bcl2 | 5′-GGAGAGCGTCAACAGGGAGA-3′ | 5′-CAGCCAGGAGAAATCAAACAGAG-3′ |
| Bim | 5′-AGTGTGACAGAGAAGGTGGACAATT-3′ | 5′-GGGATTACCTTGCGGTTCTGT-3′ |
| Rpl13a | 5′-TCTCAAGGTTGTTCGGCTGAA-3′ | 5′-GCCAGACGCCCCAGGTA-3′ |
3. Results
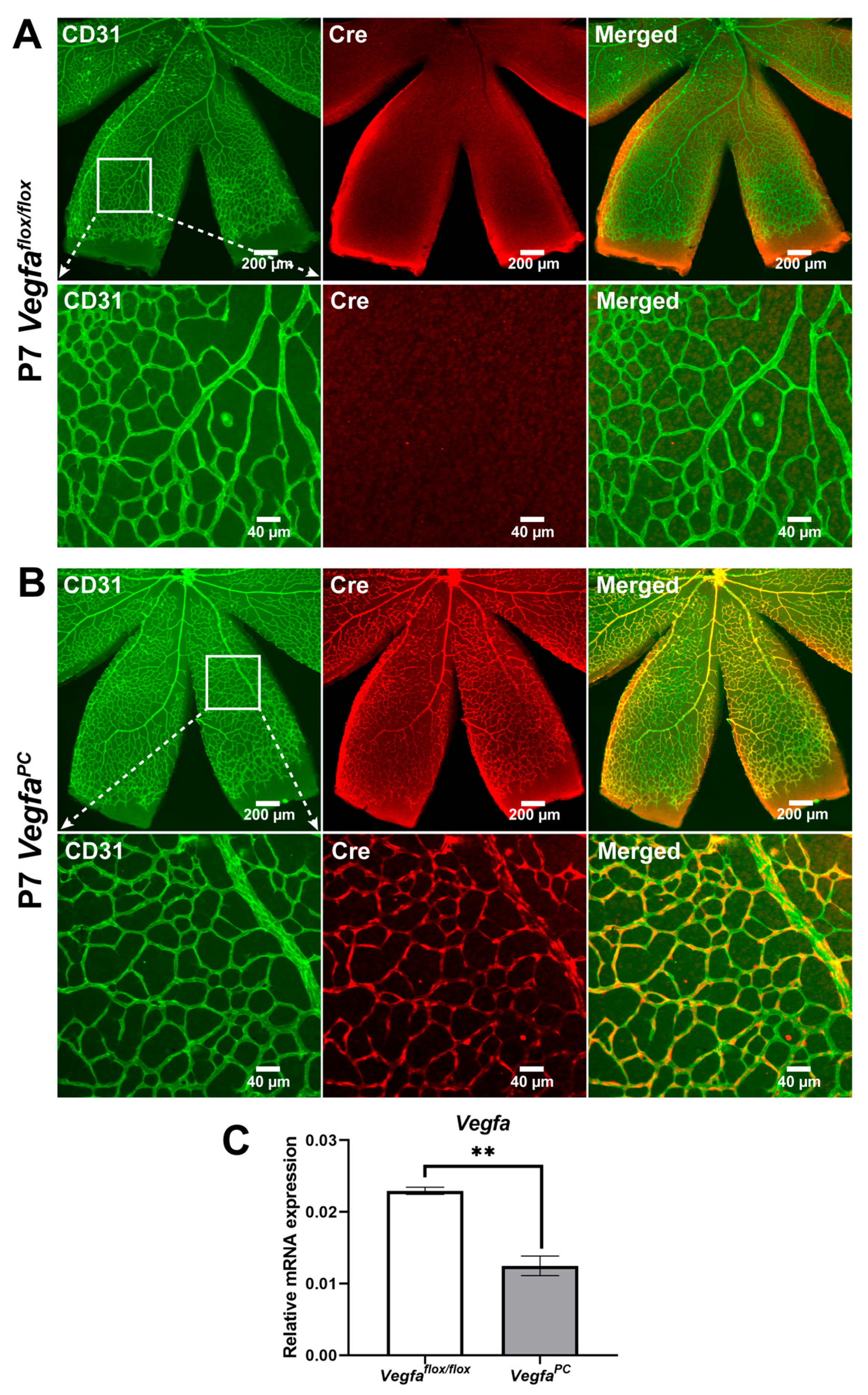
3.1. Validation of the Genetic Model for Loss of VEGF in Pericytes
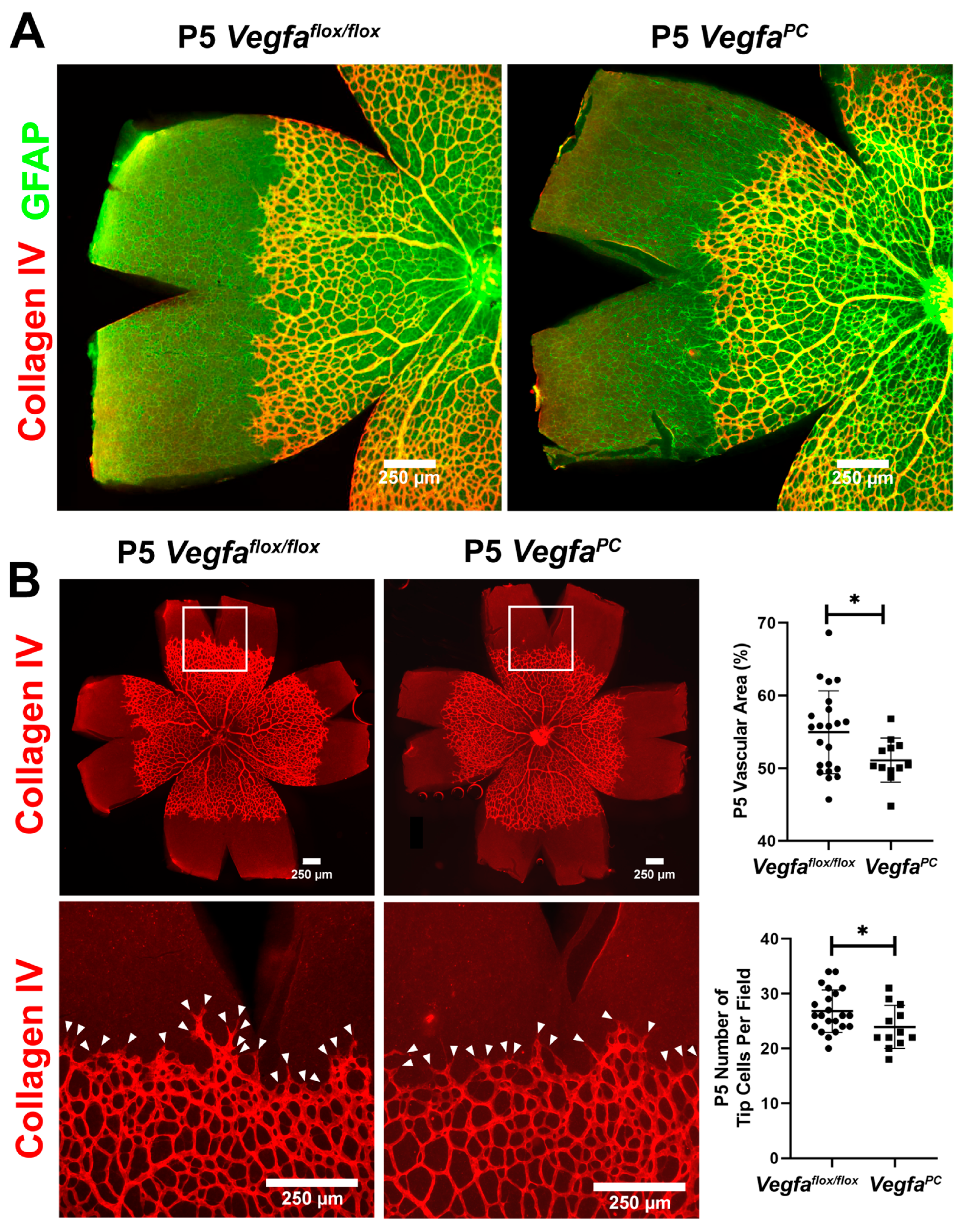
3.2. Early Retinal Vascularization Was Delayed in VegfaPC Mice
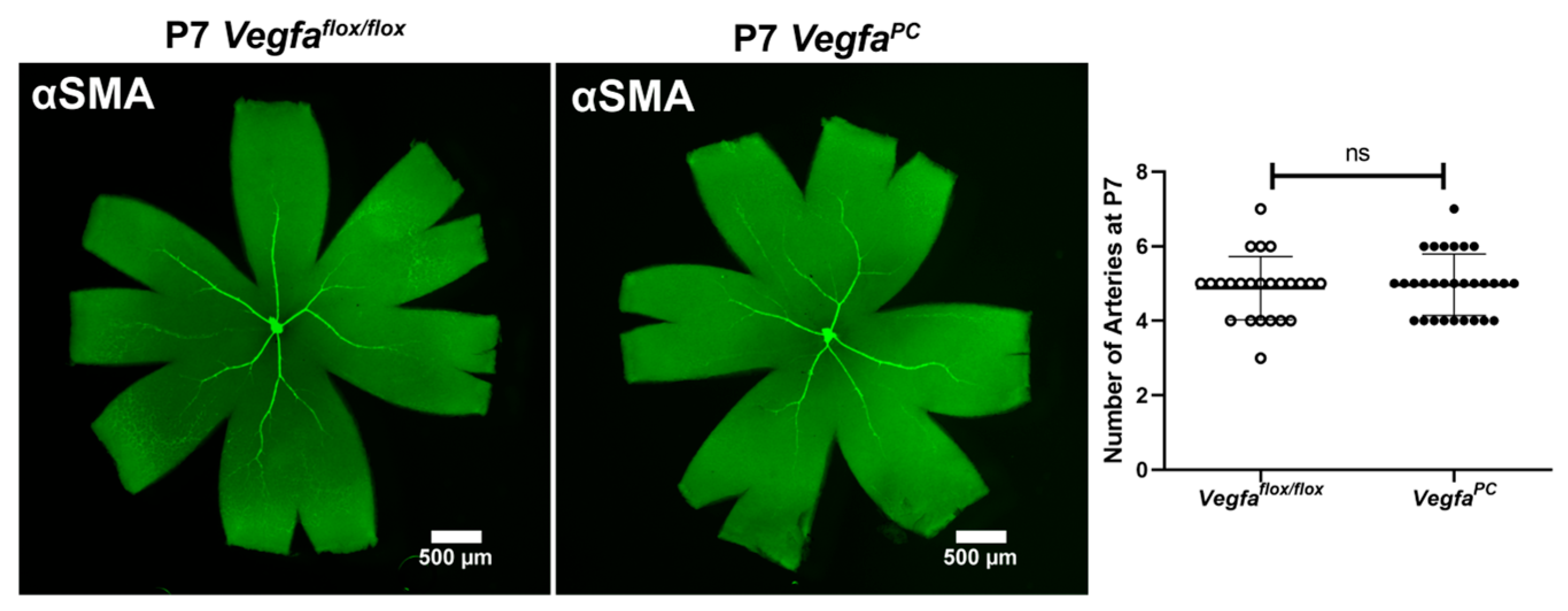
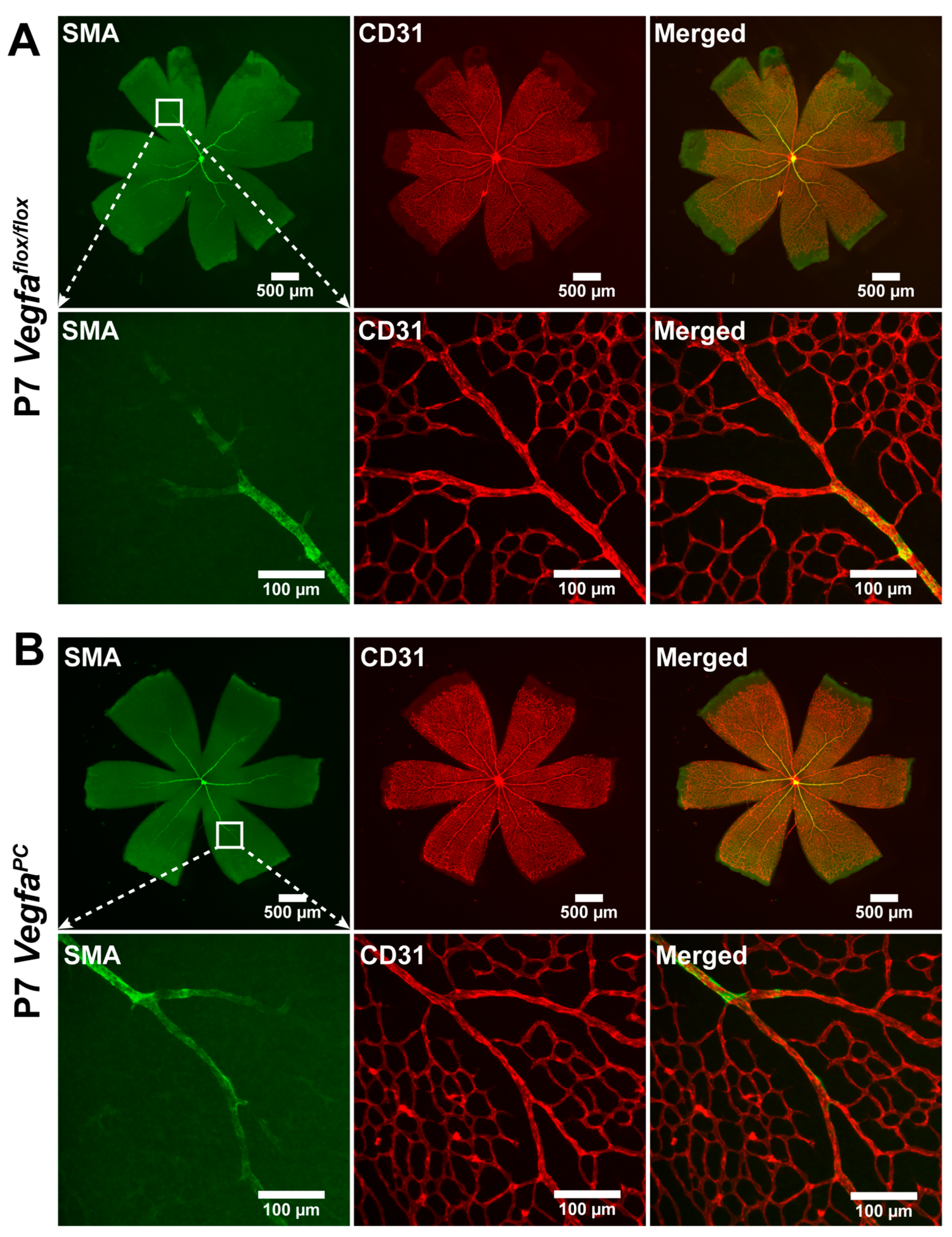
3.3. Lack of VEGF in Pericytes Does Not Impact the Integrity and Differentiation Status of Retinal Vasculature
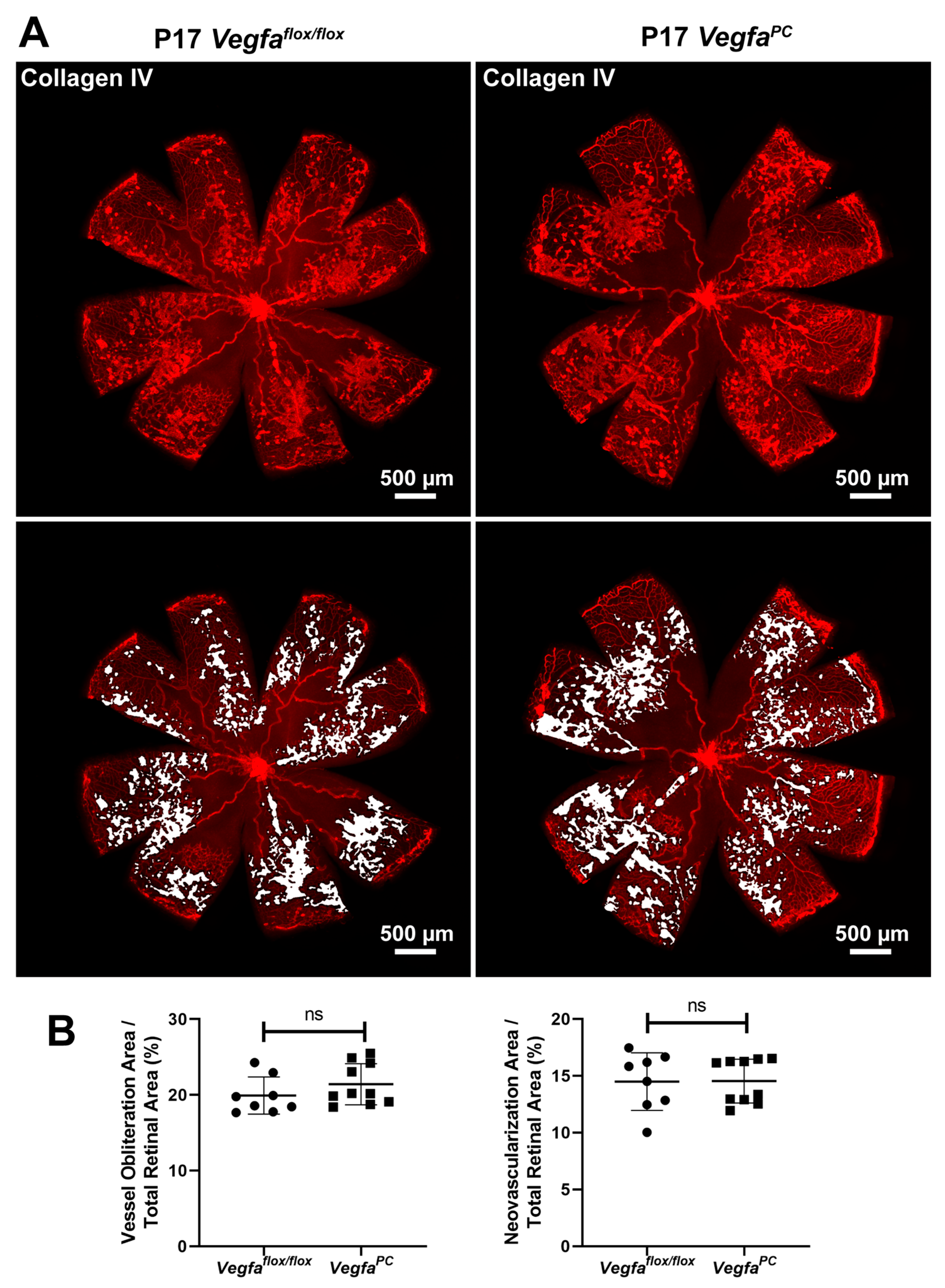
3.4. Lack of VEGF Expression in Pericytes Does Not Affect Area of Vessel Obliteration and Retinal Neovascularization During OIR
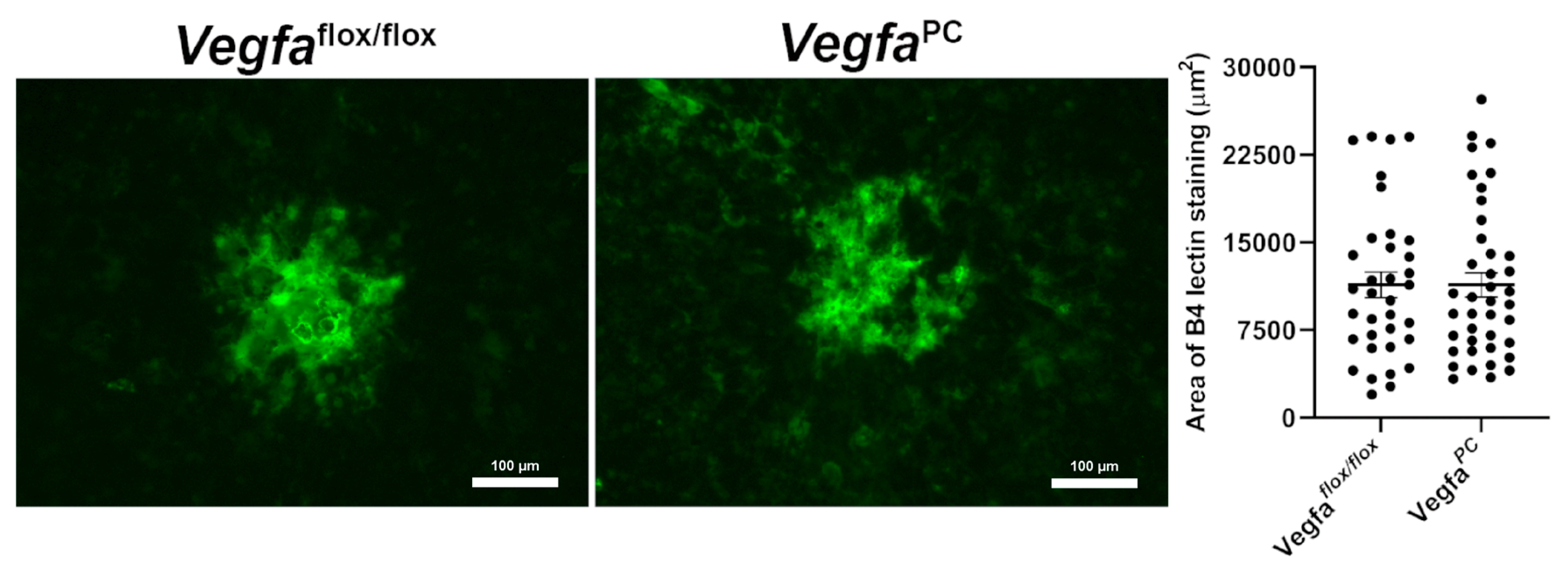
3.5. Lack of VEGF Expression in Pericytes Did Not Impact Choroidal Neovascularization
3.6. The Impact of Pericyte VEGF Expression on Angioregulatory Gene Expression in the Retina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gerber, H.-P.; Hillan, K.J.; Ryan, A.M.; Kowalski, J.; Keller, G.-A.; Rangell, L.; Wright, B.D.; Radtke, F.; Aguet, M.; Ferrara, N. VEGF is required for growth and survival in neonatal mice. Development 1999, 126, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’SHea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Akhlaq, A. Sustained suppression of VEGF for treatment of retinal/choroidal vascular diseases. Prog. Retin. Eye Res. 2021, 83, 100921. [Google Scholar] [CrossRef]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Qaum, T.; Xu, Q.; Joussen, A.M.; Clemens, M.W.; Qin, W.; Miyamoto, K.; Hassessian, H.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2408–2413. [Google Scholar]
- Lee, S.; Chen, T.T.; Barber, C.L.; Jordan, M.C.; Murdock, J.; Desai, S.; Ferrara, N.; Nagy, A.; Roos, K.P.; Iruela-Arispe, M.L. Autocrine VEGF Signaling Is Required for Vascular Homeostasis. Cell 2007, 130, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Froger, N.; Matonti, F.; Roubeix, C.; Forster, V.; Ivkovic, I.; Brunel, N.; Baudouin, C.; Sahel, J.-A.; Picaud, S. VEGF is an autocrine/paracrine neuroprotective factor for injured retinal ganglion neurons. Sci. Rep. 2020, 10, 12409. [Google Scholar] [CrossRef]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, D.; Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 2011, 118, 2906–2917. [Google Scholar] [CrossRef]
