Updated Applications of Stem Cells in Hypoplastic Left Heart Syndrome
Abstract
1. Introduction
2. Key Pathophysiological Basis and Signalling Molecules of HLHS
2.1. Key Signalling Pathways for Heart Development and HLHS
2.2. Transcription Factors Underpinning Cardiac Development and HLHS
3. Application of Stem Cell in HLHS as an Adjunct Treatment
3.1. Umbilical Cord Derived Stem Cells
3.2. Bone Marrow Stem Cells
3.3. Cardiac Stem and Cardiosphere-Derived Cells
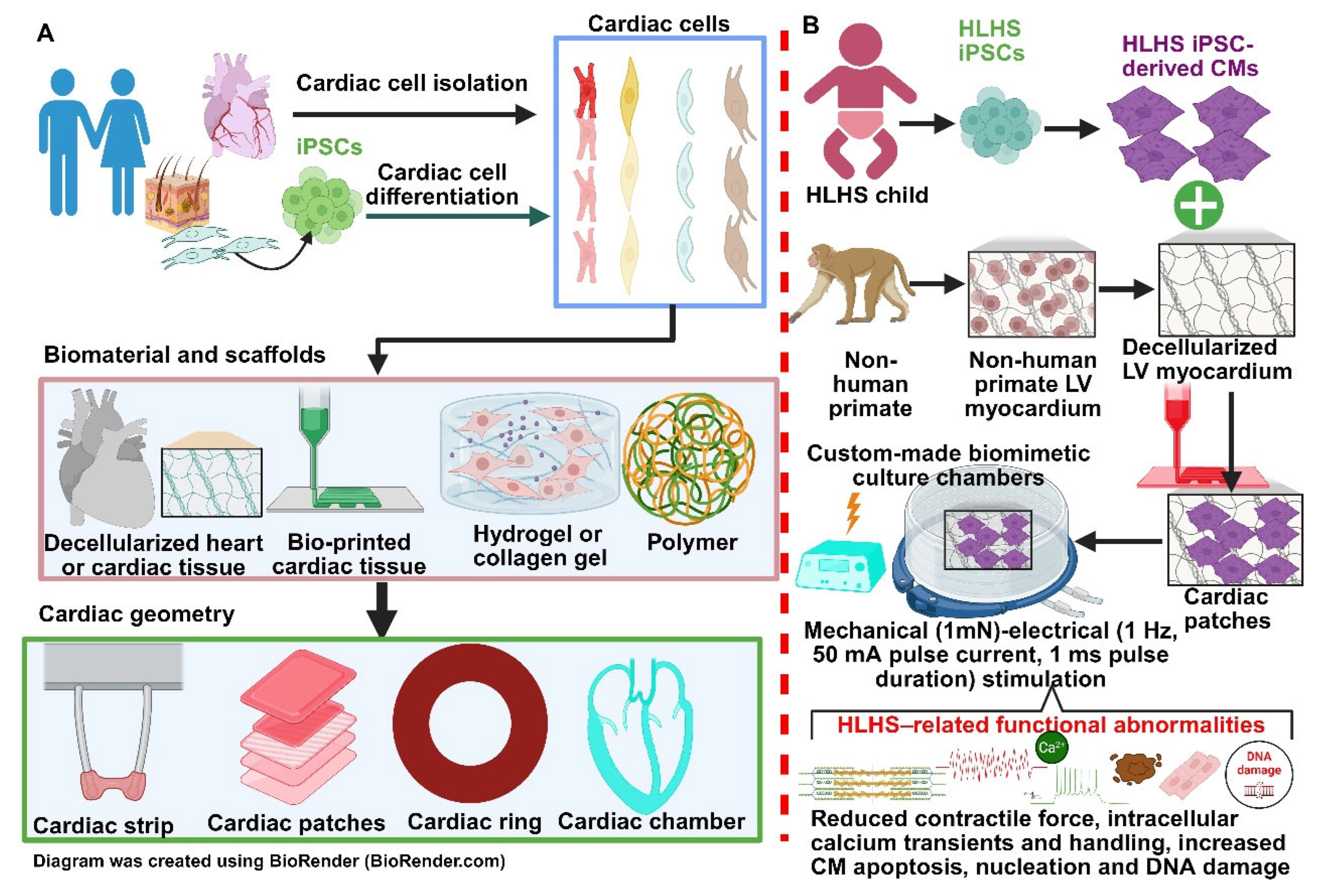
4. PSC Derived Cardiomyocytes for Studying HLHS
4.1. PSC-Derived Cardiomyocytes from HLHS Patients
4.2. Human iPSC-Derived Cardiomyocytes as Disease Modelling for HLHS
4.3. HLHS iPSC-CMs: A Platform for Drug Discovery and Evaluating Drug Toxicity
4.4. Limitations of iPSC-CMs 2D Model
5. Implications of HiPSC-Derived 3D Cardiac Patches for Treating HLHS
6. Future Directions
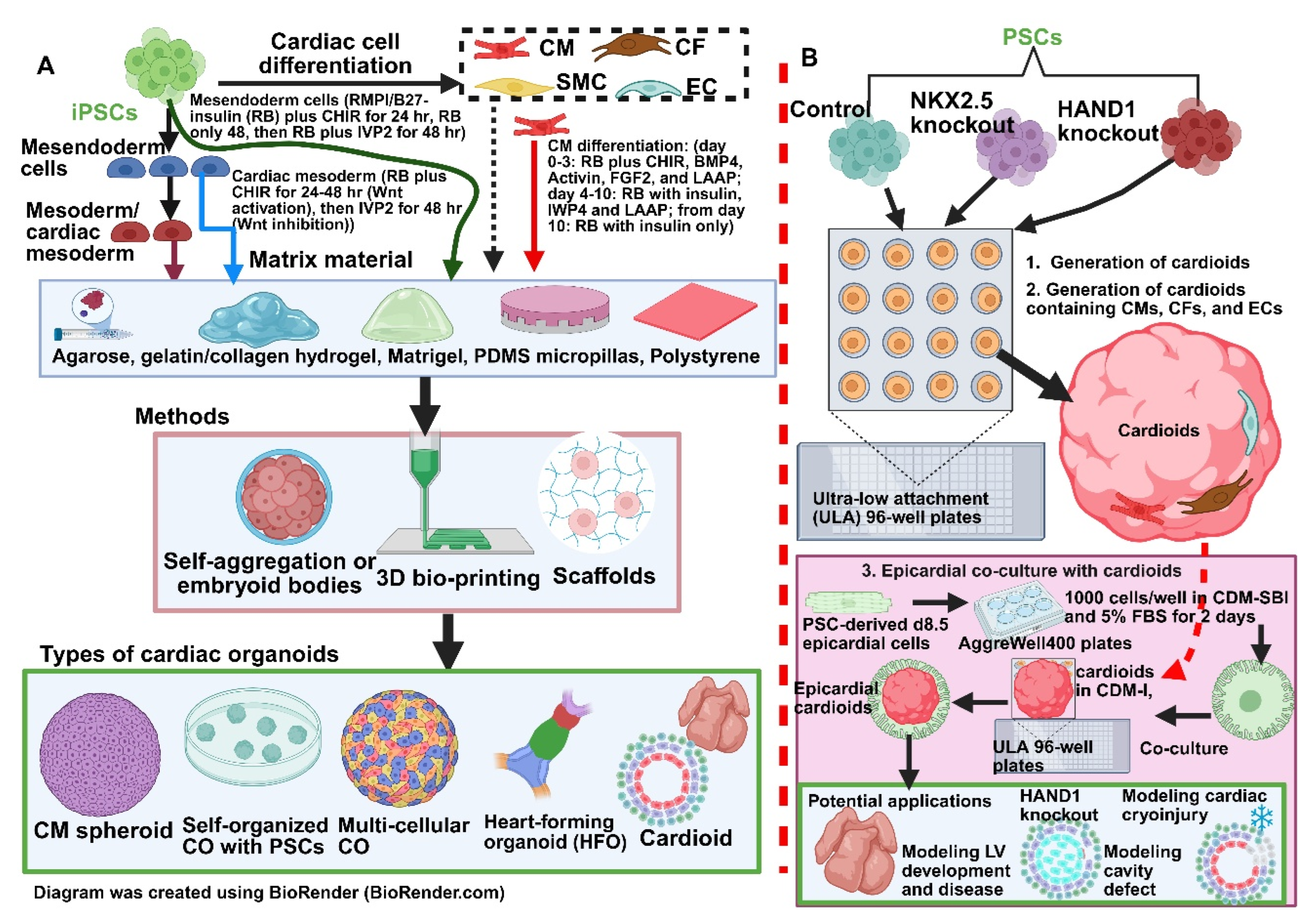
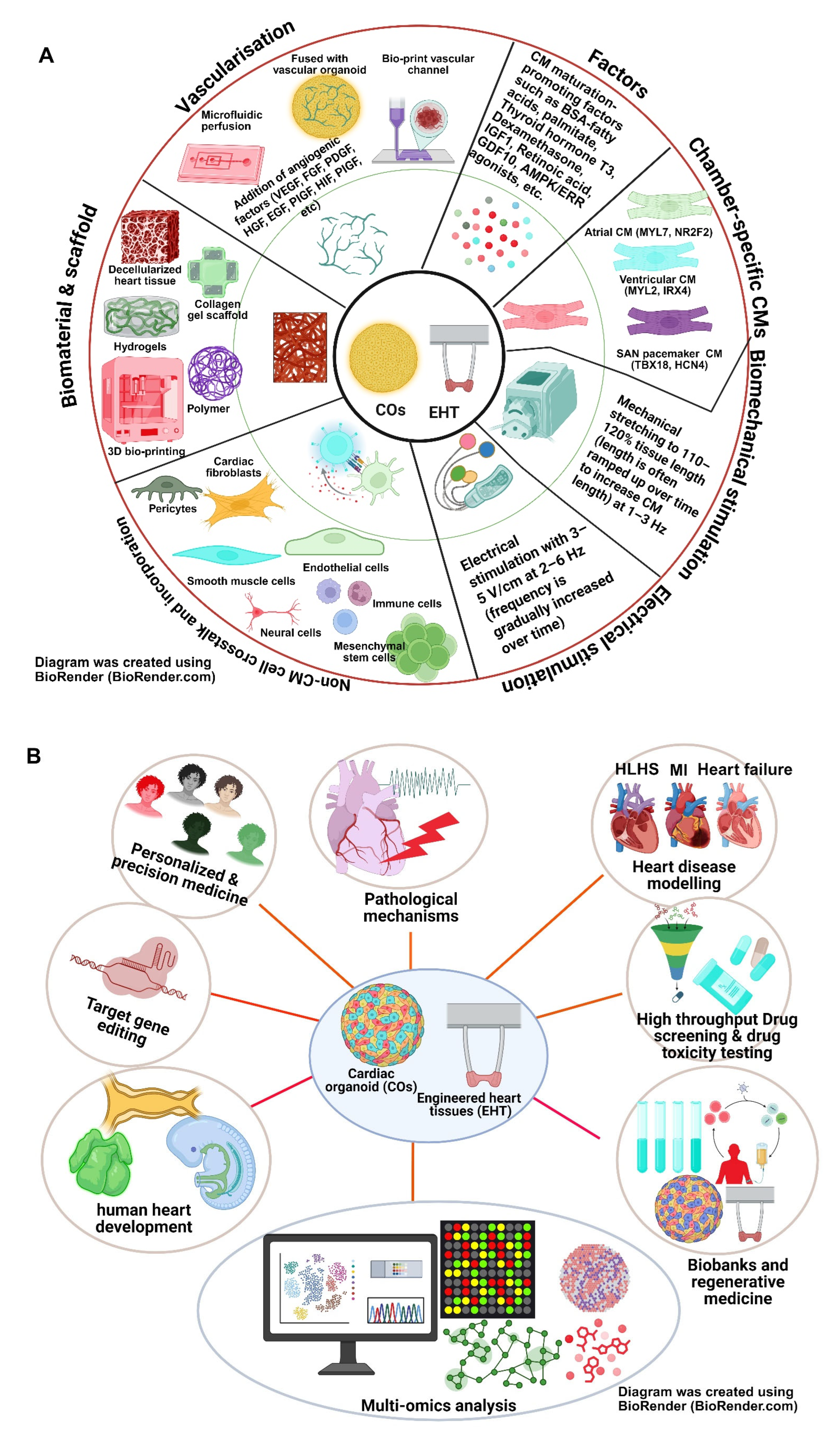
6.1. Cardiac Organoids: A Better Model for HLHS?
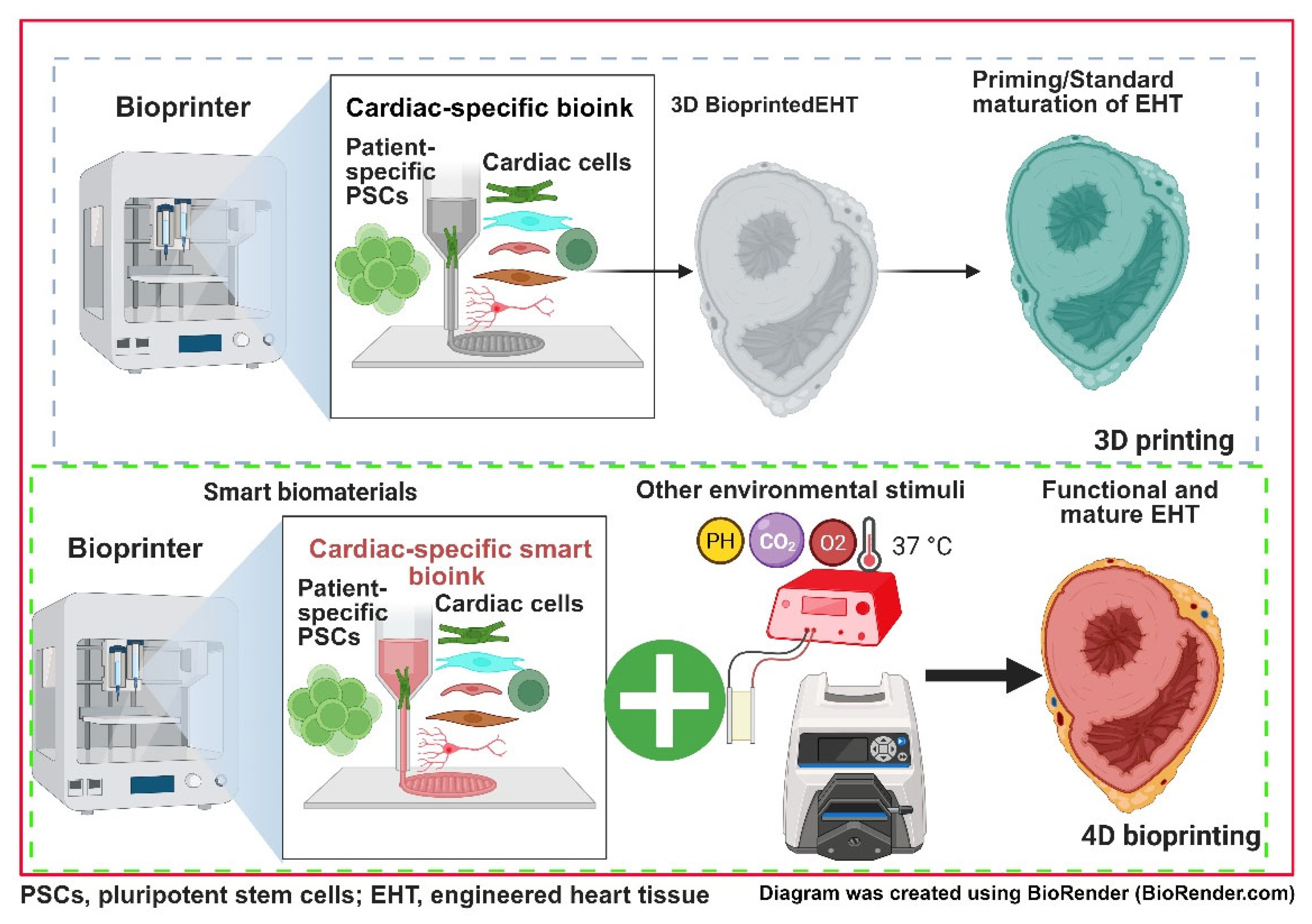
6.2. Three-Dimensional/Four-Dimensional Bioprinting PSC-Derived Cardiac Tissues for Studying HLHS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HLHS | hypoplastic left heart syndrome |
| PSC | pluripotent stem cell |
| iPSC | induced pluripotent stem cell |
| CHD | congenital heart disease |
| CM | cardiomyocyte |
| RV | right ventricle |
| LV | left ventricle |
| FHF | first heart field |
| SHF | second heart field |
| EF | ejection fraction |
| BM-MSC | bone marrow-derived mesenchymal stem cell |
| BM-MNC | bone marrow-derived mononuclear cells |
| CDC | cardiosphere-derived cells |
| UCB-MSC | umbilical cord blood-derived mesenchymal stem cells |
| UCB-MNC | umbilical cord blood-derived mononuclear cells |
| NKX2 | NK homeobox 2 |
References
- Stallings, E.B.; Isenburg, J.L.; Rutkowski, R.E.; Kirby, R.S.; Nembhard, W.N.; Sandidge, T.; Villavicencio, S.; Nguyen, H.H.; McMahon, D.M.; Nestoridi, E.; et al. National population-based estimates for major birth defects, 2016–2020. Birth Defects Res. 2024, 116, e2301. [Google Scholar] [CrossRef]
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafane, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic left heart syndrome: Current considerations and expectations. J. Am. Coll. Cardiol. 2012, 59, S1–S42. [Google Scholar] [CrossRef]
- Hoshino, K.; Ogawa, K.; Hishitani, T.; Kitazawa, R.; Uehara, R. Hypoplastic left heart syndrome: Duration of survival without surgical intervention. Am. Heart J. 1999, 137, 535–542. [Google Scholar] [CrossRef]
- Alphonso, N.; Angelini, A.; Barron, D.J.; Bellsham-Revell, H.; Blom, N.A.; Brown, K.; Davis, D.; Duncan, D.; Fedrigo, M.; Galletti, L.; et al. Guidelines for the management of neonates and infants with hypoplastic left heart syndrome: The European Association for Cardio-Thoracic Surgery (EACTS) and the Association for European Paediatric and Congenital Cardiology (AEPC) Hypoplastic Left Heart Syndrome Guidelines Task Force. Eur. J. Cardiothorac. Surg. 2020, 58, 416–499. [Google Scholar] [CrossRef]
- Ohye, R.G.; Schranz, D.; D’Udekem, Y. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation 2016, 134, 1265–1279. [Google Scholar] [CrossRef]
- Norwood, W.I.; Lang, P.; Casteneda, A.R.; Campbell, D.N. Experience with operations for hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 1981, 82, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Geoffrion, T.R. Norwood Procedure. In StatPearls; Treasure Island (FL) ineligible companies; Disclosure: Tracy Geoffrion declares no relevant financial relationships with ineligible companies; National Library of Medicine: Bethesda, MD, USA, 2025. [Google Scholar]
- Glenn, W.W. Circulatory bypass of the right side of the heart. IV. Shunt between superior vena cava and distal right pulmonary artery; report of clinical application. N. Engl. J. Med. 1958, 259, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef]
- Steele, M.M.; Zahr, R.A.; Kirshbom, P.M.; Kopf, G.S.; Karimi, M. Quality of Life for Historic Cavopulmonary Shunt Survivors. World J. Pediatr. Congenit. Heart Surg. 2016, 7, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.H.; D’Udekem, Y.; Sholler, G.F.; Opotowsky, A.R.; Costa, D.S.J.; Sharpe, L.; Celermajer, D.S.; Winlaw, D.S.; Newburger, J.W.; Kasparian, N.A. Health-Related Quality of Life in Children, Adolescents, and Adults with a Fontan Circulation: A Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014172. [Google Scholar] [CrossRef] [PubMed]
- Dib, N.; Poirier, N.; Samuel, M.; Hermann Honfo, S.; Zaidi, A.; Opotowsky, A.R.; Mongeon, F.P.; Mondesert, B.; Kay, J.; Ibrahim, R.; et al. Cardiovascular Outcomes Associated with Hypoplastic Left Heart Syndrome Versus Other Types of Single Right Ventricle: A Multicenter Study. J. Am. Heart Assoc. 2024, 13, e034757. [Google Scholar] [CrossRef]
- Yimer, M.A.; Tisma-Dupanovic, S.; Malloy-Walton, L.; Connelly, D.; Noel-Macdonnell, J.; Brien, J.O.; Papagiannis, J. Post-operative arrhythmias in patients with hypoplastic left heart syndrome and anatomic variants: Incidence, type, and course. Cardiol. Young 2021, 31, 1412–1418. [Google Scholar] [CrossRef]
- Poh, C.L.; d’Udekem, Y. Life After Surviving Fontan Surgery: A Meta-Analysis of the Incidence and Predictors of Late Death. Heart Lung Circ. 2018, 27, 552–559. [Google Scholar] [CrossRef]
- Chrisant, M.R.; Naftel, D.C.; Drummond-Webb, J.; Chinnock, R.; Canter, C.E.; Boucek, M.M.; Boucek, R.J.; Hallowell, S.C.; Kirklin, J.K.; Morrow, W.R.; et al. Fate of infants with hypoplastic left heart syndrome listed for cardiac transplantation: A multicenter study. J. Heart Lung Transplant. 2005, 24, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef]
- Bittle, G.J.; Morales, D.; Deatrick, K.B.; Parchment, N.; Saha, P.; Mishra, R.; Sharma, S.; Pietris, N.; Vasilenko, A.; Bor, C.; et al. Stem Cell Therapy for Hypoplastic Left Heart Syndrome: Mechanism, Clinical Application, and Future Directions. Circ. Res. 2018, 123, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Hare, J.M.; Hoffman, J.R.; Boyd, R.M.; Ramdas, K.N.; Pietris, N.; Kutty, S.; Tweddell, J.S.; Husain, S.A.; Menon, S.C.; et al. Intramyocardial cell-based therapy with Lomecel-B during bidirectional cavopulmonary anastomosis for hypoplastic left heart syndrome: The ELPIS phase I trial. Eur. Heart J. Open 2023, 3, oead002. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed]
- van Mil, A.; Balk, G.M.; Neef, K.; Buikema, J.W.; Asselbergs, F.W.; Wu, S.M.; Doevendans, P.A.; Sluijter, J.P.G. Modelling inherited cardiac disease using human induced pluripotent stem cell-derived cardiomyocytes: Progress, pitfalls, and potential. Cardiovasc. Res. 2018, 114, 1828–1842. [Google Scholar] [CrossRef]
- Caspi, O.; Huber, I.; Gepstein, A.; Arbel, G.; Maizels, L.; Boulos, M.; Gepstein, L. Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ. Cardiovasc. Genet. 2013, 6, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, M.; Hsueh, B.; Jia, X.; Pasca, A.M.; Bernstein, J.A.; Hallmayer, J.; Dolmetsch, R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 2011, 471, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Lee, A.S.; Liang, P.; Sanchez-Freire, V.; Nguyen, P.K.; Wang, L.; Han, L.; Yen, M.; Wang, Y.; Sun, N.; et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013, 12, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.T.; He, W.; Kim, M.S.; Liang, H.L.; Shradhanjali, A.; Jurkiewicz, H.; Freudinger, B.P.; Greene, A.S.; LaDisa, J.F., Jr.; Tayebi, L.; et al. 3D-bioprinting of patient-derived cardiac tissue models for studying congenital heart disease. Front. Cardiovasc. Med. 2023, 10, 1162731. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Contreras, J.; Bering, J.; Zhao, M.T. In Vivo and In Vitro Approaches to Modeling Hypoplastic Left Heart Syndrome. Curr. Cardiol. Rep. 2024, 26, 1221–1229. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Deng, S.; Liu, B.; Jing, X.; Yan, Y.; Liu, Y.; Wang, J.; Zhou, X.; She, Q. The molecular mechanisms of cardiac development and related diseases. Signal Transduct. Target. Ther. 2024, 9, 368. [Google Scholar] [CrossRef]
- Van Vliet, P.; Wu, S.M.; Zaffran, S.; Puceat, M. Early cardiac development: A view from stem cells to embryos. Cardiovasc. Res. 2012, 96, 352–362. [Google Scholar] [CrossRef]
- Brand, T. Heart development: Molecular insights into cardiac specification and early morphogenesis. Dev. Biol. 2003, 258, 1–19. [Google Scholar] [CrossRef]
- Kirby, M.L.; Waldo, K.L. Neural crest and cardiovascular patterning. Circ. Res. 1995, 77, 211–215. [Google Scholar] [CrossRef]
- Gitler, A.D.; Lu, M.M.; Jiang, Y.Q.; Epstein, J.A.; Gruber, P.J. Molecular markers of cardiac endocardial cushion development. Dev. Dyn. 2003, 228, 643–650. [Google Scholar] [CrossRef]
- Boselli, F.; Freund, J.B.; Vermot, J. Blood flow mechanics in cardiovascular development. Cell. Mol. Life Sci. 2015, 72, 2545–2559. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Chaturvedi, R.R.; Sled, J.G. Flow-Mediated Factors in the Pathogenesis of Hypoplastic Left Heart Syndrome. J. Cardiovasc. Dev. Dis. 2022, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cao, W.; Skillman, M.; Wu, M. Hypoplastic Left Heart Syndrome: Signaling & Molecular Perspectives, and the Road Ahead. Int. J. Mol. Sci. 2023, 24, 15249. [Google Scholar] [CrossRef] [PubMed]
- Niessen, K.; Karsan, A. Notch signaling in cardiac development. Circ. Res. 2008, 102, 1169–1181. [Google Scholar] [CrossRef]
