Prenatal Choline Attenuates the Elevated Adiposity and Glucose Intolerance Caused by Prenatal Alcohol Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Alcohol Exposure and Choline Intervention
2.3. Body Composition
2.4. Glucose Tolerance Testing
2.5. Statistical Analysis
3. Results
3.1. PAE and Choline Affect Body Weight Gains and Brain and Hepatic Weight in Aged Male and Female Mice
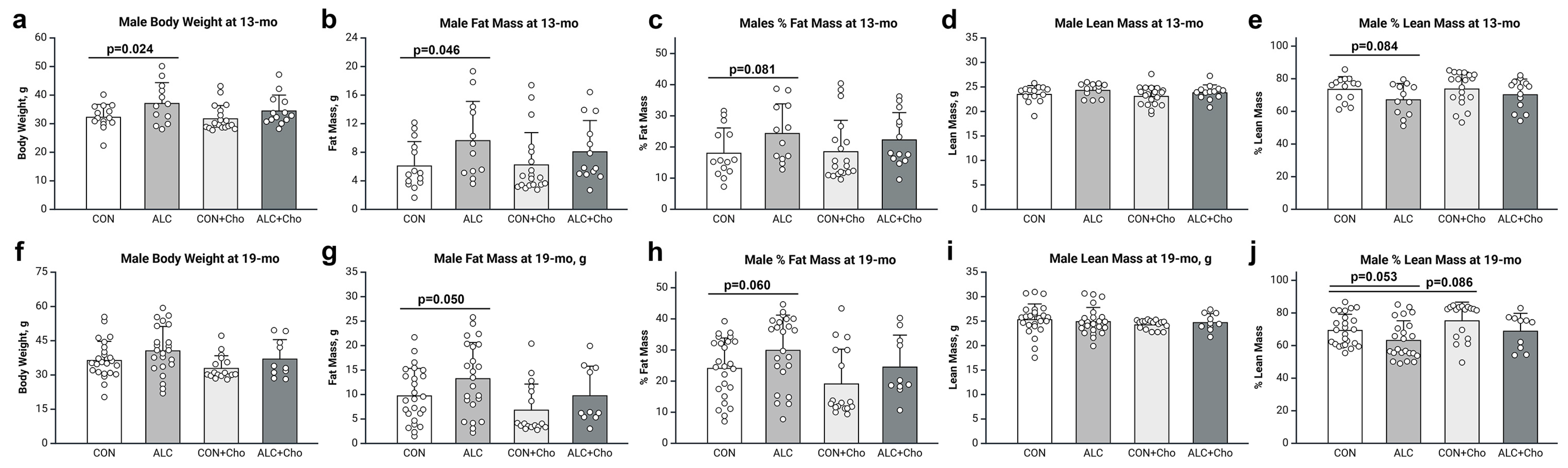
3.2. PAE Increases Adiposity in Male and Female Mice
3.3. Prenatal Choline Attenuates PAE-Dependent Adiposity in Male and Female Mice
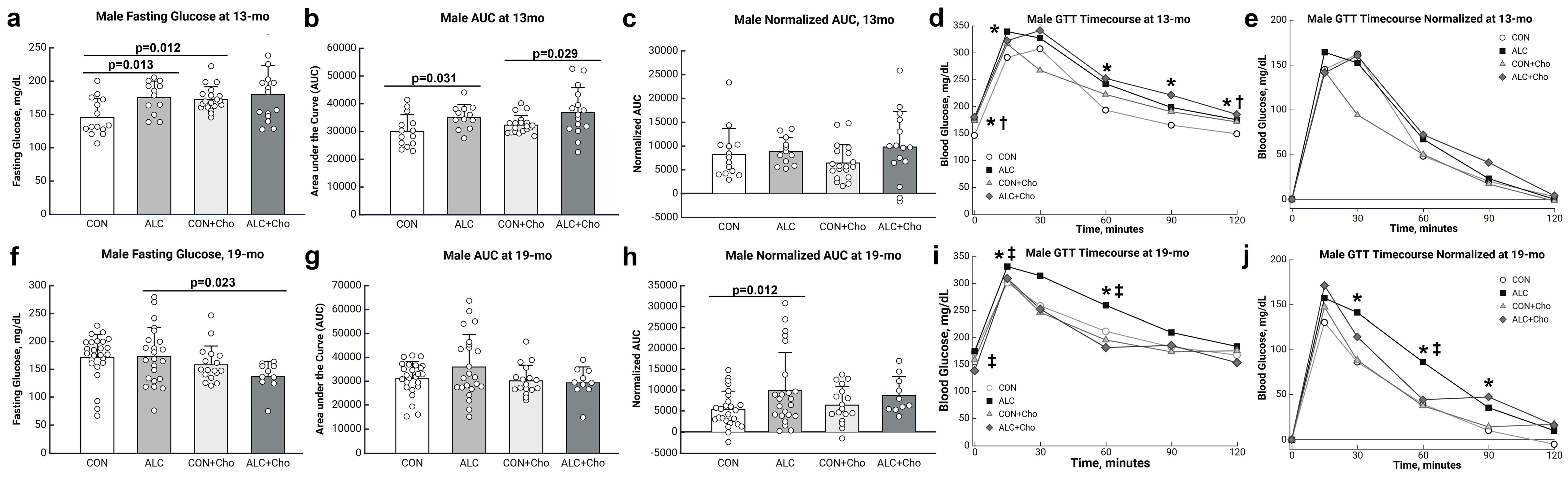
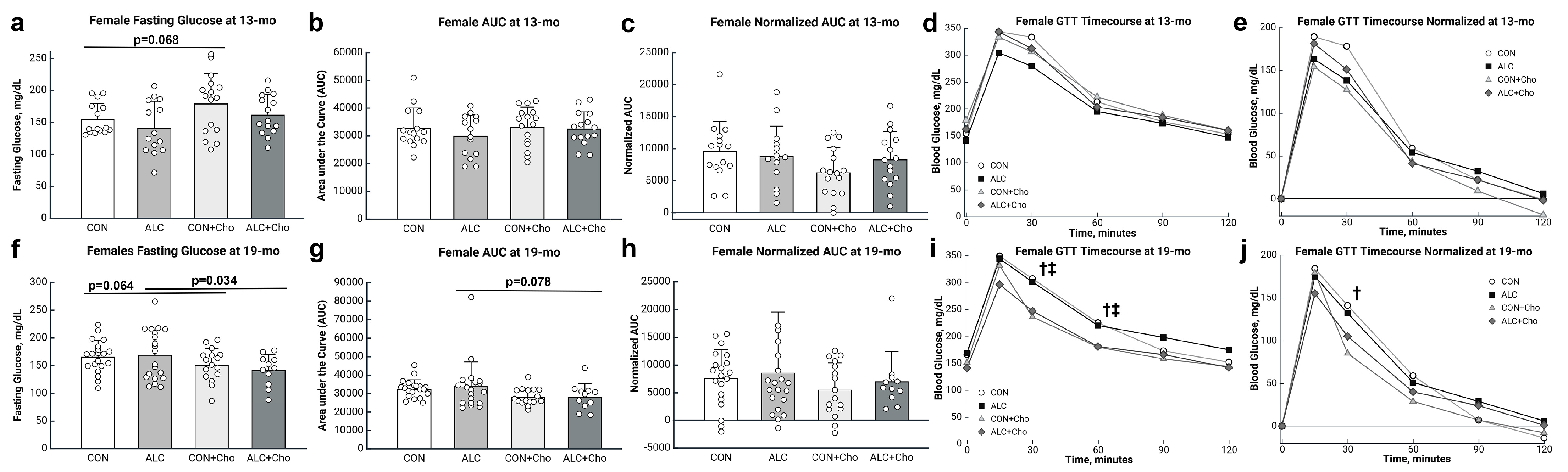
3.4. PAE Worsens Glucose Tolerance in Male but Not Female Mice
3.5. Prenatal Choline Improves Glucose Tolerance in Male and Female PAE Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area-under-the-curve |
| IPGTT | Interperitoneal glucose tolerance test |
| PAE | Prenatal alcohol exposure |
| PCS | Prenatal choline supplementation |
| SAH | S-adenosyl-homocysteine |
| SAM | S-adenosyl-methionine |
| T2DM | Type-2 diabetes mellitus |
References
- Wozniak, J.R.; Riley, E.P.; Charness, M.E. Clinical presentation, diagnosis, and management of Fetal Alcohol Spectrum Disorder. Lancet Neurol. 2019, 18, 760–770. [Google Scholar] [CrossRef]
- Himmelreich, M.; Lutke, C.J.; Hargrove, E.T. The lay of the land: Fetal alcohol spectrum disorder (FASD) as a whole-body diagnosis. In The Routledge Handbook of Social Work and Addictive Behaviors; Routledge: London, UK, 2020; pp. 191–215. [Google Scholar] [CrossRef]
- Cook, J.C.; Lynch, M.E.; Coles, C.D. Association analysis: Fetal alcohol spectrum disorder and hypertension status in children and adolescents. Alcohol Clin. Exp. Res. 2019, 43, 1727–1733. [Google Scholar] [CrossRef]
- Dylag, K.A.; Wieczorek-Stawinska, W.; Burkot, K.; Drzewiecki, L.; Przybyszewska, K.; Tokarz, A.; Dumnicka, P. Exploring nutritional status and metabolic imbalances in children with FASD: A cross-sectional study. Nutrients 2024, 16, 3401. [Google Scholar] [CrossRef]
- Vanderpeet, C.; Akison, L.; Moritz, K.; Hayes, N.; Reid, N. Beyond the brain: The physical health and whole-body impact of fetal alcohol spectrum disorders. Alcohol Res. 2025, 45, 5. [Google Scholar] [CrossRef]
- Hayes, N.; Reid, N.; Akison, L.K.; Moritz, K.M. The effect of heavy prenatal alcohol exposure on adolescent body mass index and waist-to-height ratio at 12–13 years. Int. J. Obes. 2021, 45, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Kable, J.A.; Mehta, P.K.; Coles, C.D. Alterations in insulin levels in adults with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2021, 45, 500–506. [Google Scholar] [CrossRef]
