Physiological and Molecular Responses of the Flag Leaf Under L-Phenylalanine Ammonia-Lyase Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. PAL Inhibition
2.3. Measurements
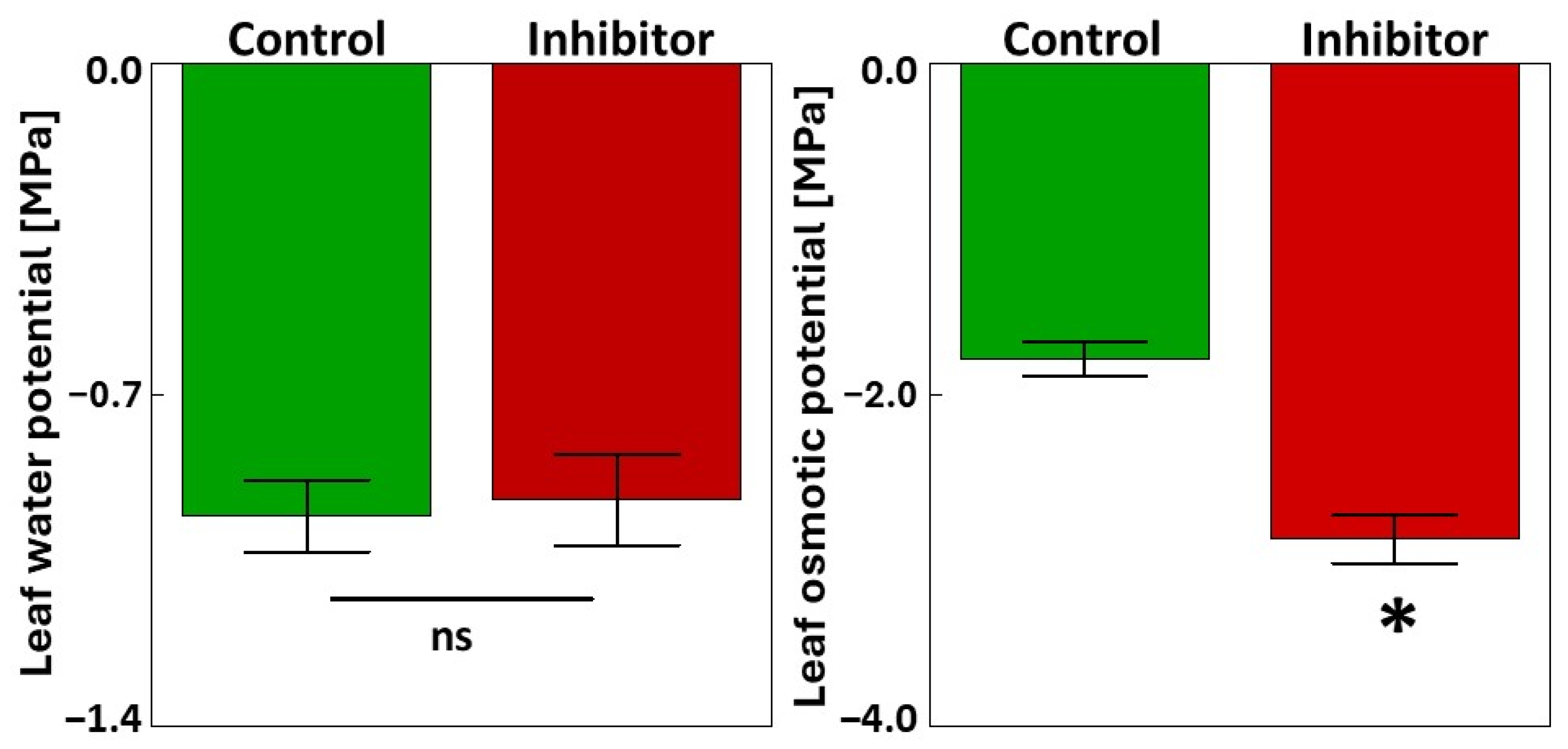
2.3.1. Leaf Water Potential (ΨW) and Leaf Osmotic Potential (ΨO)
2.3.2. Hydroxybenzoic Acid Hydrazide Levels
2.3.3. Soluble Phenolics (SPh)
2.3.4. Activity of L-Phenylalanine Ammonia-Lyase (PAL) and L-Tyrosine Ammonia-Lyase (TAL)
2.3.5. Photosynthetic Activity
2.3.6. Chlorophyll Fluorescence Measurements
2.3.7. Chlorophyll Content
2.3.8. Soluble Carbohydrate Content
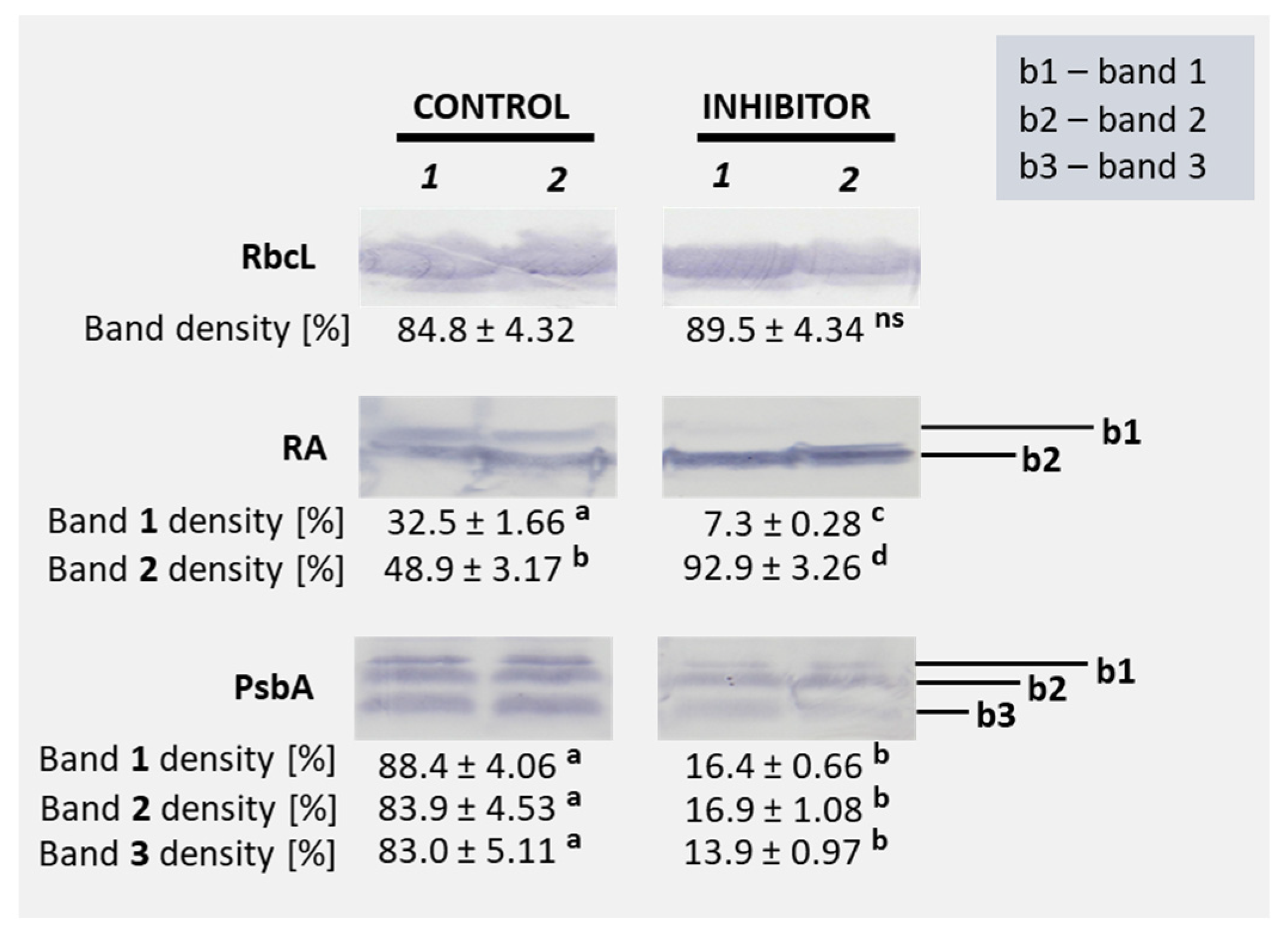
2.3.9. Western Blot Analysis
2.3.10. Analysis of Antioxidant Capacity
Superoxide Dismutase (SOD, EC 1.15.1.1)
Total Soluble Peroxidase (POX, EC 1.11.1.x)
Catalase (CAT, EC 1.11.1.6)
Glutathione Peroxidase (GPx, EC 1.11.1.9)
Glutathione Reductase (GR, EC 1.6.4.2)
L-Ascorbate Peroxidase (AsPOX, EC 1.11.1.11)
Soluble Protein Content
Total Extractable Antioxidants
Non-Enzymatic Antioxidant Capacities of Soluble Proteins
Total Antioxidant Capacity of Insoluble and Matrix-Bound Matter
Hydrogen Peroxide Content
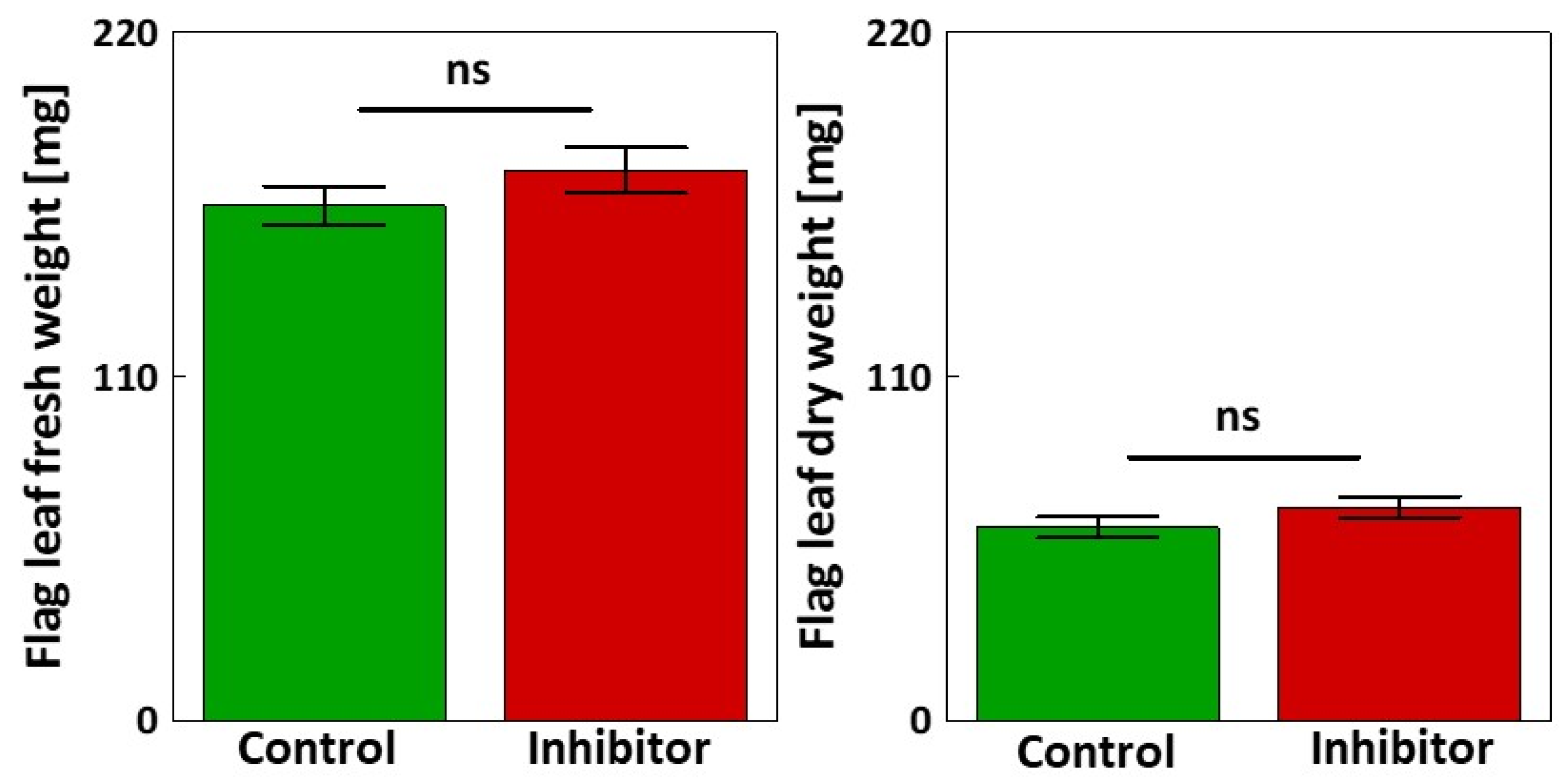
2.3.11. Determination of Fresh/Dry Weight and Yield Components
2.4. Statistical Analyses
3. Results
3.1. Soluble Phenolics, PAL and TAL Activities, Carbohydrates, and the Water Status of the Flag Leaves
3.2. Photosynthesis, Chlorophyll Fluorescence, Chlorophyll Content, and Accumulation of Selected Proteins
3.3. Non-Enzymatic and Enzymatic Antioxidants and Hydrogen Peroxide
3.4. Fresh and Dry Weight and Yield-Related Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Tilman, D.; Jin, Z.; Smith, P.; Barrett, C.B.; Zhu, Y.-G.; Burney, J.; D’Odorico, P.; Fantke, P.; Fargione, J.; et al. Climate change exacerbates the environmental impacts of agriculture. Science 2024, 385, eadn3747. [Google Scholar] [CrossRef]
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Urban, K.; Hura, T. The use of L-phenylalanine ammonia lyase inhibitors in plant ecophysiological studies. Postępy Biochem. 2023, 69, 11–17. [Google Scholar]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? Plant Physiol. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Wang, R.; Li, J.; Wang, X. Conservation tillage rotation enhanced soil structure and soil nutrients in long-term dryland agriculture. Eur. J. Agron. 2021, 131, 126379. [Google Scholar] [CrossRef]
- Anjum, M.N.; Cheema, M.J.M.; Hussain, F.; Wu, R.S. Precision Irrigation. In Precision Agriculture; Elsevier: Amsterdam, The Netherlands, 2023; pp. 85–101. [Google Scholar]
