Trisomy 21 Disrupts Thyroid Hormones Signaling During Human iPSC-Derived Neural Differentiation In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintenance of Human iPSC
2.2. Generation of hiPSC-Derived Neuroprogenitor Cells, Neurons, and Astrocytes
2.3. Immunocytochemistry
2.4. Digital Karyotyping
2.5. RT-qPCR Assay
2.6. Multi-Electrode Array
2.7. Proliferation Assay
2.8. Cell Cycle Analysis
2.9. Analysis of Publicly Available Single-Nucleus RNA Sequencing Data
2.9.1. Gene Expression Dataset Acquisition
2.9.2. Data Preprocessing and Gene Categorization
2.9.3. Differential Expression Visualization
2.9.4. Gene Set Expression Trend Analysis
2.9.5. Computational Environment
2.10. Statistical Analysis
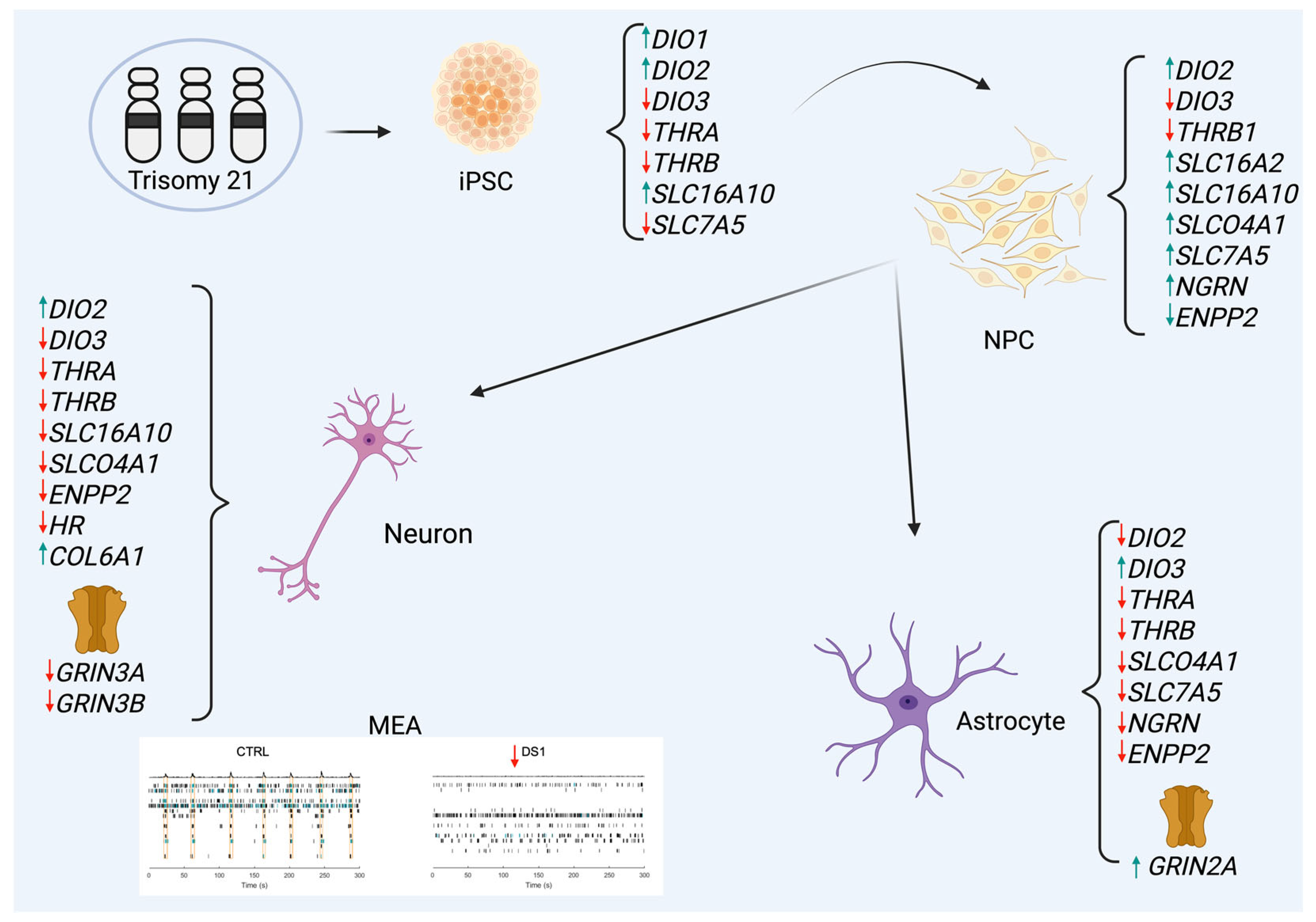
3. Results
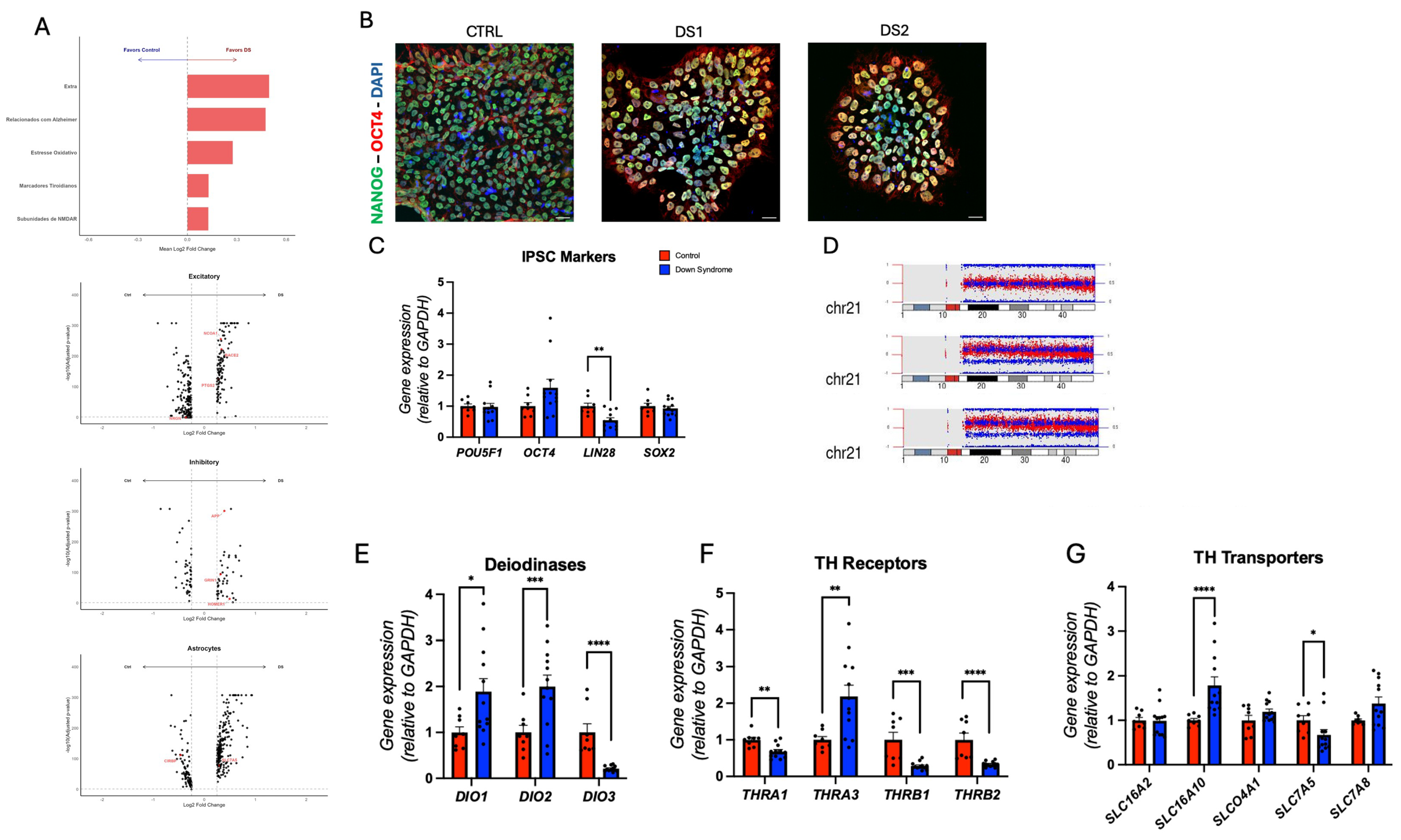
3.1. Expression of Thyroid Hormones Signaling Genes in the Cortical Brain of DS Subjects
3.2. Altered Thyroid Hormones Maintenance in Down Syndrome hiPSCs
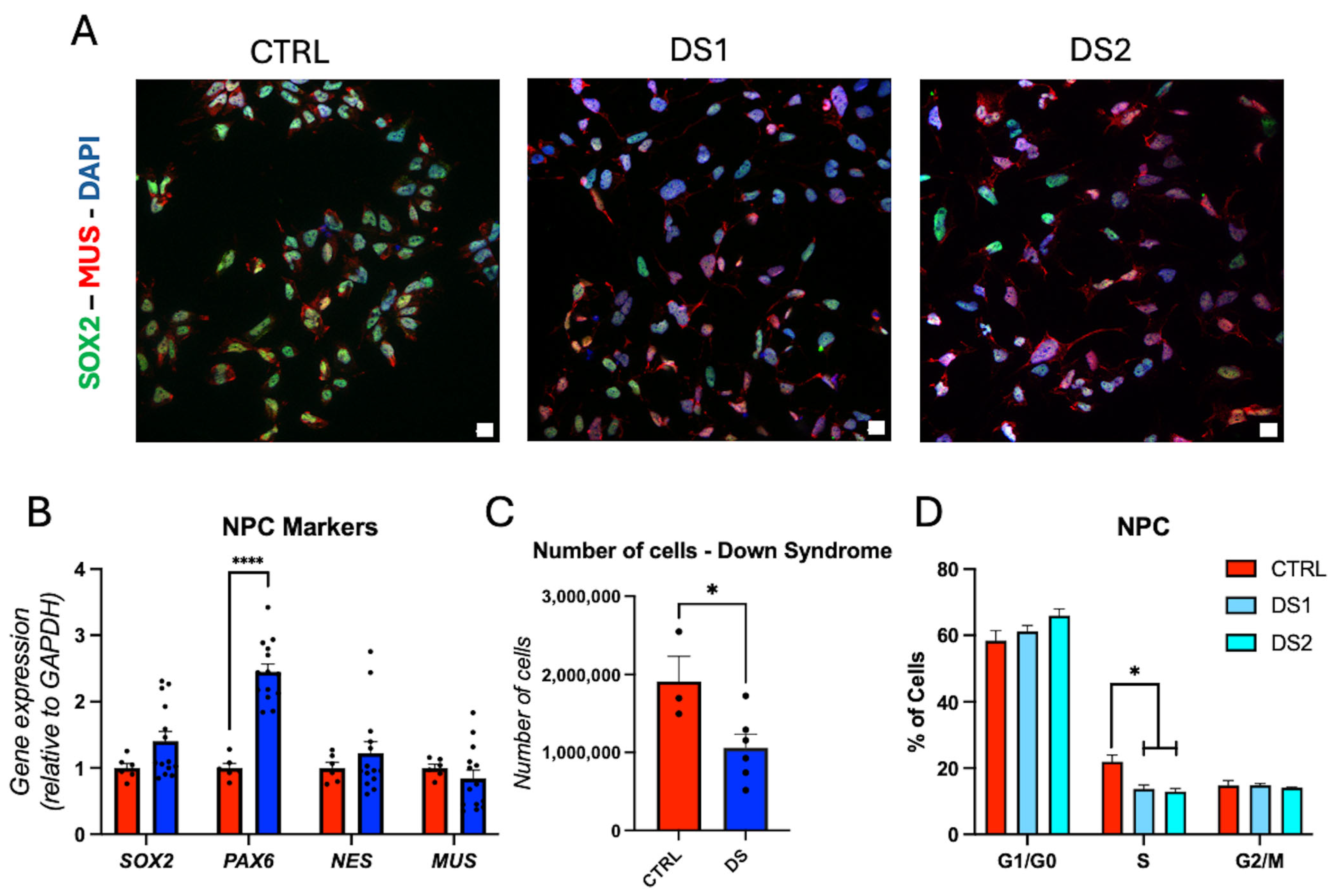
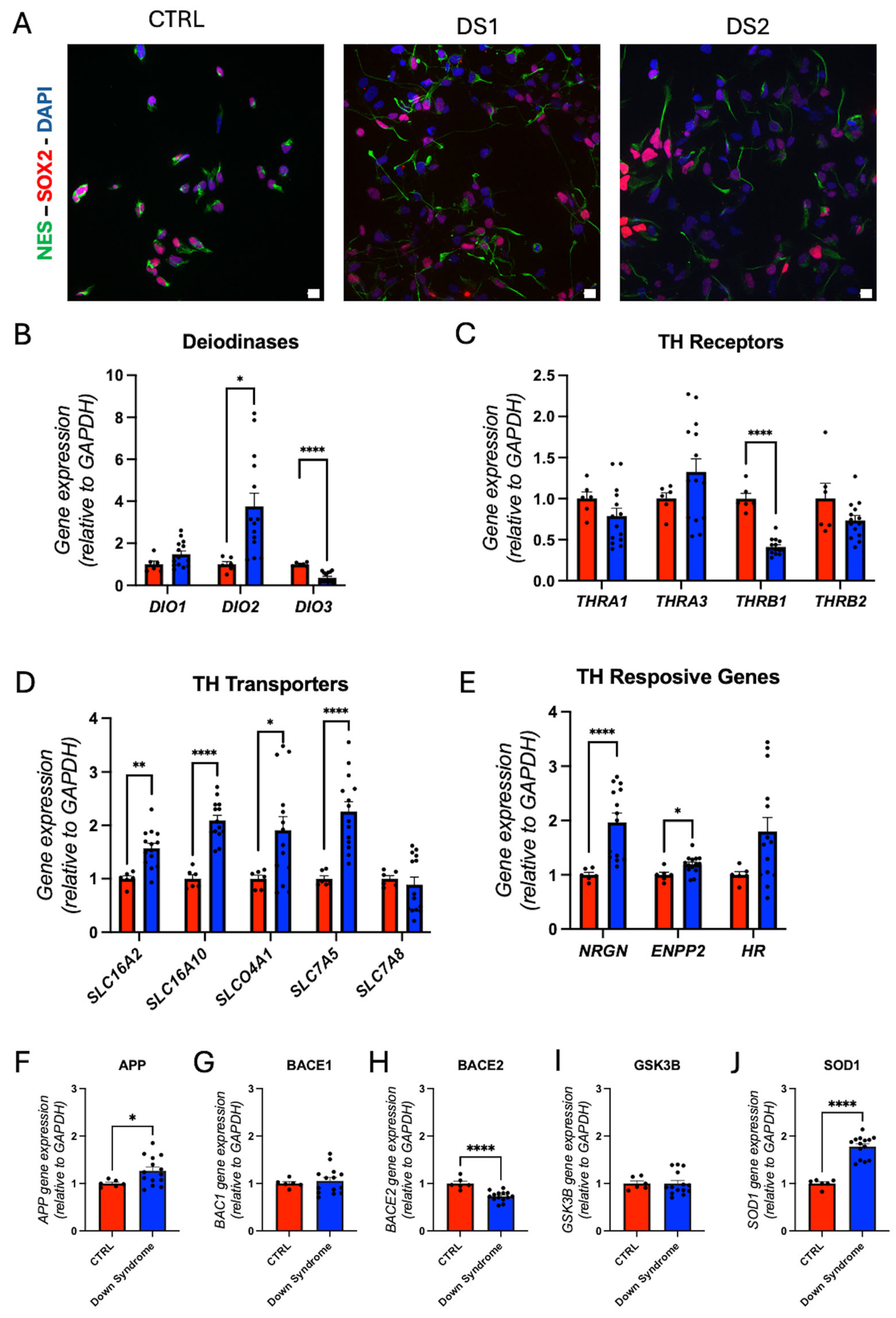
3.3. Altered Thyroid Hormones Maintenance in Down Syndrome Hid-NPCs
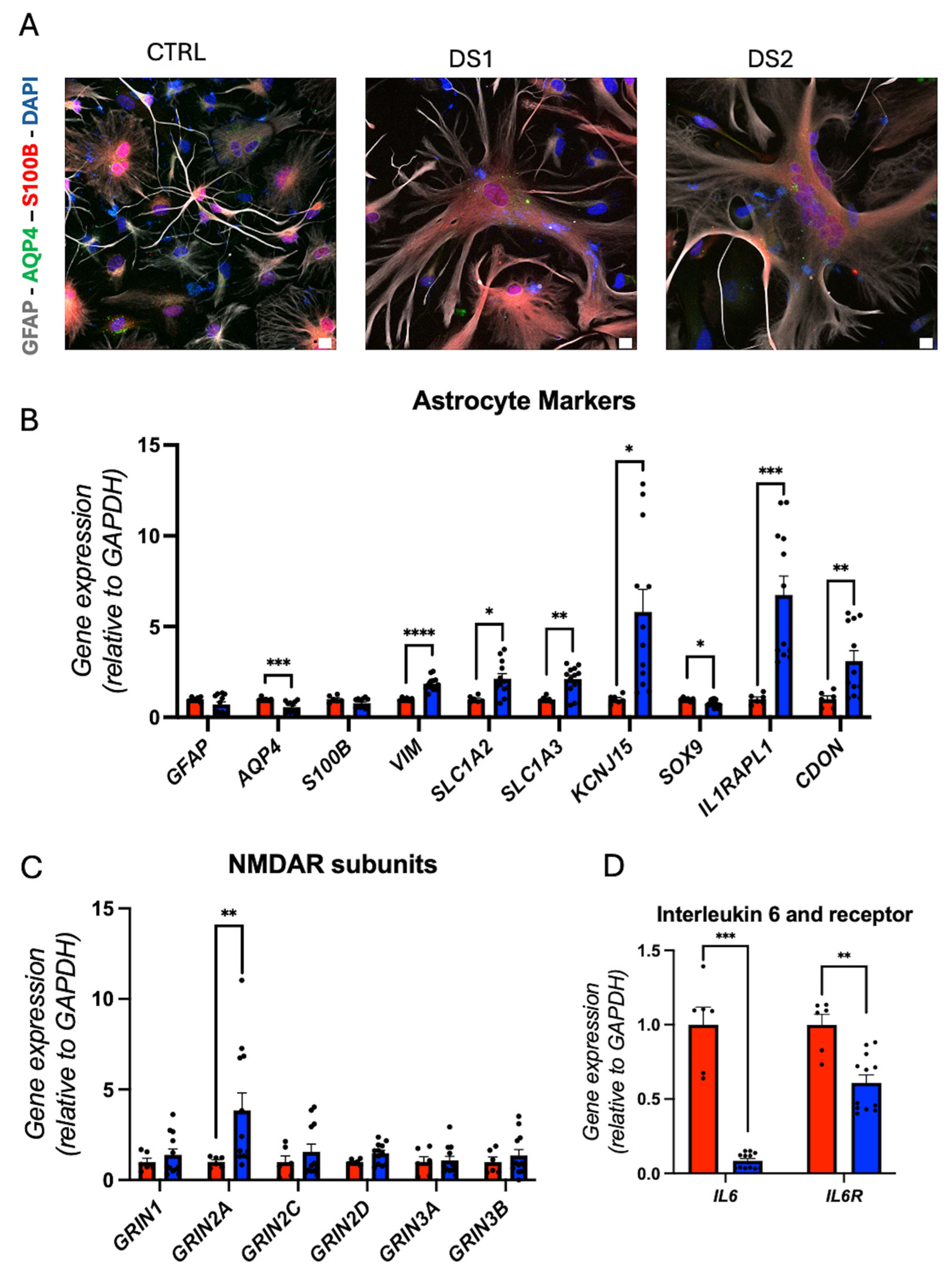
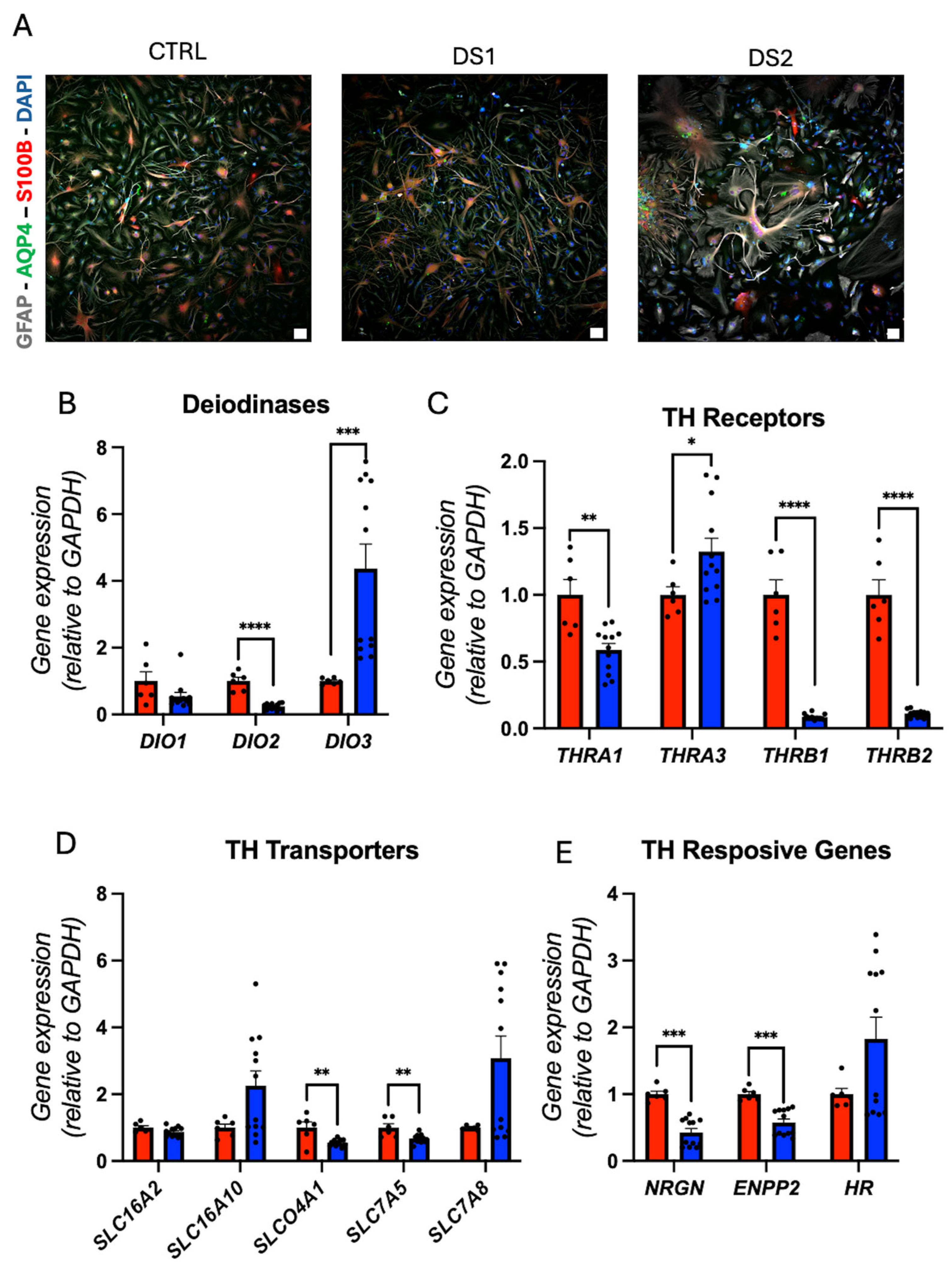
3.4. Altered Thyroid Hormones Maintenance in Down Syndrome Hid-Astrocytes
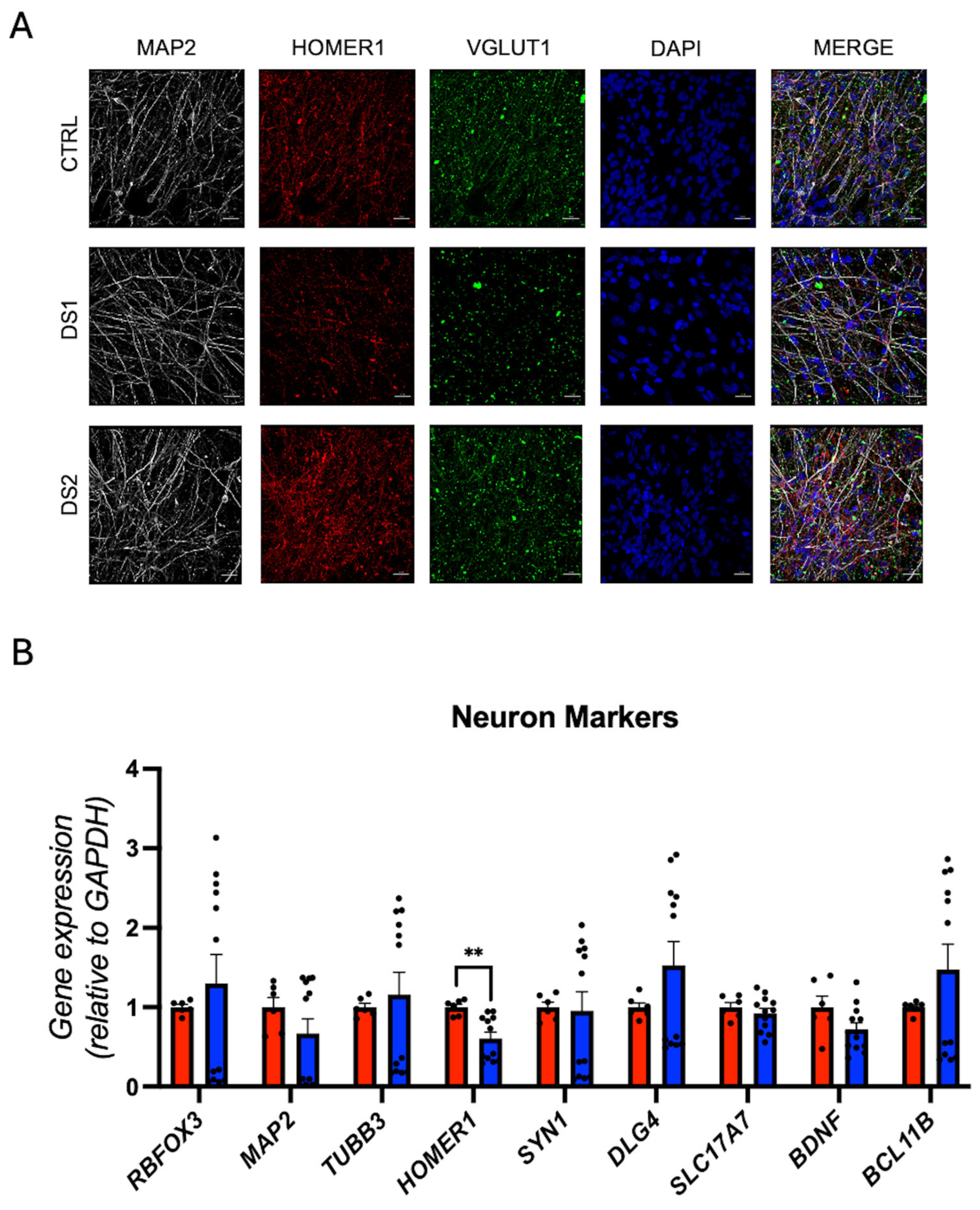
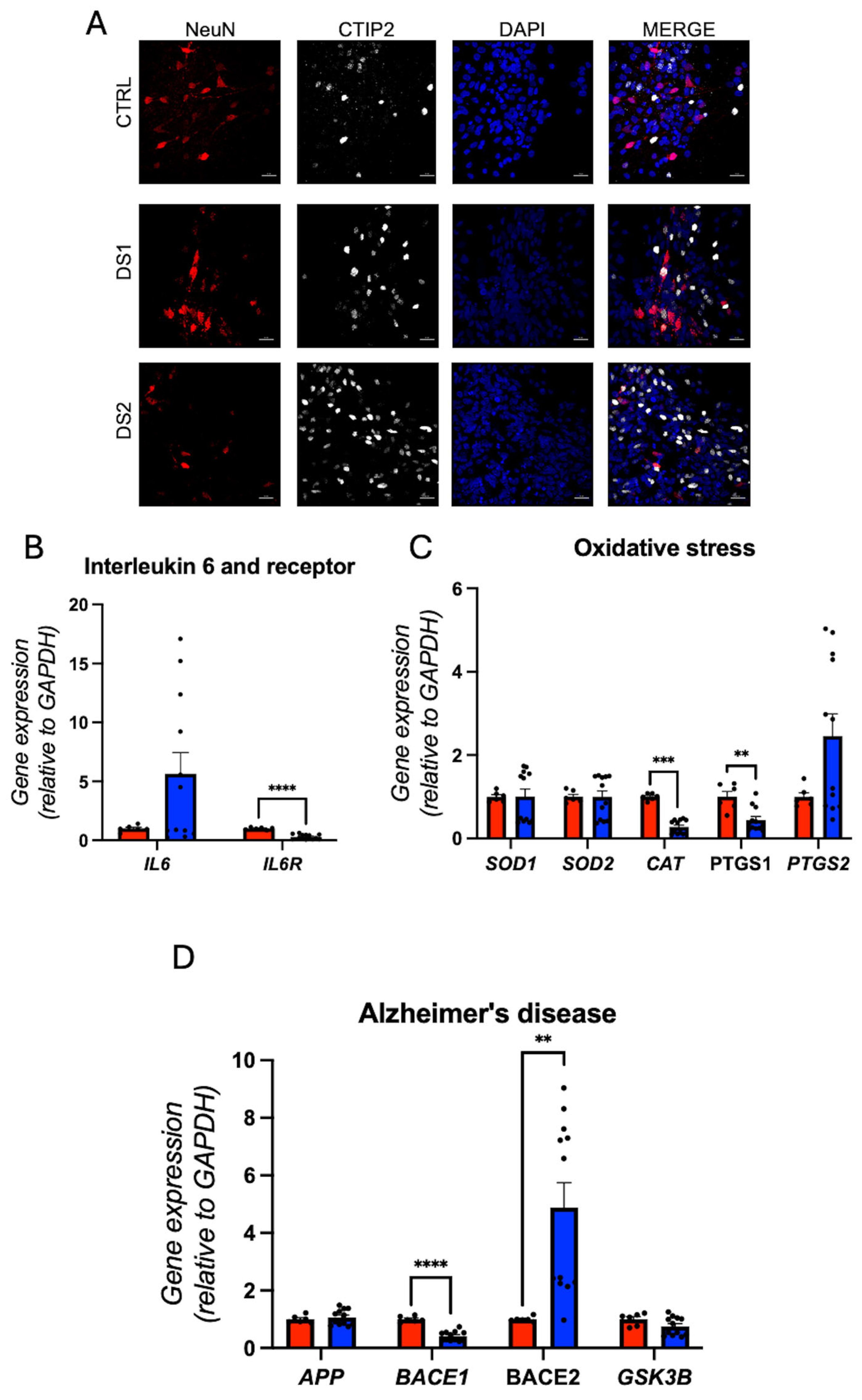
3.5. Altered Thyroid Hormones Maintenance in Down Syndrome Hid-Neurons
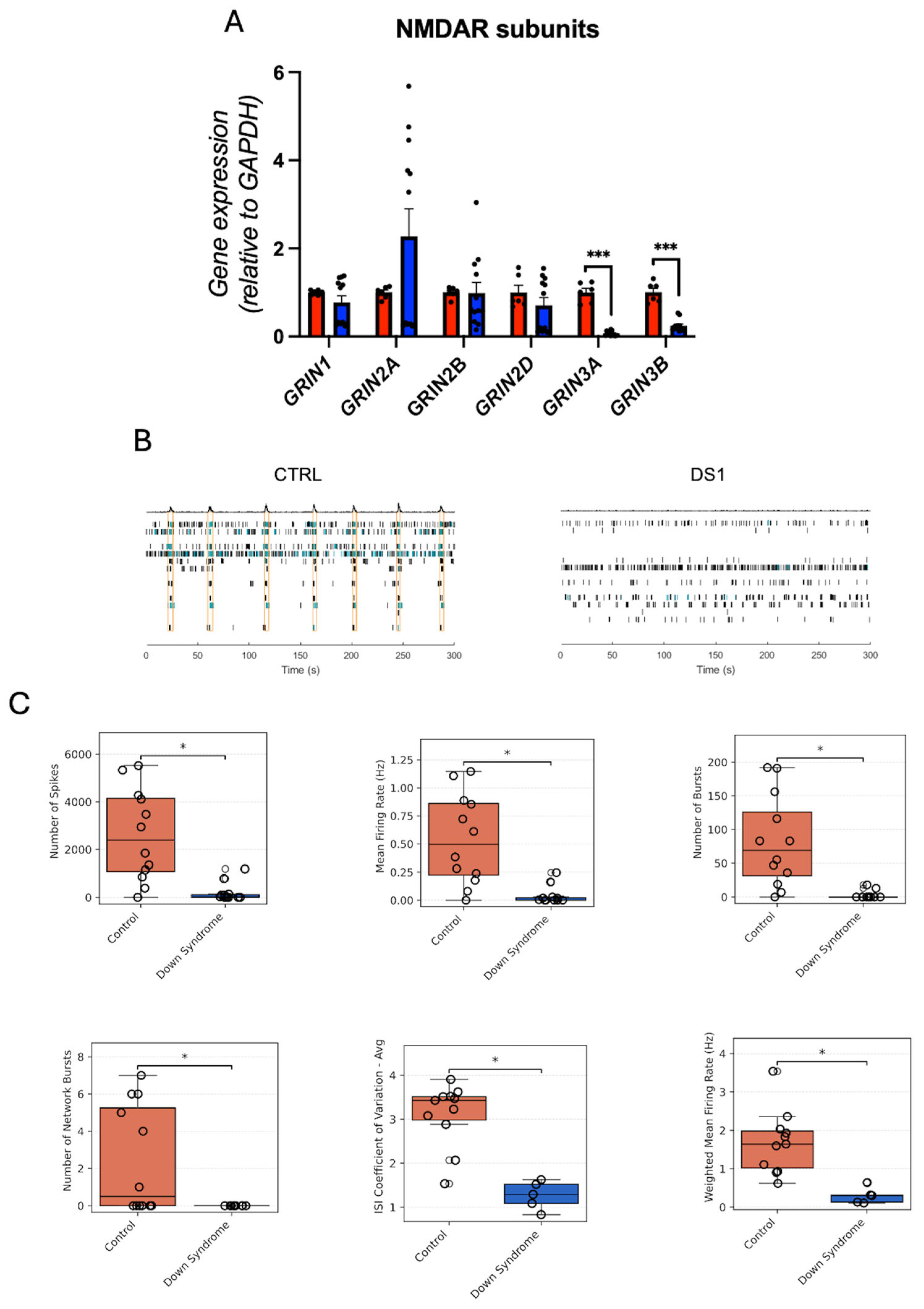
3.6. NMDAR Subunits Gene Expression and Network Activity in Down Syndrome Hid-Neurons Are Altered
4. Discussion
5. Limitations and Outlook
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Souza, J.S. Thyroid hormone biosynthesis and its role in brain development and maintenance. Adv. Protein Chem. Struct. Biol. 2024, 142, 329–365. [Google Scholar] [CrossRef] [PubMed]