- Evensen, L.; Micklem, D.R.; Blois, A.; Berge, S.V.; Aarsæther, N.; Littlewood-Evans, A.; Wood, J.; Lorens, J.B.; Cao, Y. Mural Cell Associated VEGF Is Required for Organotypic Vessel Formation. PLoS ONE 2009, 4, e5798. [Google Scholar] [CrossRef]
- Darland, D.; Massingham, L.; Smith, S.; Piek, E.; Saint-Geniez, M.; D’AMore, P. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 2003, 264, 275–288. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, J.; Guo, J.; Wang, J.; Zhu, M.; Chen, Y.; Le, Y. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 2009, 219, 446–454. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, X.; Chao, J.; Zhang, Y.; Qian, C.; Li, P.; Liu, D.; Han, B.; Zhao, L.; Zhang, J.; et al. Pericytes Contribute to the Disruption of the Cerebral Endothelial Barrier via Increasing VEGF Expression: Implications for Stroke. PLoS ONE 2015, 10, e0124362. [Google Scholar] [CrossRef]
- Shepro, D.; Morel, N.M.L. Pericyte physiology. FASEB J. 1993, 7, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.; Croes, A.A.; Prevaes, E.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. Development of Immunostaining Protocols for 3D Visualization of Pericytes in Human Retinal Flatmounts. J. Histochem. Cytochem. 2025, 73, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Darden, J.; Payne, L.B.; Zhao, H.; Chappell, J.C. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis 2018, 22, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Nör, J.E.; Christensen, J.; Mooney, D.J.; Polverini, P.J. Vascular Endothelial Growth Factor (VEGF)-Mediated Angiogenesis Is Associated with Enhanced Endothelial Cell Survival and Induction of Bcl-2 Expression. Am. J. Pathol. 1999, 154, 375–384. [Google Scholar] [CrossRef]
- Nör, J.E.; Christensen, J.; Liu, J.; Peters, M.; Mooney, D.J.; Strieter, R.M.; Polverini, P.J. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001, 61, 2183–2188. [Google Scholar]