- Grover, D.; Mishra, A.K.; Rani, P.; Kalonia, N.; Chaudhary, A.; Sharma, S. Soil management in Sustainable agriculture: Principles and techniques. In Technological Approaches for Climate Smart Agriculture; Springer: Cham, Switzerland, 2024; pp. 41–77. [Google Scholar]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J. Exp. Bot. 2009, 60, 509–521. [Google Scholar] [CrossRef]
- Sharkey, T.D. The end game(s) of photosynthetic carbon metabolism. Plant Physiol. 2023, 195, 67–78. [Google Scholar] [CrossRef]
- Shende, V.V.; Bauman, K.D.; Moore, B.S. The shikimate pathway: Gateway to metabolic diversity. Nat. Prod. Rep. 2024, 41, 604–648. [Google Scholar] [CrossRef]
- Li, G.H.; Song, C.; Manzoor, M.A.; Li, D.Y.; Cao, Y.P.; Cai, Y.P. Functional and kinetics of two efficient phenylalanine ammonia lyase from Pyrus bretschneideri. BMC Plant Biol. 2023, 23, 612. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Ramegowda, V.; Ramakrishnan, P.; Nataraja, K.N.; Sheshshayee, M.S. Comparative metabolite profiling of rice contrasts reveal combined drought and heat stress signatures in flag leaf and spikelets. Plant Sci. 2022, 320, 111262. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, J.; Lu, Y.; Yang, S.; Zhang, Y.; Chai, H.; Jiang, D.; Dai, T.; Tian, Z. Rubisco and sucrose synthesis and translocation are involved in the regulation of photosynthesis in wheat with different source-sink relationships. Physiol. Plant. 2024, 176, e14196. [Google Scholar] [CrossRef]
- Tiryakioğlu, M. The relationship between flag leaf senescence and grain yield of some durum wheat varieties under drought stress during grain filling period. J. Agric. Sci. 2015, 21, 382–393. [Google Scholar]
- Xie, Z.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of exogenous ABA and cytokinin on leaf photosynthesis and grain protein accumulation in wheat ears cultured in vitro. Plant Growth Regul. 2004, 44, 25–32. [Google Scholar] [CrossRef]
- Mergoum, M.; Sapkota, S.; ElDoliefy AE, A.; Naraghi, S.M.; Pirseyedi, S.; Alamri, M.S.; Abu Hammad, W. Triticale (× Triticosecale wittmack) breeding. In Advances in Plant Breeding Strategies: Cereals; Springer: Cham, Switzerland, 2019; pp. 405–451. [Google Scholar]
- McGoverin, C.M.; Snyders, F.; Muller, N.; Botes, W.; Fox, G.; Manley, M. A review of triticale uses and the effect of growth environment on grain quality. J. Sci. Food Agric. 2011, 91, 1155–1165. [Google Scholar] [CrossRef]