- Giannocco, G.; Kizys, M.M.L.; Maciel, R.M.; de Souza, J.S. Thyroid hormone, gene expression, and Central Nervous System: Where we are. Semin. Cell Dev. Biol. 2021, 114, 47–56. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- McAninch, E.A.; Bianco, A.C. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann. N. Y. Acad. Sci. 2014, 1311, 77–87. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Zwahlen, J.; Gairin, E.; Vianello, S.; Mercader, M.; Roux, N.; Laudet, V. The ecological function of thyroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2024, 379, 20220511. [Google Scholar] [CrossRef]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 10. [Google Scholar] [CrossRef]
- Dsouki, N.A.; Pereira, B.F.; da Silva, R.G.; Rodrigues, V.G.; Brito, R.d.S.; Kizys, M.M.L.; Chiamolera, M.I.; Maciel, R.M.; Serrano-Nascimento, C.; Giannocco, G. The Interplay of the Mammalian Brain and Thyroid Hormones, and the Threat of Endocrine-Disrupting Chemicals. Endocrines 2024, 5, 501–515. [Google Scholar] [CrossRef]
- Brent, G.A. A Historical Reflection on Scientific Advances in Understanding Thyroid Hormone Action. Thyroid 2023, 33, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A. Thyroid Hormone Action by Genomic and Nongenomic Molecular Mechanisms. Methods Mol. Biol. 2025, 2876, 17–34. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Transcriptional Cofactors for Thyroid Hormone Receptors. Endocrinology 2025, 166, bqae164. [Google Scholar] [CrossRef]
- Sreenivasan, V.K.A.; Dore, R.; Resch, J.; Maier, J.; Dietrich, C.; Henck, J.; Balachandran, S.; Mittag, J.; Spielmann, M. Single-cell RNA-based phenotyping reveals a pivotal role of thyroid hormone receptor alpha for hypothalamic development. Development 2023, 150, dev201228. [Google Scholar] [CrossRef]
