Development and Characterization of a Novel Lineage of Renal Progenitor Cells for Potential Use in Feline Chronic Kidney Disease: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Acquisition and In Vitro Culture of Mesonephric and Metanephric Cells
2.3. Cell Isolation and Culture
2.4. Morphological Analysis of Metanephric and Mesonephric Cultures
2.5. Cell Viability Test and Cryopreservation
2.6. Cell Metabolism Evaluation
2.7. Cell Characterization by Flow Cytometry
2.8. Differentiation of Cultured Metanephric and Mesonephric Cells into Adipogenic, Osteogenic, and Chondrogenic Cells
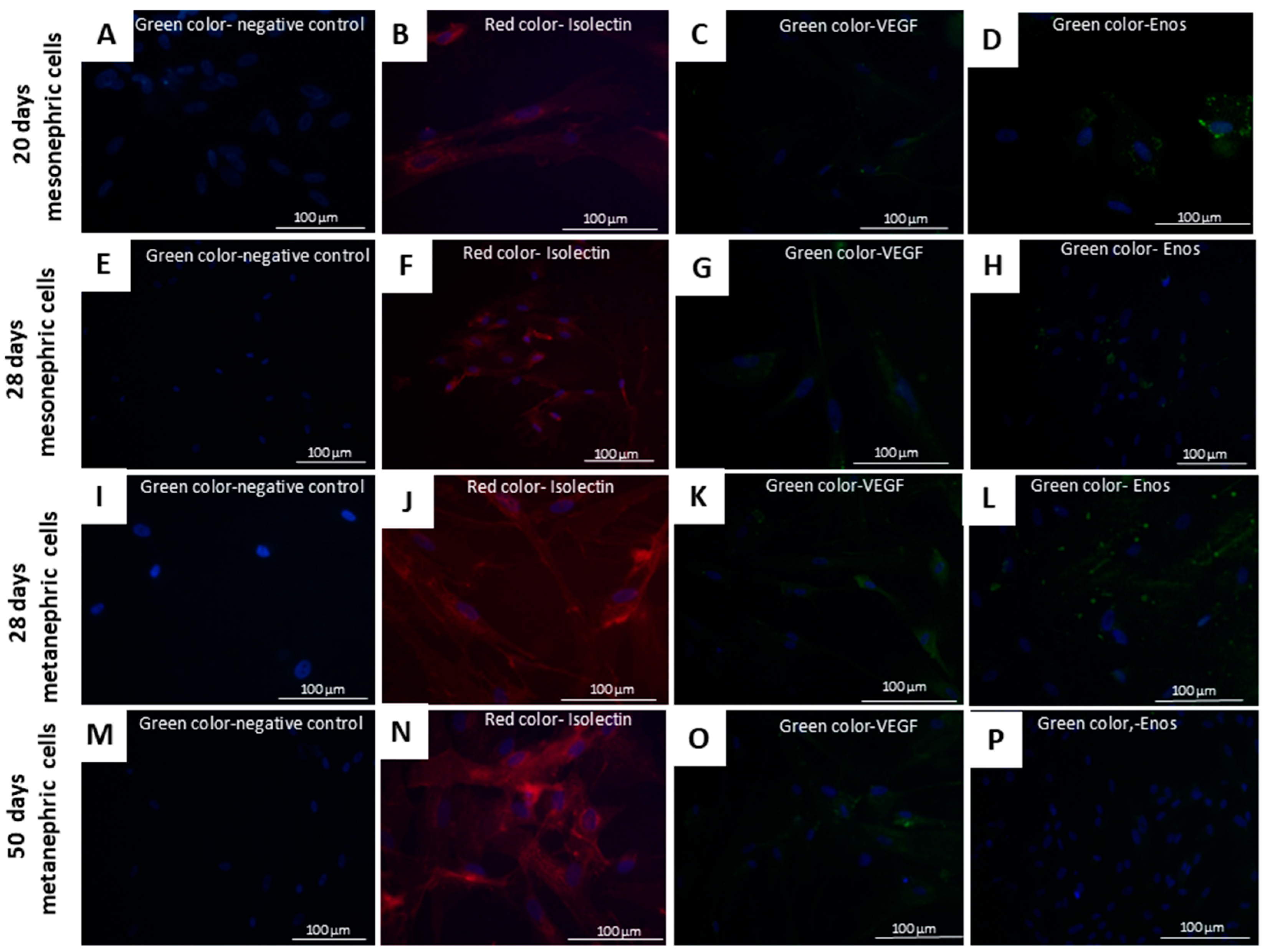
2.9. Differentiation of Mesonephric and Metanephric Cells into Endothelial Cells
2.10. Evaluation of the Tumorigenic Potential of Cultured Mesonephric and Metanephric Cells
2.11. Feline CKD Treatment Using Metanephric Cells
2.12. Intraperitoneal Cell Injection for Cell Therapy
2.13. Laboratory Monitoring and Clinical Evaluation
2.14. Statistical Analysis
3. Results
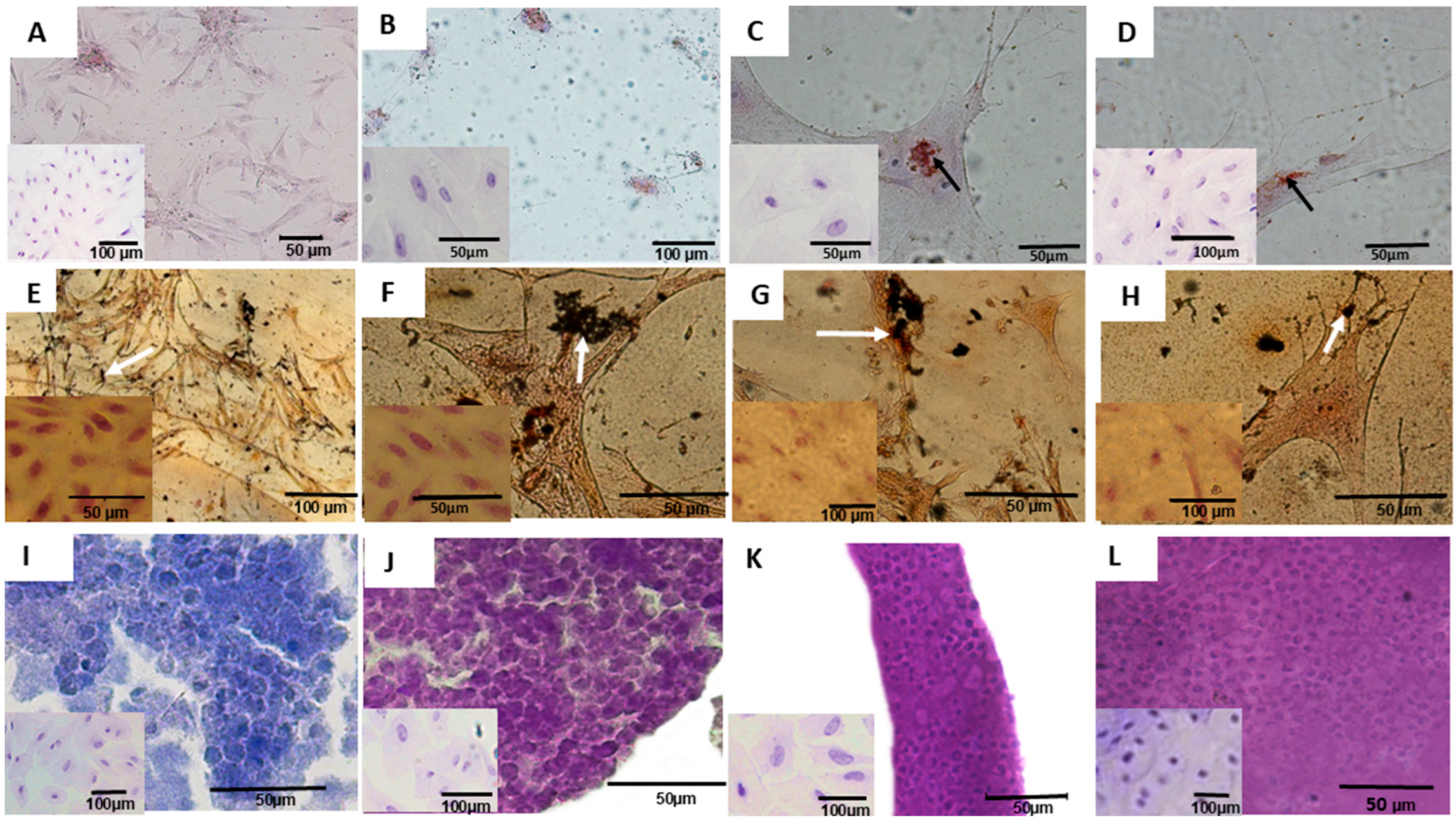
3.1. Culture, Differentiation, Characterization, and Assessment of Tumorigenic Potential of Mesonephric and Metanephric Cells
3.2. Cellular Application—Pilot Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chew, D.J.; DiBartola, S.P.; Schenck, P.A. Urologia e Nefrologia Do Cão e Do Gato; Elsevier Health Sciences Brazil: Amsterdam, The Netherlands, 2012; ISBN 8535247009. [Google Scholar]
- Zacharias, S.; Welty, M.B.; Sand, T.T.; Black, L.L. Impact of Allogeneic Feline Uterine-Derived Mesenchymal Stromal Cell Intravenous Treatment on Renal Function of Nephrectomized Cats with Chronic Kidney Disease. Res. Vet. Sci. 2021, 141, 33–41. [Google Scholar] [CrossRef]
- Jung, Y.R.; Yim, J.H.; Lee, Y.J.; Lee, S.B.; Heo, S.Y.; Bae, S.G.; Kim, K.T.; Kwon, Y.S.; Park, S.J.; Park, J.K.; et al. Decreased SMP30 Expression Is Related with EMT in the Kidneys of Two Siberian Tigers with CKD. In Vivo 2024, 38, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.A.; Kim, T.; Ju, J. Case Reports of Amniotic Membrane Derived-Cell Treatment for Feline Chronic Renal Failure. J. Anim. Reprod. Biotechnol. 2021, 36, 116–120. [Google Scholar] [CrossRef]
- Krofič Žel, M.; Nemec Svete, A.; Tozon, N.; Pavlin, D. Hemogram-Derived Inflammatory Markers in Cats with Chronic Kidney Disease. Animals 2024, 14, 1813. [Google Scholar] [CrossRef]
- Waki, M.F.; Martorelli, C.R.; Mosko, P.E.; Kogika, M.M. Classificação Em Estágios Da Doença Renal Crônica Em Cães e Gatos: Abordagem Clínica, Laboratorial e Terapêutica. Ciência Rural 2010, 40, 2226–2234. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Randall, E.; Marolf, A.; Valdes-Martinez, A.; Dow, S.W. Assessment of Intravenous Adipose-Derived Allogeneic Mesenchymal Stem Cells for the Treatment of Feline Chronic Kidney Disease: A Randomized, Placebo-Controlled Clinical Trial in Eight Cats. J. Feline Med. Surg. 2016, 18, 165–171. [Google Scholar] [CrossRef]
- Hansen, B.; Vigani, A. Maintenance Fluid Therapy: Isotonic versus Hypotonic Solutions. Vet. Clin. Small Anim. Pract. 2017, 47, 383–395. [Google Scholar] [CrossRef]