- Bekele, S. Impacts of climate change on livestock production: A review. J. Nat. Sci. Res. 2017, 7, 53–59. [Google Scholar]
- Hura, T.; Dziurka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. Different allocation of carbohydrates and phenolics in dehydrated leaves of triticale. J. Plant Physiol. 2016, 202, 1–9. [Google Scholar] [CrossRef]
- Hoagland, D.R. Lectures on the Inorganic Nutrition of Plants; Chronica Botanica Co.: Waltham, MA, USA, 1948. [Google Scholar]
- Hura, T.; Hura, K.; Ostrowska, A.; Gadzinowska, J.; Fiust, A. Water stress-induced flag leaf senescence may be accelerated by rehydration. J. Plant Physiol. 2019, 236, 109–116. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–157. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Dziurka, K.; Ostrowska, A.; Bączek-Kwinta, R.; Grzesiak, M. An increase in the content of cell wall-bound phenolics correlates with the productivity of triticale under soil drought. J. Plant Physiol. 2012, 169, 1728–1736. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A.; Grzesiak, M.; Dziurka, K. The cell wall-bound phenolics as a biochemical indicator of soil drought resistance in winter triticale. Plant Soil Environ. 2013, 59, 189–195. [Google Scholar] [CrossRef]
- Peltonen, S.; Karjalainen, R. Phenylalanine ammonia-lyase activity in barley after infection with Bipolaris sorokiniana or treatment with its purified xylanase. J. Phytopathol. 1995, 143, 239–245. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Berry, J.A.; Downton, W. Environmental regulation of photosynthesis. In Photosynthesis, Development, Carbon Metabolism, and Plant Productivity; Academic Press: New York, NY, USA, 1982; Volume 2, pp. 263–343. [Google Scholar]
- Li, X.; Xu, K. Effects of exogenous hormones on leaf photosynthesis of panax ginseng. Photosynthetica 2014, 52, 152–156. [Google Scholar] [CrossRef]
- Gadzinowska, J.; Ostrowska, A.; Hura, K.; Dziurka, M.; Pawłowska, B.; Hura, T. Physiological traits determining high adaptation potential of sweet briar (Rosa rubiginosa L.) at early stage of growth to dry lands. Sci. Rep. 2019, 9, 19390. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A.; Gadzinowska, J.; Grzesiak, M.T.; Dziurka, K.; Dubas, E. Rieske iron-sulfur protein of cytochrome-b6f is involved in plant recovery after drought stress. Environ. Exp. Bot. 2018, 156, 228–239. [Google Scholar] [CrossRef]