- Kable, J.A.; Mehta, P.K.; Rashid, F.; Coles, C.D. Path analysis of the impact of prenatal alcohol on adult vascular function. Alcohol Clin. Exp. Res. 2023, 47, 116–126. [Google Scholar] [CrossRef]
- Weeks, O.; Bossé, G.D.; Oderberg, I.M.; Akle, S.; Houvras, Y.; Wrighton, P.J.; LaBella, K.; Iversen, I.; Tavakoli, S.; Adatto, I.; et al. Fetal alcohol spectrum disorder predisposes to metabolic abnormalities in adulthood. J. Clin. Investig. 2020, 130, 2252–2269. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nyomba, B.L. Whole body insulin resistance in rat offspring of mothers consuming alcohol during pregnancy or lactation: Comparing prenatal and postnatal exposure. J. Appl. Physiol. 2004, 96, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.C.; Mongillo, D.L.; Brien, D.C.; Stepita, R.; Poklewska-Koziell, M.; Winterborn, A.; Holloway, A.C.; Brien, J.F.; Reynolds, J.N. Chronic prenatal ethanol exposure increases adiposity and disrupts pancreatic morphology in adult guinea pig offspring. Nutr. Diabetes 2012, 2, e57. [Google Scholar] [CrossRef]
- Gårdebjer, E.M.; Cuffe, J.S.M.; Ward, L.C.; Steane, S.; Anderson, S.T.; Dorey, E.S.; Kalisch-Smith, J.I.; Pantaleon, M.; Chong, S.; Yamada, L.; et al. Effects of periconceptional maternal alcohol intake and a postnatal high-fat diet on obesity and liver disease in male and female rat offspring. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E694–E704. [Google Scholar] [CrossRef]
- Gårdebjer, E.M.; Anderson, S.T.; Pantaleon, M.; Wlodek, M.E.; Moritz, K.M. Maternal alcohol intake around the time of conception causes glucose intolerance and insulin insensitivity in rat offspring, which is exacerbated by a postnatal high-fat diet. FASEB J. 2015, 29, 2690–2701. [Google Scholar] [CrossRef]
- Nguyen, T.M.T.; Steane, S.E.; Moritz, K.M.; Akison, L.K. Prenatal alcohol exposure programmes offspring disease: Insulin resistance in adult males in a rat model of acute exposure. J. Physiol. 2019, 597, 5619–5637. [Google Scholar] [CrossRef] [PubMed]
- Probyn, M.E.; Parsonson, K.R.; Gårdebjer, E.M.; Ward, L.C.; Wlodek, M.E.; Anderson, S.T.; Moritz, K.M. Impact of low dose prenatal ethanol exposure on glucose homeostasis in Sprague-Dawley rats aged up to eight months. PLoS ONE 2013, 8, e59718. [Google Scholar] [CrossRef]