- Davis, H.; Jensen, T.; Johnson, A.; Knowles, P.; Meyer, R.; Rucinsky, R.; Shafford, H. 2013 AAHA/AAFP Fluid Therapy Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2013, 49, 149–159. [Google Scholar] [CrossRef]
- Li, M.; Guo, X.; Cheng, L.; Zhang, H.; Zhou, M.; Zhang, M.; Yin, Z.; Guo, T.; Zhao, L.; Liu, H.; et al. Porcine Kidney Organoids Derived from Naïve-like Embryonic Stem Cells. Int. J. Mol. Sci. 2024, 25, 682. [Google Scholar] [CrossRef]
- Thomson, A.L.; Berent, A.C.; Weisse, C.; Langston, C.E. Intra-Arterial Renal Infusion of Autologous Mesenchymal Stem Cells for Treatment of Chronic Kidney Disease in Cats: Phase I Clinical Trial. J. Vet. Intern. Med. 2019, 33, 1353–1361. [Google Scholar] [CrossRef]
- Fujimoto, T.; Yamanaka, S.; Tajiri, S.; Takamura, T.; Saito, Y.; Matsumoto, N.; Matsumoto, K.; Tachibana, T.; Okano, H.J.; Yokoo, T. Generation of Human Renal Vesicles in Mouse Organ Niche Using Nephron Progenitor Cell Replacement System. Cell Rep. 2020, 32, 108130. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, M.; Guo, Q.; Shen, L.; Liu, X.; Pan, J.; Zhang, Y.; Xu, T.; Zhang, D.; Wei, G. Human Umbilical Cord Mesenchymal Stem Cell Exosome-Derived MiR-874-3p Targeting RIPK1/PGAM5 Attenuates Kidney Tubular Epithelial Cell Damage. Cell. Mol. Biol. Lett. 2023, 28, 12. [Google Scholar] [CrossRef]
- Djedovic, V. RCSI Smj Staff Review Bioengineered Transplants: Stem Cell Reversal of Chronic Kidney Disease. RCSI Stud. Med. J. 2023, 12, 2018–2019. [Google Scholar]
- Hong, I.S. Enhancing Stem Cell-Based Therapeutic Potential by Combining Various Bioengineering Technologies. Front. Cell Dev. Biol. 2022, 10, 901661. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.G.; Ferreira, P.I.; Krause, A. Mesenchymal Stem Cell Transplantation: Systematic Review, Meta-Analysis and Clinical Applications for Acute Kidney Injury and Chronic Kidney Disease in Dogs and Cats. Res. Vet. Sci. 2024, 175, 105313. [Google Scholar] [CrossRef] [PubMed]
- Quimby, J.M.; Webb, T.L.; Habenicht, L.M.; Dow, S.W. Safety and Efficacy of Intravenous Infusion of Allogeneic Cryopreserved Mesenchymal Stem Cells for Treatment of Chronic Kidney Disease in Cats: Results of Three Sequential Pilot Studies. Stem Cell Res. Ther. 2013, 4, 48. [Google Scholar] [CrossRef]
- Santos, E.J.C.; Poppi, F.P.; Braga, C.L. Células progenitoras adultas multipotentes alogênicas no tratamento de doença renal em felinos. Sci. Anim. Health 2018, 6, 266–285. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Marino, C.L.; Lascelles, B.D.X.; Vaden, S.L.; Gruen, M.E.; Marks, S.L. Prevalence and Classification of Chronic Kidney Disease in Cats Randomly Selected from Four Age Groups and in Cats Recruited for Degenerative Joint Disease Studies. J. Feline Med. Surg. 2014, 16, 465–472. [Google Scholar] [CrossRef]
- Quimby, J.M.; Borjesson, D.L. Mesenchymal Stem Cell Therapy in Cats: Current Knowledge and Future Potential. J. Feline Med. Surg. 2018, 20, 208–216. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Zhao, J.; Bouchentouf, M.; Forner, K.; Birman, E.; Yuan, S.; Boivin, M.-N.; Martineau, D. Human Marrow-Derived Mesenchymal Stromal Cells Decrease Cisplatin Renotoxicity in Vitro and in Vivo and Enhance Survival of Mice Post-Intraperitoneal Injection. Am. J. Physiol.-Ren. Physiol. 2010, 299, F1288–F1298. [Google Scholar] [CrossRef]