- Dziurka, M.; Maksymowicz, A.; Ostrowska, A.; Biesaga-Kościelniak, J. The Interaction effect of drought and exogenous application of zearalenone on the physiological, biochemical parameters and yield of legumes. J. Plant Growth Regul. 2021, 40, 1824–1835. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovic, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Lück, H. Methoden der Enzymatischen Analyse; Von Bergmeyer, H.U., Ed.; Chemie: Weinheim, Germany, 1962. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1984; pp. 21–126. [Google Scholar]
- Wendel, A. Glutathione peroxidase. In Enzymatic Basis of Detoxication; Jakoby, W., Ed.; Academic Press: New York, NY, USA, 1980; Volume 1, pp. 333–353. [Google Scholar]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Bektaşoğlu, B.; Apak, R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids–La(III) complexes. Anal. Chim. Acta 2007, 588, 88–95. [Google Scholar] [CrossRef]
- Dziurka, M.; Kubica, P.; Kwiecień, I.; Biesaga-Kościelniak, J.; Ekiert, H.; Abdelmohsen, S.A.M.; Al-Harbi, F.F.; El-Ansary, D.O.; Elansary, H.O.; Szopa, A. In vitro cultures of some medicinal plant species (Cistus × incanus, Verbena officinalis, Scutellaria lateriflora, and Scutellaria baicalensis) as a rich potential source of antioxidants—Evaluation by CUPRAC and QUENCHER-CUPRAC assays. Plants 2021, 10, 454. [Google Scholar] [CrossRef]
- Cekiç, S.D.; Başkan, K.S.; Tütem, E.; Apak, R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Tufan, A.N.; Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Direct measurement of total antioxidant capacity of cereals: QUENCHER-CUPRAC method. Talanta 2013, 108, 136–142. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takeda, T.; Shigeoka, S.; Hirayama, O.; Mitsunaga, T. Hydrogen peroxide generation in organelles of Euglena gracilis. Phytochemistry 1993, 33, 1297–1299. [Google Scholar] [CrossRef]
- Morelló, J.R.; Romero, M.P.; Ramo, T.; Motilva, M.J. Evaluation of L-phenylalanine ammonia-lyase activity and phenolic profile in olive drupe (Olea europaea L.) from fruit setting period to harvesting time. Plant Sci. 2005, 168, 65–72. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Peiser, G.; López-Gálvez, G.; Cantwell, M.; Saltveit, M.E. Phenylalanine ammonia-lyase inhibitors do not prevent russet spotting lesion development in lettuce midribs. J. Am. Soc. Hortic. Sci. 1998, 123, 687–691. [Google Scholar] [CrossRef]
- Cline, E.I.; Adesanya, S.A.; Ogundana, S.K.; Roberts, M.F. Induction of PAL activity and dihydrostilbene phytoalexins in Dioscorea alata and their plant growth inhibitory properties. Phytochemistry 1989, 28, 2621–2625. [Google Scholar] [CrossRef]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2016, 2, 16050. [Google Scholar] [CrossRef]