- Smith, S.M.; Pjetri, E.; Friday, W.B.; Presswood, B.H.; Ricketts, D.K.; Walter, K.R.; Mooney, S.M. Aging-related behavioral, adiposity, and glucose impairments and their association following prenatal alcohol exposure in the C57BL/6J Mouse. Nutrients 2022, 14, 1438. [Google Scholar] [CrossRef]
- Walter, K.R.; Ricketts, D.K.; Presswood, B.H.; Smith, S.M.; Mooney, S.M. Prenatal alcohol exposure causes persistent microglial activation and age- and sex- specific effects on cognition and metabolic outcomes in an Alzheimer’s Disease mouse model. Am. J. Drug Alcohol Abus. 2023, 49, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Gosdin, L.K.; Deputy, N.P.; Kim, S.Y.; Dang, E.P.; Denny, C.H. Alcohol consumption and binge drinking during pregnancy among adults aged 18–49 years-United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 10–13. [Google Scholar] [CrossRef]
- Lunde, E.R.; Washburn, S.E.; Golding, M.C.; Bake, S.; Miranda, R.C.; Ramadoss, J. Alcohol-induced developmental origins of adult-onset diseases. Alcohol Clin. Exp. Res. 2016, 40, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Wainwright, H.; Molteno, C.D.; Georgieff, M.K.; Dodge, N.C.; Warton, F.; Meintjes, E.M.; Jacobson, J.L.; Jacobson, S.W. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin. Exp. Res. 2016, 40, 753–764. [Google Scholar] [CrossRef]
- Kwan, S.T.C.; Presswood, B.H.; Helfrich, K.K.; Baulch, J.W.; Mooney, S.M.; Smith, S.M. An interaction between fetal sex and placental weight and efficiency predicts intrauterine growth in response to maternal protein insufficiency and gestational exposure window in a mouse model of FASD. Biol. Sex Differ. 2020, 11, 40. [Google Scholar] [CrossRef]
- Steane, S.E.; Edwards, C.; Cavanagh, E.; Vanderpeet, C.; Kubler, J.M.; Akison, L.K.; Cuffe, J.S.; Gallo, L.A.; Moritz, K.M.; Clifton, V.L. Prenatal alcohol exposure is associated with altered feto-placental blood flow and sex-specific placental changes. J. Clin. Investig. 2025, 10, e186096. [Google Scholar] [CrossRef]
- Gualdoni, G.S.; Jacobo, P.V.; Barril, C.; Ventureira, M.R.; Cebral, E. Early abnormal placentation and evidence of vascular endothelial growth factor system dysregulation at the feto-maternal interface after periconceptional alcohol consumption. Front. Physiol. 2022, 12, 815760. [Google Scholar] [CrossRef]
- Jakoubek, V.; Hampl, V. Alcohol and fetoplacental vasoconstrictor reactivity. Physiol. Res. 2018, 67, 509–513. [Google Scholar] [CrossRef]
- Tai, M.; Piskorski, A.; Kao, J.C.; Hess, L.A.; M de la Monte, S.; Gündoğan, F. Placental morphology in Fetal Alcohol Spectrum Disorders. Alcohol Alcohol. 2017, 52, 138–144. [Google Scholar] [CrossRef]
