Multispectral Pulsed Photobiomodulation Enhances Re-Epithelialization via Keratinocyte Activation in Full-Thickness Skin Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Multispectral PBM System Setup
2.2. Keratinocyte Culture and PBM Exposure Protocol
2.3. Assessment of Keratinocyte Proliferation
2.4. In Vitro Migration Analysis
2.5. RNA Extraction and qPCR Analysis
2.6. Protein Extraction and Western Blotting
2.7. In Vivo Photobiomodulation Treatment in Murine Wound Healing
2.8. Quantitative Assessment of Wound Closure
2.9. Tissue Processing and Histological Evaluation
2.10. Stastical Analysis
3. Results
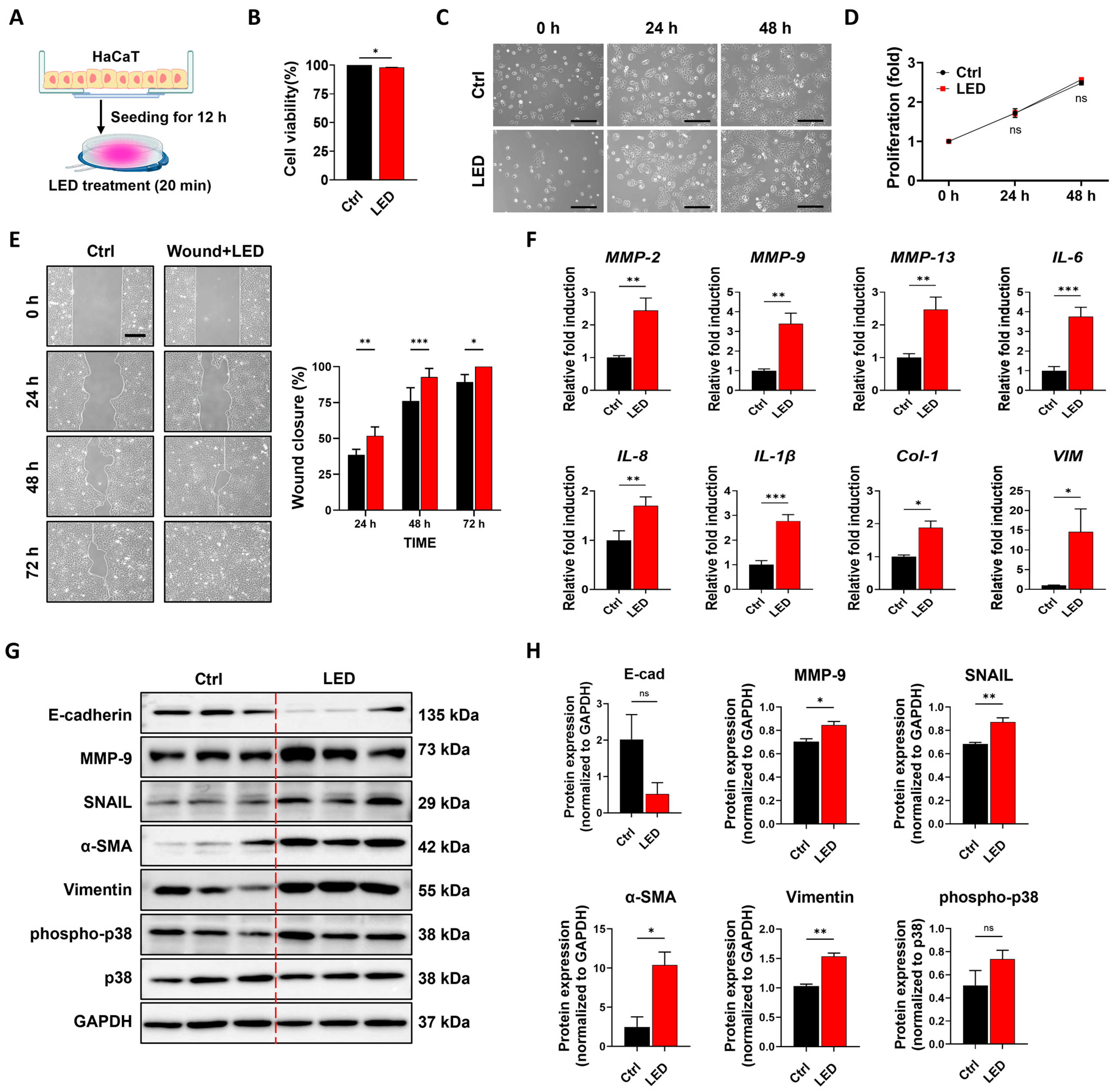
3.1. PBM Enhances Keratinocyte Migration While Maintaining Proliferative Capacity
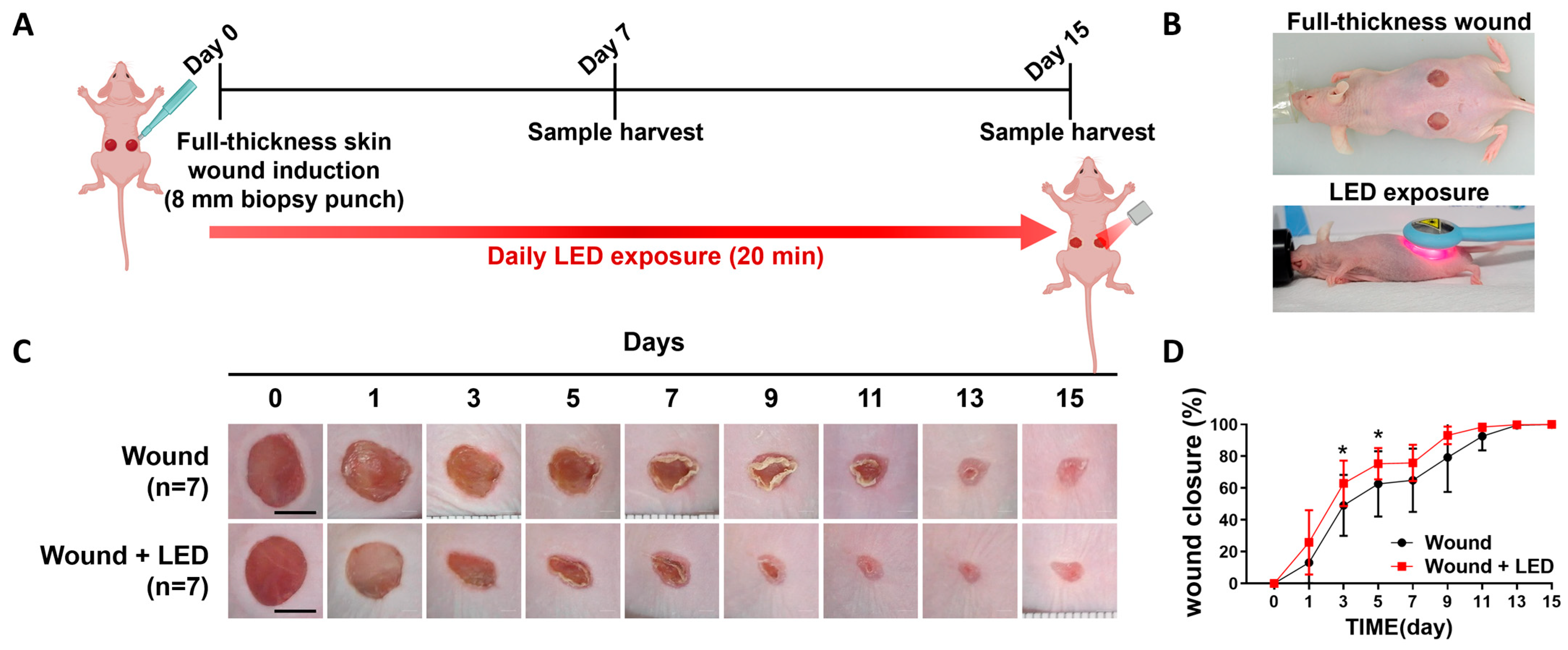
3.2. PBM Accelerates Early Wound Closure and Promotes Re-Epithelialization In Vivo
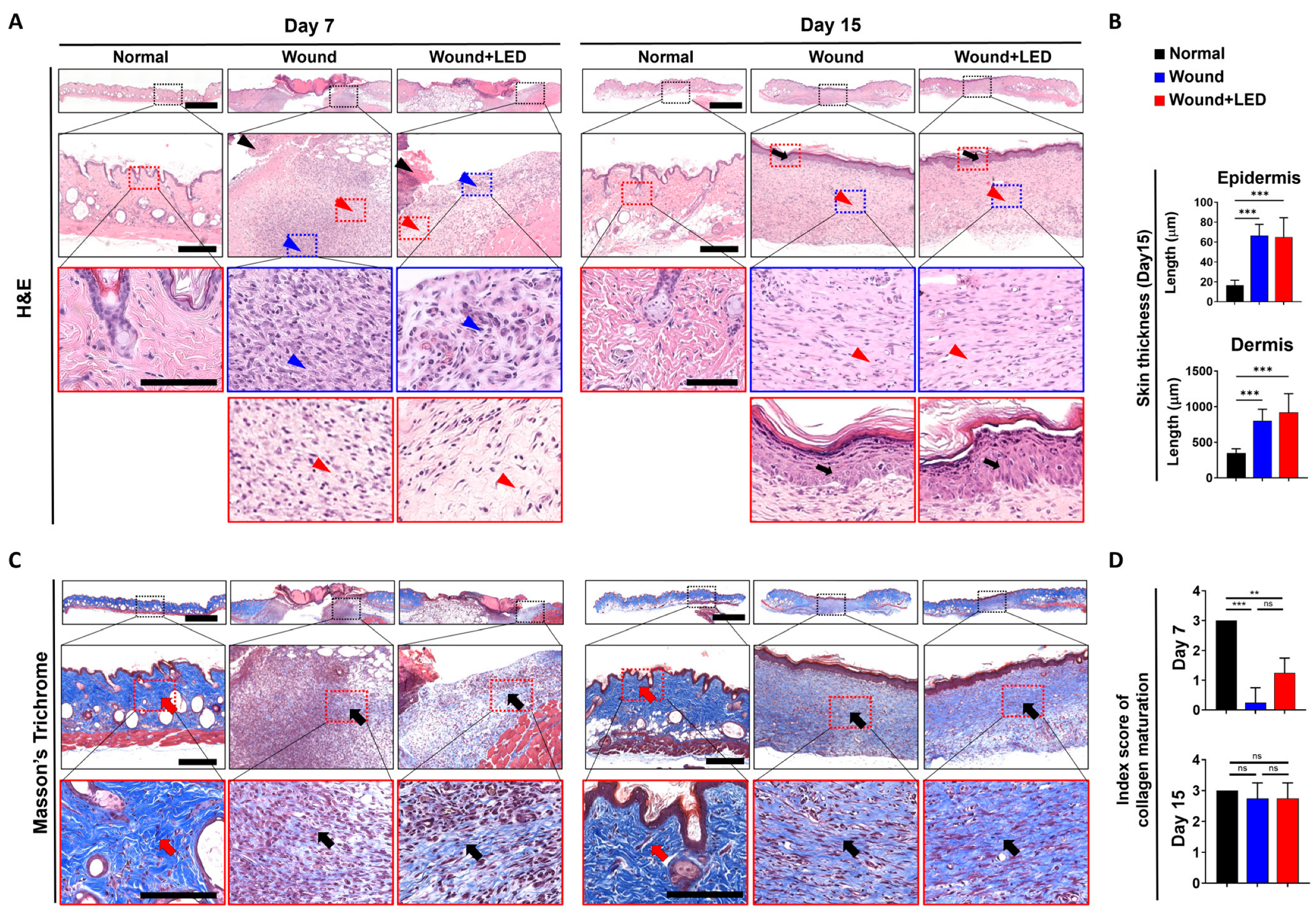
3.3. PBM Enhances Epidermal Regeneration and Collagen Remodeling During Wound Healing
| Skin | Groups | Day 7 | Day 15 | |||||
|---|---|---|---|---|---|---|---|---|
| Normal | Wound | Wound + LED | Normal | Wound | Wound + LED | |||
| No. of Animals | 2 | 4 | 4 | 2 | 4 | 4 | ||
| Epidermis | Necrosis with crust | ++ | 0 | 0 | 2 | 0 | 0 | 0 |
| +++ | 0 | 3 | 2 | 0 | 0 | 0 | ||
| Regeneration | + | 0 | 3 | 3 | 0 | 0 | 0 | |
| ++ | 0 | 0 | 1 | 0 | 3 | 2 | ||
| +++ | 0 | 0 | 0 | 0 | 1 | 2 | ||
| Dermis | Maturation, fibroblast to fibrocyte | ± | 0 | 3 | 0 | 0 | 0 | 0 |
| + | 0 | 1 | 2 | 0 | 0 | 0 | ||
| ++ | 0 | 0 | 2 | 0 | 1 | 2 | ||
| +++ | 0 | 0 | 0 | 0 | 3 | 3 | ||
| Collagen maturation | ± | 0 | 3 | 0 | 0 | 0 | 0 | |
| + | 0 | 1 | 3 | 0 | 0 | 0 | ||
| ++ | 0 | 0 | 1 | 0 | 1 | 1 | ||
| +++ | 2 | 0 | 0 | 2 | 3 | 3 | ||
| Vascularization | ± | 0 | 0 | 0 | 0 | 1 | 0 | |
| + | 0 | 1 | 0 | 0 | 0 | 0 | ||
| Infiltrate, inflammatory cell | ± | 0 | 1 | 1 | 0 | 2 | 3 | |
| + | 0 | 2 | 0 | 0 | 0 | 0 | ||
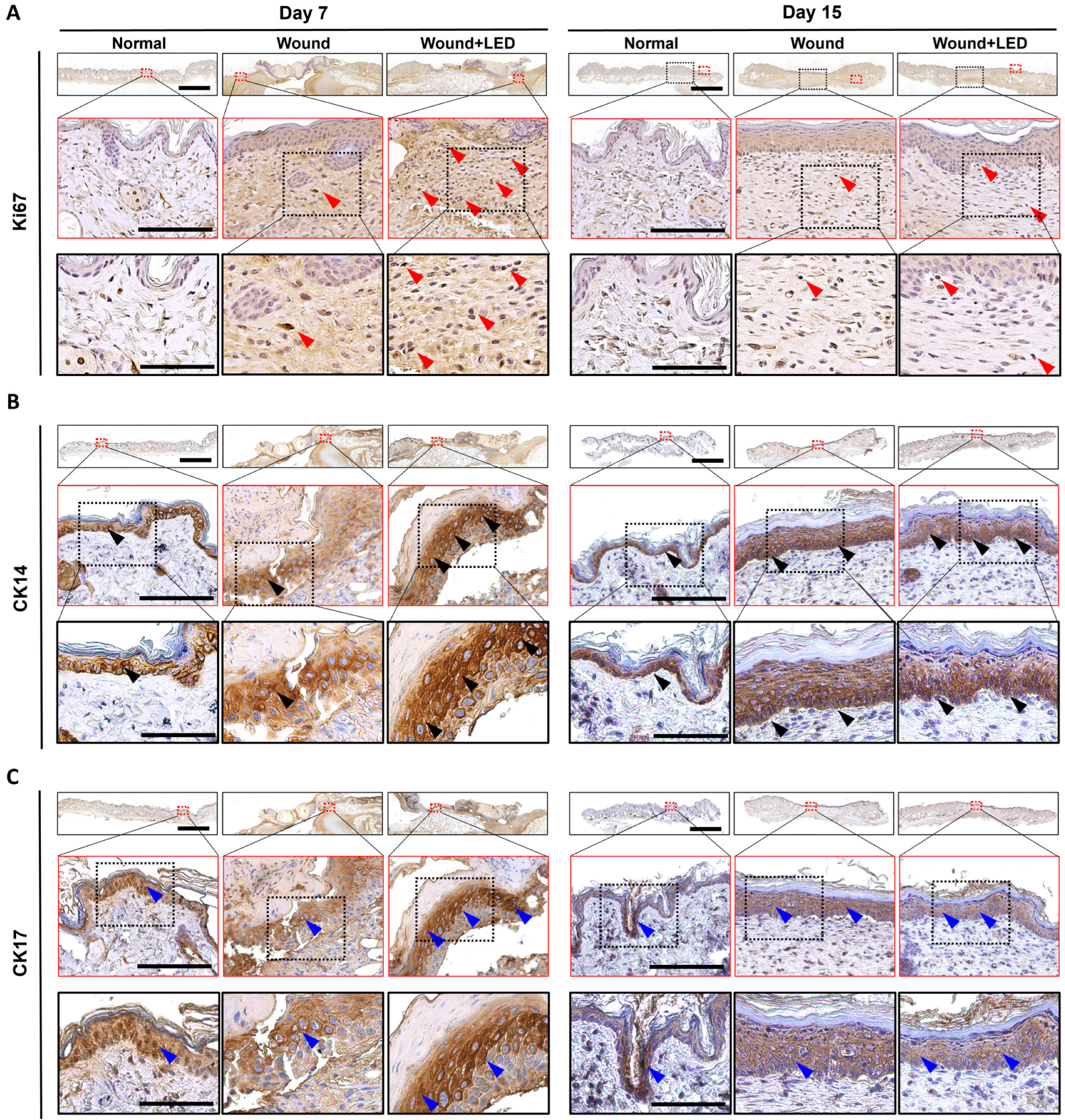
3.4. PBM Enhances Epidermal Proliferation and Re-Epithelialization in a Murine Full-Thickness Wound Model