- Ribeiro, P.d.C.; Lojudice, F.H.; Fernandes-Charpiot, I.M.M.; Baptista, M.A.S.F.; Araújo, S.d.A.; Mendes, G.E.F.; Sogayar, M.C.; Abbud-Filho, M.; Caldas, H.C. Therapeutic Potential of Human Induced Pluripotent Stem Cells and Renal Progenitor Cells in Experimental Chronic Kidney Disease. Stem Cell Res. Ther. 2020, 11, 530. [Google Scholar] [CrossRef]
- Rafii, S.; Lyden, D. Therapeutic Stem and Progenitor Cell Transplantation for Organ Vascularization and Regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wan, J.-X.; Yuan, J.; Dong, R.; Da, J.-J.; Sun, Z.-L.; Zha, Y. Effects of Calcitriol on Peripheral Endothelial Progenitor Cells and Renal Renovation in Rats with Chronic Renal Failure. J. Steroid Biochem. Mol. Biol. 2021, 214, 105956. [Google Scholar] [CrossRef] [PubMed]
- Peired, A.J.; Melica, M.E.; Molli, A.; Nardi, C.; Romagnani, P.; Lasagni, L. Molecular Mechanisms of Renal Progenitor Regulation: How Many Pieces in the Puzzle? Cells 2021, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.B.; Gomes, S.A.; Dulce, R.A.; Premer, C.; Rodrigues, C.O.; Kanashiro-Takeuchi, R.M.; Oskouei, B.; Carvalho, D.A.; Ruiz, P.; Reiser, J.; et al. C-Kit+ Cells Isolated from Developing Kidneys Are a Novel Population of Stem Cells with Regenerative Potential. Stem Cells 2013, 31, 1644–1656. [Google Scholar] [CrossRef]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The Dynamic in Vivo Distribution of Bone Marrow-Derived Mesenchymal Stem Cells after Infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef]
- Stommel, M.W.J.; Strik, C.; van Goor, H. Response to Pathological Processes in the Peritoneal Cavity-Sepsis, Tumours, Adhesions, and Ascites. Semin. Pediatr. Surg. 2014, 23, 331–335. [Google Scholar] [CrossRef]
- Dekel, B.; Zangi, L.; Shezen, E.; Reich-Zeliger, S.; Eventov-Friedman, S.; Katchman, H.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Margalit, R.; et al. Isolation and Characterization of Nontubular Sca-1+Lin—Multipotent Stem/Progenitor Cells from Adult Mouse Kidney. J. Am. Soc. Nephrol. 2006, 17, 3300–3314. [Google Scholar] [CrossRef]
- Johnson, R.A.; Snyder, L.B.; Schroeder, C. Canine and Feline Anesthesia Canine Anesthesia and Co-Existing Disease; Johnson, R.A., Snyder, L.B., Schroeder, C., Eds.; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Maeshima, A.; Yamashita, S.; Nojima, Y. Identification of Renal Progenitor-Like Tubular Cells That Participate in the Regeneration Processes of the Kidney. J. Am. Soc. Nephrol. 2003, 14, 3138–3146. [Google Scholar] [CrossRef]
- Maeshima, A.; Sakurai, H.; Nigam, S.K. Adult Kidney Tubular Cell Population Showing Phenotypic Plasticity, Tubulogenic Capacity, and Integration Capability into Developing Kidney. J. Am. Soc. Nephrol. 2006, 17, 188–198. [Google Scholar] [CrossRef]
- Tasker, L. Methods for the Euthanasia of Dogs and Cats: Comparison and Recommendations. World Soc. Prot. Anim. 2018. Available online: https://www.icam-coalition.org/wp-content/uploads/2017/03/Methods-for-the-euthanasia-of-dogs-and-cats-English.pdf (accessed on 24 August 2025).