- Carter, R.C.; Chen, J.; Li, Q.; Deyssenroth, M.; Dodge, N.C.; Wainwright, H.C.; Molteno, C.D.; Meintjes, E.M.; Jacobson, J.L.; Jacobson, S.W. Alcohol-related alterations in placental imprinted gene expression in humans mediate effects of prenatal alcohol exposure on postnatal growth. Alcohol Clin. Exp. Res. 2018, 42, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef]
- Kuzawa, C.Y.; Quinn, E.A. Developmental Origins of Adult Function and Health: Evolutionary hypotheses. Annu. Rev. Anthropol. 2009, 38, 131–147. [Google Scholar] [CrossRef]
- Ernst, A.M.; Gimbel, B.A.; de Water, E.; Eckerle, J.K.; Radke, J.P.; Georgieff, M.K.; Wozniak, J.R. Prenatal and postnatal choline supplementation in fetal alcohol spectrum disorder. Nutrients 2022, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef]
- Carter, R.C.; Senekal, M.; Duggan, C.P.; Dodge, N.C.; Meintjes, E.M.; Molteno, C.D.; Jacobson, J.L.; Jacobson, S.W. Gestational weight gain and dietary energy, iron, and choline intake predict severity of fetal alcohol growth restriction in a prospective birth cohort. Am. J. Clin. Nutr. 2022, 116, 460–469. [Google Scholar] [CrossRef]
- Thomas, J.D.; La Fiette, M.H.; Quinn, V.R.; Riley, E.P. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol. Teratol. 2000, 22, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Kwan, S.T.C.; Ricketts, D.K.; Presswood, B.H.; Smith, S.M.; Mooney, S.M. Prenatal choline supplementation during mouse pregnancy has differential effects in alcohol-exposed fetal organs. Alcohol Clin. Exp. Res. 2021, 45, 2471–2484. [Google Scholar] [CrossRef] [PubMed]
- Steane, S.E.; Fielding, A.M.; Kent, N.L.; Andersen, I.; Browne, D.J.; Tejo, E.N.; Gårdebjer, E.M.; Kalisch-Smith, J.I.; Sullivan, M.A.; Moritz, K.M.; et al. Maternal choline supplementation in a rat model of periconceptional alcohol exposure: Impacts on the fetus and placenta. Alcohol Clin. Exp. Res. 2021, 45, 2130–2146. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Mooney, S.M.; Pjetri, E.; Friday, W.B.; Smith, S.M. Growth and behavioral differences in a C57BL/6J mouse model of prenatal alcohol exposure. Alcohol 2021, 97, 51–57. [Google Scholar] [CrossRef]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P.; NIH Mouse Metabolic Phenotyping Center Consortium. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- Amos-Kroohs, R.M.; Fink, B.A.; Smith, C.J.; Chin, L.; Van Calcar, S.C.; Wozniak, J.R.; Smith, S.M. Abnormal eating behaviors are common in children with fetal alcohol spectrum disorder. J. Pediatr. 2016, 169, 194–200.e1. [Google Scholar] [CrossRef] [PubMed]