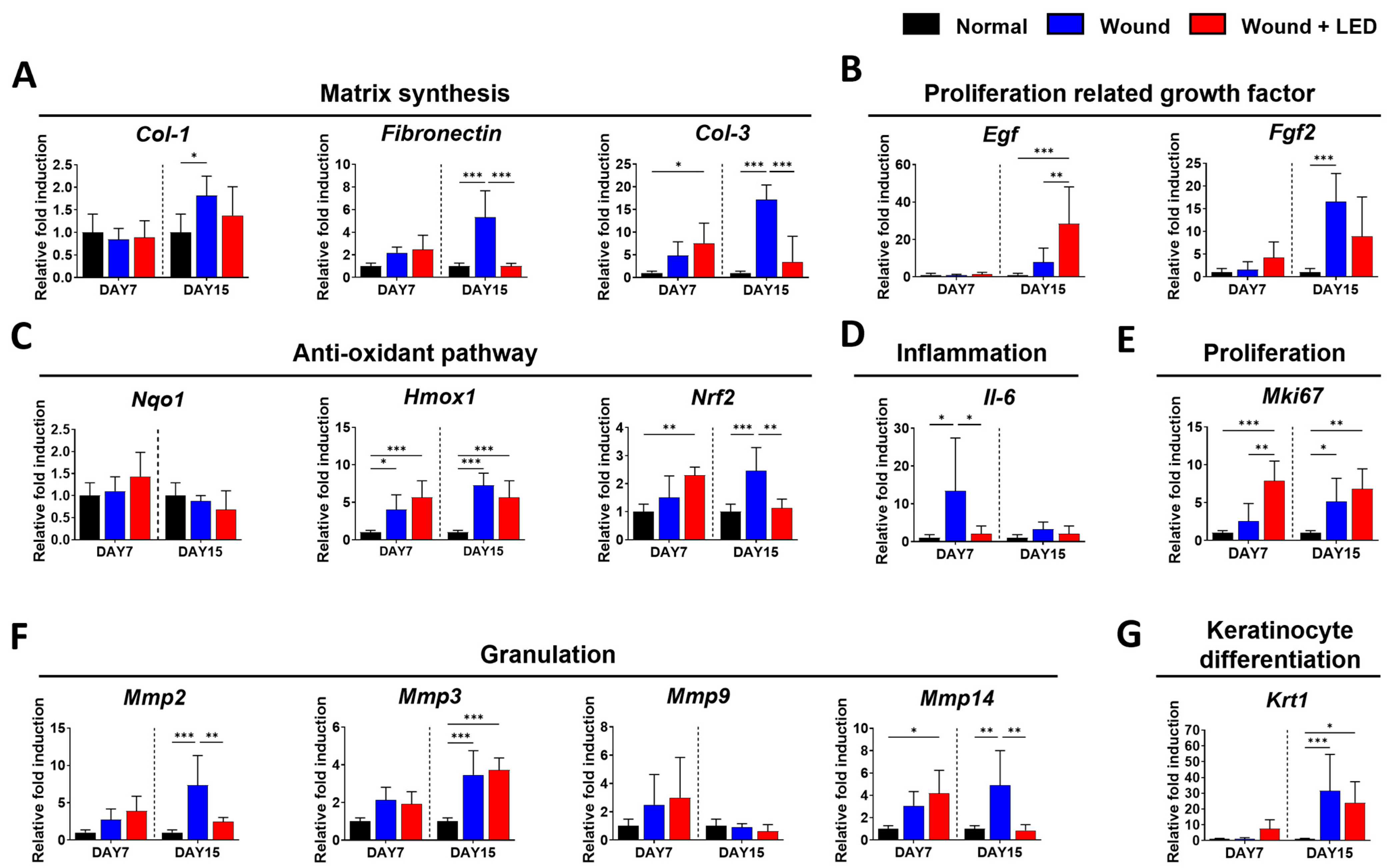
3.5. PBM Regulates Key Gene Expression Pathways Involved in Wound Healing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LED | light-emitting diode |
| ECM | extracellular matrix |
| LLLT | Low-level light therapy |
| TGF-β1 | transforming growth factor-beta 1 |
| PBM | photobiomodulation |
| ATP | adenosine triphosphate |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| α-SMA | alpha smooth muscle actin |
References
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Anggradita, L.D.; Lee, S.J.; Hur, S.S.; Bae, J.; Hwang, N.S.; Nam, S.M.; Hwang, Y. Ameliorating Fibrotic Phenotypes of Keloid Dermal Fibroblasts Through an Epidermal Growth Factor-Mediated Extracellular Matrix Remodeling. Int. J. Mol. Sci. 2021, 22, 2198. [Google Scholar] [CrossRef] [PubMed]
- Vizely, K.; Wagner, K.T.; Mandla, S.; Gustafson, D.; Fish, J.E.; Radisic, M. Angiopoietin-1 derived peptide hydrogel promotes molecular hallmarks of regeneration and wound healing in dermal fibroblasts. iScience 2023, 26, 105984. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Subramaniam, M.D.; Bae, J.S.; Son, J.; Anggradita, L.D.; Kim, M.K.; Lee, M.Y.; Jang, S.; Choi, K.; Lee, J.C.; Nam, S.M.; et al. Floating electrode-dielectric barrier discharge-based plasma promotes skin regeneration in a full-thickness skin defect mouse model. Biomed. Eng. Lett. 2024, 14, 605–616. [Google Scholar] [CrossRef]
- Oyebode, O.A.; Jere, S.W.; Houreld, N.N. Current Therapeutic Modalities for the Management of Chronic Diabetic Wounds of the Foot. J. Diabetes Res. 2023, 2023, 1359537. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Stapley, S. Debridement of diabetic foot ulcers. Cochrane Database Syst. Rev. 2010, 2010, CD003556. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Li, H.; Chen, X.; Feng, S.; Mei, Z. How Effective are Nano-Based Dressings in Diabetic Wound Healing? A Comprehensive Review of Literature. Int. J. Nanomed. 2022, 17, 2097–2119. [Google Scholar] [CrossRef]