- Salas-Lucia, F.; Fekete, C.; Sinkó, R.; Egri, P.; Rada, K.; Ruska, Y.; Gereben, B.; Bianco, A.C. Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain. Elife 2023, 12, e82683. [Google Scholar] [CrossRef]
- Schroeder, A.C.; Privalsky, M.L. Thyroid hormones, t3 and t4, in the brain. Front. Endocrinol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Salas-Lucia, F.; Bianco, A.C. T3 levels and thyroid hormone signaling. Front. Endocrinol. 2022, 13, 1044691. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R. Neurodevelopmental and neurophysiological actions of thyroid hormone. J. Neuroendocrinol. 2008, 20, 784–794. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Sanchez-Huerta, K.; Wood, C. Wood, Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology 2016, 157, 774–787. [Google Scholar] [CrossRef]
- Moran, C.; Schoenmakers, N.; Visser, W.E.; Schoenmakers, E.; Agostini, M.; Chatterjee, K. Genetic disorders of thyroid development, hormone biosynthesis and signalling. Clin. Endocrinol. 2022, 97, 502–514. [Google Scholar] [CrossRef]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; O Ribeiro, M.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B.M.L.C. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef] [PubMed]
- Krieger, T.G.; Moran, C.M.; Frangini, A.; Visser, W.E.; Schoenmakers, E.; Muntoni, F.; Clark, C.A.; Gadian, D.; Chong, W.K.; Kuczynski, A.; et al. Mutations in thyroid hormone receptor α1 cause premature neurogenesis and progenitor cell depletion in human cortical development. Proc. Natl. Acad. Sci. USA 2019, 116, 22754–22763. [Google Scholar] [CrossRef]