- Fuglestad, A.J.; Boys, C.J.; Chang, P.N.; Miller, B.S.; Eckerle, J.K.; Deling, L.; Fink, B.A.; Hoecker, H.L.; Hickey, M.K.; Jimenez-Vega, J.M.; et al. Overweight and obesity among children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin. Exp. Res. 2014, 38, 2502–2508. [Google Scholar] [CrossRef]
- Tangen, A.R.; Tice, A.L.; McNeill, A.; Jessup, M.; McCarthy, D.M.; Schatschneider, C.; Wang, Y.; Steiner, J.L. Minimal impact of prenatal alcohol exposure on metabolism and physical performance in adult FVB/NJ mice. Alcohol Alcohol. 2025, 60, agaf035. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; Nyomba, B.L. Abnormal glucose homeostasis in adult female rat offspring after intrauterine ethanol exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1926–R1933. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Hales, C.N. Role of fetal and infant growth in programming metabolism in later life. Biol. Rev. Camb. Philos. Soc. 1997, 72, 329–348. [Google Scholar] [CrossRef]
- Alur, P. Sex differences in nutrition, growth, and metabolism in preterm infants. Front. Pediatr. 2019, 7, 22. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Rosati, A.; Sarti, R.D.; Cruciani, L.; Cutuli, A.M. Does fetal sex affect pregnancy outcome? Gend. Med. 2007, 4, 19–30. [Google Scholar] [CrossRef]
- Nordin, M.; Bergman, D.; Halje, M.; Engström, W.; Ward, A. Epigenetic regulation of the Igf2/H19 gene cluster. Cell Prolif. 2014, 47, 189–199. [Google Scholar] [CrossRef]
- Bestry, M.; Symons, M.; Larcombe, A.; Muggli, E.; Craig, J.M.; Hutchinson, D.; Halliday, J.; Martino, D. Association of prenatal alcohol exposure with offspring DNA methylation in mammals: A systematic review of the evidence. Clin. Epigenetics 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Ouko, L.A.; Shantikumar, K.; Knezovich, J.; Haycock, P.; Schnugh, D.J.; Ramsay, M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: Implications for fetal alcohol spectrum disorders. Alcohol Clin. Exp. Res. 2009, 33, 1615–1627. [Google Scholar] [CrossRef]
- Amos-Kroohs, R.M.; Nelson, D.W.; Hacker, T.A.; Yen, C.E.; Smith, S.M. Does prenatal alcohol exposure cause a metabolic syndrome? (Non-)evidence from a mouse model of fetal alcohol spectrum disorder. PLoS ONE 2018, 13, e0199213. [Google Scholar] [CrossRef]
- Dobson, C.C.; Thevasundaram, K.; Mongillo, D.L.; Winterborn, A.; Holloway, A.C.; Brien, J.F.; Reynolds, J.N. Chronic prenatal ethanol exposure alters expression of central and peripheral insulin signaling molecules in adult guinea pig offspring. Alcohol 2014, 48, 687–693. [Google Scholar] [CrossRef]