- Mansouri, V.; Arjmand, B.; Rezaei Tavirani, M.; Razzaghi, M.; Rostami-Nejad, M.; Hamdieh, M. Evaluation of Efficacy of Low-Level Laser Therapy. J. Lasers Med. Sci. 2020, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.H.; Son, J.W.; Eun, Y.S.; Yang, N.G.; Kim, J.Y.; Lee, S.; Heo, N.H.; Rhee, J.; Lee, S.Y.; Hwang, Y.; et al. A Novel, Hand-Held, and Low-Level Light Therapy Device for the Treatment of Acne Vulgaris: A Single-Arm, Prospective Clinical Study. Dermatol. Ther. 2023, 2023, 8846620. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, H.J.; Kim, J.Y.; Ban, M.J.; Son, J.; Hwang, Y.; Cho, S.B. Immediate and Late Effects of Pulse Widths and Cycles on Bipolar, Gated Radiofrequency-Induced Tissue Reactions in In Vivo Rat Skin. Clin. Cosmet. Investig. Dermatol. 2023, 16, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Panda, P.K.; Su, C.; Lin, Y.C.; Sakthivel, R.; Chen, S.L.; Chung, R.J. Near-infrared-driven upconversion nanoparticles with photocatalysts through water-splitting towards cancer treatment. J. Mater. Chem. B 2024, 12, 3881–3907. [Google Scholar] [CrossRef]
- Wang, T.; Song, Y.; Yang, L.; Liu, W.; He, Z.; Shi, Y.; Song, B.; Yu, Z. Photobiomodulation Facilitates Rat Cutaneous Wound Healing by Promoting Epidermal Stem Cells and Hair Follicle Stem Cells Proliferation. Tissue Eng. Regen. Med. 2024, 21, 65–79. [Google Scholar] [CrossRef]
- Wiegand, C.; Dirksen, A.; Tittelbach, J. Treatment with a red-laser-based wound therapy device exerts positive effects in models of delayed keratinocyte and fibroblast wound healing. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12926. [Google Scholar] [CrossRef]
- Choi, J.; Ban, M.J.; Gil, C.H.; Hur, S.S.; Anggradita, L.D.; Kim, M.K.; Son, J.W.; Kim, J.E.; Hwang, Y. Multispectral Pulsed Photobiomodulation Enhances Diabetic Wound Healing via Focal Adhesion-Mediated Cell Migration and Extracellular Matrix Remodeling. Int. J. Mol. Sci. 2025, 26, 6232. [Google Scholar] [CrossRef]