- Beaver, B.V.; Reed, W.; Leary, S.; McKiernan, B.; Bain, F.; Schultz, R.; Bennet, B.T.; Pascoe, P.; Shull, E.; Cork, L.C.; et al. 2000 Report of the AVMA Panel on Euthanasia. J. Am. Vet. Med. Assoc. 2001, 218, 669–696. [Google Scholar] [CrossRef]
- Evans, H.E.; Sack, W.O. Prenatal Development of Domestic and Laboratory Mammals: Growth Curves, External Features and Selected References. Anat. Histol. Embryol. 1973, 2, 11–45. [Google Scholar] [CrossRef] [PubMed]
- Mario, L.C.; Morais, M.P.; Borghesi, J.; Favaron, P.O.; Oliveira, F.D.; Anunciação, A.R.A.; Agopian, R.G.; Gomes, S.A.; Miglino, M.A. Development of Urinary Organs in Domestic Cat during the Embryonic and Fetal Periods. Microsc. Res. Tech. 2018, 81, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, J.; Ferreira, H.d.S.; Favaron, P.O.; Mario, L.C.; Anunciação, A.R.d.A.d.; de Oliveira, F.D.; Hayashi, R.G.; Morini, A.C.; Miglino, M.A. Characterization of the Extracellular Matrix in the Chorioallantoic Membrane of Water Buffalo (Bubalus Bubalis) in Early Gestation. Reprod. Domest. Anim. 2019, 54, 1313–1321. [Google Scholar] [CrossRef]
- International Renal Interest Society. Diagnosing, Staging, and Treating Chronic Kidney Disease in Dog and Cats; International Renal Interest Society: Cambridge, UK, 2023; p. 3. [Google Scholar]
- Doit, H.; Dean, R.S.; Duz, M.; Finch, N.C.; Brennan, M.L. What Outcomes Should Be Measured in Feline Chronic Kidney Disease Treatment Trials? Establishing a Core Outcome Set for Research. Prev. Vet. Med. 2021, 192, 105348. [Google Scholar] [CrossRef]
- Kinomura, M.; Kitamura, S.; Tanabe, K.; Ichinose, K.; Hirokoshi, K.; Takazawa, Y.; Kitayama, H.; Nasu, T.; Sugiyama, H.; Yamasaki, Y.; et al. Amelioration of Cisplatin-Induced Acute Renal Injury by Renal Progenitor-like Cells Derived from the Adult Rat Kidney. Cell Transpl. 2008, 17, 143–158. [Google Scholar] [CrossRef]
- Langworthy, M.; Zhou, B.; De Caestecker, M.; Moeckel, G.; Baldwin, H.S. NFATc1 Identifies a Population of Proximal Tubule Cell Progenitors. J. Am. Soc. Nephrol. 2009, 20, 311–321. [Google Scholar] [CrossRef]
- Paretsis, B.F.; Mario, L.C.; Sasahara, T.H.D.C.; da Silva, L.C.G.; Dos Santos, J.M.; Kfoury Junior, J.R.; Leandro, R.M. Stereological Analysis of Metanephros from Domestic Cat (Felis Catus, Linnaeus 1798) Embryos and Fetus. Anat. Histol. Embryol. 2021, 50, 965–973. [Google Scholar] [CrossRef]
- Plavsic, M. Q5D Derivation and Characterization of Cell Substrates Used for Production of Biotechnological/Biological Products; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Stolzing, A.; Coleman, N.; Scutt, A. Glucose-Induced Replicative Senescence in Mesenchymal Stem Cells. Rejuvenation Res. 2006, 9, 31–35. [Google Scholar] [CrossRef]
- Maga, G.; Hübscher, U. Proliferating Cell Nuclear Antigen (PCNA): A Dancer with Many Partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef]
- Zhao, F.; Franco, H.L.; Rodriguez, K.F.; Brown, P.R.; Tsai, M.J.; Tsai, S.Y.; Yao, H.H.C. Elimination of the Male Reproductive Tract in the Female Embryo Is Promoted by COUP-TFII in Mice. Science 2017, 357, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Jericó, M.M.; Andrade Neto, J.P.D.; Kogika, M.M. Tratado de Medicina Interna de Cães e Gatos; Amazon.Com.Br; ROCA: Barcelona, Spain, 2014; Volume 2, ISBN 8527726432. [Google Scholar]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical Expression of Endothelial Markers CD31, CD34, von Willebrand Factor, and Fli-1 in Normal Human Tissues. J. Histochem. Cytochem. 2006, 54, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Kisselbach, L.; Merges, M.; Bossie, A.; Boyd, A. CD90 Expression on Human Primary Cells and Elimination of Contaminating Fibroblasts from Cell Cultures. Cytotechnology 2009, 59, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.L.; Terzic, A.; Conley, B.A.; Oxburgh, L.H.; Nicola, T.; Vary, C.P.H. Endoglin Plays Distinct Roles in Vascular Smooth Muscle Cell Recruitment and Regulation of Arteriovenous Identity during Angiogenesis. Dev. Dyn. 2009, 238, 2479–2493. [Google Scholar] [CrossRef]