- Yuen, R.K.C.; Thiruvahindrapuram, B.; Merico, D.; Walker, S.; Tammimies, K.; Hoang, N.; Chrysler, C.; Nalpathamkalam, T.; Pellecchia, G.; Liu, Y.; et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat. Med. 2015, 21, 185–191. [Google Scholar] [CrossRef]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Prim. 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Feldman, P.M.; Rodriguez, N.; Morrison, E.; Barton, B.; Lee, M.M. Prospective study of thyroid function in the first year of life in infants with Down syndrome. Eur. J. Pediatr. 2023, 182, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Giannocco, G.; Maglione, A.V.; Henrique, J.S.; DE Souza, J.S. Down syndrome: The prevalence of Alzheimer’s disease and the role of thyroid hormone. Cad. De Pós-Grad. Em Distúrbios Do Desenvolv. 2020, 20, 116–131. [Google Scholar] [CrossRef]
- Purdy, I.B.; Singh, N.; Brown, W.L.; Vangala, S.; Devaskar, U.P. Revisiting early hypothyroidism screening in infants with Down syndrome. J. Perinatol. 2014, 34, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; Lucaccioni, L.; Fugetto, F.; Mason, A.; Predieri, B. Thyroid function in Down syndrome. Expert. Rev. Endocrinol. Metab. 2015, 10, 525–532. [Google Scholar] [CrossRef]
- Amr, N.H. Thyroid Disorders in Subjects with Down Syndrome: An Update. Acta Biomed. 2018, 89, 132–139. [Google Scholar] [CrossRef]
- Malle, M.; Patel, R.S.; Martin-Fernandez, M.; Stewart, O.J.; Philippot, Q.; Buta, S.; Richardson, A.; Barcessat, V.; Taft, J.; Bastard, P.; et al. Autoimmunity in Down’s syndrome via cytokines, CD4 T cells and CD11c. Nature 2023, 615, 305–314. [Google Scholar] [CrossRef]
- Gorini, F.; Coi, A.; Pierini, A.; Assanta, N.; Bottoni, A.; Santoro, M. Hypothyroidism in Patients with Down Syndrome: Prevalence and Association with Congenital Heart Defects. Children 2024, 11, 513. [Google Scholar] [CrossRef]