- Khadra, M.; Lyngstadaas, S.P.; Haanaes, H.R.; Mustafa, K. Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 2005, 26, 3503–3509. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Carpena, N.; Kang, B.; Lee, M.Y. Effects of Photobiomodulation on Stem Cells Important for Regenerative Medicine. Med. Lasers 2020, 9, 134–141. [Google Scholar] [CrossRef]
- Mineroff, J.; Maghfour, J.; Ozog, D.M.; Lim, H.W.; Kohli, I.; Jagdeo, J. Photobiomodulation CME Part II: Clinical applications in dermatology. J. Am. Acad. Dermatol. 2024, 91, 805–815. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Houreld, N.N.; Abrahamse, H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol. Ther. 2012, 14, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Chen, S.; Luo, Y.; He, Y.; Li, M.; Liu, Y.; Zhou, X.; Hou, J.; Zhou, S. In-situ-sprayed therapeutic hydrogel for oxygen-actuated Janus regulation of postsurgical tumor recurrence/metastasis and wound healing. Nat. Commun. 2024, 15, 814. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-enhanced immunoregulation hydrogel for oxygenation and ROS neutralization in diabetic foot ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Rhea, L.; Dunnwald, M. Murine Excisional Wound Healing Model and Histological Morphometric Wound Analysis. J. Vis. Exp. 2020, 162, 61616. [Google Scholar] [CrossRef]
- Wang, Y.; Jeong, Y.; Jhiang, S.M.; Yu, L.; Menq, C.H. Quantitative characterization of cell behaviors through cell cycle progression via automated cell tracking. PLoS ONE 2014, 9, e98762. [Google Scholar] [CrossRef]
- Keenan, C.M.; Baker, J.F.; Bradley, A.E.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; Mahler, B.; Meseck, E.; et al. International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) progress to date and future plans. J. Toxicol. Pathol. 2015, 28, 51–53. [Google Scholar] [CrossRef][Green Version]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Simoes, A.; Correa, L.; Aranha, A.C.; Giudice, F.S.; Hamblin, M.R.; Sousa, S.C. Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J. Biophotonics 2015, 8, 795–803. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and In Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; He, Y.; Ni, W.; Zhang, Y.; Zhu, Y.; Cao, M.; He, R.; Yao, M. LLLT accelerates experimental wound healing under microgravity conditions via PI3K/AKT-CCR2 signal axis. Front. Bioeng. Biotechnol. 2024, 12, 1387474. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.C.; Pandya, R.P.; Leffler, K.A.; Yoshida, T.; Beck, L.A.; Brewer, M.G. Characterization of Human Keratinocyte Cell Lines for Barrier Studies. JID Innov. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Blanchard, G.; Pich, C.; Hohl, D. HaCaT cells as a model system to study primary cilia in keratinocytes. Exp. Dermatol. 2022, 31, 1276–1280. [Google Scholar] [CrossRef]
- Sutterby, E.; Chheang, C.; Thurgood, P.; Khoshmanesh, K.; Baratchi, S.; Pirogova, E. Investigating the effects of low intensity visible light on human keratinocytes using a customized LED exposure system. Sci. Rep. 2022, 12, 18907. [Google Scholar] [CrossRef] [PubMed]
- Raja; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Shabestani Monfared, G.; Ertl, P.; Rothbauer, M. An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure. Sci. Rep. 2020, 10, 16192. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in Relation to Photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef]
- Huang, X.H.; Ma, Y.; Lou, H.; Chen, N.; Zhang, T.; Wu, L.Y.; Chen, Y.J.; Zheng, M.M.; Lou, Y.L.; Xie, D.L. The Role of TSC1 in the Macrophages Against Vibrio vulnificus Infection. Front. Cell Infect. Microbiol. 2020, 10, 596609. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Abate, M.; Citro, M.; Pisanti, S.; Caputo, M.; Martinelli, R. Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol. Int. J. Mol. Sci. 2021, 22, 2438. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, C.; Liu, X.; Yang, Y.; Cao, L.; Xiang, G.; Liu, F.; Wang, S.; Liu, J.; Meng, Q.; et al. TGF-β1 stimulates epithelial-mesenchymal transition and cancer-associated myoepithelial cell during the progression from in situ to invasive breast cancer. Cancer Cell Int. 2019, 19, 343. [Google Scholar] [CrossRef]