- Kerstjens-Frederikse, W.S.; van de Laar, I.M.; Vos, Y.J.; Verhagen, J.M.; Berger, R.M.; Lichtenbelt, K.D.; Klein Wassink-Ruiter, J.S.; van der Zwaag, P.A.; du Marchie Sarvaas, G.J.; Bergman, K.A.; et al. Cardiovascular malformations caused by NOTCH1 mutations do not keep left: Data on 428 probands with left-sided CHD and their families. Genet. Med. 2016, 18, 914–923. [Google Scholar] [CrossRef]
- McBride, K.L.; Riley, M.F.; Zender, G.A.; Fitzgerald-Butt, S.M.; Towbin, J.A.; Belmont, J.W.; Cole, S.E. NOTCH1 mutations in individuals with left ventricular outflow tract malformations reduce ligand-induced signaling. Hum. Mol. Genet. 2008, 17, 2886–2893. [Google Scholar] [CrossRef]
- Iascone, M.; Ciccone, R.; Galletti, L.; Marchetti, D.; Seddio, F.; Lincesso, A.R.; Pezzoli, L.; Vetro, A.; Barachetti, D.; Boni, L.; et al. Identification of de novo mutations and rare variants in hypoplastic left heart syndrome. Clin. Genet. 2012, 81, 542–554. [Google Scholar] [CrossRef]
- Durbin, M.D.; Cadar, A.G.; Williams, C.H.; Guo, Y.; Bichell, D.P.; Su, Y.R.; Hong, C.C. Hypoplastic Left Heart Syndrome Sequencing Reveals a Novel NOTCH1 Mutation in a Family with Single Ventricle Defects. Pediatr. Cardiol. 2017, 38, 1232–1240. [Google Scholar] [CrossRef]
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995, 9, 2105–2116. [Google Scholar] [CrossRef]
- Beppu, H.; Kawabata, M.; Hamamoto, T.; Chytil, A.; Minowa, O.; Noda, T.; Miyazono, K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev. Biol. 2000, 221, 249–258. [Google Scholar] [CrossRef]
- Jiao, K.; Kulessa, H.; Tompkins, K.; Zhou, Y.; Batts, L.; Baldwin, H.S.; Hogan, B.L. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003, 17, 2362–2367. [Google Scholar] [CrossRef]
- Uchimura, T.; Komatsu, Y.; Tanaka, M.; McCann, K.L.; Mishina, Y. Bmp2 and Bmp4 genetically interact to support multiple aspects of mouse development including functional heart development. Genesis 2009, 47, 374–384. [Google Scholar] [CrossRef]
- Liu, W.; Selever, J.; Wang, D.; Lu, M.F.; Moses, K.A.; Schwartz, R.J.; Martin, J.F. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc. Natl. Acad. Sci. USA 2004, 101, 4489–4494. [Google Scholar] [CrossRef]
- Bobos, D.; Soufla, G.; Angouras, D.C.; Lekakis, I.; Georgopoulos, S.; Melissari, E. Investigation of the Role of BMP2 and -4 in ASD, VSD and Complex Congenital Heart Disease. Diagnostics 2023, 13, 2717. [Google Scholar] [CrossRef]
- Stefanovic, S.; Zaffran, S. Mechanisms of retinoic acid signaling during cardiogenesis. Mech. Dev. 2017, 143, 9–19. [Google Scholar] [CrossRef]
- Vermot, J.; Niederreither, K.; Garnier, J.M.; Chambon, P.; Dolle, P. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1763–1768. [Google Scholar] [CrossRef]
- Jiang, X.; Choudhary, B.; Merki, E.; Chien, K.R.; Maxson, R.E.; Sucov, H.M. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mech. Dev. 2002, 117, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Vermot, J.; Messaddeq, N.; Schuhbaur, B.; Chambon, P.; Dolle, P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 2001, 128, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Horitani, K.; Shiojima, I. Wnt signaling in cardiac development and heart diseases. Vitr. Cell. Dev. Biol.-Anim. 2024, 60, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, A.; Calore, M.; Poloni, G.; De Windt, L.J.; Braghetta, P.; Rampazzo, A. Wnt/beta-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget 2017, 8, 60640–60655. [Google Scholar] [CrossRef]
- Briggs, L.E.; Burns, T.A.; Lockhart, M.M.; Phelps, A.L.; Van den Hoff, M.J.; Wessels, A. Wnt/beta-catenin and sonic hedgehog pathways interact in the regulation of the development of the dorsal mesenchymal protrusion. Dev. Dyn. 2016, 245, 103–113. [Google Scholar] [CrossRef]
- Theis, J.L.; Vogler, G.; Missinato, M.A.; Li, X.; Nielsen, T.; Zeng, X.I.; Martinez-Fernandez, A.; Walls, S.M.; Kervadec, A.; Kezos, J.N.; et al. Patient-specific genomics and cross-species functional analysis implicate LRP2 in hypoplastic left heart syndrome. eLife 2020, 9, e59554. [Google Scholar] [CrossRef]
- Washington Smoak, I.; Byrd, N.A.; Abu-Issa, R.; Goddeeris, M.M.; Anderson, R.; Morris, J.; Yamamura, K.; Klingensmith, J.; Meyers, E.N. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev. Biol. 2005, 283, 357–372. [Google Scholar] [CrossRef]
- Goddeeris, M.M.; Schwartz, R.; Klingensmith, J.; Meyers, E.N. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development 2007, 134, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Heallen, T.; Martin, J.F. The Hippo pathway in the heart: Pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, L.; Zhao, B.; Guan, K.L. The hippo pathway in heart development, regeneration, and diseases. Circ. Res. 2015, 116, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jin, K.; Bais, A.S.; Zhu, W.; Yagi, H.; Feinstein, T.N.; Nguyen, P.K.; Criscione, J.D.; Liu, X.; Beutner, G.; et al. Uncompensated mitochondrial oxidative stress underlies heart failure in an iPSC-derived model of congenital heart disease. Cell Stem Cell 2022, 29, 840–855.e7. [Google Scholar] [CrossRef]
- Khosravi, F.; Ahmadvand, N.; Bellusci, S.; Sauer, H. The Multifunctional Contribution of FGF Signaling to Cardiac Development, Homeostasis, Disease and Repair. Front. Cell Dev. Biol. 2021, 9, 672935. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, J.Y.; Huang, Y.; Lin, X.; Luo, Y.; Schwartz, R.J.; Martin, J.F.; Wang, F. The FGF-BMP signaling axis regulates outflow tract valve primordium formation by promoting cushion neural crest cell differentiation. Circ. Res. 2010, 107, 1209–1219. [Google Scholar] [CrossRef]
- Reuter, M.S.; Sokolowski, D.J.; Diaz-Mejia, J.J.; Keunen, J.; de Vrijer, B.; Chan, C.; Wang, L.; Ryan, G.; Chiasson, D.A.; Ketela, T.; et al. Decreased left heart flow in fetal lambs causes left heart hypoplasia and pro-fibrotic tissue remodeling. Commun. Biol. 2023, 6, 770. [Google Scholar] [CrossRef]
- Han, Z.; Yi, P.; Li, X.; Olson, E.N. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development 2006, 133, 1175–1182. [Google Scholar] [CrossRef]
- Riley, P.; Anson-Cartwright, L.; Cross, J.C. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 1998, 18, 271–275. [Google Scholar] [CrossRef]
- McFadden, D.G.; Barbosa, A.C.; Richardson, J.A.; Schneider, M.D.; Srivastava, D.; Olson, E.N. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development 2005, 132, 189–201. [Google Scholar] [CrossRef]
- Togi, K.; Kawamoto, T.; Yamauchi, R.; Yoshida, Y.; Kita, T.; Tanaka, M. Role of Hand1/eHAND in the dorso-ventral patterning and interventricular septum formation in the embryonic heart. Mol. Cell. Biol. 2004, 24, 4627–4635. [Google Scholar] [CrossRef]
- Reamon-Buettner, S.M.; Ciribilli, Y.; Inga, A.; Borlak, J. A loss-of-function mutation in the binding domain of HAND1 predicts hypoplasia of the human hearts. Hum. Mol. Genet. 2008, 17, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Firulli, B.A.; Toolan, K.P.; Harkin, J.; Millar, H.; Pineda, S.; Firulli, A.B. The HAND1 frameshift A126FS mutation does not cause hypoplastic left heart syndrome in mice. Cardiovasc. Res. 2017, 113, 1732–1742. [Google Scholar] [CrossRef]
- Akazawa, H.; Komuro, I. Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases. Pharmacol. Ther. 2005, 107, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Lyons, I.; Parsons, L.M.; Hartley, L.; Li, R.; Andrews, J.E.; Robb, L.; Harvey, R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Biben, C.; Weber, R.; Kesteven, S.; Stanley, E.; McDonald, L.; Elliott, D.A.; Barnett, L.; Koentgen, F.; Robb, L.; Feneley, M.; et al. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2-5. Circ. Res. 2000, 87, 888–895. [Google Scholar] [CrossRef]
- Elliott, D.A.; Kirk, E.P.; Yeoh, T.; Chandar, S.; McKenzie, F.; Taylor, P.; Grossfeld, P.; Fatkin, D.; Jones, O.; Hayes, P.; et al. Cardiac homeobox gene NKX2-5 mutations and congenital heart disease: Associations with atrial septal defect and hypoplastic left heart syndrome. J. Am. Coll. Cardiol. 2003, 41, 2072–2076. [Google Scholar] [CrossRef]
- Schott, J.J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef]
- Doering, L.; Cornean, A.; Thumberger, T.; Benjaminsen, J.; Wittbrodt, B.; Kellner, T.; Hammouda, O.T.; Gorenflo, M.; Wittbrodt, J.; Gierten, J. CRISPR-based knockout and base editing confirm the role of MYRF in heart development and congenital heart disease. Dis. Model. Mech. 2023, 16, dmm-049811. [Google Scholar] [CrossRef]
- Qi, H.; Yu, L.; Zhou, X.; Wynn, J.; Zhao, H.; Guo, Y.; Zhu, N.; Kitaygorodsky, A.; Hernan, R.; Aspelund, G.; et al. De novo variants in congenital diaphragmatic hernia identify MYRF as a new syndrome and reveal genetic overlaps with other developmental disorders. PLoS Genet. 2018, 14, e1007822. [Google Scholar] [CrossRef] [PubMed]
- Watt, A.J.; Battle, M.A.; Li, J.; Duncan, S.A. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 12573–12578. [Google Scholar] [CrossRef]
- Misra, C.; Sachan, N.; McNally, C.R.; Koenig, S.N.; Nichols, H.A.; Guggilam, A.; Lucchesi, P.A.; Pu, W.T.; Srivastava, D.; Garg, V. Congenital heart disease-causing Gata4 mutation displays functional deficits in vivo. PLoS Genet. 2012, 8, e1002690. [Google Scholar] [CrossRef]