- Szeliga, K.; Antosz, A.; Skrzynska, K.; Kalina-Faska, B.; Januszek-Trzciakowska, A.; Gawlik, A. Subclinical Hypothyroidism as the Most Common Thyroid Dysfunction Status in Children with Down’s Syndrome. Front. Endocrinol. 2021, 12, 782865. [Google Scholar] [CrossRef]
- Kariyawasam, D.; Rachdi, L.; Carré, A.; Martin, M.; Houlier, M.; Janel, N.; Delabar, J.-M.; Scharfmann, R.; Polak, M. DYRK1A BAC transgenic mouse: A new model of thyroid dysgenesis in Down syndrome. Endocrinology 2015, 156, 1171–1180. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-E.; Yang, Y.-C.; Chen, S.-M.; Su, H.-L.; Huang, P.-C.; Tsai, M.-S.; Wang, T.-H.; Tseng, C.-P.; Hwang, S.-M. Modeling neurogenesis impairment in Down syndrome with induced pluripotent stem cells from Trisomy 21 amniotic fluid cells. Exp. Cell Res. 2013, 319, 498–505. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.S.; Carromeu, C.; Torres, L.B.; Araujo, B.H.S.; Cugola, F.R.; Maciel, R.M.; Muotri, A.R.; Giannocco, G. IGF1 neuronal response in the absence of MECP2 is dependent on TRalpha 3. Hum. Mol. Genet. 2017, 26, 270–281. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.S.; Ferreira, D.R.; Herai, R.; Carromeu, C.; Torres, L.B.; Araujo, B.H.S.; Cugola, F.; Maciel, R.M.B.; Muotri, A.R.; Giannocco, G. Altered Gene Expression of Thyroid Hormone Transporters and Deiodinases in iPS MeCP2-Knockout Cells-Derived Neurons. Mol. Neurobiol. 2019, 56, 8277–8295. [Google Scholar] [CrossRef]
- Marchetto, M.C.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef]
- Araujo, B.H.S.; Kaid, C.; De Souza, J.S.; da Silva, S.G.; Goulart, E.; Caires, L.C.J.; Musso, C.M.; Torres, L.B.; Ferrasa, A.; Herai, R.; et al. Down Syndrome iPSC-Derived Astrocytes Impair Neuronal Synaptogenesis and the mTOR Pathway In Vitro. Mol. Neurobiol. 2018, 55, 5962–5975. [Google Scholar] [CrossRef]