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Kalgudde Gopal, S.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 5653. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Sabino, F.; auf dem Keller, U. Matrix metalloproteinases in impaired wound healing. Met. Med. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ko, E.; Ryu, Y.H.; Lee, S.J.; Jun, Y.J. Hyaluronic Acid Based Adipose Tissue-Derived Extracellular Matrix Scaffold in Wound Healing: Histological and Immunohistochemical Study. Tissue Eng. Regen. Med. 2024, 21, 829–842. [Google Scholar] [CrossRef]
- Lee, Y.B.; Lee, D.H.; Kim, Y.C.; Bhang, S.H. Enhancing Skin Regeneration Efficacy of Human Dermal Fibroblasts Using Carboxymethyl Cellulose-Coated Biodegradable Polymer. Tissue Eng. Regen. Med. 2025, 22, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Kuo, J.C. Mechanotransduction at focal adhesions: Integrating cytoskeletal mechanics in migrating cells. J. Cell Mol. Med. 2013, 17, 704–712. [Google Scholar] [CrossRef]
- Mavrakis, M.; Juanes, M.A. The compass to follow: Focal adhesion turnover. Curr. Opin. Cell Biol. 2023, 80, 102152. [Google Scholar] [CrossRef]
- Jones, G.E.; Prigmore, E.; Calvez, R.; Hogan, C.; Dunn, G.A.; Hirsch, E.; Wymann, M.P.; Ridley, A.J. Requirement for PI 3-kinase gamma in macrophage migration to MCP-1 and CSF-1. Exp. Cell Res. 2003, 290, 120–131. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D. Macrophage Polarization: A Novel Target and Strategy for Pathological Scarring. Tissue Eng. Regen. Med. 2024, 21, 1109–1124. [Google Scholar] [CrossRef]
- Statha, D.; Sfiniadakis, I.; Rallis, M.; Anastassopoulou, J.; Alexandratou, E. Investigating the wound healing potential of low-power 661 nm laser light in a pigmented hairless murine model. Photochem. Photobiol. Sci. 2025, 24, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Fleckner, M.; Döhmen, N.K.; Salz, K.; Christophers, T.; Windolf, J.; Suschek, C.V.; Oezel, L. Exposure of Primary Human Skin Fibroblasts to Carbon Dioxide-Containing Solution Significantly Reduces TGF-β-Induced Myofibroblast Differentiation In Vitro. Int. J. Mol. Sci. 2024, 25, 13013. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, B.; Zhai, M.; Hull, L.; Cui, W.; Xiao, M. Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury. Int. J. Mol. Sci. 2024, 25, 12498. [Google Scholar] [CrossRef]