- Ang, Y.S.; Rivas, R.N.; Ribeiro, A.J.S.; Srivas, R.; Rivera, J.; Stone, N.R.; Pratt, K.; Mohamed, T.M.A.; Fu, J.D.; Spencer, C.I.; et al. Disease Model of GATA4 Mutation Reveals Transcription Factor Cooperativity in Human Cardiogenesis. Cell 2016, 167, 1734–1749.E22. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Kathiriya, I.S.; Barnes, R.; Schluterman, M.K.; King, I.N.; Butler, C.A.; Rothrock, C.R.; Eapen, R.S.; Hirayama-Yamada, K.; Joo, K.; et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 2003, 424, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Ghorbani, S.; Adriani, M.R.; Bahaloo, S.; Naeini, M.A.; Heidari, M.M.; Hadadzadeh, M. Novel Point Mutations in 3′-Untranslated Region of GATA4 Gene Are Associated with Sporadic Non-syndromic Atrial and Ventricular Septal Defects. Curr. Med. Sci. 2022, 42, 129–143. [Google Scholar] [CrossRef]
- Chen, L.; Fulcoli, F.G.; Ferrentino, R.; Martucciello, S.; Illingworth, E.A.; Baldini, A. Transcriptional control in cardiac progenitors: Tbx1 interacts with the BAF chromatin remodeling complex and regulates Wnt5a. PLoS Genet. 2012, 8, e1002571. [Google Scholar] [CrossRef]
- Xu, H.; Morishima, M.; Wylie, J.N.; Schwartz, R.J.; Bruneau, B.G.; Lindsay, E.A.; Baldini, A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development 2004, 131, 3217–3227. [Google Scholar] [CrossRef]
- Rauch, A.; Devriendt, K.; Koch, A.; Rauch, R.; Gewillig, M.; Kraus, C.; Weyand, M.; Singer, H.; Reis, A.; Hofbeck, M. Assessment of association between variants and haplotypes of the remaining TBX1 gene and manifestations of congenital heart defects in 22q11.2 deletion patients. J. Med. Genet. 2004, 41, e40. [Google Scholar] [CrossRef]
- Patel, C.; Silcock, L.; McMullan, D.; Brueton, L.; Cox, H. TBX5 intragenic duplication: A family with an atypical Holt-Oram syndrome phenotype. Eur. J. Hum. Genet. 2012, 20, 863–869. [Google Scholar] [CrossRef]
- Bruneau, B.G.; Nemer, G.; Schmitt, J.P.; Charron, F.; Robitaille, L.; Caron, S.; Conner, D.A.; Gessler, M.; Nemer, M.; Seidman, C.E.; et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 2001, 106, 709–721. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Song, F.F.; Packham, E.A.; Buxton, S.; Robinson, T.E.; Ronksley, J.; Self, T.; Bonser, A.J.; Brook, J.D. Physical interaction between TBX5 and MEF2C is required for early heart development. Mol. Cell. Biol. 2009, 29, 2205–2218. [Google Scholar] [CrossRef]
- von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Crucean, A.; Alqahtani, A.; Barron, D.J.; Brawn, W.J.; Richardson, R.V.; O’Sullivan, J.; Anderson, R.H.; Henderson, D.J.; Chaudhry, B. Re-evaluation of hypoplastic left heart syndrome from a developmental and morphological perspective. Orphanet J. Rare Dis. 2017, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.M.; Harris, I.S.; Jaehnig, E.J.; Sauls, K.; Sinha, T.; Rojas, A.; Schachterle, W.; McCulley, D.J.; Norris, R.A.; Black, B.L. MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1. Development 2016, 143, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.H.; Wang, F.; Zhang, X.L.; Huang, R.T.; Xue, S.; Wang, J.; Qiu, X.B.; Liu, X.Y.; Yang, Y.Q. MEF2C loss-of-function mutation contributes to congenital heart defects. Int. J. Med. Sci. 2017, 14, 1143–1153. [Google Scholar] [CrossRef]
- Gao, R.; Liang, X.; Cheedipudi, S.; Cordero, J.; Jiang, X.; Zhang, Q.; Caputo, L.; Gunther, S.; Kuenne, C.; Ren, Y.; et al. Pioneering function of Isl1 in the epigenetic control of cardiomyocyte cell fate. Cell Res. 2019, 29, 486–501. [Google Scholar] [CrossRef]
- Stevens, K.N.; Hakonarson, H.; Kim, C.E.; Doevendans, P.A.; Koeleman, B.P.; Mital, S.; Raue, J.; Glessner, J.T.; Coles, J.G.; Moreno, V.; et al. Common variation in ISL1 confers genetic susceptibility for human congenital heart disease. PLoS ONE 2010, 5, e10855. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, J.; Li, L.; Qiao, Q.; Di, R.M.; Li, X.M.; Xu, Y.J.; Zhang, M.; Li, R.G.; Qiu, X.B.; et al. ISL1 loss-of-function mutation contributes to congenital heart defects. Heart Vessel. 2019, 34, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Tomita-Mitchell, A.; Stamm, K.D.; Mahnke, D.K.; Kim, M.S.; Hidestrand, P.M.; Liang, H.L.; Goetsch, M.A.; Hidestrand, M.; Simpson, P.; Pelech, A.N.; et al. Impact of MYH6 variants in hypoplastic left heart syndrome. Physiol. Genom. 2016, 48, 912–921. [Google Scholar] [CrossRef]
- Theis, J.L.; Hu, J.J.; Sundsbak, R.S.; Evans, J.M.; Bamlet, W.R.; Qureshi, M.Y.; O’Leary, P.W.; Olson, T.M. Genetic Association Between Hypoplastic Left Heart Syndrome and Cardiomyopathies. Circ. Genom. Precis. Med. 2021, 14, e003126. [Google Scholar] [CrossRef]
- Quintanilla Anfinson, M.; Creighton, S.; Simpson, P.M.; James, J.M.; Lim, P.; Frommelt, P.C.; Tomita-Mitchell, A.; Mitchell, M.E. MYH6 Variants Are Associated with Atrial Dysfunction in Neonates with Hypoplastic Left Heart Syndrome. Genes 2024, 15, 1449. [Google Scholar] [CrossRef]
- Kim, M.S.; Fleres, B.; Lovett, J.; Anfinson, M.; Samudrala, S.S.K.; Kelly, L.J.; Teigen, L.E.; Cavanaugh, M.; Marquez, M.; Geurts, A.M.; et al. Contractility of Induced Pluripotent Stem Cell-Cardiomyocytes With an MYH6 Head Domain Variant Associated With Hypoplastic Left Heart Syndrome. Front. Cell Dev. Biol. 2020, 8, 440. [Google Scholar] [CrossRef]
- Christ, A.; Christa, A.; Klippert, J.; Eule, J.C.; Bachmann, S.; Wallace, V.A.; Hammes, A.; Willnow, T.E. LRP2 Acts as SHH Clearance Receptor to Protect the Retinal Margin from Mitogenic Stimuli. Dev. Cell 2015, 35, 36–48. [Google Scholar] [CrossRef]
- Kantarci, S.; Al-Gazali, L.; Hill, R.S.; Donnai, D.; Black, G.C.; Bieth, E.; Chassaing, N.; Lacombe, D.; Devriendt, K.; Teebi, A.; et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat. Genet. 2007, 39, 957–959. [Google Scholar] [CrossRef]
- Kalcheva, N.; Qu, J.; Sandeep, N.; Garcia, L.; Zhang, J.; Wang, Z.; Lampe, P.D.; Suadicani, S.O.; Spray, D.C.; Fishman, G.I. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc. Natl. Acad. Sci. USA 2007, 104, 20512–20516. [Google Scholar] [CrossRef]
- Britz-Cunningham, S.H.; Shah, M.M.; Zuppan, C.W.; Fletcher, W.H. Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N. Engl. J. Med. 1995, 332, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.; Martinez, A.M.; Zuppan, C.W.; Shah, M.M.; Bailey, L.L.; Fletcher, W.H. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE). Mutat. Res. 2001, 479, 173–186. [Google Scholar] [CrossRef]
- Asp, M.; Giacomello, S.; Larsson, L.; Wu, C.; Furth, D.; Qian, X.; Wardell, E.; Custodio, J.; Reimegard, J.; Salmen, F.; et al. A Spatiotemporal Organ-Wide Gene Expression and Cell Atlas of the Developing Human Heart. Cell 2019, 179, 1647–1660.E19. [Google Scholar] [CrossRef]
- Gessert, S.; Kuhl, M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ. Res. 2010, 107, 186–199. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Tzahor, E. Wnt/beta-catenin signaling and cardiogenesis: Timing does matter. Dev. Cell 2007, 13, 10–13. [Google Scholar] [CrossRef]
- Buikema, J.W.; Mady, A.S.; Mittal, N.V.; Atmanli, A.; Caron, L.; Doevendans, P.A.; Sluijter, J.P.; Domian, I.J. Wnt/beta-catenin signaling directs the regional expansion of first and second heart field-derived ventricular cardiomyocytes. Development 2013, 140, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Fu, X.; Wang, J.; Lu, M.F.; Chen, L.; Baldini, A.; Klein, W.H.; Martin, J.F. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc. Natl. Acad. Sci. USA 2007, 104, 9319–9324. [Google Scholar] [CrossRef] [PubMed]
- Nagy, I.I.; Railo, A.; Rapila, R.; Hast, T.; Sormunen, R.; Tavi, P.; Rasanen, J.; Vainio, S.J. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc. Res. 2010, 85, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yuan, L.; Goss, A.M.; Wang, T.; Yang, J.; Lepore, J.J.; Zhou, D.; Schwartz, R.J.; Patel, V.; Cohen, E.D.; et al. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev. Cell 2010, 18, 275–287. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, L.; Majumdar, A.; Li, X.; Zhang, X.; Liu, W.; Etheridge, L.; Shi, Y.; Martin, J.; Van de Ven, W.; et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat. Genet. 2007, 39, 1225–1234. [Google Scholar] [CrossRef]
- Li, D.; Angermeier, A.; Wang, J. Planar cell polarity signaling regulates polarized second heart field morphogenesis to promote both arterial and venous pole septation. Development 2019, 146, dev181719. [Google Scholar] [CrossRef] [PubMed]
- Ryckebusch, L.; Wang, Z.; Bertrand, N.; Lin, S.C.; Chi, X.; Schwartz, R.; Zaffran, S.; Niederreither, K. Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. USA 2008, 105, 2913–2918. [Google Scholar] [CrossRef]
- Sirbu, I.O.; Zhao, X.; Duester, G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008, 237, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef]
- Gruber, P.J.; Kubalak, S.W.; Pexieder, T.; Sucov, H.M.; Evans, R.M.; Chien, K.R. RXR alpha deficiency confers genetic susceptibility for aortic sac, conotruncal, atrioventricular cushion, and ventricular muscle defects in mice. J. Clin. Investig. 1996, 98, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.B.; Holowiecki, A.; Waxman, J.S. Retinoic acid signaling restricts the size of the first heart field within the anterior lateral plate mesoderm. Dev. Biol. 2021, 473, 119–129. [Google Scholar] [CrossRef]
- Griffin, A.H.C.; Small, A.M.; Johnson, R.D.; Medina, A.M.; Kollar, K.T.; Nazir, R.A.; McGuire, A.M.; Schumacher, J.A. Retinoic acid promotes second heart field addition and regulates ventral aorta patterning in zebrafish. Dev. Biol. 2025, 522, 143–155. [Google Scholar] [CrossRef]
- Rones, M.S.; McLaughlin, K.A.; Raffin, M.; Mercola, M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development 2000, 127, 3865–3876. [Google Scholar] [CrossRef]