- Saini, N.; Mooney, S.M.; Smith, S.M. Alcohol blunts pregnancy-mediated insulin resistance and reduces fetal brain glucose despite elevated fetal gluconeogenesis, and these changes associate with fetal weight outcomes. FASEB J. 2023, 37, e23172. [Google Scholar] [CrossRef]
- Yao, X.H.; Chen, L.; Nyomba, B.L. Adult rats prenatally exposed to ethanol have increased gluconeogenesis and impaired insulin response of hepatic gluconeogenic genes. J. Appl. Physiol. 2006, 100, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Thomas, J.D.; Tarale, P.; Soja, J.; Inkelis, S.; Chambers, C.; Sarkar, D.K. Altered circadian expression of clock genes and clock-regulatory epigenetic modifiers in saliva of children with fetal alcohol spectrum disorders. Sci. Rep. 2024, 14, 19886. [Google Scholar] [CrossRef]
- Agapito, M.A.; Zhang, C.; Murugan, S.; Sarkar, D.K. Fetal alcohol exposure disrupts metabolic signaling in hypothalamic proopiomelanocortin neurons via a circadian mechanism in male mice. Endocrinology 2014, 155, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: Implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef]
- Silva, L.L.S.E.; Fernandes, M.S.S.; Lima, E.A.; Stefano, J.T.; Oliveira, C.P.; Jukemura, J. Fatty pancreas: Disease or finding? Clinics 2021, 76, e2439. [Google Scholar] [CrossRef]
- Jack-Roberts, C.; Joselit, Y.; Nanobashvili, K.; Bretter, R.; Malysheva, O.V.; Caudill, M.A.; Saxena, A.; Axen, K.; Gomaa, A.; Jiang, X. Choline supplementation normalizes fetal adiposity and reduces lipogenic gene expression in a mouse model of maternal obesity. Nutrients 2017, 9, 899. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Edwards, K.; Dave, B.; Jack-Roberts, C.; Yu, H.; Saxena, A.; Salvador, M.; Dembitzer, M.; Phagoora, J.; Jiang, X. Prenatal choline supplementation during high-fat feeding improves long-term blood glucose control in male mouse offspring. Nutrients 2020, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Shelp, G.V.; Poole, E.M.; Cook, W.J.J.; Michaud, J.; Cho, C.E. Prenatal choline supplementation enhances metabolic outcomes with differential impact on DNA methylation in Wistar rat offspring and dams. J. Nutr. Biochem. 2025, 136, 109806. [Google Scholar] [CrossRef]
- Brown, W.E.; Holdorf, H.T.; Johnson, S.J.; Kendall, S.J.; Green, S.E.; White, H.M. In utero choline exposure alters growth, metabolism, feed efficiency, and carcass characteristics of Holstein × Angus cattle from weaning to slaughter. J. Anim. Sci. 2023, 101, skad186. [Google Scholar] [CrossRef]