- Del Bufalo, D.; Lervolino, A.; Trisciuoglio, D.; Ribatti, D.; Candiloro, A.; Biroccio, A.; Zupi, G. Bcl-2 Overexpression in Human Melanoma Cells Increases Angiogenesis through Vegf Mrna Stabilization and Hif-1-mediated Transcriptional Activity. Tumori J. 2002, 88, 1453–1455. [Google Scholar] [CrossRef]
- Kondo, S.; Tang, Y.; Scheef, E.A.; Sheibani, N.; Sorenson, C.M. Attenuation of retinal endothelial cell migration and capillary morphogenesis in the absence of bcl-2. Am. J. Physiol. Physiol. 2008, 294, C1521–C1530. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Gabellini, C.; Desideri, M.; Ragazzoni, Y.; De Luca, T.; Ziparo, E.; Del Bufalo, D. Involvement of BH4 domain of bcl-2 in the regulation of HIF-1-mediated VEGF expression in hypoxic tumor cells. Cell Death Differ. 2011, 18, 1024–1035. [Google Scholar] [CrossRef]
- Park, S.; DiMaio, T.A.; Scheef, E.A.; Sorenson, C.M.; Sheibani, N. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am. J. Physiol. Physiol. 2010, 299, C1468–C1484. [Google Scholar] [CrossRef] [PubMed]
- Palenski, T.L.; Sorenson, C.M.; Jefcoate, C.R.; Sheibani, N. Lack of Cyp1b1 promotes the proliferative and migratory phenotype of perivascular supporting cells. Mod. Pathol. 2013, 93, 646–662. [Google Scholar] [CrossRef]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Powner, M.B.; Gandhi, P.; Clarkin, C.; Gutmann, D.H.; Johnson, R.S.; Ferrara, N.; Fruttiger, M.; Connon, C.J. Astrocyte-Derived Vascular Endothelial Growth Factor Stabilizes Vessels in the Developing Retinal Vasculature. PLoS ONE 2010, 5, e11863. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Williams, J.; Nathans, J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Investig. 2019, 129, 3807–3820. [Google Scholar] [CrossRef]
- Liyanage, S.E.; Fantin, A.; Villacampa, P.; Lange, C.A.; Denti, L.; Cristante, E.; Smith, A.J.; Ali, R.R.; Luhmann, U.F.; Bainbridge, J.W.; et al. Myeloid-Derived Vascular Endothelial Growth Factor and Hypoxia-Inducible Factor Are Dispensable for Ocular Neovascularization—Brief Report. Arter. Thromb. Vasc. Biol. 2016, 36, 19–24. [Google Scholar] [CrossRef]
- Stockmann, C.; Doedens, A.; Weidemann, A.; Zhang, N.; Takeda, N.; Greenberg, J.I.; Cheresh, D.A.; Johnson, R.S. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 2008, 456, 814–818. [Google Scholar] [CrossRef]