- Rösler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef]
- Watts, K.T.; Mijts, B.N.; Lee, P.C.; Manning, A.J.; Schmidt-Dannert, C. Discovery of a substrate selectivity switch in tyrosine ammonia-lyase, a member of the aromatic amino acid lyase family. Chem. Biol. 2006, 13, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.; Appel, H.; Patel, V.; Stocum, E.; Kavalier, A.; Schultz, J. Carbohydrate translocation determines the phenolic content of Populus foliage: A test of the sink–source model of plant defense. New Phytol. 2004, 164, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon Fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef]
- Ross, J.; Li, Y.; Lim, E.K.; Bowles, D.J. Higher plant glycosyltransferases. Genome Biol. 2001, 2, 1–6. [Google Scholar] [CrossRef]
- Lim, E.K.; Bowles, D.J. A class of plant glycosyltransferases involved in cellular homeostasis. EMBO J. 2004, 23, 2915–2922. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef]
- Sapes, G.; Demaree, P.; Lekberg, Y.; Sala, A. Plant carbohydrate depletion impairs water relations and spreads via ectomycorrhizal networks. New Phytol. 2021, 229, 3172–3183. [Google Scholar] [CrossRef]
- Blumstein, M.; Sala, A.; Weston, D.J.; Holbrook, N.M.; Hopkins, R. Plant carbohydrate storage: Intra-and inter-specific trade-offs reveal a major life history trait. New Phytol. 2022, 235, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.G.; Lim, E.-K.; Li, Y.; Kowalczyk, M.; Sandberg, G.; Hoggett, J.; Ashford, D.A.; Bowles, D.J. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J. Biol. Chem. 2001, 276, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Priest, D.M.; Ambrose, S.J.; Vaistij, F.E.; Elias, L.; Higgins, G.S.; Ross, A.R.S.; Abrams, S.R.; Bowles, D.J. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 2006, 46, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Havlová, M.; Dobrev, P.I.; Motyka, V.; Štorchová, H.; Libus, J.; Dobrá, J.; Malbeck, J.; Gaudinová, A.; Vanková, R. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ. 2008, 31, 341–353. [Google Scholar] [CrossRef]
- Poppenberger, B.; Fujioka, S.; Soeno, K.; George, G.L.; Vaistij, F.E.; Hiranuma, S.; Seto, H.; Takatsuto, S.; Adam, G.; Yoshida, S.; et al. The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proc. Natl. Acad. Sci. USA 2005, 102, 15253–15258. [Google Scholar] [CrossRef]