- Migliario, M.; Yerra, P.; Gino, S.; Sabbatini, M.; Reno, F. Laser Biostimulation Induces Wound Healing-Promoter Beta2-Defensin Expression in Human Keratinocytes via Oxidative Stress. Antioxidants 2023, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Wilson, C.H.; Harding, K.G.; Finlay, A.Y.; Bowden, P.E. Numerous keratinocyte subtypes involved in wound re-epithelialization. J. Investig. Dermatol. 2006, 126, 497–502. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequences (5′–3′) |
|---|---|
| GAPDH_Forward | CACTCCACCTTTGACGC |
| GAPDH_Reverse | GGTCCAGGGGTCTTACTCC |
| MMP2_Forward | GATACCCCTTTGACGGTAAGGA |
| MMP2_Reverse | CCTTCTCCCAAGGTCCATAGC |
| MMP9_Forward | GGGACGCAGACATCGTCATC |
| MMP9_Reverse | TCGTCATCGTCGAAATGGGC |
| MMP13_Forward | TCGTCATCGTCGAAATGGGC |
| MMP13_Reverse | TCGTCATCGTCGAAATGGGC |
| IL-6_Forward | ACTCACCTCTTCAGAACGAATTG |
| IL-6_Reverse | CCATCTTTGGAAGGTTCAGGTTG |
| IL-8_Forward | ACTGAGAGTGATTGAGAGTGGAC |
| IL-8_Reverse | AACCCTCTGCACCCAGTTTTC |
| IL-1β_Forward | ATGATGGCTTATTACAGTGGCAA |
| IL-1β_Reverse | GTCGGAGATTCGTAGCTGGA |
| COL-1_Forward | CAAGACAG TGATTGAATACAAAACCA |
| COL-1_Reverse | ACGTCGAAGCCGAATTCCT |
| Vimentin_Forward | AATCCAAGTTTGCTGACCTCTCTGA |
| Vimentin_Reverse | ACTGCACCTGTCTCCGGTACTC |
| Primer | Sequences (5′–3′) |
|---|---|
| Gapdh_Forward | AAGGTCATCCCAGAGCTGAA |
| Gapdh_Reverse | CTGCTTCACCACCTTCTTGA |
| Col-1_Forward | GCT CCT CTT AGG GGC CAC T |
| Col-1_Reverse | CCT TTGTCA GAA TAC TGA GCA GC |
| Fibronectin_Forward | ATGTGGACCCCTCCTGATAGT |
| Fibronectin_Reverse | GCCCAGTGATTTCAGCAAAGG |
| Egf_Forward | AGCATCTCTCGGATTGACCCA |
| Egf_Reverse | CCTGTCCCGTTAAGGAAAACTCT |
| Fgf2_Forward | GCGACCCACACGTCAAACTA |
| Fgf2_Reverse | CCGTCCATCTTCCTTCATAGC |
| Nqo1_Forward | AGGATGGGAGGTACTCGAATC |
| Nqo1_Reverse | AGGCGTCCTTCCTTATATGCTA |