- Stockmann, C.; Kerdiles, Y.; Nomaksteinsky, M.; Weidemann, A.; Takeda, N.; Doedens, A.; Torres-Collado, A.X.; Iruela-Arispe, L.; Nizet, V.; Johnson, R.S. Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 4329–4334. [Google Scholar] [CrossRef]
- Deyama, S.; Li, X.-Y.; Duman, R.S. Neuron-specific deletion of VEGF or its receptor Flk-1 impairs recognition memory. Eur. Neuropsychopharmacol. 2020, 31, 145–151. [Google Scholar] [CrossRef]
- Hill, C.; Jacobs, B.; Kennedy, L.; Rohde, S.; Zhou, B.; Baldwin, S.; Goudy, S. Cranial neural crest deletion of VEGFa causes cleft palate with aberrant vascular and bone development. Cell Tissue Res. 2015, 361, 711–722. [Google Scholar] [CrossRef]
- Okabe, K.; Kobayashi, S.; Yamada, T.; Kurihara, T.; Tai-Nagara, I.; Miyamoto, T.; Mukouyama, Y.-S.; Sato, T.N.; Suda, T.; Ema, M.; et al. Neurons Limit Angiogenesis by Titrating VEGF in Retina. Cell 2014, 159, 584–596. [Google Scholar] [CrossRef]
- Marneros, A.G.; Fan, J.; Yokoyama, Y.; Gerber, H.P.; Ferrara, N.; Crouch, R.K.; Olsen, B.R. Vascular Endothelial Growth Factor Expression in the Retinal Pigment Epithelium Is Essential for Choriocapillaris Development and Visual Function. Am. J. Pathol. 2005, 167, 1451–1459. [Google Scholar] [CrossRef]
- Eilken, H.M.; Diéguez-Hurtado, R.; Schmidt, I.; Nakayama, M.; Jeong, H.-W.; Arf, H.; Adams, S.; Ferrara, N.; Adams, R.H. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat. Commun. 2017, 8, 1574. [Google Scholar] [CrossRef]
- Hellström, M.; Gerhardt, H.; Kalén, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. Lack of Pericytes Leads to Endothelial Hyperplasia and Abnormal Vascular Morphogenesis. J. Cell Biol. 2001, 153, 543–554. [Google Scholar] [CrossRef]
- Fazio, A.; Neri, I.; Koufi, F.-D.; Marvi, M.V.; Galvani, A.; Evangelisti, C.; McCubrey, J.A.; Cocco, L.; Manzoli, L.; Ratti, S. Signaling Role of Pericytes in Vascular Health and Tissue Homeostasis. Int. J. Mol. Sci. 2024, 25, 6592. [Google Scholar] [CrossRef] [PubMed]
- Zaitoun, I.S.; Wintheiser, C.M.; Jamali, N.; Wang, S.; Suscha, A.; Darjatmoko, S.R.; Schleck, K.; Hanna, B.A.; Lindner, V.; Sheibani, N.; et al. Bcl-2 Expression in Pericytes and Astrocytes Impacts Vascular Development and Homeostasis. Sci. Rep. 2019, 9, 9700. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, A.S.; LeClair, R.J.; Stohn, J.P.; Wang, Q.; Sorenson, C.M.; Liaw, L.; Lindner, V. Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis 2011, 49, 673–680. [Google Scholar] [CrossRef]