- Grego-Bessa, J.; Luna-Zurita, L.; del Monte, G.; Bolos, V.; Melgar, P.; Arandilla, A.; Garratt, A.N.; Zang, H.; Mukouyama, Y.S.; Chen, H.; et al. Notch signaling is essential for ventricular chamber development. Dev. Cell 2007, 12, 415–429. [Google Scholar] [CrossRef]
- Luxan, G.; D’Amato, G.; MacGrogan, D.; de la Pompa, J.L. Endocardial Notch Signaling in Cardiac Development and Disease. Circ. Res. 2016, 118, e1–e18. [Google Scholar] [CrossRef]
- MacGrogan, D.; Nus, M.; de la Pompa, J.L. Notch signaling in cardiac development and disease. Curr. Top. Dev. Biol. 2010, 92, 333–365. [Google Scholar] [CrossRef]
- del Monte, G.; Casanova, J.C.; Guadix, J.A.; MacGrogan, D.; Burch, J.B.; Perez-Pomares, J.M.; de la Pompa, J.L. Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ. Res. 2011, 108, 824–836. [Google Scholar] [CrossRef]
- Klaus, A.; Saga, Y.; Taketo, M.M.; Tzahor, E.; Birchmeier, W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18531–18536. [Google Scholar] [CrossRef]
- Chen, H.; Shi, S.; Acosta, L.; Li, W.; Lu, J.; Bao, S.; Chen, Z.; Yang, Z.; Schneider, M.D.; Chien, K.R.; et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 2004, 131, 2219–2231. [Google Scholar] [CrossRef]
- McCulley, D.J.; Kang, J.O.; Martin, J.F.; Black, B.L. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev. Dyn. 2008, 237, 3200–3209. [Google Scholar] [CrossRef]
- Luna-Zurita, L.; Stirnimann, C.U.; Glatt, S.; Kaynak, B.L.; Thomas, S.; Baudin, F.; Samee, M.A.; He, D.; Small, E.M.; Mileikovsky, M.; et al. Complex Interdependence Regulates Heterotypic Transcription Factor Distribution and Coordinates Cardiogenesis. Cell 2016, 164, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.G.; Seidman, C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, B.G. Transcriptional regulation of vertebrate cardiac morphogenesis. Circ. Res. 2002, 90, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, B.G. The developmental genetics of congenital heart disease. Nature 2008, 451, 943–948. [Google Scholar] [CrossRef]
- Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 2006, 313, 1922–1927. [Google Scholar] [CrossRef]
- Oka, T.; Maillet, M.; Watt, A.J.; Schwartz, R.J.; Aronow, B.J.; Duncan, S.A.; Molkentin, J.D. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ. Res. 2006, 98, 837–845. [Google Scholar] [CrossRef]
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Stennard, F.A.; Harvey, R.P. T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development 2005, 132, 4897–4910. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Schwarz, J.; Bucana, C.; Olson, E.N. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 1997, 276, 1404–1407. [Google Scholar] [CrossRef]
- Tanaka, M.; Chen, Z.; Bartunkova, S.; Yamasaki, N.; Izumo, S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 1999, 126, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Durocher, D.; Charron, F.; Warren, R.; Schwartz, R.J.; Nemer, M. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. EMBO J. 1997, 16, 5687–5696. [Google Scholar] [CrossRef]
- Targoff, K.L.; Colombo, S.; George, V.; Schell, T.; Kim, S.H.; Solnica-Krezel, L.; Yelon, D. Nkx genes are essential for maintenance of ventricular identity. Development 2013, 140, 4203–4213. [Google Scholar] [CrossRef]
- Pashmforoush, M.; Lu, J.T.; Chen, H.; Amand, T.S.; Kondo, R.; Pradervand, S.; Evans, S.M.; Clark, B.; Feramisco, J.R.; Giles, W.; et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 2004, 117, 373–386. [Google Scholar] [CrossRef]
- Benson, D.W.; Silberbach, G.M.; Kavanaugh-McHugh, A.; Cottrill, C.; Zhang, Y.; Riggs, S.; Smalls, O.; Johnson, M.C.; Watson, M.S.; Seidman, J.G.; et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J. Clin. Investig. 1999, 104, 1567–1573. [Google Scholar] [CrossRef]
- Kobayashi, J.; Yoshida, M.; Tarui, S.; Hirata, M.; Nagai, Y.; Kasahara, S.; Naruse, K.; Ito, H.; Sano, S.; Oh, H. Directed differentiation of patient-specific induced pluripotent stem cells identifies the transcriptional repression and epigenetic modification of NKX2-5, HAND1, and NOTCH1 in hypoplastic left heart syndrome. PLoS ONE 2014, 9, e102796. [Google Scholar] [CrossRef]
- Dai, Y.S.; Cserjesi, P.; Markham, B.E.; Molkentin, J.D. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J. Biol. Chem. 2002, 277, 24390–24398. [Google Scholar] [CrossRef] [PubMed]
- Risebro, C.A.; Smart, N.; Dupays, L.; Breckenridge, R.; Mohun, T.J.; Riley, P.R. Hand1 regulates cardiomyocyte proliferation versus differentiation in the developing heart. Development 2006, 133, 4595–4606. [Google Scholar] [CrossRef]
- Firulli, B.A.; George, R.M.; Harkin, J.; Toolan, K.P.; Gao, H.; Liu, Y.; Zhang, W.; Field, L.J.; Liu, Y.; Shou, W.; et al. HAND1 loss-of-function within the embryonic myocardium reveals survivable congenital cardiac defects and adult heart failure. Cardiovasc. Res. 2020, 116, 605–618. [Google Scholar] [CrossRef]
- Vincentz, J.W.; Toolan, K.P.; Zhang, W.; Firulli, A.B. Hand factor ablation causes defective left ventricular chamber development and compromised adult cardiac function. PLoS Genet. 2017, 13, e1006922. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Basson, C.T.; Benson, D.W., Jr.; Gelb, B.D.; Giglia, T.M.; Goldmuntz, E.; McGee, G.; Sable, C.A.; Srivastava, D.; Webb, C.L.; et al. Genetic basis for congenital heart defects: Current knowledge: A scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: Endorsed by the American Academy of Pediatrics. Circulation 2007, 115, 3015–3038. [Google Scholar] [CrossRef]
- Liu, X.; Yagi, H.; Saeed, S.; Bais, A.S.; Gabriel, G.C.; Chen, Z.; Peterson, K.A.; Li, Y.; Schwartz, M.C.; Reynolds, W.T.; et al. The complex genetics of hypoplastic left heart syndrome. Nat. Genet. 2017, 49, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.C.; Yagi, H.; Xu, X.; Lo, C.W. Novel Insights into the Etiology, Genetics, and Embryology of Hypoplastic Left Heart Syndrome. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Liu, X.; Gabriel, G.C.; Wu, Y.; Peterson, K.; Murray, S.A.; Aronow, B.J.; Martin, L.J.; Benson, D.W.; Lo, C.W. The Genetic Landscape of Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2018, 39, 1069–1081. [Google Scholar] [CrossRef]
- Birla, A.K.; Brimmer, S.; Short, W.D.; Olutoye, O.O., 2nd; Shar, J.A.; Lalwani, S.; Sucosky, P.; Parthiban, A.; Keswani, S.G.; Caldarone, C.A.; et al. Current state of the art in hypoplastic left heart syndrome. Front. Cardiovasc. Med. 2022, 9, 878266. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Tarui, S.; Ousaka, D.; Eitoku, T.; Kondo, M.; Okuyama, M.; Kobayashi, J.; Baba, K.; Arai, S.; et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: The TICAP prospective phase 1 controlled trial. Circ. Res. 2015, 116, 653–664. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef]
- Burkhart, H.M.; Qureshi, M.Y.; Rossano, J.W.; Cantero Peral, S.; O’Leary, P.W.; Hathcock, M.; Kremers, W.; Nelson, T.J.; Wanek, H.C.C.P. Autologous stem cell therapy for hypoplastic left heart syndrome: Safety and feasibility of intraoperative intramyocardial injections. J. Thorac. Cardiovasc. Surg. 2019, 158, 1614–1623. [Google Scholar] [CrossRef]
- Brizard, C.P.; Elwood, N.J.; Kowalski, R.; Horton, S.B.; Jones, B.O.; Hutchinson, D.; Zannino, D.; Sheridan, B.J.; Butt, W.; Cheung, M.M.H.; et al. Safety and feasibility of adjunct autologous cord blood stem cell therapy during the Norwood heart operation. J. Thorac. Cardiovasc. Surg. 2023, 166, 1746–1755. [Google Scholar] [CrossRef]
- Gallego-Navarro, C.; Jaggers, J.; Burkhart, H.M.; Carlo, W.F.; Morales, D.L.; Qureshi, M.Y.; Rossano, J.W.; Hagen, C.E.; Seisler, D.K.; Peral, S.C.; et al. Autologous umbilical cord blood mononuclear cell therapy for hypoplastic left heart syndrome: A nonrandomized control trial of the efficacy and safety of intramyocardial injections. Stem Cell Res. Ther. 2025, 16, 215. [Google Scholar] [CrossRef]
- Ren, H.; Sang, Y.; Zhang, F.; Liu, Z.; Qi, N.; Chen, Y. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016, 2016, 3516574. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.; Elwood, N.J.; Li, S.; Cullinane, F.; Edwards, G.A.; Newgreen, D.F.; Brizard, C.P. Human cord blood stem cells enhance neonatal right ventricular function in an ovine model of right ventricular training. Ann. Thorac. Surg. 2010, 89, 585–593.e4. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Rajamarthandan, S.; Francis, B.; O’Leary-Kelly, M.K.; Sinha, P. Update on stem cell technologies in congenital heart disease. J. Card. Surg. 2020, 35, 174–179. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Ascheim, D.D.; Gelijns, A.C.; Goldstein, D.; Moye, L.A.; Smedira, N.; Lee, S.; Klodell, C.T.; Szady, A.; Parides, M.K.; Jeffries, N.O.; et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014, 129, 2287–2296. [Google Scholar] [CrossRef]
- Oswald, J.; Boxberger, S.; Jorgensen, B.; Feldmann, S.; Ehninger, G.; Bornhauser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Ma, S.; Gao, Y.; Xu, T.; Chang, P.; Dong, L. Mesenchymal Stem Cell-Derived Exosomes: Biological Function and Their Therapeutic Potential in Radiation Damage. Cells 2020, 10, 42. [Google Scholar] [CrossRef]
- Wehman, B.; Sharma, S.; Pietris, N.; Mishra, R.; Siddiqui, O.T.; Bigham, G.; Li, T.; Aiello, E.; Murthi, S.; Pittenger, M.; et al. Mesenchymal stem cells preserve neonatal right ventricular function in a porcine model of pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1816–H1826. [Google Scholar] [CrossRef]