- Palmer, C.R.; Liu, C.S.; Romanow, W.J.; Lee, M.-H.; Chun, J. Altered cell and RNA isoform diversity in aging Down syndrome brains. Proc. Natl. Acad. Sci. USA 2021, 118, e2114326118. [Google Scholar] [CrossRef]
- Song, H.; Xu, W.; Song, J.; Liang, Y.; Fu, W.; Zhu, X.-C.; Li, C.; Peng, J.-S.; Zheng, J.-N. Overexpression of Lin28 inhibits the proliferation, migration and cell cycle progression and induces apoptosis of BGC-823 gastric cancer cells. Oncol. Rep. 2015, 33, 997–1003. [Google Scholar] [CrossRef]
- Ouyang, J.; Shen, Y.-C.; Yeh, L.-K.; Li, W.; Coyle, B.M.; Liu, C.-Y.; Fini, M.E. Pax6 overexpression suppresses cell proliferation and retards the cell cycle in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2006, 47, 2397–2407. [Google Scholar] [CrossRef]
- Sheng, L.; Luo, Q.; Chen, L. Amino Acid Solute Carrier Transporters in Inflammation and Autoimmunity. Drug Metab. Dispos. 2022, 50, 1228–1237. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, P.; Xue, H.; Peterson, S.E.; Tran, H.T.; McCann, A.E.; Parast, M.M.; Li, S.; Pleasure, D.E.; Laurent, L.; et al. Role of astroglia in Down’s syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat. Commun. 2014, 5, 4430. [Google Scholar] [CrossRef]
- Harrison, P.J.; Bannerman, D.M. GRIN2A (NR2A): A gene contributing to glutamatergic involvement in schizophrenia. Mol. Psychiatry 2023, 28, 3568–3572. [Google Scholar] [CrossRef] [PubMed]
- Keller, E.T.; Ershler, W.B. Effect of IL-6 receptor antisense oligodeoxynucleotide on in vitro proliferation of myeloma cells. J. Immunol. 1995, 154, 4091–4098. [Google Scholar] [CrossRef] [PubMed]
- Güven, A.; Kalebic, N.; Long, K.R.; Florio, M.; Vaid, S.; Brandl, H.; Stenzel, D.; Huttner, W.B. Extracellular matrix-inducing Sox9 promotes both basal progenitor proliferation and gliogenesis in developing neocortex. Elife 2020, 9, e49808. [Google Scholar] [CrossRef] [PubMed]