| Hmox1_Forward | AAGCCGAGAATGCTGAGTTCA |
| Hmox1_Reverse | GCCGTGTAGATATGGTACAAGGA |
| Nrf2_Forward | CTGAACTCCTGGACGGGACTA |
| Nrf2_Reverse | CGGTGGGTCTCCGTAAATGG |
| Il-6_Forward | TAGTCCTTCCTACCCCAATTTCC |
| Il-6_Reverse | TTGGTCCTTAGCCACTCCTTC |
| Mki-67_Forward | CTGCCTCAGATGGCTCAAAGA |
| Mki-67_Reverse | GAAGACTTCGGTTCCCTGTAAC |
| Mmp2_Forward | CAAGTTCCCCGGCGATGTC |
| Mmp2_Reverse | TTCTGGTCAAGGTCACCTGTC |
| Mmp3_Forward | GATGAGCACACAACCACACAC |
| Mmp3_Reverse | GGTACAGAGCTGTGGGAAGTC |
| Mmp9_Forward | GGGACGCAGACATCGTCATC |
| Mmp9_Reverse | CCCACATTTGACGTCCAGAGAAGAA |
| Mmp14_Forward | CAGTATGGCTACCTACCTCCAG |
| Mmp14_Reverse | GCCTTGCCTGTCACTTGTAAA |
| Krt1_Forward | TGGGAGATTTTCAGGAGGAGG |
| Krt1_Reverse | GCCACACTCTTGGAGATGCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Baatar, D.; Ban, M.J.; Son, J.W.; Choi, J.; Gil, C.H.; Kim, M.-K.; Hur, S.S.; Kim, J.E.; Hwang, Y. Multispectral Pulsed Photobiomodulation Enhances Re-Epithelialization via Keratinocyte Activation in Full-Thickness Skin Wounds. Cells 2025, 14, 1415. https://doi.org/10.3390/cells14181415
Kim JH, Baatar D, Ban MJ, Son JW, Choi J, Gil CH, Kim M-K, Hur SS, Kim JE, Hwang Y. Multispectral Pulsed Photobiomodulation Enhances Re-Epithelialization via Keratinocyte Activation in Full-Thickness Skin Wounds. Cells. 2025; 14(18):1415. https://doi.org/10.3390/cells14181415
Chicago/Turabian StyleKim, Joo Hyun, Delgerzul Baatar, Myung Jin Ban, Ji Won Son, Jihye Choi, Chan Hee Gil, Min-Kyu Kim, Sung Sik Hur, Jung Eun Kim, and Yongsung Hwang. 2025. "Multispectral Pulsed Photobiomodulation Enhances Re-Epithelialization via Keratinocyte Activation in Full-Thickness Skin Wounds" Cells 14, no. 18: 1415. https://doi.org/10.3390/cells14181415
APA StyleKim, J. H., Baatar, D., Ban, M. J., Son, J. W., Choi, J., Gil, C. H., Kim, M.-K., Hur, S. S., Kim, J. E., & Hwang, Y. (2025). Multispectral Pulsed Photobiomodulation Enhances Re-Epithelialization via Keratinocyte Activation in Full-Thickness Skin Wounds. Cells, 14(18), 1415. https://doi.org/10.3390/cells14181415