- Bouvier, J.W.; Emms, D.M.; Kelly, S. Rubisco is evolving for improved catalytic efficiency and CO2 assimilation in plants. Proc. Natl. Acad. Sci. USA 2024, 121, e2321050121. [Google Scholar] [CrossRef]
- Degen, G.E.; Orr, D.J.; Carmo-Silva, E. Heat-induced changes in the abundance of wheat Rubisco activase isoforms. New Phytol. 2021, 229, 1298–1311. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Scales, J.C.; Lee, W.S.; Kanyuka, K.; Carmo-Silva, E. Down-regulation of wheat Rubisco activase isoforms expression by virus-induced gene silencing. Plant Direct 2024, 8, e583. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Buchner, P.; Carmo-Silva, E. The relative abundance of wheat Rubisco activase isoforms is post-transcriptionally regulated. Photosynth. Res. 2021, 148, 47–56. [Google Scholar] [CrossRef]
- Khan, N.; Choi, S.H.; Lee, C.H.; Qu, M.; Jeon, J.S. Photosynthesis: Genetic strategies adopted to gain higher efficiency. Int. J. Mol. Sci. 2024, 25, 8933. [Google Scholar] [CrossRef]
- Jia, H.; Lu, C. Effects of abscisic acid on photoinhibition in maize plants. Plant Sci. 2003, 165, 1403–1410. [Google Scholar] [CrossRef]
- Gu, L. Optimizing the electron transport chain to sustainably improve photosynthesis. Plant Physiol. 2023, 193, 2398–2412. [Google Scholar] [CrossRef]
- Bethmann, S.; Melzer, M.; Schwarz, N.; Jahns, P. The zeaxanthin epoxidase is degraded along with the D1 protein during photoinhibition of photosystem II. Plant Direct 2019, 3, e00185. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C. Phenolics as antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; Blackwell Publishing: Hoboken, NJ, USA, 2005; Volume 141, p. 168. [Google Scholar]
- Emus-Medina, A.; Contreras-Angulo, L.A.; Ambriz-Perez, D.L.; Vazquez-Olivo, G.; Heredia, J.B. UV light stress induces phenolic compounds in plants. In Plant Phenolics in Abiotic Stress Management; Springer Nature: Singapore, 2023; pp. 415–440. [Google Scholar]
- Trchounian, A.; Petrosyan, M.; Sahakyan, N. Plant cell redox homeostasis and reactive oxygen species. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–50. [Google Scholar]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Machado, J.; Vasconcelos, M.W.; Soares, C.; Fidalgo, F.; Heuvelink, E.; Carvalho, S.M.P. Enzymatic and non-enzymatic antioxidant responses of young tomato plants (cv. Micro-tom) to single and combined mild nitrogen and water deficit: Not the sum of the parts. Antioxidants 2023, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Kolahi, M.; Kazemi, E.M.; Yazdi, M.; Goldson-Barnaby, A. Oxidative stress induced by cadmium in lettuce (Lactuca sativa Linn.): Oxidative stress indicators and prediction of their genes. Plant Physiol. Biochem. 