- Bastide, P.; Darido, C.; Pannequin, J.; Kist, R.; Robine, S.; Marty-Double, C.; Bibeau, F.; Scherer, G.; Joubert, D.; Hollande, F.; et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 2007, 178, 635–648. [Google Scholar] [CrossRef]
- Yoon, S.; Piguel, N.H.; Khalatyan, N.; Dionisio, L.E.; Savas, J.N.; Penzes, P. Homer1 promotes dendritic spine growth through ankyrin-G and its loss reshapes the synaptic proteome. Mol. Psychiatry 2021, 26, 1775–1789. [Google Scholar] [CrossRef]
- Hardy, O.; Worley, G.; Lee, M.M.; Chaing, S.; Mackey, J.; Crissman, B.; Kishnani, P.S. Hypothyroidism in Down syndrome: Screening guidelines and testing methodology. Am. J. Med. Genet. Part A 2004, 124A, 436–437. [Google Scholar] [CrossRef]
- Salloum-Asfar, S.; Shin, K.C.; Taha, R.Z.; Khattak, S.; Park, Y.; Abdulla, S.A. The Potential Role of Thyroid Hormone Therapy in Neural Progenitor Cell Differentiation and Its Impact on Neurodevelopmental Disorders. Mol. Neurobiol. 2024, 61, 3330–3342. [Google Scholar] [CrossRef]
- Bernal, J.; Guadaño-Ferraz, A.; Morte, B. Thyroid hormone transporters-functions and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Remaud, S.; Gothié, J.D.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid hormone signaling and adult neurogenesis in mammals. Front. Endocrinol. 2014, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Osumi, N.; Shinohara, H.; Numayama-Tsuruta, K.; Maekawa, M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 2008, 26, 1663–1672. [Google Scholar] [CrossRef]
- Martin, A.A.; Mayerl, S. Local Thyroid Hormone Action in Brain Development. Int. J. Mol. Sci. 2023, 24, 12352. [Google Scholar] [CrossRef]