- Domigan, C.K.; Warren, C.M.; Antanesian, V.; Happel, K.; Ziyad, S.; Lee, S.; Krall, A.; Duan, L.; Torres-Collado, A.X.; Castellani, L.W.; et al. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J. Cell Sci. 2015, 128, 2236–2248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zaitoun, I.S.; Johnson, R.P.; Jamali, N.; Gurel, Z.; Wintheiser, C.M.; Strasser, A.; Lindner, V.; Sheibani, N.; Sorenson, C.M.; et al. Bim expression in endothelial cells and pericytes is essential for regression of the fetal ocular vasculature. PLoS ONE 2017, 12, e0178198. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Wang, S.; Wu, Z.; Sorenson, C.M.; Lawler, J.; Sheibani, N. Thrombospondin-1–deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev. Dyn. 2003, 228, 630–642. [Google Scholar] [CrossRef]
- Fruttiger, M. Development of the retinal vasculature. Angiogenesis 2007, 10, 77–88. [Google Scholar] [CrossRef]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Chen, J.; Guerin, K.I.; Smith, L.E.H. Computer-aided quantification of retinal neovascularization. Angiogenesis 2009, 12, 297–301. [Google Scholar] [CrossRef]
- Shchuko, A.G.; Zaitseva, N.V.; Yurieva, T.N.; Chernykh, E.R.; Mikhalevich, I.M.; Shevela, E.Y.; Grigorieva, A.V. Intraocular Cytokines and Their Correlations with Clinical Parameters in Patients with Myopic Choroidal Neovascularization. Ophthalmologica 2017, 237, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Pachydaki, S.I.; Jakobiec, F.A.; Bhat, P.; Sobrin, L.; Michaud, N.A.; Seshan, S.V.; D’aMico, D.J. Surgical management and ultrastructural study of choroidal neovascularization in punctate inner choroidopathy after bevacizumab. J. Ophthalmic Inflamm. Infect. 2011, 2, 29–37. [Google Scholar] [CrossRef]
- Galvagni, F.; Nardi, F.; Spiga, O.; Trezza, A.; Tarticchio, G.; Pellicani, R.; Andreuzzi, E.; Caldi, E.; Toti, P.; Tosi, G.M.; et al. Dissecting the CD93-Multimerin 2 interaction involved in cell adhesion and migration of the activated endothelium. Matrix Biol. 2017, 64, 112–127. [Google Scholar] [CrossRef]