- Liufu, R.; Shi, G.; He, X.; Lv, J.; Liu, W.; Zhu, F.; Wen, C.; Zhu, Z.; Chen, H. The therapeutic impact of human neonatal BMSC in a right ventricular pressure overload model in mice. Stem Cell Res. Ther. 2020, 11, 96. [Google Scholar] [CrossRef]
- Wittenberg, R.E.; Gauvreau, K.; Leighton, J.; Moleon-Shea, M.; Borow, K.M.; Marx, G.R.; Emani, S.M. Prospective randomized controlled trial of the safety and feasibility of a novel mesenchymal precursor cell therapy in hypoplastic left heart syndrome. JTCVS Open 2023, 16, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef]
- Gratwohl, A.; Hermans, J.; Goldman, J.M.; Arcese, W.; Carreras, E.; Devergie, A.; Frassoni, F.; Gahrton, G.; Kolb, H.J.; Niederwieser, D.; et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet 1998, 352, 1087–1092. [Google Scholar] [CrossRef]
- Burnett, A.K.; Goldstone, A.H.; Stevens, R.M.; Hann, I.M.; Rees, J.K.; Gray, R.G.; Wheatley, K. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: Results of MRC AML 10 trial. UK Medical Research Council Adult and Children’s Leukaemia Working Parties. Lancet 1998, 351, 700–708. [Google Scholar] [CrossRef]
- Qureshi, M.Y.; Cabalka, A.K.; Khan, S.P.; Hagler, D.J.; Haile, D.T.; Cannon, B.C.; Olson, T.M.; Cantero-Peral, S.; Dietz, A.B.; Radel, D.J.; et al. Cell-Based Therapy for Myocardial Dysfunction After Fontan Operation in Hypoplastic Left Heart Syndrome. Mayo Clin. Proc. Innov. Qual. Outcomes 2017, 1, 185–191. [Google Scholar] [CrossRef][Green Version]
- van Berlo, J.H.; Kanisicak, O.; Maillet, M.; Vagnozzi, R.J.; Karch, J.; Lin, S.C.; Middleton, R.C.; Marban, E.; Molkentin, J.D. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014, 509, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Zhang, L.; Yan, J.; Chen, J.; Cai, W.; Razzaque, S.; Jeong, D.; Sheng, W.; Bu, L.; Xu, M.; et al. Resident c-kit+ cells in the heart are not cardiac stem cells. Nat. Commun. 2015, 6, 8701. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef]
- Hasan, A.S.; Luo, L.; Yan, C.; Zhang, T.X.; Urata, Y.; Goto, S.; Mangoura, S.A.; Abdel-Raheem, M.H.; Zhang, S.; Li, T.S. Cardiosphere-Derived Cells Facilitate Heart Repair by Modulating M1/M2 Macrophage Polarization and Neutrophil Recruitment. PLoS ONE 2016, 11, e0165255. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.A.; Stuckey, D.J.; Tan, J.J.; Tan, S.C.; Gomes, R.S.; Camelliti, P.; Messina, E.; Giacomello, A.; Ellison, G.M.; Clarke, K. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks—An MRI study. PLoS ONE 2011, 6, e25669. [Google Scholar] [CrossRef]
- Lee, S.T.; White, A.J.; Matsushita, S.; Malliaras, K.; Steenbergen, C.; Zhang, Y.; Li, T.S.; Terrovitis, J.; Yee, K.; Simsir, S.; et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 455–465. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef]
- Bittle, G.J.; Morales, D.; Pietris, N.; Parchment, N.; Parsell, D.; Peck, K.; Deatrick, K.B.; Rodriguez-Borlado, L.; Smith, R.R.; Marban, L.; et al. Exosomes isolated from human cardiosphere-derived cells attenuate pressure overload-induced right ventricular dysfunction. J. Thorac. Cardiovasc. Surg. 2021, 162, 975–986.e6. [Google Scholar] [CrossRef]
- Sano, T.; Ousaka, D.; Goto, T.; Ishigami, S.; Hirai, K.; Kasahara, S.; Ohtsuki, S.; Sano, S.; Oh, H. Impact of Cardiac Progenitor Cells on Heart Failure and Survival in Single Ventricle Congenital Heart Disease. Circ. Res. 2018, 122, 994–1005. [Google Scholar] [CrossRef]
- Tarui, S.; Ishigami, S.; Ousaka, D.; Kasahara, S.; Ohtsuki, S.; Sano, S.; Oh, H. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: Three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients with Single-Ventricle Physiology (TICAP) trial. J. Thorac. Cardiovasc. Surg. 2015, 150, 1198–1208.E2. [Google Scholar] [CrossRef]
- Hirai, K.; Sawada, R.; Hayashi, T.; Araki, T.; Nakagawa, N.; Kondo, M.; Yasuda, K.; Hirata, T.; Sato, T.; Nakatsuka, Y.; et al. Eight-Year Outcomes of Cardiosphere-Derived Cells in Single Ventricle Congenital Heart Disease. J. Am. Heart Assoc. 2024, 13, e038137. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yu, Y.; Zhao, Z.A.; Zhao, D.; Ni, X.; Wang, Y.; Fang, X.; Yu, M.; Wang, Y.; Tang, J.M.; et al. Patient-specific iPSC-derived cardiomyocytes reveal abnormal regulation of FGF16 in a familial atrial septal defect. Cardiovasc. Res. 2022, 118, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Thareja, S.K.; Anfinson, M.; Cavanaugh, M.; Kim, M.S.; Lamberton, P.; Radandt, J.; Brown, R.; Liang, H.L.; Stamm, K.; Afzal, M.Z.; et al. Altered contractility, Ca2+ transients, and cell morphology seen in a patient-specific iPSC-CM model of Ebstein’s anomaly with left ventricular noncompaction. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H149–H162. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Buganim, Y.; Faddah, D.A.; Cheng, A.W.; Itskovich, E.; Markoulaki, S.; Ganz, K.; Klemm, S.L.; van Oudenaarden, A.; Jaenisch, R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 2012, 150, 1209–1222. [Google Scholar] [CrossRef]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Yang, X.; Pabon, L.; Murry, C.E. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.E22. [Google Scholar] [CrossRef]
- Jiang, Y.; Habibollah, S.; Tilgner, K.; Collin, J.; Barta, T.; Al-Aama, J.Y.; Tesarov, L.; Hussain, R.; Trafford, A.W.; Kirkwood, G.; et al. An induced pluripotent stem cell model of hypoplastic left heart syndrome (HLHS) reveals multiple expression and functional differences in HLHS-derived cardiac myocytes. Stem Cells Transl. Med. 2014, 3, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, C.; Xu, Z.; Lin, H.; Wan, X.; Yu, Y.; Adhicary, S.; Zhang, J.Z.; Zhou, Y.; Liu, C.; et al. Impaired Human Cardiac Cell Development due to NOTCH1 Deficiency. Circ. Res. 2023, 132, 187–204. [Google Scholar] [CrossRef]
- Lewis, J.L.T.; Schlegel, B.; Woods, D.; Rao, K.; Sentis, A.; Tan, J.; Jagannathan, R.; Javed, Z.; Boudreau, A.N.; Nelson, T.; et al. Hypomorphic NOTCH1 Expression Alters Cardiomyocyte Cellular Architecture in Hypoplastic Left Heart Syndrome. BioRxiv 2024. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Yu, M.; Lee, D.; Alharti, S.; Hellen, N.; Ahmad Shaik, N.; Banaganapalli, B.; Sheikh Ali Mohamoud, H.; Elango, R.; et al. Induced pluripotent stem cell modelling of HLHS underlines the contribution of dysfunctional NOTCH signalling to impaired cardiogenesis. Hum. Mol. Genet. 2017, 26, 3031–3045. [Google Scholar] [CrossRef]
- Krane, M.; Dressen, M.; Santamaria, G.; My, I.; Schneider, C.M.; Dorn, T.; Laue, S.; Mastantuono, E.; Berutti, R.; Rawat, H.; et al. Sequential Defects in Cardiac Lineage Commitment and Maturation Cause Hypoplastic Left Heart Syndrome. Circulation 2021, 144, 1409–1428. [Google Scholar] [CrossRef] [PubMed]
- Hrstka, S.C.; Li, X.; Nelson, T.J.; Wanek Program Genetics Pipeline, G. NOTCH1-Dependent Nitric Oxide Signaling Deficiency in Hypoplastic Left Heart Syndrome Revealed Through Patient-Specific Phenotypes Detected in Bioengineered Cardiogenesis. Stem Cells 2017, 35, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.L.; Hrstka, S.C.; Evans, J.M.; O’Byrne, M.M.; de Andrade, M.; O’Leary, P.W.; Nelson, T.J.; Olson, T.M. Compound heterozygous NOTCH1 mutations underlie impaired cardiogenesis in a patient with hypoplastic left heart syndrome. Hum. Genet. 2015, 134, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Paige, S.L.; Galdos, F.X.; Lee, S.; Chin, E.T.; Ranjbarvaziri, S.; Feyen, D.A.M.; Darsha, A.K.; Xu, S.; Ryan, J.A.; Beck, A.L.; et al. Patient-Specific Induced Pluripotent Stem Cells Implicate Intrinsic Impaired Contractility in Hypoplastic Left Heart Syndrome. Circulation 2020, 142, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Contreras, J.; Ye, S.; Lin, H.; Hernandez-Rosario, L.; McBride, K.L.; Texter, K.; Garg, V.; Zhao, M.T. Characterization of an iPSC line NCHi006-A from a patient with hypoplastic left heart syndrome (HLHS). Stem Cell Res. 2022, 64, 102892. [Google Scholar] [CrossRef]
- Adhicary, S.; Ye, S.; Lin, H.; Texter, K.; Garg, V.; Zhao, M.T. Establishment of NCHi009-A, an iPSC line from a patient with hypoplastic left heart syndrome (HLHS) carrying a heterozygous NOTCH1 mutation. Stem Cell Res. 2023, 66, 103013. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Ye, S.; Qin, H.; Alonzo, M.; Onorato, A.; Argall, A.; Texter, K.; Ma, Q.; Garg, V.; et al. Common and divergent cellular aetiologies underlying hypoplastic left heart syndrome and hypoplastic right heart syndrome. Eur. Heart J. 2025, 46, 1946–1949. [Google Scholar] [CrossRef]
- Kussauer, S.; David, R.; Lemcke, H. hiPSCs Derived Cardiac Cells for Drug and Toxicity Screening and Disease Modeling: What Micro- Electrode-Array Analyses Can Tell Us. Cells 2019, 8, 1331. [Google Scholar] [CrossRef]
- Sinnecker, D.; Laugwitz, K.L.; Moretti, A. Induced pluripotent stem cell-derived cardiomyocytes for drug development and toxicity testing. Pharmacol. Ther. 2014, 143, 246–252. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Fang, C.; Zhong, C.; Li, J.; Xiao, Q. Recent advances in pluripotent stem cell-derived cardiac organoids and heart-on-chip applications for studying anti-cancer drug-induced cardiotoxicity. Cell Biol. Toxicol. 2023, 39, 2527–2549. [Google Scholar] [CrossRef]
- Colatsky, T.; Fermini, B.; Gintant, G.; Pierson, J.B.; Sager, P.; Sekino, Y.; Strauss, D.G.; Stockbridge, N. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative—Update on progress. J. Pharmacol. Toxicol. Methods 2016, 81, 15–20. [Google Scholar] [CrossRef]