- Li, X.Z.; He, J.C. An Update: The Role of Nephrin inside and Outside the Kidney. Sci. China Life Sci. 2015, 58, 649–657. [Google Scholar] [CrossRef]
- Kreidberg, J.A. WT1 and Kidney Progenitor Cells. Organogenesis 2010, 6, 61–70. [Google Scholar] [CrossRef]
- Aggarwal, S.; Moggio, A.; Bussolati, B. Concise Review: Stem/Progenitor Cells for Renal Tissue Repair: Current Knowledge and Perspectives. Stem Cells Transl. Med. 2013, 2, 1011–1019. [Google Scholar] [CrossRef]
- Hong, X.; Nie, H.; Deng, J.; Liang, S.; Chen, L.; Li, J.; Gong, S.; Wang, G.; Zuo, W.; Hou, F.; et al. WT1+ Glomerular Parietal Epithelial Progenitors Promote Renal Proximal Tubule Regeneration after Severe Acute Kidney Injury. Theranostics 2023, 13, 1311. [Google Scholar] [CrossRef]
- Gomes, S.A.; Hare, J.M.; Rangel, E.B. Kidney-Derived C-Kit+ Cells Possess Regenerative Potential. Stem Cells Transl. Med. 2018, 7, 317. [Google Scholar] [CrossRef]
- Rangel, E.B.; Gomes, S.A.; Kanashiro-Takeuchi, R.; Saltzman, R.G.; Wei, C.; Ruiz, P.; Reiser, J.; Hare, J.M. Kidney-Derived c-Kit+ Progenitor/Stem Cells Contribute to Podocyte Recovery in a Model of Acute Proteinuria. Sci. Rep. 2018, 8, 14723. [Google Scholar] [CrossRef] [PubMed]
- Dorison, A.; Ghobrial, I.; Graham, A.; Peiris, T.; Forbes, T.A.; See, M.; Das, M.; Saleem, M.A.; Quinlan, C.; Lawlor, K.T.; et al. Kidney Organoids Generated Using an Allelic Series of NPHS2 Point Variants Reveal Distinct Intracellular Podocin Mistrafficking. J. Am. Soc. Nephrol. 2023, 34, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.K.; Arif, E.; Srivastava, P.; Furcht, C.M.; Rahman, B.; Wen, P.; Singh, A.; Holzman, L.B.; Fitzgibbon, W.R.; Budisavljevic, M.N.; et al. Phosphorylation of Slit Diaphragm Proteins NEPHRIN and NEPH1 upon Binding of HGF Promotes Podocyte Repair. J. Biol. Chem. 2021, 297, 101079. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.; Ho, J.; Pandey, P.; MacIsaac, K.; Taglienti, M.; Xiang, M.; Alterovitz, G.; Ramoni, M.; Fraenkel, E.; Kreidberg, J.A. Genomic Characterization of Wilms’ Tumor Suppressor 1 Targets in Nephron Progenitor Cells during Kidney Development. Development 2010, 137, 1189–1203. [Google Scholar] [CrossRef]
- Arcolino, F.O.; Hosgood, S.; Akalay, S.; Jordan, N.; Herman, J.; Elliott, T.; Veys, K.; Vermeire, K.; Sprangers, B.; Nicholson, M.; et al. De Novo SIX2 Activation in Human Kidneys Treated with Neonatal Kidney Stem/Progenitor Cells. Am. J. Transplant. 2022, 22, 2791–2803. [Google Scholar] [CrossRef]
- Boyle, S.; Shioda, T.; Perantoni, A.O.; de Caestecker, M. Cited1 and Cited2 Are Differentially Expressed in the Developing Kidney but Are Not Required for Nephrogenesis. Dev. Dyn. 2007, 236, 2321–2330. [Google Scholar] [CrossRef]
- Arcolino, F.O.; Zia, S.; Held, K.; Papadimitriou, E.; Theunis, K.; Bussolati, B.; Raaijmakers, A.; Allegaert, K.; Voet, T.; Deprest, J.; et al. Urine of Preterm Neonates as a Novel Source of Kidney Progenitor Cells. J. Am. Soc. Nephrol. 2016, 27, 2762–2770. [Google Scholar] [CrossRef]