2020, 146, 71–89. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Santos, M.C.B.; Lima, L.R.d.S.; Nascimento, F.R.; Nascimento, T.P.D.; Cameron, L.C.; Ferreira, M.S.L. Metabolomic approach for characterization of phenolic compounds in different wheat genotypes during grain development. Food Res. Int. 2019, 124, 118–128. [Google Scholar] [CrossRef]
- Wagay, N.A.; Lone, R.; Rafiq, S.; Bashir, S.U. Phenolics: A game changer in the life cycle of plants. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 241–275. [Google Scholar]
- Slewinski, T.L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Heloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trda, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]

| Measurements | Control | Inhibitor [10−3 M] |
|---|---|---|
| 4-hydroxybenzoic hydrazide (HBH) | 0.00 ± 0.00 | 4.81 ± 0.44 * |
| Soluble phenolics | 15.9 ± 0.81 | 8.7 ± 0.91 * |
| L-phenylalanine ammonia-lyase (PAL) | 2.42 ± 0.21 | 2.32 ± 0.22 |
| L-tyrosine ammonia-lyase (TAL) | 0.235 ± 0.038 | 0.548 ± 0.074 * |
| Measurements | Control | Inhibitor [10−3 M] |
|---|---|---|
| Net photosynthesis rate—PN | 20.84 ± 0.48 | 17.82 ± 0.46 * |
| Transpiration rate—E | 6.60 ± 0.06 | 6.39 ± 0.19 |
| Stomatal conductance—gS | 639.8 ± 31.4 | 496.4 ± 55.2 * |
| Intercellular concentration of CO2—Ci | 329.6 ± 2.4 | 328.3 ± 3.1 |
| Stomatal limitation value—LS | 0.161 ± 0.008 | 0.177 ± 0.012 |
| Apparent carboxylation efficiency—PN/Ci | 0.063 ± 0.002 | 0.054 ± 0.002 * |
| Intrinsic water use efficiency—WUEintr. | 0.033 ± 0.002 | 0.039 ± 0.003 |
| Instantaneous water use efficiency—WUEinst. | 3.16 ± 0.09 | 2.80 ± 0.08 * |
| Measurements | Control | Inhibitor [10−3 M] |
|---|---|---|
| Quantum yield of PSII—Fv/Fm | 0.844 ± 0.006 | 0.859 ± 0.001 * |
| Maximum efficiency of PSII—Fv’/Fm’ | 0.546 ± 0.008 | 0.561 ± 0.008 |
| PSII quantum efficiency—ΦPSII | 0.282 ± 0.012 | 0.328 ± 0.011 * |
| Photochemical quenching coefficient—qP | 0.515 ± 0.017 | 0.584 ± 0.012 * |
| Non-photochemical quenching—qN | 0.806 ± 0.006 | 0.818 ± 0.007 |
| Electron transport rate—ETR | 1.48 ± 0.19 | 1.81 ± 0.25 |
| Chlorophyll level—Chl | 22.06 ± 0.97 | 22.39 ± 1.48 |
| Antioxidant Capacities | Control | Inhibitor [10−3 M] |
|---|---|---|
| Non-enzymatic soluble proteins | 7.79 ± 0.79 | 5.16 ± 0.31 * |
| H2O fraction | 45.0 ± 3.84 | 63.1 ± 1.28 * |
| MeOH fraction | 28.8 ± 1.06 | 30.1 ± 1.13 |