- Khan, K.A.; Naylor, A.J.; Khan, A.; Noy, P.J.; Mambretti, M.; Lodhia, P.; Athwal, J.; Korzystka, A.; Buckley, C.D.; Willcox, B.E.; et al. Multimerin-2 is a ligand for group 14 family C-type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene 2017, 36, 6097–6108. [Google Scholar] [CrossRef]
- Tosi, G.M.; Neri, G.; Barbera, S.; Mundo, L.; Parolini, B.; Lazzi, S.; Lugano, R.; Poletto, E.; Leoncini, L.; Pertile, G.; et al. The Binding of CD93 to Multimerin-2 Promotes Choroidal Neovascularization. Investig. Opthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef]
- Fejza, A.; Poletto, E.; Carobolante, G.; Camicia, L.; Andreuzzi, E.; Capuano, A.; Pivetta, E.; Pellicani, R.; Colladel, R.; Marastoni, S.; et al. Multimerin-2 orchestrates the cross-talk between endothelial cells and pericytes: A mechanism to maintain vascular stability. Matrix Biol. Plus 2021, 11, 100068. [Google Scholar] [CrossRef] [PubMed]
- Colladel, R.; Pellicani, R.; Andreuzzi, E.; Paulitti, A.; Tarticchio, G.; Todaro, F.; Colombatti, A.; Mongiat, M. MULTIMERIN2 binds VEGF-A primarily via the carbohydrate chains exerting an angiostatic function and impairing tumor growth. Oncotarget 2015, 7, 2022–2037. [Google Scholar] [CrossRef]
- Pellicani, R.; Poletto, E.; Andreuzzi, E.; Paulitti, A.; Doliana, R.; Bizzotto, D.; Braghetta, P.; Colladel, R.; Tarticchio, G.; Sabatelli, P.; et al. Multimerin-2 maintains vascular stability and permeability. Matrix Biol. 2020, 87, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Jamali, N.; Song, Y.; Sorenson, C.M.; Sheibani, N. 1,25(OH)2D3regulates the proangiogenic activity of pericyte through VDR-mediated modulation of VEGF production and signaling of VEGF and PDGF receptors. FASEB BioAdv. 2019, 1, 415–434. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Wells, A. Differential regulation of pericyte function by the CXC receptor 3. Wound Repair Regen. 2015, 23, 785–796. [Google Scholar] [CrossRef]