- Pan, D.; Li, B.; Wang, S. Establishment and validation of a torsade de pointes prediction model based on human iPSC-derived cardiomyocytes. Exp. Ther. Med. 2023, 25, 61. [Google Scholar] [CrossRef]
- Blinova, K.; Dang, Q.; Millard, D.; Smith, G.; Pierson, J.; Guo, L.; Brock, M.; Lu, H.R.; Kraushaar, U.; Zeng, H.; et al. International Multisite Study of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Proarrhythmic Potential Assessment. Cell Rep. 2018, 24, 3582–3592. [Google Scholar] [CrossRef]
- Bedut, S.; Seminatore-Nole, C.; Lamamy, V.; Caignard, S.; Boutin, J.A.; Nosjean, O.; Stephan, J.P.; Coge, F. High-throughput drug profiling with voltage- and calcium-sensitive fluorescent probes in human iPSC-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H44–H53. [Google Scholar] [CrossRef]
- Huang, C.Y.; Nicholson, M.W.; Wang, J.Y.; Ting, C.Y.; Tsai, M.H.; Cheng, Y.C.; Liu, C.L.; Chan, D.Z.H.; Lee, Y.C.; Hsu, C.C.; et al. Population-based high-throughput toxicity screen of human iPSC-derived cardiomyocytes and neurons. Cell Rep. 2022, 39, 110643. [Google Scholar] [CrossRef]
- Miao, Y.; Tian, L.; Martin, M.; Paige, S.L.; Galdos, F.X.; Li, J.; Klein, A.; Zhang, H.; Ma, N.; Wei, Y.; et al. Intrinsic Endocardial Defects Contribute to Hypoplastic Left Heart Syndrome. Cell Stem Cell 2020, 27, 574–589.e578. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; DeYoung, T.; Cahill, L.S.; Yee, Y.; Debebe, S.K.; Botelho, O.; Seed, M.; Chaturvedi, R.R.; Sled, J.G. A mouse model of hypoplastic left heart syndrome demonstrating left heart hypoplasia and retrograde aortic arch flow. Dis. Model. Mech. 2021, 14, dmm049077. [Google Scholar] [CrossRef]
- Li, W.; Luo, X.; Strano, A.; Arun, S.; Gamm, O.; Poetsch, M.S.; Hasse, M.; Steiner, R.P.; Fischer, K.; Poche, J.; et al. Comprehensive promotion of iPSC-CM maturation by integrating metabolic medium with nanopatterning and electrostimulation. Nat. Commun. 2025, 16, 2785. [Google Scholar] [CrossRef] [PubMed]
- Goversen, B.; van der Heyden, M.A.G.; van Veen, T.A.B.; de Boer, T.P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on I(K1). Pharmacol. Ther. 2018, 183, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Koivumaki, J.T.; Naumenko, N.; Tuomainen, T.; Takalo, J.; Oksanen, M.; Puttonen, K.A.; Lehtonen, S.; Kuusisto, J.; Laakso, M.; Koistinaho, J.; et al. Structural Immaturity of Human iPSC-Derived Cardiomyocytes: In Silico Investigation of Effects on Function and Disease Modeling. Front. Physiol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Vuckovic, S.; Dinani, R.; Nollet, E.E.; Kuster, D.W.D.; Buikema, J.W.; Houtkooper, R.H.; Nabben, M.; van der Velden, J.; Goversen, B. Characterization of cardiac metabolism in iPSC-derived cardiomyocytes: Lessons from maturation and disease modeling. Stem Cell Res. Ther. 2022, 13, 332. [Google Scholar] [CrossRef]
- Tu, C.; Chao, B.S.; Wu, J.C. Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2018, 123, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- Parikh, S.S.; Blackwell, D.J.; Gomez-Hurtado, N.; Frisk, M.; Wang, L.; Kim, K.; Dahl, C.P.; Fiane, A.; Tonnessen, T.; Kryshtal, D.O.; et al. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2017, 121, 1323–1330. [Google Scholar] [CrossRef]
- Yang, X.; Rodriguez, M.L.; Leonard, A.; Sun, L.; Fischer, K.A.; Wang, Y.; Ritterhoff, J.; Zhao, L.; Kolwicz, S.C., Jr.; Pabon, L.; et al. Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 13, 657–668. [Google Scholar] [CrossRef]
- Carey, A.S.; Liang, L.; Edwards, J.; Brandt, T.; Mei, H.; Sharp, A.J.; Hsu, D.T.; Newburger, J.W.; Ohye, R.G.; Chung, W.K.; et al. Effect of copy number variants on outcomes for infants with single ventricle heart defects. Circ. Cardiovasc. Genet. 2013, 6, 444–451. [Google Scholar] [CrossRef]
- Mills, J.L.; Troendle, J.; Conley, M.R.; Carter, T.; Druschel, C.M. Maternal obesity and congenital heart defects: A population-based study. Am. J. Clin. Nutr. 2010, 91, 1543–1549. [Google Scholar] [CrossRef]
- Nicoll, R. Environmental Contaminants and Congenital Heart Defects: A Re-Evaluation of the Evidence. Int. J. Environ. Res. Public Health 2018, 15, 2096. [Google Scholar] [CrossRef]
- Roland, T.J.; Song, K. Advances in the Generation of Constructed Cardiac Tissue Derived from Induced Pluripotent Stem Cells for Disease Modeling and Therapeutic Discovery. Cells 2024, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Li, G.; Gokulnath, P.; Xiao, J. Generating 3D human cardiac constructs from pluripotent stem cells. EBioMedicine 2022, 76, 103813. [Google Scholar] [CrossRef]
- Min, S.; Cho, S.W. Engineered human cardiac tissues for modeling heart diseases. BMB Rep. 2023, 56, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Crawford, F.A., Jr.; Sade, R.M.; Spinale, F. Bovine pericardium for correction of congenital heart defects. Ann. Thorac. Surg. 1986, 41, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Jebran, A.F.; Seidler, T.; Tiburcy, M.; Daskalaki, M.; Kutschka, I.; Fujita, B.; Ensminger, S.; Bremmer, F.; Moussavi, A.; Yang, H.; et al. Engineered heart muscle allografts for heart repair in primates and humans. Nature 2025, 639, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.; Fukunishi, T.; Bai, Y.; Bedja, D.; Pitaktong, I.; Mattson, G.; Jeyaram, A.; Lui, C.; Ong, C.S.; Inoue, T.; et al. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef]
- Miyagawa, S.; Kawamura, T.; Ito, E.; Takeda, M.; Iseoka, H.; Yokoyama, J.; Harada, A.; Mochizuki-Oda, N.; Imanishi-Ochi, Y.; Li, J.; et al. Pre-clinical evaluation of the efficacy and safety of human induced pluripotent stem cell-derived cardiomyocyte patch. Stem Cell Res. Ther. 2024, 15, 73. [Google Scholar] [CrossRef]
- Mahle, W.T.; Hu, C.; Trachtenberg, F.; Menteer, J.; Kindel, S.J.; Dipchand, A.I.; Richmond, M.E.; Daly, K.P.; Henderson, H.T.; Lin, K.Y.; et al. Heart failure after the Norwood procedure: An analysis of the Single Ventricle Reconstruction Trial. J. Heart Lung Transplant. 2018, 37, 879–885. [Google Scholar] [CrossRef]
- Querdel, E.; Reinsch, M.; Castro, L.; Kose, D.; Bahr, A.; Reich, S.; Geertz, B.; Ulmer, B.; Schulze, M.; Lemoine, M.D.; et al. Human Engineered Heart Tissue Patches Remuscularize the Injured Heart in a Dose-Dependent Manner. Circulation 2021, 143, 1991–2006. [Google Scholar] [CrossRef]
- Miyagawa, S.; Kainuma, S.; Kawamura, T.; Suzuki, K.; Ito, Y.; Iseoka, H.; Ito, E.; Takeda, M.; Sasai, M.; Mochizuki-Oda, N.; et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 950829. [Google Scholar] [CrossRef]
- Liu, C.; Niu, K.; Xiao, Q. Updated perspectives on vascular cell specification and pluripotent stem cell-derived vascular organoids for studying vasculopathies. Cardiovasc. Res. 2022, 118, 97–114. [Google Scholar] [CrossRef]
- Cheruku, G.R.; Wilson, C.V.; Raviendran, S.; Xiao, Q. Recent Advances and Future Perspectives in Vascular Organoids and Vessel-on-Chip. Organoids 2024, 3, 203–246. [Google Scholar] [CrossRef]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Drakhlis, L.; Biswanath, S.; Farr, C.M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef]
- Yang, J.; Lei, W.; Xiao, Y.; Tan, S.; Yang, J.; Lin, Y.; Yang, Z.; Zhao, D.; Zhang, C.; Shen, Z.; et al. Generation of human vascularized and chambered cardiac organoids for cardiac disease modelling and drug evaluation. Cell Prolif. 2024, 57, e13631. [Google Scholar] [CrossRef]
- Song, M.; Choi, D.B.; Im, J.S.; Song, Y.N.; Kim, J.H.; Lee, H.; An, J.; Kim, A.; Choi, H.; Kim, J.C.; et al. Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids. Cell Death Dis. 2024, 15, 308. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ming, C.L.C.; Gentile, C. In vitro modeling of myocardial ischemia/reperfusion injury with murine or human 3D cardiac spheroids. STAR Protoc. 2022, 3, 101751. [Google Scholar] [CrossRef]
- Pocock, M.W.; Reid, J.D.; Robinson, H.R.; Charitakis, N.; Krycer, J.R.; Foster, S.R.; Fitzsimmons, R.L.; Lor, M.; Devilee, L.A.C.; Batho, C.A.P.; et al. Maturation of human cardiac organoids enables complex disease modeling and drug discovery. Nat. Cardiovasc. Res. 2025, 4, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, G.; Ma, T.; Simmons, C.A.; Santerre, J.P. A critical review on advances and challenges of bioprinted cardiac patches. Acta Biomater. 2024, 189, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.Y.; Xiang, Y.; Tang, M.; Chen, S. 3D Printing Approaches to Engineer Cardiac Tissue. Curr. Cardiol. Rep. 2023, 25, 505–514. [Google Scholar] [CrossRef]
- Faber, L.; Yau, A.; Chen, Y. Translational biomaterials of four-dimensional bioprinting for tissue regeneration. Biofabrication 2023, 16, 012001. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Zengjie, F.; Suthiwanich, K.; Lorestani, F.; Orive, G.; Ostrovidov, S.; Khademhosseini, A. Advances and Future Perspectives in 4D Bioprinting. Biotechnol. J. 2018, 13, e1800148. [Google Scholar] [CrossRef] [PubMed]
| Signalling Pathway | Normal Function | Disease Secondary to Defect | Association with HLHS |
|---|---|---|---|
| NOTCH1 | Valve formation. Ventricular septation. Left–right patterning. Regulation of progenitor differentiation [36]. | Bicuspid aortic valve. Right ventricular hypoplasia. VSD [37]. | Strong: Rare genetic variants (G661S, R1279H, A683T) and de novo mutations associated with HLHS found in patients and relatives [38,39]. Targeted and whole exome sequencing analysis revealed an association between a novel germline frameshift/stop-gain mutation in NOTCH1 and HLHS [40]. |