- Murphy, A.J.; Pierce, J.; de Caestecker, C.; Taylor, C.; Anderson, J.R.; Perantoni, A.O.; de Caestecker, M.P.; Lovvorn, H.N. SIX2 and CITED1, Markers of Nephronic Progenitor Self-Renewal, Remain Active in Primitive Elements of Wilms’ Tumor. J. Pediatr. Surg. 2012, 47, 1239–1249. [Google Scholar] [CrossRef]
- Lindström, N.O.; Tran, T.; Guo, J.; Rutledge, E.; Parvez, R.K.; Thornton, M.E.; Grubbs, B.; McMahon, J.A.; McMahon, A.P. Conserved and Divergent Molecular and Anatomic Features of Human and Mouse Nephron Patterning. J. Am. Soc. Nephrol. 2018, 29, 825–840. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, P.; Sharma, R. The Major Histocompatibility Complex: A Review. Asian J. Pharm. Clin. Res. 2017, 10, 33–36. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Gauthaman, K.; Bongso, A. Teratomas from Pluripotent Stem Cells: A Clinical Hurdle. J. Cell. Biochem. 2010, 111, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.B.; Sims-Lucas, S. Unwrapping the Origins and Roles of the Renal Endothelium. Pediatr. Nephrol. 2015, 30, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.A.; Martins, J.F.P.; Maria, D.A.; Wenceslau, C.V.; De Souza, D.M.; Kerkis, A.; Câmara, N.O.S.; Balieiro, J.C.C.; Kerkis, I. Immature Dental Pulp Stem Cells Showed Renotropic and Pericyte-like Properties in Acute Renal Failure in Rats. Cell Med. 2015, 7, 95–108. [Google Scholar] [CrossRef]
- Bazhanov, N.; Ylostalo, J.H.; Bartosh, T.J.; Tiblow, A.; Mohammadipoor, A.; Foskett, A.; Prockop, D.J. Intraperitoneally Infused Human Mesenchymal Stem Cells Form Aggregates with Mouse Immune Cells and Attach to Peritoneal Organs. Stem Cell Res. Ther. 2016, 7, 27. [Google Scholar] [CrossRef]
- Geddes, R.F.; Finch, N.C.; Syme, H.M.; Elliott, J. The Role of Phosphorus in the Pathophysiology of Chronic Kidney Disease. J. Vet. Emerg. Crit. Care 2013, 23, 122–133. [Google Scholar] [CrossRef]
- Braff, J.; Obare, E.; Yerramilli, M.; Elliott, J.; Yerramilli, M. Relationship between Serum Symmetric Dimethylarginine Concentration and Glomerular Filtration Rate in Cats. J. Vet. Intern. Med. 2014, 28, 1699–1701. [Google Scholar] [CrossRef]
- Holt, D.; Agnello, K.A. Peritoneum; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; ISBN 9780702043369. [Google Scholar]
- Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Kim, D.R. Effect of Intraperitoneal Local Anesthetic on Pain Characteristics after Laparoscopic Cholecystectomy. World J. Gastroenterol. 2015, 21, 13386–13395. [Google Scholar] [CrossRef]
- Michael, H.; Szlosek, D.; Clements, C.; Mack, R. Symmetrical Dimethylarginine: Evaluating Chronic Kidney Disease in the Era of Multiple Kidney Biomarkers. Vet. Clin. Small Anim. Pract. 2022, 52, 609–629. [Google Scholar] [CrossRef]
- Perini-Perera, S.; Del-Ángel-Caraza, J.; Pérez-Sánchez, A.P.; Quijano-Hernández, I.A.; Recillas-Morales, S. Evaluation of Chronic Kidney Disease Progression in Dogs with Therapeutic Management of Risk Factors. Front. Vet. Sci. 2021, 8, 621084. [Google Scholar] [CrossRef]
- Sosnar, M.; Kohout, P.; Růžička, M.; Vrbasová, L. Retrospective Study of Renal Failure in Dogs and Cats Admitted to University of Veterinary and Pharmaceutical Sciences Brno during 1999–2001. Acta Vet. Brno 2003, 72, 593–598. [Google Scholar] [CrossRef]
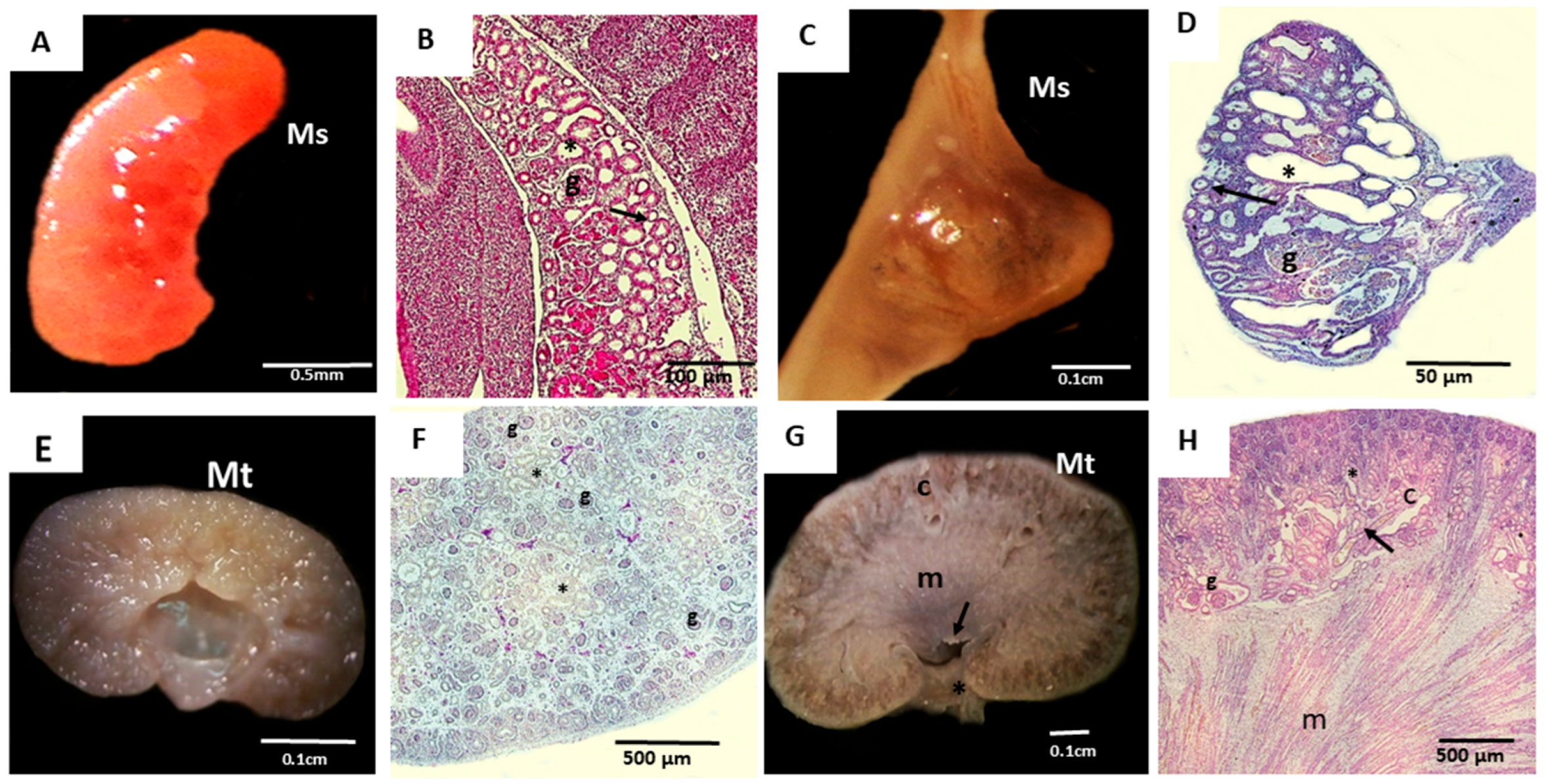



| Cat Mesonephros | Cat Metanephros | ||
|---|---|---|---|
| 15th to 21st Days of Gestation | 22nd to 30th Days of Gestation | 24th to 30th Days of Gestation | 50th to 60th Days of Gestation |
| Early gestation | Mid-gestation | Mid-gestation | Late gestation |
| CR = 0.5 − 2.1 cm | CR = 2.5 − 6.5 cm | CR = 3.1 − 6.5 cm | CR = 9.3 − 13 cm |