| Insoluble fraction (IF) | 18.8 ± 0.36 | 18.0 ± 0.37 |
| H2O + MeOH + IF | 92. 6 ± 4.42 | 111.3 ± 2.28 * |
| Measurements | Control | Inhibitor [10−3 M] |
|---|---|---|
| Superoxide dismutase—SOD | 1361.5 ± 149.9 | 2843.7 ± 222.8 * |
| Total soluble peroxidase—POX | 1067.3 ± 67.9 | 3315.3 ± 387.3 * |
| Catalase—CAT | 179.5 ± 18.9 | 360.5 ± 33.6 * |
| L-Ascorbate peroxidase—AsPOX | 2286.6 ± 287.5 | 5283.1 ± 706.3 * |
| Glutathione peroxidase—GPx | 14.30 ± 1.60 | 25.75 ± 1.62 * |
| Glutathione reductase—GR | 1230.6 ± 85.5 | 2195.9 ± 101.0 * |
| Hydrogen peroxide—H2O2 | 1.21 ± 0.11 | 0.81 ± 0.13 * |
| Measurements | Control | Inhibitor [10−3 M] |
|---|---|---|
| Main shoot length [cm] | 77.5 ± 1.86 | 77.7 ± 2.40 |
| Number of lateral shoots/per plant | 0.56 ± 0.11 | 0.70 ± 0.15 |
| Straw biomass [g] | 3.96 ± 0.21 | 3.51 ± 0.24 |
| Length of the main shoot ear [cm] | 9.42 ± 0.15 | 8.58 ± 0.19 * |
| Ear weight—main shoot [g] | 1.99 ± 0.09 | 1.83 ± 0.13 |
| Grain number—main shoot ear | 36.50 ± 2.64 | 35.30 ± 2.39 |
| Grain weight from the ear—main shoot [g] | 1.51 ± 0.09 | 1.42 ± 0.09 |
| Weight of the de-grained ear—main shoot [g] | 0.481 ± 0.011 | 0.396 ± 0.016 * |
| Number of grains—lateral shoots | 26.6 ± 6.52 | 27.0 ± 6.53 |
| Grain weight—lateral shoots [g] | 0.79 ± 0.22 | 0.82 ± 0.21 |
| Total number of grains | 81.2 ± 4.01 | 75.1 ± 4.28 |
| Total grain weight [g] | 2.87 ± 0.14 | 2.60 ± 0.20 |
| Thousand-grain weight [g] | 35.7 ± 1.12 | 34.6 ± 1.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hura, K.; Dziurka, M.; Hura, T.; Wójcik-Jagła, M.; Zieliński, A. Physiological and Molecular Responses of the Flag Leaf Under L-Phenylalanine Ammonia-Lyase Inhibition. Cells 2025, 14, 1368. https://doi.org/10.3390/cells14171368
Hura K, Dziurka M, Hura T, Wójcik-Jagła M, Zieliński A. Physiological and Molecular Responses of the Flag Leaf Under L-Phenylalanine Ammonia-Lyase Inhibition. Cells. 2025; 14(17):1368. https://doi.org/10.3390/cells14171368
Chicago/Turabian StyleHura, Katarzyna, Michał Dziurka, Tomasz Hura, Magdalena Wójcik-Jagła, and Andrzej Zieliński. 2025. "Physiological and Molecular Responses of the Flag Leaf Under L-Phenylalanine Ammonia-Lyase Inhibition" Cells 14, no. 17: 1368. https://doi.org/10.3390/cells14171368
APA StyleHura, K., Dziurka, M., Hura, T., Wójcik-Jagła, M., & Zieliński, A. (2025). Physiological and Molecular Responses of the Flag Leaf Under L-Phenylalanine Ammonia-Lyase Inhibition. Cells, 14(17), 1368. https://doi.org/10.3390/cells14171368