| Bone Morphogenetic Protein (BMP) | Mesoderm induction and regulation [41]. FHF formation. Proliferation of CMs. Development of cardiac cushion [42]. | Pulmonary arterial hypertension. AV canal defects [43]. | Nill or weak: Although genetic animal study showed that Bmp2/4 and Bmp4/7 play a potential role in ventricular septal defects [44] and OFT septation [45], respectively, human study failed to confirm a causal relationship between BMP2/4 gene mutations with ASD, VSD, and complex CHD [46]. |
| Retinoic Acid (RA) | Anterior–posterior patterning. FHF and SHF development. Mesoderm formation and induction [47]. | DiGeorge syndrome [48]. AV cushion defects. Truncus arteriosus [49]. heart morphogenesis (posterior chamber developmental impairment and OFT septation defects) [50]. | No causal genetic association between RA signalling and HLHS was reported. |
| Wnt/Beta-catenin | Mesoderm induction. CM differentiation. SHF expansion and patterning [51]. | VSD. Truncus arteriosus. ASD. Familial exudative vitreoretinopathy. Arrhythmogenic cardiomyopathy [52,53]. | HLHS family-based WGS, variant filtering, and transcriptional profiling identified 10 candidate genes including LRP2 (p.N3205D and p.A57V), and data from multi-disciplinary platforms confirmed that LRP2 is required for cardiomyocyte proliferation and differentiation [54]. |
| Sonic Hedgehog Pathway (SHH) | Heart tube development. SHF proliferation, essential for SHF and OFT development which contribute to normal development of LV [55]. | Compromised DMP formation and AVSD [53]. OFT defect [56]. | No causal genetic association between RA signalling and HLHS was reported. |
| Hippo Pathway | Organ size. Regulates myocardial thickness [57]. | Abnormal heart size [58]. | HLHS iPSC-CMs showed defects in YAP-regulated antioxidant response which is associated with heart failure outcome [59]. |
| Fibroblast Growth Factor (FGF) Family | Mesoderm induction. Outflow tracts development. SHF proliferation [60]. | OFT defects. Overriding aorta [61]. | Although dysregulated FGF signal pathways were observed in HLHS foetal lamb model [62], no causal genetic association between FGF signalling and HLHS was reported. |
| Transcription Factors | |||
| HAND1 | Ventricle development. LV specification. Ventricular trabeculation [63,64]. | Defects in the left ventricle and endocardial cushions [65]. Defects in dorso-ventral patterning and interventricular septum formation [66]. | A126fs frameshift mutation is identified in HLHS patient cardiac tissue [67], but this mutation does not cause HLHS in mice [68]. |
| NKX2-5 | Differentiation of CMs and ventricle formation. Purkinje fibre network, AV node, and bundle branch development [69]. | Impaired looping morphogenesis [70] and atrial septal dysmorphogenesis [71]. | Moderate: Cohort studies show a genetic variant (T178M) in a subset of HLHS [72]. Three different NKX2-5 mutations were identified in patients with ASD and VSD [73]. |
| MYRF | Ventricular formation. Transcriptional regulation [74]. | Hypoplastic ventricle [74]. | Moderate: Associated with syndromic presentations of HLHS [75]. |
| GATA4 | Septation of chambers. CM differentiation. Valve formation [76]. | GATA4 mutation (G295S) leads to thin ventricular myocardium and CM proliferation [77], and GATA4 has significant synergism with TBX5 required for early cardiogenesis [78]. | Moderate: A heterozygous G296S missense mutation of GATA4 caused AVSDs and pulmonary valve stenosis in humans [79]. Additional novel point mutations in 3′-untranslated region of GATA4 gene were reported to be associated with sporadic non-syndromic AVSDs [80]. |
| TBX1 [28] | Transcriptional control in cardiac progenitors in SHF [81]. | Severe hypoplasia of SHF-dependent segments of the heart [81]. Aortic arch patterning defects and OFT defects in individuals with DiGeorge syndrome [82]. | Weak: A 9 bp deletion DAGG379-381 was found to segregate with VSD [83]. |
| TBX5 | Cardiac septation. Conduction system. Chamber specification. | Holt–Oram syndrome (HOS) [84,85]. Dual knockdown of Tbx5 and Mef2c causes severe defects in heart tube looping [86]. | Moderate: An intragenic duplication of TBX5 was reported in HOS patients presented with HLHS, AVSD, valve disease, and pulmonary stenosis [84]. |
| WT1 | Epicardial-to-mesenchymal transition [87]. | Wilms tumour. Denys–Drash syndrome. | No causal genetic association between FGF signalling and HLHS was reported. |
| MEF2C | MEF2+ cardiac progenitor cells contribute to the endocardium and myocardium of the right ventricle, as well as the aortic and mitral valves during early cardiogenesis [88]. | Loss of Mef2c function in the anterior second heart field results in a spectrum of outflow tract alignment defects [89]. | A novel heterozygous missense mutation (pL38P) in MEF2C was identified in patients with PDA and VSD [90]. |
| ISL1 | SHF formation and regulation. Coronary development. CM lineage commitment [91]. | OFT septation abnormalities, ASD, and VSD [91]. | Moderate: Variations can increase susceptibility to CHD including HLHS [92]. A novel heterozygous missense mutation (pE137X) in ISL1 was identified in patients with PDA and VSD [93]. |
| Structural/Regulatory Proteins | |||
| MYH6 | Encodes Alpha-myosin heavy chain. Predominantly controls atrial contractile function. | ASD. Late-onset hypertrophic cardiomyopathy. | Moderate: Multiple rare genetic variants (R443) found in HLHS cohorts [94,95]. MYH6 variant carriers exhibit impaired RA contractility [96]. HiPSC-CMs carrying an MYH6-R443P head domain variant display reduced contractility [97]. |
| LRP2 | Encodes endocytic receptors responsible for developmental signalling (SHH pathway) [98]. | Neural tube defects. Donnai–Barrow syndrome [99]. | Moderate: Multiple rare genetic variants found in HLHS genomic and transcriptomic studies [54]. |
| GJA1 (Connexin43) | Encodes gap junctions. Facilitates electrical and metabolic communications between CMs. | Arrhythmogenic cardiomyopathy. Cardiac conduction disorders. Oculodentodigital dysplasia [100]. | Emerging: One or more mutations were found in children with congenital heart malformation including HLHS [101]. Altered expression of GJA1 in HLHS heart tissue [102]. |
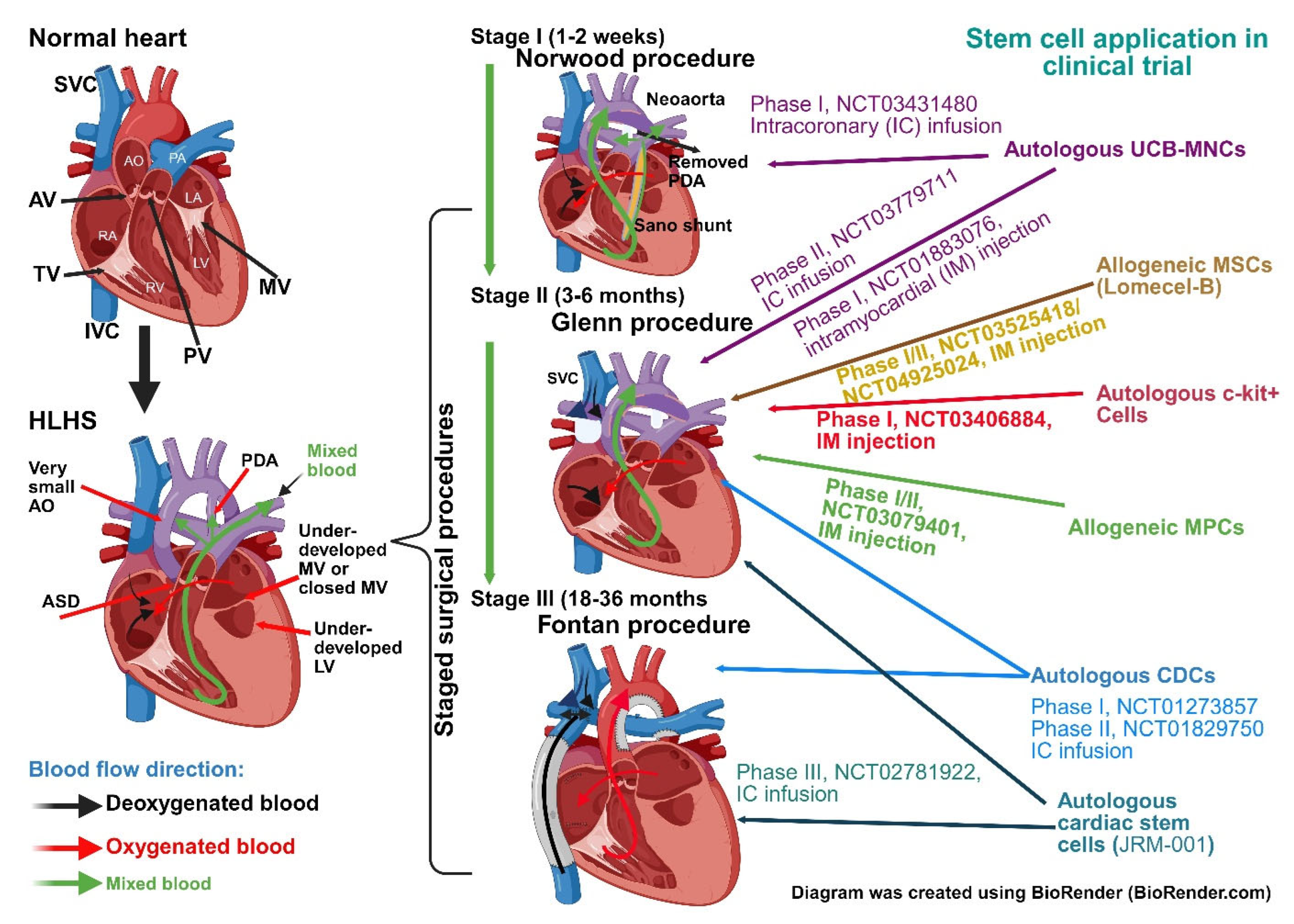
| Study Name (NCT Number) | Stem Cell Type | Study Timeline | Study Stage | Enrolment: Total (Control/Treatment) | Route and Timing of Administration | Key Findings | Limitations | Status/Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single Ventricle Physiology (TICAP) (NCT01273857) | Autologous CDC | 2011–2013 | Phase I | 14 (7/7) | IC at Stage II/III surgical palliation | ↑ RV function over 18 month period. ↑ HF status. Safety and feasibility of CDCs. Long-standing benefits on follow-up analysis. | Non-randomised; open-label; small sample size; variable timing of intervention. | Completed; Ishigami et al. [151]. |
| Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease (PERSEUS) (NCT01829750) | Autologous CDC | 2013–2016 | Phase II | 34 (17/17) | IC at Stage II/III surgical palliation | ↑ RV function; ↑ quality of life and somatic growth. Safety and feasibility of CDCs; long-standing benefits on follow-up analysis. | Limited long-term conclusion; open-label; single-ventricle; disease heterogeneity. | Completed; Ishigami et al. [152]. |
| Safety Study of Autologous Umbilical Cord Blood Cells for Treatment of Hypoplastic Left Heart Syndrome (NCT01883076) | Autologous UCB-MNCs | 2013–2021 | Phase I | Phase I: 10 (0/10) | IM during Glenn operation (Stage II) | Preserved RV. Safety and feasibility. | Single-centre study; no control group; short-term follow-up; heterogeneity of UCB-MNCs. | Completed; Burkhart et al. [153]. |
| Safety of Autologous Cord Blood Cells in HLHS Patients During Norwood Heart Surgery (NCT03431480) | Autologous UCB-MNCs | 2018–2022 | Phase I | 10 (0/10) | IC during Norwood procedure | Preserved RV. Safety and feasibility. | Open-label; no control group; heterogeneity of UCB-MNCs. | Completed; Brizard et al. [154]. |
| Intramyocardial Injection of Autologous Umbilical Cord Blood Derived Mononuclear Cells During Surgical Repair of Hypoplastic Left Heart Syndrome (NCT03779711) | Autologous UCB-MNCs | 2019–2026 | Phase IIb | 95 (45/50) | IM at Stage II surgical repair | An unfavourable change in longitudinal cardiac strain and a greater incidence (20%) of at least one severe adverse event in treatment group. Failed to enhance cardiac functions. | Multicentre, open-label, non-randomised study; negative results. | Completed; Gallego-Navarro et al. [155]. |
| Cardiac Stem/Progenitor Cell Infusion in Univentricular Physiology (APOLLON) (NCT02781922) | Autologous cardiac stem cells | 2016–2023 | Phase III | 40 (NR/NR) | IC after Stage II/III surgical palliation | NR. | NR | NR. |
| Lomecel-B Injection in Patients With Hypoplastic Left Heart Syndrome: A Phase I/II Study (ELPIS) (NCT03525418/NCT04925024) | Allogeneic MSCs (Lomecel-B) | 2018–2025 | Phase I Phase II | Phase I: 10 (0/10) Phase II: 20 (10/10) | IM during Glenn operation | Phase I: Safety and feasibility. No alloimmune sensitisation. Phase II: NR. | Phase I: Open-label; no control; small sample size; non-randomised. | Phase I: Completed; Kaushal et al. [20]. Phase II: Ongoing. |
| Autologous Cardiac Stem Cell Injection in Patients With Hypoplastic Left Heart Syndrome: An Open Label Pilot Study (CHILD Trial) (NCT03406884) | Autologous c-kit+ | 2019–2024 | Phase I/II | 10 (Phase I); 22 (Phase II) | IM during Glenn operation | NR | Open-label. | Completed; pending report. |