| 3 embryos | 3 embryos and fetuses | 3 fetuses | 3 fetuses |
| Antibody | Catalog Number, Manufacturer | Function |
|---|---|---|
| CD34 | ab8158, Abcam, Nova Analítica, SP, Brazil | Hematopoietic progenitors |
| CD117 | A4502, Dako, CiteAB, Bath, Somerset, UK | Renal precursor |
| CD105 | ab53321, Abcam, Nova Analítica, SP, Brazil | Endothelial cells |
| PCNA | sc-46, Santa Cruz Biotechnology, Interprise, Paulinia, SP, Brazil | Cellular proliferation |
| CD90 | WS0809D-100, Kingfisher Biotech, Quimigen, Alverca do Ribatejo, Lisboa, Portugal | Mesenchymal cells |
| MHC I | 18067213, Thermo Fisher Scientific, São Paulo, SP, Brazil | Expression of major histocompatibility complex (MHC I) proteins |
| MHC II | 17092082, Thermo Fisher Scientific, São Paulo, SP, Brazil | Expression of major histocompatibility complex (MHC II) proteins |
| WT1 | ab89901, Abcam Nova Analítica, SP, Brazil | Kidney progenitor cells during development |
| Nephrin | ab216692, Abcam Nova Analítica, SP, Brazil | Kidney progenitor cells for podocytes |
| Anima l | Group | Description | Inclusion Criteria | Internship CKD (IRIS) | |||
|---|---|---|---|---|---|---|---|
| Creatinine Level | DU | Morphological Change | FIV/ FeLV | ||||
| 1 | E | 8 years CF/UB | 2.25 mg/dL | 1.016 | Yes | 2 | |
| 2 | E | 9 years CF/UB | 2.89 mg/dL | 1.021 | Yes | 3 | |
| 3 | E | 6 years CF/SRD | 1.5 mg/dL | 1.050 | Yes | 1 | |
| 4 | E | 7 years CM/UB | 1.70 mg/dL | 1.017 | Yes | 1 | |
| 1 | C | 7 years CF/UB | 1.6 mg/dL | 1.050 | Yes | 1 | |
| 2 | C | 8 years CF/UB | 4.1 mg/dL | 1.019 | Yes | 3 | |
| 3 | C | 9 years CM/UB | 2.57 mg/dL | 1.021 | Yes | 2 | |
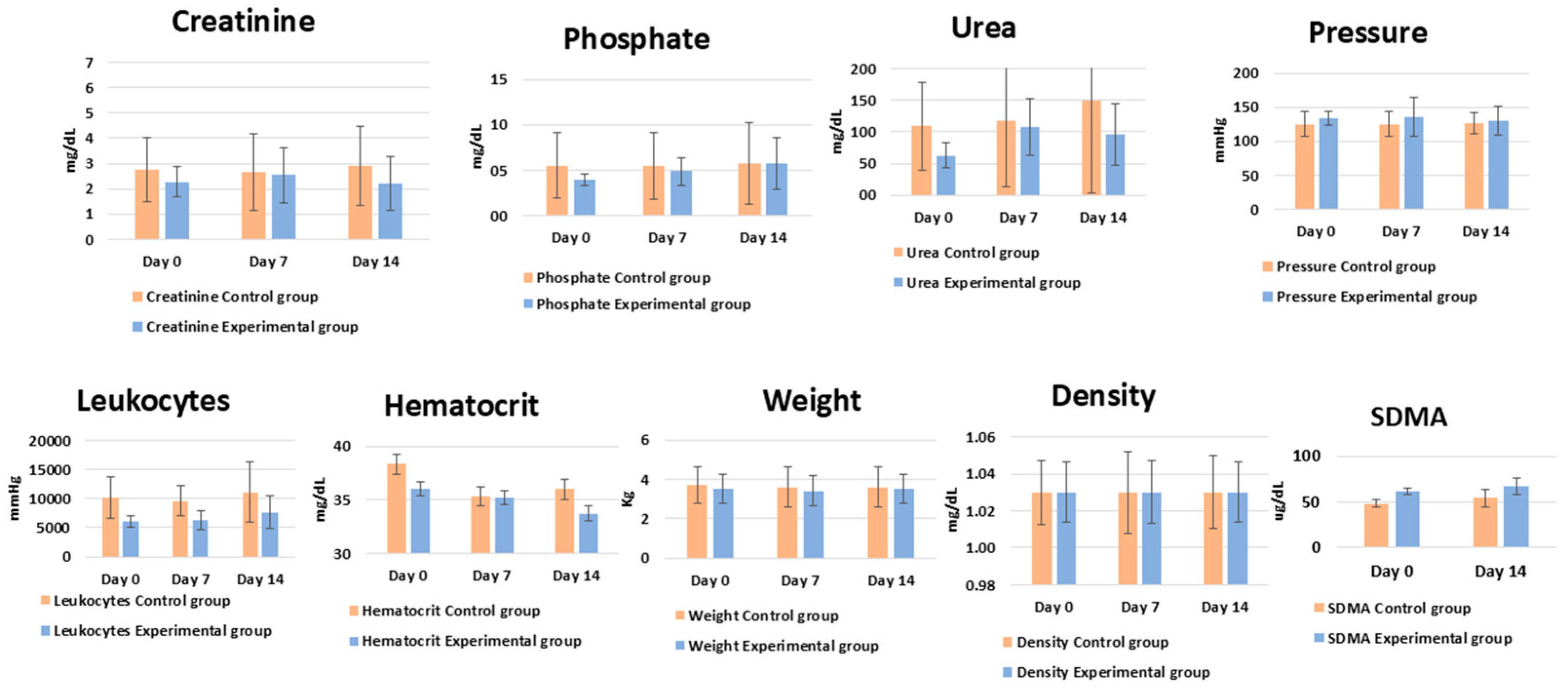
| Experimental Group—Average | Control Group—Average | t-Test Day by Day | t-Test Control vs. Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 days | 7 days | 14 days | 0 days | 7 days | 14 days | 0 days | 7 days | 14 days | ||
| Creatinine | 2.3 | 2.5 | 2.2 | 2.8 | 2.7 | 3.2 | 0.59 | 0.90 | 0.38 | 0.415 |
| Phosphate | 4.0 | 4.9 | 5.8 | 5.5 | 5.5 | 5.7 | 0.43 | 0.78 | 0.97 | 0.888 |
| Urea | 62.8 | 107.6 | 96.0 | 109.0 | 116.9 | 148.8 | 0.25 | 0.88 | 0.52 | 0.490 |
| Pressure | 133.8 | 136.3 | 130.0 | 125.0 | 125.0 | 126.7 | 0.44 | 0.58 | 0.82 | 0.517 |
| Leukocytes | 6100.0 | 6325.0 | 7675.0 | 10,200.0 | 9600.0 | 11,100.0 | 0.08 | 0.09 | 0.31 | 0.060 |
| Hematocrit | 36.0 | 35.3 | 33.8 | 38.3 | 35.3 | 36.0 | 0.61 | 0.99 | 0.75 | 0.772 |
| Weight | 3.5 | 3.4 | 3.5 | 3.7 | 3.6 | 3.6 | 0.76 | 0.79 | 0.90 | 0.750 |
| Density | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.77 | 0.85 | 0.86 | 0.775 |
| Mesonephros (21 Days) | Mesonephros (28 Days) | Metanephros (28 Days) | Metanephros (50 Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hour | DMEM HIGH | DMEM HAM’S F12 | ALPHA MEM | DMEM HIGH | DMEM HAM’S F12 | ALPHA MEM | DMEM HIGH | DMEM HAM’S F12 | ALPHA MEM | DMEM HIGH | DMEM HAM’S F12 | ALPHA MEM |
| 24 | 0.17 | 0.07 | 0.13 | 0.049 | 0.048 | 0.048 | 0.049 | 0.049 | 0.064 | 0.100 | 0.090 | 0.120 |
| 0.15 | 0.07 | 0.12 | 0.124 | 0.120 | 0.147 | 0.131 | 0.085 | 0.123 | 0.170 | 0.080 | 0.090 | |
| 0.14 | 0.07 | 0.13 | 0.140 | 0.113 | 0.151 | 0.129 | 0.083 | 0.124 | 0.110 | 0.070 | 0.090 | |
| 0.16 | 0.07 | 0.19 | 0.137 | 0.104 | 0.134 | 0.119 | 0.126 | 0.125 | 0.100 | 0.100 | 0.130 | |
| 0.09 | 0.06 | 0.07 | 0.153 | 0.153 | 0.170 | 0.116 | 0.088 | 0.110 | 0.050 | 0.070 | 0.080 | |
| 48 | 0.190 | 0.11 | 0.10 | 0.048 | 0.048 | 0.049 | 0.049 | 0.049 | 0.048 | 0.090 | 0.060 | 0.150 |
| 0.350 | 0.09 | 0.19 | 0.156 | 0.101 | 0.172 | 0.116 | 0.082 | 0.111 | 0.130 | 0.100 | 0.080 | |