- Teng, Y.W.; Ellis, J.M.; Coleman, R.A.; Zeisel, S.H. Mouse betaine-homocysteine S-methyltransferase deficiency reduces body fat via increasing energy expenditure and impairing lipid synthesis and enhancing glucose oxidation in white adipose tissue. J. Biol. Chem. 2012, 287, 16187–16198. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, J.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Caudill, M.A. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J. 2012, 26, 3563–3574. [Google Scholar] [CrossRef]
- Hammoud, R.; Pannia, E.; Kubant, R.; Wasek, B.; Bottiglieri, T.; Malysheva, O.V.; Caudill, M.A.; Anderson, G.H. Choline and folic acid in diets consumed during pregnancy interact to program food intake and metabolic regulation of male Wistar rat offspring. J. Nutr. 2021, 151, 857–865. [Google Scholar] [CrossRef]
- Warrier, M.; Paules, E.M.; Silva-Gomez, J.; Friday, W.B.; Bramlett, F.; Kim, H.; Zhang, K.; Trujillo-Gonzalez, I. Homocysteine-induced endoplasmic reticulum stress activates FGF21 and is associated with browning and atrophy of white adipose tissue in Bhmt knockout mice. Heliyon 2023, 9, e13216. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Kadam, I.; Reaz, A.; Bretter, R.; Saxena, A.; Johnson, C.H.; Caviglia, J.M.; Kiang, X. Prenatal choline supplement in a maternal obesity model modulates offspring hepatic lipidomes. Nutrients 2023, 15, 965. [Google Scholar] [CrossRef]
- Seitz, H.K.; Moreira, B.; Neuman, M.G. Pathogenesis of alcoholic fatty liver: A narrative review. Life 2023, 13, 1662. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Schön, C.; Derbyshire, E.; Jiang, X.; Mellott, T.J.; Blusztajn, J.K.; Zeisel, S.H. A narrative review on maternal choline intake and liver function of the fetus and the infant; implications for research, policy, and practice. Nutrients 2024, 16, 260. [Google Scholar] [CrossRef]
- Petry, H.G.; Saini, N.; Smith, S.M.; Mooney, S.M. Alcohol exposure may increase prenatal choline needs through redirection of choline into lipid synthesis rather than methyl donation. Metabolites 2025, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Steane, S.E.; Kumar, V.; Cuffe, J.S.M.; Moritz, K.M.; Akison, L.K. Prenatal choline supplementation alters one carbon metabolites in a rat model of periconceptional alcohol exposure. Nutrients 2022, 14, 1874. [Google Scholar] [CrossRef]
- Gutherz, O.R.; Li, Q.; Deyssenroth, M.; Wainwright, H.; Jacobson, J.L.; Meintjes, E.M.; Chen, J.; Jacobson, S.W.; Carter, R.C. The roles of maternal one-carbon metabolism and placental imprinted gene expression in placental development and somatic growth in a longitudinal birth cohort. Placenta 2025, 167, 109–121. [Google Scholar] [CrossRef]