| Mesoblast Stem Cell Therapy for Patients With Single Ventricle and Borderline Left Ventricle (NCT03079401) | Allogeneic MPCs | 2017–2024 | Phase I Phase II | 19 (9/10) | IM during Glenn operation | NR | NR | Completed; pending report. |
| Cell Source for iPSC Reprogramming | Reprogramming System | Genetic Mutations | Methodology (Medium, Small Molecular) for CM Differentiation | Key Findings | Applications | Reference |
|---|---|---|---|---|---|---|
| H7/H9 ESCs; WTC iPSCs; 176/1 iPSC 176/5 iPSC 176/8 iPSC | WiCell Research Institute; WiCell Research Institute; IMBA Stem Cell Core Facility | HAND1 knockout or NKX2.5 knockout PSCs | Day 0–2: CDM plus 30 ng/mL FGF2, 5 µM LY294002, 50 ng/mL AA, 10 ng/mL BMP4, and 1–4 µM CHIR99021 (Wnt-activation). Day 3–6: CDM plus BMP4 (10 ng/mL), FGF2 (8 ng/mL), insulin (10 µg/mL), 5 µM IWP2 (Wnt inhibition), and 0.5 µM RA. Day 7–8: CDM plus BMP4 (10 ng/mL), FGF2 (8 ng/mL), and insulin (10 µg/mL). Day 9 onwards: CDM plus insulin (10 µg/mL) for CM maintenance. | Differentiated CMs can be used to generate hollow, beating 3D structures, which was applied to confirm stage-specific regulation of HAND1 and NKX2.5 during cardiac development in the context of HLHS. | Cardioids generation, molecular insights into cardiac cavity morphogenesis, disease modelling such as cryoinjury (mimicking myocardial infarct) and HLHS | Hofbauer et al. [196] |
| Cardiac progenitor cells | Retroviruses to deliver OKSM factors | NR (not reported) | Matrigel-based monolayer: Day 0–1: RPMI/B27 medium supplemented with 100 ng/mL AA. Day 2–5: RPMI/B27 medium plus 10 ng/mL BMP 4. | HLHS iPSC-CMs exhibit a lower cardiomyogenic differentiation potential; decreased NKX2.5, TBX2, NOTCH/HEY signalling, and HAND1/2; and reduced H3K4 dimethylation and histone H3 acetylation but increased H3K27 trimethylation. | Provide molecular insights into complex transcriptional and epigenetic mechanisms underlying HLHS | Kobayashi et al. [141] |
| Dermal fibroblasts | Polycistronic lentiviral system for OCT4, KLF4, SOX2, and MYC (OKSM) factors | NR | EB-based protocol: Day 0–7: StemPro-34 SFM supplemented with 1 mM ascorbic acid, 4 × 10−4 M MTG, 10 ng/mL BMP4, 12.5 ng/mL bFGF, 6 ng/mL AA, 150 ng/mL DKK1, 5 ng/mL VEGF, and 5.4 μM SB-431542. Day 8 onwards: EBs were plated on 0.1% gelatin-coated 12-well culture plates (20 EBs per well) and cultured in StemPro-34 SFM supplemented with 10 ng/mL VEGF and 5 ng/mL bFGF. | HLHS iPSC-CMs display a decreased number of beating clusters; myofibrillar disorganisation, persistence of a foetal gene expression pattern, and changes in commitment to ventricular versus atrial lineages; different calcium transient patterns and electrophysiological responses. | NR | Jiang et al. [197] |
| Peripheral blood mononuclear cells (PBMCs) | CytoTune-iPSC 2.0 Sendai Reprogramming Kit | NOTCH1 knockout | Matrigel-based monolayer (over 90% confluency): Day 0–2: RPMI/B27 medium supplemented with 6 μM CHIR99021. Day 3: RPMI/B27 medium only. Day 4–5: RPMI/B27 medium plus 5 μM IWR-1. Day 6–9: Differentiated cells were incubated with RPMI1640 without glucose plus B27 supplement for 4 days to remove non-CMs. Day 10 onwards: RPMI/B27 until use. | HLHS iPSC-CMs confirm that disruption of NOTCH1 blocks human ventricular-like CM differentiation but promotes atrial-like CM generation, defective CM proliferation; impaired cell cycle progression and mitosis; and biased differentiation toward epicardial and SHF progenitors at the expense of FHF progenitors. | Possibly modelling HLHS, gaining new insights into the comprehension of the mechanisms underlying HLHS aetiology | Ye et al. [198] |
| Fibroblast (ATCC) | Episomal plasmid (ND2.0, NIH CRM control iPSC line) | Isogenic hiPSC hypomorphic NOTCH1 clones | Matrigel-based monolayer (over 90% confluency): CM differentiation media A and B. | HLHS iPSC-CMs display abnormalities in pathways associated with mitochondrial function, actin cytoskeleton, and cardiomyocyte development; skewed differentiation away from CMs and towards fibroblasts and SMCs; impaired cardiac cytoskeletal and mitochondrial architecture; and decreased CM contractility and ATP production. | Possibly modelling HLHS; high-throughput drug screening to identify potential HLHS drug such as auranofin | Lewis et al. [199] |
| Neonatal fibroblasts | Polycistronic vector encoding KLF4, OCT4, SOX2, as well as vectors encoding hc-Myc and hKlf4 | Deleterious genetic variants of NOTCH1-4 | Embryoid body (EB) based protocol: StemPro-34 SFM (basal media). Day 0–3: 10 ng/mL BMP4, 6 ng/mL AA, 5 ng/mL bFGF, 10 µM Y276321. Day 3–5: 150 ng/mL DKK1, 10 ng/mL VEGF, 5.4 µM SB431542, 0.25 µM Droso, 10 µM Y276321. Day 5–7: 150 ng/mL DKK1, 10 ng/mL VEGF, 10 µM Y276321. Day 7 onwards: EBs were plated on Matrigel-coated 12-well culture plates (20 EBs per well) and cultured in basal media plus 10 ng/mL VEGF and 5 ng/mL bFGF. | HLHS iPSC-CMs exhibit a reduced ability to give rise to mesodermal, cardiac progenitors, and mature CMs and an enhanced ability to differentiate to SMCs; lower beating rate; disorganised sarcomeres and sarcoplasmic reticulum; and blunted response to isoprenaline. | Possibly modelling HLHS; provide novel signalling and genetic insights into HLHS pathogenesis | Yang et al. [200] |
| Skin fibroblasts or PBMCs | CytoTune-iPSTM-iPS 2.0 Sendai Reprogramming Kit | heterozygous de-novo mutations in multiple genes including MYRF, BAI2, FGFR1, AIM1L, SYBU, MACF1, etc. | Matrigel-based monolayer (over 90% confluency): Day 1: CDM3 (RPMI1640 supplemented with 500 μM/mL Oryza sativa-derived recombinant human albumin and 213 μg/mL L-ascorbic acid 2-phosphate) plus 4–6 μM CHIR99021. Day 2–3: CDM3 plus 2 μM Wnt-C59. Day 4 onwards: CDM3 only. | Unique aberrations in autophagy terms were present when directing HLHS iPSCs toward early cardiac progenitors (CPs), whereas apoptosis-associated pathways appeared solely affected in later CPs (day 6) and cardiomyocytes (day 8); dysregulated lineage-specific CM differentiation; disrupted both early CM subtype lineage specification and CM differentiation and maturation in HLHS. | Possibly modelling HLHS; provide novel signalling and genetic insights into HLHS pathogenesis; use for 3D cardiac patch generation | Krane et al. [201] |
| Dermal fibroblasts | Lentiviral transduction of OKSM factors | Heterozygous NOTCH1(P1256L/P1964L) | Monolayer (over 90% confluency): Day 1: RPMI/B27 plus 40–100 ng/mL AA. Day 2–5: RPMI/B27 plus 5–20 ng/mL bFGF and BMP4. Day 5 onwards: RPMI/B27 only. | HLHS iPSC-CMS exhibit deficiency in NOTCH signalling pathway and a diminished capacity to generate CMs, as well as impaired NO signalling. | HLHS modelling; identification of small therapeutic molecules to compensate dysregulated NO signalling | Hrstka et al. [202] Theis et al. [203] |
| Dermal fibroblasts | Sendai Reprogramming Kits | MYH6-R443P variant | Geltrex-based monolayer: Day 0: mTeSR1 medium plus 5 μM ROCK inhibitor. Day 1–2: insulin-free RPMI/B27 plus 10 μM CHIR99021 and 10 ng/mL AA. Day 3–6: insulin-free RPMI/B27 with 5 μM IWP. Day 7 onwards: RPMI/B27 with insulin. | HLHS iPSC-CMs carrying the MYH6-R443P variant express beta-myosin heavy chain expression (MYH7), with impaired contractility, relaxation, and CM differentiation, as well as sarcomere disorganisation. | HLHS modelling; study how genetic variants contribute to HLHS | Kim et al. [97]; Tomita-Mitchell et al. [94] |
| NR | NR | NR | Small-molecule modulation of the canonical Wnt/β-catenin signalling pathway. | HLHS iPSC-CMs display impaired contractility; upregulation in sarcomere and cytoskeletal genes and downregulation in genes involved in mitochondrial function and metabolism; and reduced mitochondrial content, mitochondrial respiration, and oxidative metabolism. | HLHS modelling and drug screening | Paige et al. [204] |
| Fibroblasts or lymphoblastoid cells | Episomal plasmids encoding OSKM | Pathogenic variants associated with mitochondrial metabolism | Matrigel-based monolayer (over 90% confluency): Day 1–2: basal CDM3 media (RPMI 1640, BSA, B27, 213 μg/mL ascorbic acid) and 6 μM CHIR99021. Day 3–14: basal CDM3 media and 10 μM XAV939. Day 15 onwards: basal CDM3 media minus ascorbic acid. | HLHS iPSC-CMs exhibit impaired CM differentiation and contractile dysfunction; cell-cycle disturbance with metaphase arrest; increased CM apoptosis; myofibrillar disarray; mitochondrial dysfunction and perturbation of mitochondrial dynamics; and defects in YAP-regulated antioxidant response. | Modelling of HLHS and early heart failure; drug screening; identification of potential therapeutics such as sildenafil and TUDCA | Xu et al. [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, R.; Darr, H.; Khan, Z.; Xiao, Q. Updated Applications of Stem Cells in Hypoplastic Left Heart Syndrome. Cells 2025, 14, 1396. https://doi.org/10.3390/cells14171396
Xiao R, Darr H, Khan Z, Xiao Q. Updated Applications of Stem Cells in Hypoplastic Left Heart Syndrome. Cells. 2025; 14(17):1396. https://doi.org/10.3390/cells14171396
Chicago/Turabian StyleXiao, Rui, Haleema Darr, Zarif Khan, and Qingzhong Xiao. 2025. "Updated Applications of Stem Cells in Hypoplastic Left Heart Syndrome" Cells 14, no. 17: 1396. https://doi.org/10.3390/cells14171396
APA StyleXiao, R., Darr, H., Khan, Z., & Xiao, Q. (2025). Updated Applications of Stem Cells in Hypoplastic Left Heart Syndrome. Cells, 14(17), 1396. https://doi.org/10.3390/cells14171396