| 0.360 | 0.08 | 0.19 | 0.158 | 0.120 | 0.141 | 0.136 | 0.085 | 0.115 | 0.070 | 0.060 | 0.090 | |
| 0.280 | 0.07 | 0.27 | 0.128 | 0.124 | 0.159 | 0.106 | 0.112 | 0.112 | 0.130 | 0.060 | 0.100 | |
| 0.060 | 0.05 | 0.07 | 0.128 | 0.114 | 0.140 | 0.103 | 0.081 | 0.118 | 0.070 | 0.050 | 0.060 | |
| 72 | 0.17 | 0.06 | 0.11 | 0.052 | 0.063 | 0.055 | 0.055 | 0.062 | 0.052 | 0.150 | 0.090 | 0.370 |
| 0.20 | 0.06 | 0.10 | 0.105 | 0.141 | 0.256 | 0.065 | 0.064 | 0.083 | 0.070 | 0.080 | 0.250 | |
| 0.17 | 0.07 | 0.13 | 0.091 | 0.112 | 0.184 | 0.061 | 0.072 | 0.103 | 0.310 | 0.080 | 0.150 | |
| 0.21 | 0.07 | 0.12 | 0.101 | 0.123 | 0.157 | 0.066 | 0.068 | 0.075 | 0.120 | 0.120 | 0.240 | |
| 0.06 | 0.05 | 0.06 | 0.085 | 0.104 | 0.112 | 0.056 | 0.069 | 0.084 | 0.060 | 0.050 | 0.060 | |
| Antibody | Gestational Period | |||
|---|---|---|---|---|
| Mesonephros (20 Days) | Mesonephros (28 Days) | Metanephros (28 Days) | Metanephros (50 Days) | |
| PCNA | 35.7% | 25.4% | 33.7% | 48.8% |
| CD34 | 10.9% | 6.9% | 5.2% | 3.9% |
| CD105 | 16.4% | 29.4% | 65.4% | 56.7% |
| CD90 | 12.7% | 30.9% | 61.9% | 63.6% |
| MHC I | 6.7% | 13.7% | 6.5% | 16.9% |
| MHC II | 9.4% | 7.9% | 7.9% | 10.9% |
| CD117 | 30.9% | 47.9% | 49.5% | 94.6% |
| Nephrin | 30.7% | 28.9% | 17.9% | 43.3% |
| CD117 plus Nephrin | 65.1% | 65.1% | 24.9% | 48.5% |
| WT1 | 13.7% | 34.2% | 11.4% | 73.2% |
| Day 0 | Day 7 | Day 14 | ||||
|---|---|---|---|---|---|---|
| Manifestation | EG | CG | EG | CG | EG | CG |
| Languidness | - | - | - | 1 (33.3%) | - | 1 (33.3%) |
| Dehydration | - | - | 1 (25%) | - | - | 1 (33.3%) |
| Hyporexia | - | - | 1 (25%) | 1 (33.3%) | - | - |
| Polyuria | 3 (75%) | 2 (66.6%) | 3 (75%) | 2 (66.6%) | 3 (75%) | 2 (66.6%) |
| Polydipsia | 3 (75%) | 2 (66.6%) | 3 (75%) | 2 (66.6%) | 3 (75%) | 2 (66.6%) |
| Vomit | - | - | - | - | - | 1 (33.3%) |
| Diarrhea | - | - | 1 (25%) | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mario, L.C.; Nhanharelli, J.d.P.; Borghesi, J.; Ribeiro, R.R.; de Carvalho, H.J.C.; da Silva, T.S.; Sol, M.d.; Barreto, R.d.S.N.; Barbalho, S.M.; Miglino, M.A. Development and Characterization of a Novel Lineage of Renal Progenitor Cells for Potential Use in Feline Chronic Kidney Disease: A Preliminary Study. Cells 2025, 14, 1395. https://doi.org/10.3390/cells14171395
Mario LC, Nhanharelli JdP, Borghesi J, Ribeiro RR, de Carvalho HJC, da Silva TS, Sol Md, Barreto RdSN, Barbalho SM, Miglino MA. Development and Characterization of a Novel Lineage of Renal Progenitor Cells for Potential Use in Feline Chronic Kidney Disease: A Preliminary Study. Cells. 2025; 14(17):1395. https://doi.org/10.3390/cells14171395
Chicago/Turabian StyleMario, Lara Carolina, Juliana de Paula Nhanharelli, Jéssica Borghesi, Rafaela Rodrigues Ribeiro, Hianka Jasmyne Costa de Carvalho, Thamires Santos da Silva, Mariano del Sol, Rodrigo da Silva Nunes Barreto, Sandra Maria Barbalho, and Maria Angelica Miglino. 2025. "Development and Characterization of a Novel Lineage of Renal Progenitor Cells for Potential Use in Feline Chronic Kidney Disease: A Preliminary Study" Cells 14, no. 17: 1395. https://doi.org/10.3390/cells14171395
APA StyleMario, L. C., Nhanharelli, J. d. P., Borghesi, J., Ribeiro, R. R., de Carvalho, H. J. C., da Silva, T. S., Sol, M. d., Barreto, R. d. S. N., Barbalho, S. M., & Miglino, M. A. (2025). Development and Characterization of a Novel Lineage of Renal Progenitor Cells for Potential Use in Feline Chronic Kidney Disease: A Preliminary Study. Cells, 14(17), 1395. https://doi.org/10.3390/cells14171395