- Kadam, I.; Trasino, S.E.; Korsmo, H.; Lucas, J.; Pinkas, M.; Jiang, X. Prenatal choline supplementation improves glucose tolerance and reduces liver fat accumulation in mouse offspring exposed to ethanol during the prenatal and postnatal periods. Nutrients 2024, 16, 1264. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Greenwald, E.; Jack-Roberts, C.; Ajeeb, T.T.; Malysheva, O.V.; Caudill, M.A.; Axen, K.; Saxena, A.; Semernina, E.; Nanobashvili, K.; et al. Choline prevents fetal overgrowth and normalizes placental fatty acid and glucose metabolism in a mouse model of maternal obesity. J. Nutr. Biochem. 2017, 49, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Klurfeld, D.M.; Gregory, J.F.; Fiorotto, M.L. Should the AIN-93 rodent diet formulas be revised? J. Nutr. 2021, 151, 1380–1382. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Fulgoni, V.L., 3rd. Assessment of total choline intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]


| Males | Females | |||
|---|---|---|---|---|
| Response to PAE | Response to Choline | Response to PAE | Response to Choline | |
| Brain Weight | = | = | = | ↑ |
| Liver Weight | ↑ | ↓ | = | ↓ |
| Body Weight | ↑ | ↓ | ↑ | ↓ |
| Fat Mass | ↑ | ↓ | ↑ | ↓ |
| Lean Mass | = | = | = | = |
| Fasting Glucose | ↑ | ↓ | = | ↓ |
| Glucose Intolerance | ↑ | ↓ | = | ↓ (trend) |
| Glucose Intolerance Normalized | ↑ | = | = | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, S.M.; Munson, C.A.; Flentke, G.R.; Mooney, S.M. Prenatal Choline Attenuates the Elevated Adiposity and Glucose Intolerance Caused by Prenatal Alcohol Exposure. Cells 2025, 14, 1429. https://doi.org/10.3390/cells14181429
Smith SM, Munson CA, Flentke GR, Mooney SM. Prenatal Choline Attenuates the Elevated Adiposity and Glucose Intolerance Caused by Prenatal Alcohol Exposure. Cells. 2025; 14(18):1429. https://doi.org/10.3390/cells14181429
Chicago/Turabian StyleSmith, Susan M., Carolyn A. Munson, George R. Flentke, and Sandra M. Mooney. 2025. "Prenatal Choline Attenuates the Elevated Adiposity and Glucose Intolerance Caused by Prenatal Alcohol Exposure" Cells 14, no. 18: 1429. https://doi.org/10.3390/cells14181429
APA StyleSmith, S. M., Munson, C. A., Flentke, G. R., & Mooney, S. M. (2025). Prenatal Choline Attenuates the Elevated Adiposity and Glucose Intolerance Caused by Prenatal Alcohol Exposure. Cells, 14(18), 1429. https://doi.org/10.3390/cells14181429