- Lee, J.; Goeckel, M.E.; Levitas, A.; Colijn, S.; Shin, J.; Hindes, A.; Mun, G.; Burton, Z.; Chintalapati, B.; Yin, Y.; et al. CXCR3-CXCL11 Signaling Restricts Angiogenesis and Promotes Pericyte Recruitment. Arter. Thromb. Vasc. Biol. 2024, 44, 2577–2595. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Vemuri, K.; Yu, D.; Bergqvist, M.; Smits, A.; Essand, M.; Johansson, S.; Dejana, E.; Dimberg, A. CD93 promotes β1 integrin activation and fibronectin fibrillogenesis during tumor angiogenesis. J. Clin. Investig. 2018, 128, 3280–3297. [Google Scholar] [CrossRef]
- Lugano, R.; Vemuri, K.; Barbera, S.; Orlandini, M.; Dejana, E.; Claesson-Welsh, L.; Dimberg, A. CD93 maintains endothelial barrier function by limiting the phosphorylation and turnover of VE-cadherin. FASEB J. 2023, 37, e22894. [Google Scholar] [CrossRef]
- Wang, S.; Cao, C.; Chen, Z.; Bankaitis, V.; Tzima, E.; Sheibani, N.; Burridge, K. Pericytes Regulate Vascular Basement Membrane Remodeling and Govern Neutrophil Extravasation during Inflammation. PLoS ONE 2012, 7, e45499. [Google Scholar] [CrossRef]
- Girbl, T.; Lenn, T.; Perez, L.; Rolas, L.; Barkaway, A.; Thiriot, A.; del Fresno, C.; Lynam, E.; Hub, E.; Thelen, M.; et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018, 49, 1062–1076.e1066. [Google Scholar] [CrossRef]
- Monickaraj, F.; Acosta, G.; Cabrera, A.P.; Das, A. Transcriptomic Profiling Reveals Chemokine CXCL1 as a Mediator for Neutrophil Recruitment Associated With Blood-Retinal Barrier Alteration in Diabetic Retinopathy. Diabetes 2023, 72, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Joulia, R.; Guerrero-Fonseca, I.M.; Girbl, T.; Coates, J.A.; Stein, M.; Vázquez-Martínez, L.; Lynam, E.; Whiteford, J.; Schnoor, M.; Voehringer, D.; et al. Neutrophil breaching of the blood vessel pericyte layer during diapedesis requires mast cell-derived IL-17A. Nat. Commun. 2022, 13, 7029. [Google Scholar] [CrossRef] [PubMed]
- Loughna, S.; Sato, T.N. Angiopoietin and Tie signaling pathways in vascular development. Matrix Biol. 2001, 20, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ramsauer, M.; D’amore, P.A. Getting Tie(2)d up in angiogenesis. J. Clin. Investig. 2002, 110, 1615–1617. [Google Scholar] [CrossRef][Green Version]
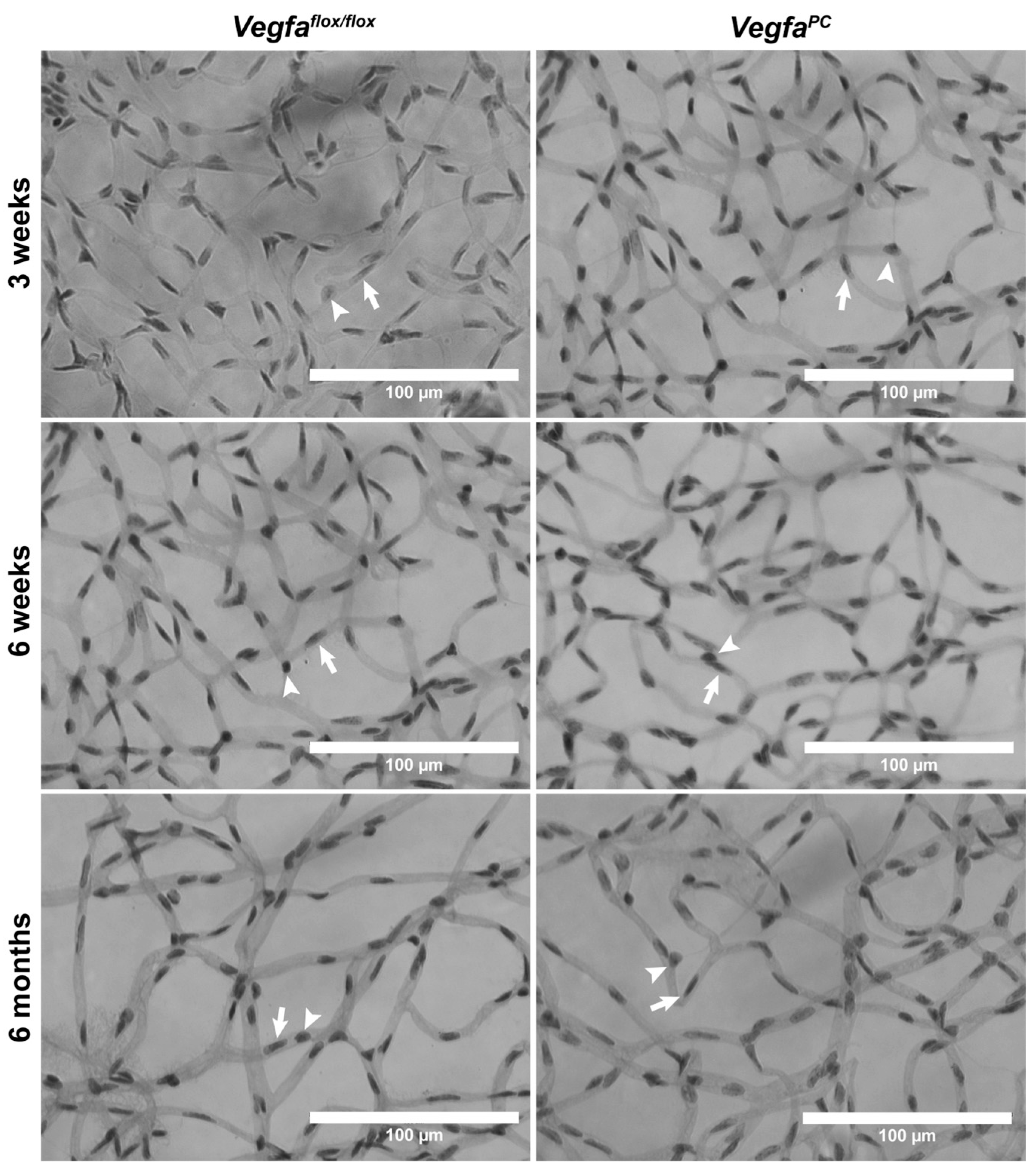



| Cell Type | Age | Vegfaflox/flox | VegfaPC |
|---|---|---|---|
| Pericyte | 3 weeks | 27.46 ± 1.61 | 21.17 ± 0.76 ** |
| Endothelial Cell | 3 weeks | 118.50 ± 4.51 | 115.60 ± 2.26 |