- Freitas, B.C.; Gereben, B.; Castillo, M.; Kalló, I.; Zeöld, A.; Egri, P.; Liposits, Z.; Zavacki, A.M.; Maciel, R.M.; Jo, S.; et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J. Clin. Investig. 2010, 120, 2206–2217. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, W.; Zhang, C.; Zhang, C.; Liu, M.-D.; Han, F.; Yao, L.-B.; Han, H.; Luo, P.; Su, N. Scaffolding protein Homer1a protects against NMDA-induced neuronal injury. Cell Death Dis. 2015, 6, e1843. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, D.; Lou, S.; Kou, Z. Structural prediction of GluN3 NMDA receptors. Front. Physiol. 2024, 15, 1446459. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, J.S.; Sanchez-Sanchez, S.; Amelinez-Robles, N.; Guerra, B.S.; Giannocco, G.; Muotri, A.R. Trisomy 21 Disrupts Thyroid Hormones Signaling During Human iPSC-Derived Neural Differentiation In Vitro. Cells 2025, 14, 1407. https://doi.org/10.3390/cells14181407
de Souza JS, Sanchez-Sanchez S, Amelinez-Robles N, Guerra BS, Giannocco G, Muotri AR. Trisomy 21 Disrupts Thyroid Hormones Signaling During Human iPSC-Derived Neural Differentiation In Vitro. Cells. 2025; 14(18):1407. https://doi.org/10.3390/cells14181407
Chicago/Turabian Stylede Souza, Janaina Sena, Sandra Sanchez-Sanchez, Nicolas Amelinez-Robles, B. S. Guerra, Gisele Giannocco, and Alysson R. Muotri. 2025. "Trisomy 21 Disrupts Thyroid Hormones Signaling During Human iPSC-Derived Neural Differentiation In Vitro" Cells 14, no. 18: 1407. https://doi.org/10.3390/cells14181407
APA Stylede Souza, J. S., Sanchez-Sanchez, S., Amelinez-Robles, N., Guerra, B. S., Giannocco, G., & Muotri, A. R. (2025). Trisomy 21 Disrupts Thyroid Hormones Signaling During Human iPSC-Derived Neural Differentiation In Vitro. Cells, 14(18), 1407. https://doi.org/10.3390/cells14181407