| Pericyte | 6 weeks | 20.43 ± 0.61 | 19.67 ± 1.62 |
| Endothelial Cell | 6 weeks | 102.40 ± 2.32 | 100.00 ± 5.27 |
| Pericyte | 6 months | 18.57 ± 0.78 | 22.13 ± 1.00 ** |
| Endothelial Cell | 6 months | 94.86 ± 2.30 | 94.06 ± 1.74 |
| Genes | P7 vs. P7 Cre− vs. Cre+ | P21 vs. P21 Cre− vs. Cre+ | P7 vs. P21 Cre− | P7 vs. P21 Cre+ |
|---|---|---|---|---|
| Vegfa | 4% # | 26% * | 38% *** | 22% |
| Vegfb | 21% * | 7% | 48% ** | 56% ** |
| Vegfc | ND | 7% * | 32% *** | 37% *** |
| Vegfr1 | 50% *** | 22% * | 87% ** | 92% ** |
| Vegfr2 | 17% | 14% | 11% | 13% |
| Vegfr3 | 11% | 11% | 50% ** | 50% ** |
| Pdgfb | 30% | 23% ** | 15% | 23% ** |
| Pdgfrb | 11% | 8% | 63% *** | 63% *** |
| Angpt1 | 20% * | 18% ** | 25% * | 24% ** |
| Angpt2 | 7% | 15% ** | 50% ** | 45% ** |
| Tek/Tie2 | 14% | 5% * | 50% *** | 45% ** |
| Acta2/αSma | 45% *** | ND | 68% *** | 42% ** |
| Cspg4/Ng2 | 4% | ND | 45% * | 47% * |
| Mmrn2 | 14% | 4% | 50% * | 43% ** |
| Cd93 | 30% ** | ND | ND | ND |
| Cxcl1 | 15% | 34% ** | 33% * | 48% ** |
| Cxcl11 | 43% * | 36% * | 47% * | 36% * |
| Cxcr3 | 31% ** | 30% * | 47% * | 36% * |
| Bcl-2 | 39% * | 11% | 71% *** | 80% ** |
| Bim | 20% * | 1% | 16% | 32% ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-S.; Wang, S.; Inampudi, S.; Risa, H.; Sorenson, C.M.; Sheibani, N. Pericyte Expression of VEGF-A Minimally Impacts Ocular Vascular Development and Neovascularization. Cells 2025, 14, 1473. https://doi.org/10.3390/cells14181473
Song Y-S, Wang S, Inampudi S, Risa H, Sorenson CM, Sheibani N. Pericyte Expression of VEGF-A Minimally Impacts Ocular Vascular Development and Neovascularization. Cells. 2025; 14(18):1473. https://doi.org/10.3390/cells14181473
Chicago/Turabian StyleSong, Yong-Seok, Shoujian Wang, Samay Inampudi, Hope Risa, Christine M. Sorenson, and Nader Sheibani. 2025. "Pericyte Expression of VEGF-A Minimally Impacts Ocular Vascular Development and Neovascularization" Cells 14, no. 18: 1473. https://doi.org/10.3390/cells14181473
APA StyleSong, Y.-S., Wang, S., Inampudi, S., Risa, H., Sorenson, C. M., & Sheibani, N. (2025). Pericyte Expression of VEGF-A Minimally Impacts Ocular Vascular Development and Neovascularization. Cells, 14(18), 1473. https://doi.org/10.3390/cells14181473






